Abstract
Acute myocardial infarction (AMI) is often underdiagnosed in women. It is therefore of interest to identify biomarkers that indicate increased risk of AMI and thereby help clinicians to have additional focus on the difficult AMI diagnosis. Type I Collagen, a component of the cardiac extracellular matrix, is cleaved by matrix metalloproteinases (MMPs) generating the neo-epitope C1M. We investigated the association between serum-C1M and AMI and evaluated whether C1M is a prognostic marker for outcome following AMI. This study is based on The Prospective Epidemiological Risk Factor (PERF) Study including postmenopausal women. 316 out of 5,450 women developed AMI within the follow-up period (14 years, median). A multivariate Cox analysis assessed association between serum-C1M and AMI, and re-infaction or death subsequent to AMI. The risk of AMI increased by 18% (p = 0.03) when serum-C1M was doubled and women in the highest quartile had a 33% increased risk compared to those in the low quartiles (p = 0.025). Serum-C1M was, however not related to reinfarction or death subsequent to AMI. In this study C1M was be an independent risk factor for AMI. Measuring MMP degraded type I collagen could be useful for prediction of increased risk of AMI if replicated in other cohorts.
Introduction
Coronary heart disease (CHD) affects mainly men but is also a leading cause of mortality in women1–3. The incidence of AMI rises after menopause and the mean age of women with first-time AMI is higher than for men (72 vs. 65 years)4. Mehta et al. reported in a Statement from American Heart Association that despite improvement in treatment modalities, women are more likely to die from acute myocardial infarction (AMI) than men1. For women AMI seems not to be studied adequately, misdiagnosed, and therefore left untreated. Additionally, evidence for prognostic factors to predict adverse outcome after AMI in women is sparse5. There is a need for novel biomarkers that assess the risk and prognosis of AMI in women to improve survival5.
Atherosclerosis is an inflammatory process characterized by accumulation of plaques and plaque calcification. Nodules erotion and rupture are complications leading to AMI and could be indicated by the epitope C1M. Plaque rupture is the most common cause of AMI by triggering the coagulation cascade whereby the risk of thrombosis is accelerated. A ruptured plaque has a necrotic core with an overlying thin fibrous cap infiltrated by inflammatory cells4,6 and expression of macrophage-released matrix metalloproteinases (MMPs) affects plaque stability7. MMP-induced proteolytic cleavage of collagen may weaken the fibrous cap and increase the risk of plaque rupture, which will trigger the thrombotic cascade leading to AMI4,8–11 and detection of plaque instability is of interest.
Cavusoglu et al.4 report elevated MMP-3 levels in plasma to be associated with five-year-risk of AMI in men. In postmenopausal women, Dragsbaek et al.12 demonstrated the MMP-2, −9, and −13 generated type I collagen degradation fragment (C1M) to be associated with cardiovascular mortality. This study evaluated whether C1M is linked to AMI in postmenopausal women. C1M data presented by Dragsbaek et al.12 were further analysed to evaluate (i) association between serum-C1M and AMI in postmenopausal women and (ii) baseline serum-C1M as a biomarker for short-term prognosis following AMI.
Materials and Methods
Study population
This evaluation is based on the cohort “The Prospective Epidemiological Risk Factor (PERF) Study” which, in a prospective observational design includes Danish postmenopausal women to investigate age-related diseases. A detailed description of the cohort have previously been published13,14. The PERF study was conducted in accordance with Good Clinical Practice and approved by the Danish National Committee on Health Research Ethics (KA99070gm) with informed consent obtained from all subjects. Women who had participated in clinical randomized placebo-controlled studies or had been screened for the studies at the Centre for Clinical and Basic Research in Denmark were invited to participate in PERF. In 1999, 8,875 postmenopausal women were invited to attend a baseline examination. 5,855 women, aged 49–89 years consented to attend baseline examination for the PERF study (between 1999–2001) with no other in- or exclusion criteria. For the present evaluation subjects without a record of AMI at baseline and with a complete dataset were selected for analysis. Thus, the study was conducted without a predefined sample size estimate.
