Abstract
Chemotherapy-induced nausea and vomiting (CINV) is one of the most feared side effects experienced by patients with cancer. The precise physiologic mechanisms responsible for acute and delayed CINV continue to be elucidated and have provided an opportunity to develop antiemetic therapies targeting these pathways. The emergence of receptor antagonists targeting serotonin and neurokinin-1 have revolutionized the prevention of CINV, significantly reducing the impact of this side effect and improving patient quality of life. However, several areas of unmet need remain, including adequate prevention of nausea, rather than just vomiting, in patients receiving chemotherapy for cancer. Prevention of delayed CINV and anticipatory CINV, as well as management of breakthrough CINV, also continues to challenge patients and clinicians. Ongoing research continues to address these areas to improve antiemetic therapies and guidelines.
Keywords: Chemotherapy-induced nausea and vomiting, CINV, NK-1 receptor antagonist, 5-HT3 receptor antagonist, Anticipatory CINV, Breakthrough CINV
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a highly unpleasant side effect associated with some treatments of cancer. The remarkable evolution of CINV prophylaxis witnessed over the last few decades can be attributed to advances in our understanding of the pathophysiology of emesis and primarily to the emergence of therapies to directly target the pathways that contribute to CINV. Despite these important advances, CINV prevention remains often suboptimal and presents a significant clinical challenge for healthcare providers and patients undergoing chemotherapy.
Pathophysiology of CINV
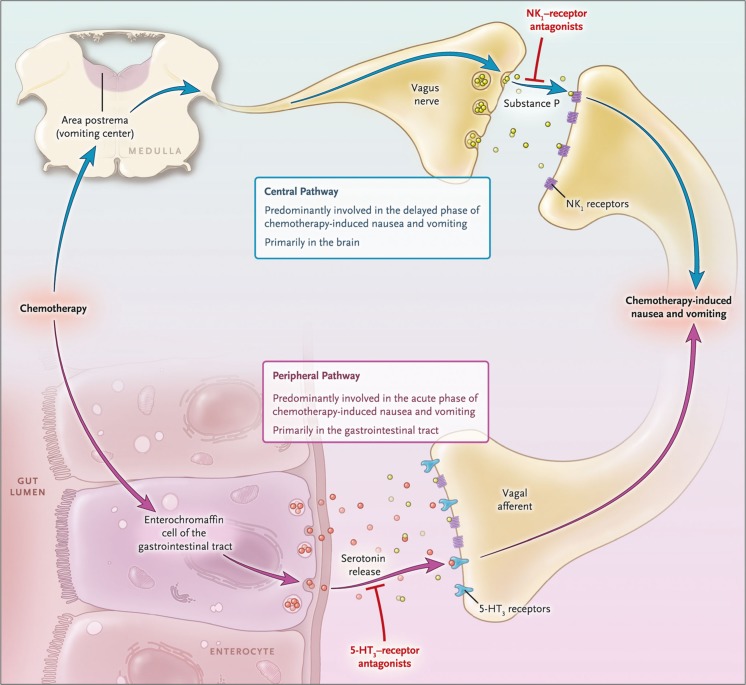
The pathophysiology of CINV is a complex multifactorial process that involves communication between several neurotransmitters and receptors in the central nervous system and gastrointestinal tract (Fig. 1) [1]. The neurotransmitters serotonin (5-hydroxytryptamine or 5-HT3) and its receptor, substance P and the neurokinin-1 (NK-1) receptor, and dopamine and its receptors play an integral role in stimulation of emesis and are the primary targets for most current antiemetic therapies. The emetic response to chemotherapy is thought to occur through two different mechanisms, the peripheral and central pathways. The peripheral 5-HT3-related pathway, which originates in the gastrointestinal tract, is activated in the first 24 h after chemotherapy administration and is primarily associated with acute emesis. The central NK-1 receptor-related pathway occurs primarily in the brain and is thought to be predominantly involved in delayed CINV, although induction of acute emesis can also occur via the central pathway.
Fig. 1.