Baseline
Fasting blood samples were collected and serum stored at −80 °C. Participants completed a physical examination by non-blinded staff including height and weight. Information in regard to physical activity, smoking, alcohol consumption, and diabetes was obtained through a structured interview questionnaire. Serum analysis, retrieval of information from The National Danish Patient Registry and The National Danish Causes of Death Registry were blinded.
Outcome Variables
In Denmark, all citizens are provided a unique personal identification number (CPR) that enables matching individuals to the Danish health registries. Data on AMI diagnosis, AMI date, cause and date of death were extracted from The National Danish Patient Registry and The National Danish Causes of Death Registry on 31/12/2014. Data from The National Danish Patient Registry and The National Danish Causes of Death Registry were matched with the PERF cohort. Diagnosis was classified in accordance with the International Classification of Diseases: Diagnosis code I21-I21.09 (AMI), tenth revision (ICD-10) or diagnosis code 410–410.9 (AMI) for the eighth revision (ICD-8). Both AMI as diagnosis and cause of death during the follow-up were included until 31/12/2014. The secondary outcomes included subsequent AMI incidents. A secondary study outcome (class A) was death (regardless of registered cause) or re-infarction within 28 days after an AMI according to the WHO definition15. Any outcome not involving death or re-infarction within 28 days was categorized as a class B outcome. For the purpose of defining classes A and B, the follow-up started on the day of the first AMI and ended at death within 28 days thereafter, re-infarction within 28 days from the AMI, or on 31/12/2014, whichever came first. Additional episodes of re-infarction or death related to a recurrent infarct were not included in the analysis.
Matrix Metalloproteinase Mediated Type I Collagen Degradation
A matrix metalloproteinase (MMP)-linked immunosorbent assay (ELISA), which detects serum fragments of type I collagen generated by MMP-2, −9, and −13 (termed C1M) was developed and validation with assay variability is published16. While other biomarkers of type I collagen fragments exist and used as measure of bone turnover17–19, the MMP generated fragment is destroyed by cathepsin-K indicating that this collagen fragment is not derived from bone turnover. The C1M fragment was analysed prior to previous studies and found to be stable after storage of serum at −80 °C for 12 years. In short, C1M stability was at the same time confirmed by seven consecutive freeze-thaw cycles with no significant variation in detected levels. Three-year stability studies were performed at the same time to validate C1M detection by measuring the same sample with a one-year interval12. The association between C1M levels and age at baseline was tested with a Pearson-correlation and found not to be significant (r = 0.01, p = 0.45). Finally, the C1M levels are comparable to those found in other cohorts. No blood samples and thus no measurements of C1M from the time of infarction were available.
Statistical Analysis
Data are presented as median with interquartile range (IQR) if not otherwise specified. Analyses were performed in MedCalc® (version 14.8.1) and RStudio® (version 3.3.1) and a p-value <0.05 was considered significant. The study population was stratified in two groups: A group included participants who were found to develop AMI and a group who did not develop AMI during the follow-up. Baseline characteristics were compared using a Mann-Whitney test for numerical variables. Categorical variables were compared using a Chi-square test. The cohort was divided into quartiles based on serum-C1M levels: Q1: 21.2–31.1 ng/mL (n = 1,406), Q2: 31.4–39.5 ng/mL (n = 1,392), Q3: 39.6–56.0 ng/mL (n = 1,384), and Q4: 56.1–2240 ng/mL (n = 1,389). A Kaplan-Meier Survival Curve was applied to demonstrate incident events of AMI over time in relation to the four quartiles of C1M-levels. Multivariate Cox proportional hazard analysis assessed association between C1M levels and incident AMI. Event-free mortality was included as a competing risk by censoring. The follow-up time since baseline was the time scale. All further analyses were conducted with C1M-quartiles, Q1–3 vs. Q4, and log2-transformed serum-C1M levels. The Q1–3 vs. Q4 cut off was chosen based on results from the Kaplan-Meier Survival Curve. The analysis was adjusted for potential confounders including “Major Risk Factors and Coronary Heart Disease” (age, BMI, diabetes mellitus, hypertension, serum-cholesterol, tobacco smoking and physical inactivity) according to American Heart Association20 and alcohol consumption ≥7 units/week cf. recommendation by the Danish Health Authority in regard to elderly21. Age, BMI, serum-cholesterol and -C1M were included in the analysis as numerical variables. The categorical variables were (i) diabetes divided into no, insulin-dependent or noninsulin-dependent, (ii) hypertension categorized as presence of hypertension or no hypertension, blood pressure systolic >139 mmHg or diastolic >89 mmHg22, (iii) smoking classified as never, previous or current, (iiii) level of physical activity grouped in relation to engagement in activities other than daily living, and (iiiii) use of alcohol defined as consumption of more or less than 7 units per week (15 mL or 12 g of pure alcohol). The multivariate Cox analysis was likewise used to evaluate serum-C1M as a prognostic marker for AMI outcome within 28 days after the event. The proportional-hazard assumption was checked by interacting covariates over time within the model adhering to STROBE guidelines.
Results
Study Population
5,855 participants were screened, but 73 subjects had a record of AMI before baseline and serum-C1M was missing for 211 women. Thus, the study population included 5,571 (Fig. 1) of whom 316 developed AMI during the follow-up. In 121 participants there were missing data (such as blood pressure, BMI, serum-cholesterol, diabetes) and the statistical analyses on a full data set involved 5,450 women with AMI occurring in 309 (5.7%) participants. In comparison, 18 (5.4%) events of AMI occurred in women who were excluded (n = 332) with no statistical difference between the two groups (p = 0.85). The median (IQR) follow-up time was 14.0 years (13.7–14.4) and time to AMI 6.5 years (2.9–10.5); for subjects in Q1 6.4 (3.2–10.9), Q2 7.3 (2.7–11.4), Q3 8.8 (3.6–10.8), and Q4 5.1 years (2.5–9.8).
Figure 1.
Flow-chart, study population.
Baseline Characteristics
Table 1 summarizes baseline characteristics for the included participants. Their age ranged from 51 to 86 years (70.9 (65.5–75.5), median with IQR) and women with AMI were older. The population had a BMI of 25.7 (23.2–28.7) kg/m2 of which the AMI group had the highest value. Additionally, serum-cholesterol was 6.3 (5.6–7.0) mmol/L with higher levels in the AMI group. Likewise, in women with AMI, 7.1% presented with diabetes and 78.3% with hypertension compared to only 2.6% and 67%, respectively, in women without AMI. In addition, the prevalence of current smokers was higher in the AMI group whereas previous smokers dominated the non-AMI group. The proportion of participants with significant alcohol consumption was similar in the two groups, but the AMI group was less physically active.
Table 1.
PERF cohort baseline characteristics: Selected variables cf. American Heart Associations “Major Risk Factor and CHD”. The variables are given as percentage or median (IQR).
| Variable | Total (n = 5,450) | AMI (n = 309) | No-AMI (n = 5,141) | P-value AMI vs no-AMI |
|---|---|---|---|---|
| Age (years) | 70.9 (65.5–75.5) | 74 (69.1–78.2) | 70.7 (65.3–75.3) | <0.001 |
| BMI (kg/m2) | 25.7 (23.2–28.7) | 26.7 (23.9–29.3) | 25.7 (23.2–28.6) | 0.005 |
| Diabetes | ||||
| Insulin-dependent | 121 (2.2%) | 16 (5.2%) | 105 (2.0%) | <0.001 |
| Noninsulin-dependent | 34 (0.6%) | 6 (2.0%) | 28 (0.5%) | <0.001 |
| Diastolic blood pressure (mmHg) | 81 (74–90) | 82 (75–91) | 81 (74–90) | 0.2823 |
| Systolic blood pressure (mmHg) | 148 (133–166) | 155 (140–174) | 148 (132–165) | <0.0001 |
| Hypertension | 3686 (67.6%) | 242 (78.3%) | 3444 (67%) | <0.001 |
| Serum-cholesterol (mmol/L) | 6.3 (5.6–7.0) | 6.4 (5.8–7.1) | 6.3 (5.6–7.0) | 0.04 |
| Smoking | ||||
| Previously | 1660 (30.5%) | 84 (27.2%) | 1576 (30.7%) | 0.009 |
| Current | 1204 (22.1%) | 91 (29.4%) | 1113 (21.6%) | 0.0016 |
| Alcohol (≥7 units/week) | 1789 (32.8%) | 86 (27.8%) | 1703 (33.1%) | 0.063 |
| Physical inactivity | 1673 (30.7%) | 118 (38.2%) | 1555 (30.2%) | 0.004 |
| Serum-C1M (ng/mL) | 39.5 (31.3–56.0) | 43.7 (32.3–66.0) | 39.4 (31.2–50.7) | 0.005 |
| Serum-C1M | ||||
| Q1 | 1374 (25.2%) | 66 (21.4%) | 1308 (25.4%) | 0.0086 |
| Q2 | 1360 (25.0%) | 71 (23.0%) | 1289 (25.1%) | |
| Q3 | 1357 (24.9%) | 70 (22.7%) | 1287 (25.0%) | |
| Q4 | 1359 (24.9%) | 102 (33.0%) | 1257 (24.5%) | |
C1M and AMI
Serum-C1M was 39.5 (31.3–56.0) ng/mL and highest in subjects with incident AMI during the study (43.7 (32.3–66.0) vs. 39.4 (31.2–50.7); p = 0.005). Hence, participants with AMI demonstrated an 11.5% higher serum-C1M when data were evaluated unfiltered by a potential influence of risk factors.