Pathophysiology of chemotherapy-induced nausea and vomiting1. From N Engl J Med, Navari RM, Aapro M, Antiemetic Prophylaxis for Chemotherapy-Induced Nausea and Vomiting, Volume No. 374, Page No. 1357, Copyright © 2016 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society
Upon exposure to chemotherapeutic agents, damaged enterochromaffin cells in the gastrointestinal tract release serotonin that subsequently binds to 5-HT3 receptors on the nearby vagal afferents in the abdomen.1 Afferent nerve fibers transmit the sensory input from the gastrointestinal tract to the emetic center of the brain. The emetic center consists of a loosely organized network of neurons in the brain stem that receives signals not only from the gastrointestinal tract but also from other structures, such as the chemotherapy trigger zone in the area postrema [2, 3]. These sensory signals are consolidated in the emetic center, leading to generation of efferent signals to the abdominal muscles, stomach, and diaphragm and subsequent emesis. The chemoreceptors of the area postrema are located outside the blood-brain barrier and can be directly activated by chemotherapeutic agents as well, triggering emesis.
The neurotransmitter substance P, which is present in the peripheral and central nervous systems, is also released upon exposure to chemotherapy and binds to NK-1 receptors [4]. While serotonin is the primary mediator of emetic signals from the gastrointestinal tract, substance P appears to most commonly bind to NK-1 receptors within the central nervous system and elicit signals directly to the chemotherapy trigger zone and the emetic center of the brain, leading to delayed emesis. However, substance P also acts in the gastrointestinal tract, potentially playing an auxiliary role in acute CINV. Crosstalk between 5-HT3 and NK-1 receptors has also been hypothesized, and may explain some differences among drugs [5]. Activation of one of these chemoreceptors may sensitize the vagus nerve to stimulation of the other receptor pathway and result in prolonged CINV.
Progress in CINV prophylaxis
Prior to the 1990s, antiemetic therapy consisted primarily of dopamine receptor antagonists such as metoclopramide, prochlorperazine, and haloperidol, and the use of glucocorticoids, such as dexamethasone [1]. Most of the research was aimed at reducing emesis during the acute phase (0 to 24 h after chemotherapy) for newly emerging, highly emetogenic chemotherapeutic agents, including high-dose cisplatin. The 1990s ushered in the development and approval of serotonin receptor antagonists and drastically improved the treatment of acute chemotherapy-induced emesis. 5-HT3 receptor antagonists have a chemical structure similar to serotonin and bind to the same site on the 5-HT3 receptor, blocking its activation. The U.S. Food and Drug Administration (FDA) approved the 5-HT3 receptor antagonist ondansetron in 1991 as prophylaxis for chemotherapy-induced emesis and approved granisetron and dolasetron for the same indication in 1997. That same year, the National Comprehensive Cancer Network (NCCN) first published guidelines for the management of CINV, [6] followed shortly by guidelines from the Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology (MASCC/ESMO) [7] and the American Society of Clinical Oncology (ASCO) [8].
The next landmark advance in CINV management came in 2003 with the approval of the second-generation 5-HT3 receptor antagonist palonosetron and the NK-1 receptor antagonist aprepitant [1]. Compared to the first-generation 5-HT3 receptor antagonists, palonosetron has a higher binding affinity to the 5-HT3 receptor and a half-life of 40 h versus 4 h, 7.3 h, and 9 h for ondansetron, dolasetron, and granisetron, respectively [9]. The chemical structure of palonosetron differs from the first-generation 5-HT3 receptor antagonists and this inhibitor not only blocks serotonin binding in an allosteric fashion but also leads to internalization of the 5-HT3 receptor and prevention of crosstalk with NK-1 receptors [5]. These characteristics may contribute to its increased efficacy against both acute and delayed emesis compared to the first-generation 5-HT3 receptor antagonists.