Figure 2 is the Kaplan-Meier Survival Curve, stratified in quartiles of C1M to illustrate the association between C1M and AMI. The plot shows a decrease in AMI-free survival rate for women with baseline serum-C1M levels in the upper quartile (Q4) compared to women with C1M levels in the lower quartiles (Q1–Q3) (p = 0.0055).
Figure 2.
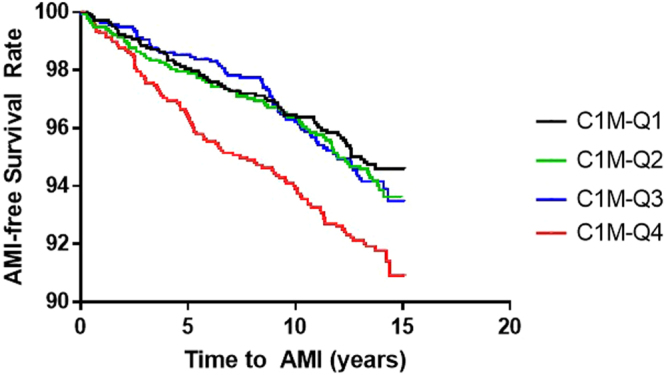
Kaplan-Meier Survival Curve of C1M quartiles. The lowest quartile, C1M-Q1, is illustrated in black, C1M-Q2 is illustrated in green, C1M-Q3 is illustrated in blue and the highest quartile of C1M-Q4 is illustrated in red. Cutoff C1M >56 ng/mL.
Based on the results from Fig. 2 and to adjust for possible confounders, a multivariate Cox proportional-hazard analysis was performed. C1M was analysed as a continuous variable (log2 transformed), divided into quartiles and using a binary outcome (Q4 vs. Q1–3) (Table 2). The independent contribution of high C1M levels to develop AMI with analysis adjusted for age, BMI, diabetes mellitus, hypertension, serum-cholesterol, tobacco smoking, physical inactivity, and alcohol consumption is evaluated. The log2-tranformed C1M analysis found that with a doubled given C1M-level, the hazard ratio for AMI increases by 18% (p = 0.03). For women with C1M-levels in the highest quartile the risk for AMI increased by 33% compared to C1M-levels in the quartile 1–3 (p = 0.025).
Table 2.
Multivariate Cox proportional-hazard analysis for C1M-levels divided into quartiles associated with AMI. Corrected for age, body mass index, diabetes mellitus, hypertension, serum-cholesterol, tobacco smoking, physical inactivity, and alcohol consumption. *p < 0.05.
| Variable | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|
| C1M-Q | |||
| Q1 Q2 Q3 Q4 C1M-cutoff |
Ref. 1.06 1.0 1.35 1.33 |
[0.76; 1.48] [0.71; 1.40] [0.98; 1.87] [1.04; 1.70] |
0.75 0.98 0.068 0.025* |
| Log2-C1M | 1.18 | [1.02; 1.38] | 0.03* |
In Table 3, evaluation on class A vs. B outcomes in relation to C1M levels are presented with 117 (A) and 199 (B) women classified. The class A outcomes included reinfarction n = 54, recurrent AMI as cause of death n = 55, and death within 28 days after recurrent AMI (registered cause other than AMI) n = 8. With analysis adjusted for age, data failed to reach statistical significance illustrating that C1M was not able to predict prognosis within 28 days following an AMI.