The first NK-1 receptor antagonist, aprepitant, was also approved in 2003 [1]. While aprepitant had good oral bioavailability, central nervous system (CNS) penetrance, and high affinity for NK-1, it was poorly water soluble and required a special formulation [5]. Changes in the chemical structure resulted in the intravenous pro-drug fosaprepitant, which is water soluble and readily converts to aprepitant in vivo. Newer oral NK-1 receptor antagonists have recently emerged, including rolapitant and netupitant (in combination with palonosetron as the regimen NEPA). Rolapitant has a high CNS penetrance, high affinity and selectivity for NK-1, and a particularly long half-life (180 h compared to 9–13 h for aprepitant) [10–12]. Netupitant also has a high binding affinity for NK-1 and a half-life of 90 h. These agents offer good tolerability and control of CINV. In addition, the antipsychotic agent olanzapine, while not approved by the FDA or European Medicines Agency in the CINV indication, has been incorporated to some extent into ESMO/MASCC guidelines and in current NCCN guidelines and is utilized for control of acute and delayed CINV, as well as breakthrough emesis. Each of these agents is discussed in further detail by Lee Schwartzberg in the third article of this supplement.
Unmet needs in CINV
Despite the substantial progress in CINV prophylaxis, as many as 40% of patients with cancer still experience nausea, vomiting, or both following receipt of chemotherapy [13]. There is a clear need for further improvements in the management of this bothersome side effect. Perhaps the greatest unmet need in CINV is the lack of complete nausea control [4]. Outside of the clinical trial setting, a study of patients with breast cancer receiving anthracycline/cyclophosphamide-based chemotherapy showed that 71% of patients experienced nausea despite being prescribed guideline-based antiemetic therapy and patients consistently ranked nausea over vomiting as the worst side effect associated with chemotherapy [14]. While nausea and vomiting are often considered a unified symptom, the precise physiology and risk factors that contribute to nausea are poorly understood and many of the antiemetic agents currently available do little to relieve chemotherapy-induced nausea [4]. In addition, nausea can only be measured subjectively and may be underreported by patients and underestimated by clinicians [15]. Commonly utilized questionnaires for patient-reported outcomes include the Functional Living Index for Emesis (FLIE) and the MASCC Antiemesis Tool (MAT). These tools need to be used consistently to gain sufficient insight to inform antiemetic treatment decisions and minimize the risk of CINV. In a survey of oncology patients and providers, 37% of oncology patients felt it was important to “be strong by not complaining” and that nausea and vomiting were perceived indications that their therapy was effective, leading to underreporting of CINV [16].
Delayed CINV also continues to be an unmet clinical need. In a prospective, observational study of 298 patients receiving chemotherapy for the first time, delayed nausea and vomiting were observed in 60 and 50%, respectively, of patients receiving highly emetogenic chemotherapy (HEC) and in 52 and 28%, respectively, of patients receiving moderately emetogenic chemotherapy (MEC) [17]. The incidence of acute nausea and vomiting was considerably less (35 and 13%, respectively.) Interestingly, over 75% of physicians and nurses evaluated in this study underestimated the incidence of delayed CINV after both HEC and MEC. A more recent study of 240 patients receiving MEC demonstrated significantly higher incidences of delayed nausea and vomiting compared to acute nausea and vomiting, with twice as many patients requiring rescue antiemetic therapy during the delayed phase [18]. It is important to note in both of these studies that although patients were receiving standard of care antiemetic therapy, at that time this did not include agents such as NK-1 receptor antagonists and olanzapine that have since demonstrated efficacy in preventing delayed CINV.
Breakthrough and refractory CINV and anticipatory CINV present substantial clinical challenges in oncology as, once established, they are often difficult to reverse [4, 19]. In a study of patients receiving HEC and prophylactic fosaprepitant, palonosetron, and dexamethasone, 41% of patients developed breakthrough CINV [20]. Breakthrough CINV despite appropriate antiemetic prophylaxis is difficult to control and can lead to treatment disruption and discontinuation. Breakthrough emesis can also lead to anticipatory CINV, with patients developing strong conditioned responses that have both a physiologic and psychologic component [4]. Anticipatory CINV can greatly interfere with a patient’s quality of life and hamper their willingness to continue treatment. A recent analysis of 991 patients receiving chemotherapy showed an incidence of anticipatory nausea of 8 to 14%, with rates increasing with each subsequent cycle [21]. Complete control of CINV during the first course of chemotherapy is the most effective strategy to prevent the development of anticipatory CINV. Patients who experienced CINV in their first cycle of chemotherapy were 3.7 times more likely to have anticipatory nausea before their second cycle of therapy. Avoidance of strong smells that may trigger symptoms, relaxation techniques, hypnosis, and acupuncture can also be helpful [22].
Perhaps the biggest barrier to effective control of CINV is the poor adherence to existing guidelines. Multiple consensus groups have published guidelines for the prevention and management of CINV, but studies suggest many patients are not receiving guideline-based antiemetic therapy [22–24]. In the Pan European Emesis Registry (PEER) prospective observational study, 55 and 46% of patients received guideline-consistent antiemetic therapy in the acute and delayed period, respectively [25]. Only 29% of patients in this study received guideline-consistent antiemetic therapy for the entire study period. A similar study in the USA showed that 57% of patients received guideline-consistent CINV prophylaxis, suggesting a continuing need to better educate clinicians, nurses, pharmacists, and patients regarding currently available guidelines and options for antiemetic prophylaxis [26].
Acknowledgements
The author would like to thank Tristin Abair, PhD, for her assistance in drafting the manuscript and Trudy Grenon Stoddert, ELS, for her editorial assistance and assistance with preparing the manuscript for submission.
Financial support
This educational activity is supported by grants from Merck and Co, Inc. and TESARO, Inc.
Compliance with ethical standards
Conflict of interest
Dr. Aapro has disclosed that he has received consulting fees or honoraria from Amgen. He has also received payment for lectures and/or support for travel fromAmgen, Helsinn, Hospira, Johnson & Johnson, Novartis, Pierre Fabre Medicament, Roche, Sandoz, TESARO, Teva, and Vifor Pharma. Dr. Aapro has received research funding from Helsinn, Hospira, Novartis, Pierre Fabre Medicament, and Sandoz. He has also disclosed receiving fees for participation in advisoryor review activities from Helsinn Healthcare, Hospira, Merck, Merck KGaA, Pierre Fabre Medicament, Sandoz, TESARO, Teva, and ViforPharma.
References
- 1.Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356–1367. doi: 10.1056/NEJMra1515442. [DOI] [PubMed] [Google Scholar]
- 2.Bayo J, Fonseca PJ, Hernando S, Servitja S, Calvo A, Falagan S, García E, González I, de Miguel MJ, Pérez Q, Milena A, Ruiz A, Barnadas A. Chemotherapy-induced nausea and vomiting: pathophysiology and therapeutic principles. Clin Transl Oncol. 2012;14:413–422. doi: 10.1007/s12094-012-0818-y. [DOI] [PubMed] [Google Scholar]
- 3.Higgins GA, Kilpatrick GH, Bunce KT, et al. 5-HT3 receptor antagonists injected into the area postrema inhibit cisplatin-induced emesis in the ferret. Br J Pharmacol. 1989;97:247–235. doi: 10.1111/j.1476-5381.1989.tb11948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janelsins MC, Tejani M, Kamen C, et al (2013) Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother 14:757–766 [DOI] [PMC free article] [PubMed]
- 5.Rojas C, Raje M, Tsukamoto T, Slusher BS. Molecular mechanisms of 5-HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol. 2014;722:26–37. doi: 10.1016/j.ejphar.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 6.(1997) NCCN antiemesis practice guidelines. Oncology (Williston Park) 11:57–89 [PubMed]
- 7.(1998) Prevention of chemotherapy- and radiotherapy-induced emesis: results of Perugia Consensus Conference. Antiemetic Subcommittee of the Multinational Association Ann Oncol 9:811–819 [PubMed]
- 8.Gralla RJ, Otsuba D, Kris MG, et al (1997) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. J Clin Oncol 17:2971–2994 [DOI] [PubMed]
- 9.Navari RM. Palonosetron for the prevention of chemotherapy-induced nausea and vomiting: approval and efficacy. Cancer Manag Res. 2009;1:167–176. doi: 10.2147/CMAR.S6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chasen MR, Rapoport BL. Rolapitant for the treatment of chemotherapy-induced nausea and vomiting: a review of the clinical evidence. Future Oncol. 2016;12:763–778. doi: 10.2217/fon.16.11. [DOI] [PubMed] [Google Scholar]