Table 3.
Multivariate Cox proportional-hazard analysis for baseline C1M as a prognostic marker for reinfarct or death within 28 days as a measure of short-term AMI outcome following the first AMI, regardless of when the first AMI occurred.
| Variable | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|
| C1M-Q | |||
| Q2 Q3 Q4 C1M-cutoff |
1.33 0.78 1.33 1.29 |
[0.77; 2.30] [0.43; 1.43] [0.79; 2.24] [0.88; 1.89] |
0.31 0.42 0.28 0.19 |
| Log2-C1M | 1.18 | [0.79; 1.26] | 0.97 |
Discussion
This study is based on data from the PERF study including Danish postmenopausal women and assessed whether serum-C1M is associated to risk of AMI. C1M was evaluated for its potential to reflect an unstable atherosclerotic plaque and consequently whether elevated C1M levels is associated with risk of plaque rupture and thrombosis. A second aim was to evaluate whether C1M correlates to outcome within a short period subsequent to AMI.
The study demonstrates that a high C1M (>56 ng/mL), as a surrogate for MMP mediated type I collagen tissue remodelling16, is associated with risk of AMI in postmenopausal women, independent of overweight, diabetes, hypertension, hyperlipidaemia, smoking, alcohol consumption, and inactivity. Within a 14-year follow-up period, postmenopausal women with high C1M levels (>56 ng/mL) demonstrated a 33% higher risk for AMI compared to women with C1M levels in the lower quartiles. Thus, the data suggest C1M as a biomarker for increased risk of AMI in postmenopausal women. This study did, however not demonstrate C1M as a prognostic marker for outcome following the first AMI, when C1M is measured prior to first AMI.
Due to an ageing population and improved therapeutic options for cardiac diseases the prevalence of cardiovascular diseases is increasing and triggers morbidity, mortality, and increased health care costs. According to the WHO 2014 Global Status Report on non-communicable diseases, CVD is the leading cause of death. In 2012, approximately 17.5 million people died from CVD and of these 7.4 million (42.3%) died because of AMI. Thus, improved methods to assess the risk of AMI are warranted.
We measured MMP-degraded type I collagen because increased levels of C1M could reflect breakdown and destabilisation of the fibrous cap of an atherosclerotic plaque. Plaque instability and subsequent rupture leading to trombosis is thought to occur due to destruction of the fibrous cap, rich in type I collagen, mediated by macrophage-derived MMPs8,23–25. This study showed that C1M, reflecting MMP-mediated breakdown of collagen type I in the fibrous cap, is associated with AMI. This hypothesis is supported by Cavusoglu et al.4 in a study of 355 male veterans referred to diagnostic coronary angiography. They found plasma MMP-3 (at a pre-specified cut-off of 20.56 ng/mL) associated to 5-year risk of AMI. The risk of plaque rupture is difficult to detect6 with plaque stability investigated by Near-Infrared Spectroscopy, laser based imaging, and Polarization-Sensitive Tomography, but success has been limited6,9,10,25.
High levels of C1M were associated with increased risk of AMI in postmenopausal women. To our knowledge we are the first to investigate MMP-degraded collagen in relation to risk of AMI. However, other extracellular matrix proteins have been investigated in relation to AMI and mortality. The endothelium is topped with glycocalyx and syndecan-1 is a biomarker of glycocalyx degradation. Ostrowski et al.26 examined plasma syndecan-1 in 571 patients with ST-myocardial infarction treated by percutaneous coronary intervention and a high syndecan-1 level was independently correlated to 30-day cardiovascular- and all-cause mortality in two univariate analyses (p = 0.012 and p = 0.002). However, when adjusting for cardiovascular risk factors, the predictive value for mortality within 30 days became insignificant and syndecan-1 did not associate to reinfarction within that time frame. The multivariate Cox analysis on the prognostic value of C1M for re-infarction or death after AMI was also not significant may be because the blood samples were collected up to 15 years prior to the AMI.