- 11.Gan TJ, Gu J, Singla N, Chung F, Pearman MH, Bergese SD, Habib AS, Candiotti KA, Mo Y, Huyck S, Creed MR, Cantillon M, Rolapitant Investigation Group Rolapitant for the prevention of postoperative nausea and vomiting: a prospective, double-blinded placebo-controlled randomized trial. Anesth Analg. 2011;112:804–812. doi: 10.1213/ANE.0b013e31820886c3. [DOI] [PubMed] [Google Scholar]
- 12.Poma A, Christensen J, Davis J, et al (2014) Phase I positron emission tomography (PET) study of the receptor occupancy of rolapitant, a novel NK-1 receptor antagonist. J Clin Oncol 32(suppl): Abstract e20690
- 13.Dranitsaris G, Molassiotis A, Clemons M, et al (2017) The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann Oncol 28:1260–1267 [DOI] [PMC free article] [PubMed]
- 14.Hernandez Torres C, Mazzarello S, Ng T, et al (2015) Defining optimal control of chemotherapy-induced nausea and vomiting-based on patients’ experience. Support Care Cancer 23:3341–3359 [DOI] [PubMed]
- 15.Di Maio M, Gallo C, Leighl NB, et al (2015) Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol 33:910–915 [DOI] [PubMed]
- 16.Salsman JM, Grunberg SM, Beaumont JL, Rogers M, Paul D, Clayman ML, Cella D. Communicating about chemotherapy-induced nausea and vomiting: a comparison of patient and provider perspectives. J Natl Compr Cancer Netw. 2012;10:149–157. doi: 10.6004/jnccn.2012.0018. [DOI] [PubMed] [Google Scholar]
- 17.Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, de Pouvourville G, Rubenstein EB, Daugaard G (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100:2261–2268. 10.1002/cncr.20230 [DOI] [PubMed]
- 18.Escobar Y, Cajaraville G, Virizuela JA, et al (2015) Incidence of chemotherapy-induced nausea and vomiting with moderately emetogenic chemotherapy: ADVICE (Actual Data of Vomiting Incidence by Chemotherapy Evaluation) study. Support Care Cancer 23:2833–2840 [DOI] [PMC free article] [PubMed]
- 19.Einhorn LH, Rapoport B, Navari RM, Herrstedt J, Brames MJ (2017) 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following multiple-day chemotherapy, high-dose chemotherapy, and breakthrough nausea and vomiting. Support Care Cancer 25:303–308 [DOI] [PubMed]
- 20.Navari RM, Nagy CK, Gray SE. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer. 2013;21:1655–1663. doi: 10.1007/s00520-012-1710-6. [DOI] [PubMed] [Google Scholar]
- 21.Molassiotis A, Lee PH, Burke TA, Dicato M, Gascon P, Roila F, Aapro M. Anticipatory nausea, risk factors, and its impact on chemotherapy-induced nausea and vomiting: results from the Pan European Emesis Registry study. J Pain Symptom Manag. 2016;51:987–993. doi: 10.1016/j.jpainsymman.2015.12.317. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network (NCCC) NCCN clinical practice guidelines in oncology. Antiemesis Version 2. 2017. Available at: www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed 1 July 2017
- 23.Roila F, Molassiotis A, Herrstedt J, participants of the MASCC/ESMO Consensus Conference Copenhagen 2015 et al (2016) 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27(suppl 5):v119–v133. 10.1093/annonc/mdw270 [DOI] [PubMed]
- 24.Hesketh PJ, Kris MG, Basch E, et al (2017) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:3240–3261 [DOI] [PubMed]
- 25.Aapro M, Molassiotis A, Dicato M et al (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol 23:1986–1992. 10.1093/annonc/mds021 [DOI] [PubMed]
- 26.Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community practice: INSPIRE Study. J Onc Pract. 2013;10:68–74. doi: 10.1200/JOP.2012.000816. [DOI] [PubMed] [Google Scholar]