Plasma-MMP-3, syndecan-1, and here serum-C1M demonstrate potential new biomarkers for identification of subjects with elevated risk of AMI, but C1M was not a prognostic marker for risk of death or re-infarction subsequent to AMI. Yet, Dragsbaek et al.12 found C1M associated to mortality and the level of C1M at the time of AMI may be related to outcome, e.g. mortality and could be determined immediately after AMI.
The strength of this paper includes a large and homogenous population without inclusion- or exclusion criteria and the participants are considered to be representative for Danish postmenopausal women. Additionally, the access to Danish patient registries is valuable; registry-linkage collected outcome information implies limited loss of data. Limitations are also identified. Autopsies are seldom performed when elderly Danes die even unexpectedly and some cases of AMI may not have been identified rendering a potential bias in the age composition of the population. Additionally, information about medication was not available and blood samples were collected up to 14.4 years prior to AMI. Thus, analysis of C1M association with short-term outcome following AMI would likely improve had C1M been obtained at that time. Thus, in this study C1M is a novel marker for long-term risk assessment. CRP measurements were unavailable for this cohort. Moreover, the potential link between cardiovascular- and connective tissue disease was not addressed and diagnostic criteria for AMI have changed during the follow-up27. Finally, Danish geography and organization of health care may not necessarily apply to other countries and as the data are restricted to postmenopausal Danish Caucasian women, the conclusions may not be generalized to the overall population.
In conclusion, in this study including postmenopausal women serum-C1M was associated to development of AMI and high levels seems to be an independent risk factor prior to AMI. External validation is warranted to confirm the results of this paper.
Acknowledgements
We acknowledge the Danish Research Foundation (“Den Danske Forskningfond”) for providing the funding to this research.
Author Contributions
D.B. and J.N. analysed data. D.B., J.N., C.B., S.N., N.S., J.S., and H.N. wrote the manuscript. A.B., J.A., M.K. and C.C. developed the biomarker and conducted the PERF study. All authors reviewed the manuscript.
Competing Interests
Signe Holm Nielsen, Morten Karsdal, Asger R. Bihlet, Jeppe R. Andersen are full time employees. Claus Christiansen is a full time employee and holds stock at Nordic Bioscience. Cecilie Bager and Henning Bay Nielsen are full time employees at ProScion. All other authors have no conflicts of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ditte Marie Bertelsen, Email: jlp988@alumni.ku.dk.
Henning Bay Nielsen, Email: hbn@nordicbio.com.
References
- 1.Gholizadeh L, Davidson P. More similarities than differences: an international comparison of CVD mortality and risk factors in women. Health Care Women Int. 2008;29:3–22. doi: 10.1080/07399330701723756. [DOI] [PubMed] [Google Scholar]
- 2.Roeters Van Lennep JE, Westerveld HT, Erkelens DW, Van Der Wall EE. Risk factors for coronary heart disease: Implications of gender. Cardiovasc. Res. 2002;53:538–549. doi: 10.1016/S0008-6363(01)00388-1. [DOI] [PubMed] [Google Scholar]
- 3.Mosca L, et al. Cardiovascular Disease in Women. A Statement for Healthcare Professionals From the American Heart Association. Circulation. 1997;96:2468–2482. doi: 10.1161/01.CIR.96.7.2468. [DOI] [PubMed] [Google Scholar]
- 4.Cavusoglu E, et al. Usefulness of Plasma Matrix Metalloproteinase-3 Levels to Predict Myocardial Infarction in Men With and Without Acute Coronary Syndrome. Am. J. Cardiol. 2016;117:881–886. doi: 10.1016/j.amjcard.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Mehta L, et al. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, et al. Near-infrared spectroscopic characterization of human advanced atherosclerotic plaques. J. Am. Coll. Cardiol. 2002;39:1305–1313. doi: 10.1016/S0735-1097(02)01767-9. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JL, et al. Effect of broad-spectrum matrix metalloproteinase inhibition on atherosclerotic plaque stability. Cardiovasc. Res. 2006;71:586–595. doi: 10.1016/j.cardiores.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Newby AC, Zaltsman A. Fibrous cap formation or destruction–the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc. Res. 1999;41:345–360. doi: 10.1016/S0008-6363(98)00286-7. [DOI] [PubMed] [Google Scholar]
- 9.Nadkarni S, et al. Measurement of collagen and smooth muscle cell content in atherosclerotic plaques using polarization-sensitive optical coherence tomography. J. Am. Coll. Cariol. 2007;49:1474–1481. doi: 10.1016/j.jacc.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumeister V, Scheibe M, Lattke P, Jaross W. Determination of the cholesterol-collagen ratio of arterial atherosclerotic plaques using near infrared spectroscopy as a possible measure of plaque stability. Atherosclerosis. 2002;165:251–257. doi: 10.1016/S0021-9150(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 11.Schafers M, Schober O, Hermann S. Matrix-metalloproteinases as imaging targets for inflammatory activity in atherosclerotic plaques. J. Nucl. Med. 2010;51:663–666. doi: 10.2967/jnumed.109.065698. [DOI] [PubMed] [Google Scholar]
- 12.Dragsbæk K, et al. Matrix Metalloproteinase Mediated Type I Collagen Degradation — An Independent Risk Factor for Mortality in Women. EBioMedicine. 2015;2:723–729. doi: 10.1016/j.ebiom.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neergaard JS, et al. Cohort Profile: The Prospective Epidemiological Risk Factor (PERF) study. Int. J. Epidemiol. 2017;46:1104–1104i. doi: 10.1093/ije/dyw251. [DOI] [PubMed] [Google Scholar]
- 14.Neergaard J, et al. Late-Life Risk Factors for All-Cause Dementia and Differential Dementia Diagnoses in Women: A Prospective Cohort Study. Med. 2016;95:e3112. doi: 10.1097/MD.0000000000003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendis S, et al. World Health Organization definition of myocardial infarction: 2008-09 revision. Int. J. Epidemiol. 2011;40:139–146. doi: 10.1093/ije/dyq165. [DOI] [PubMed] [Google Scholar]
- 16.Leeming D, et al. A novel marker for assessment of liver matrix remodeling: an enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M) Biomarkers. 2011;16:616–628. doi: 10.3109/1354750X.2011.620628. [DOI] [PubMed] [Google Scholar]
- 17.Garnero P, et al. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J. Bone Miner. Res. 2003;18:859–867. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 18.Leeming DJ, et al. Enzyme-linked immunosorbent serum assays (ELISAs) for rat and human N-terminal pro-peptide of collagen type I (PINP) - Assessment of corresponding epitopes. Clin. Biochem. 2010;43:1249–1256. doi: 10.1016/j.clinbiochem.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Rosenquist C, Qvist P, Bjarnason N, Christiansen C. Measurement of a more stable region of osteocalcin in serum by ELISA with two monoclonal antibodies. Clin. Chem. 1995;14:1439–1445. [PubMed] [Google Scholar]
- 20.Coronary Artery Disease - Coronary Heart Disease. American Heart Association. Available at: http://www.heart.org/HEARTORG/Conditions/More/MyHeartandStrokeNews/Coronary-Artery-Disease–Coronary-Heart-Disease_UCM_436416_Article.jsp#.WmHE9KjiZPY (2016).
- 21.Alcohol. Danish Health Authority Available at: https://www.sst.dk/en/health-and-lifestyle/alcohol (2014).
- 22.The Facts about blood pressure 2016. American Heart Association Available at: https://www.heart.org/HEARTORG/Conditions/HighBloodPressure/AboutHighBloodPressure/The-Facts-About-High-Blood-Pressure_UCM_002050_Article.jsp (2016).
- 23.Davies M. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation. 1996;94:2013–2020. doi: 10.1161/01.CIR.94.8.2013. [DOI] [PubMed] [Google Scholar]
- 24.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 25.Nadkarni S, Bouma B, de Boer J, Tearney G. Evaluation of collagen in atherosclerotic plaques: the use of two coherent laser-based imaging methods. Lasers Med. Sci. 2009;24:439–445. doi: 10.1007/s10103-007-0535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrowski S, Pedersen S, Jensen J, Mogelvang R, Johansson P. Acute myocardial infarction is associated with endothelial glycocalyx and cell damage and a parallel increase in circulating catecholamines. Crit. care. 2013;14:R32. doi: 10.1186/cc12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thygesen K, et al. Third Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]


