Abstract
Purpose
The role of uveitis, an uncommon ocular disease, is often neglected in research and treatment of autoimmune conditions. The study described the spectrum of uveitis at a referral center in North Italy, and compared that to a previously published series of patients.
Methods
We reviewed all patients with uveitis diagnosed from 2013 to 2015 at the Immunology Eye Unit, Arcispedale S. M. Nuova-IRCCS, Reggio Emilia, Italy. We examined patient characteristics, disease spectrum, and etiologies.
Results
In total, 990 cases of uveitis were identified, who were mostly female (59%) with a median age at presentation of 44 years (interquartile range = 29–57). Anterior uveitis was most frequent (53.5%), followed by panuveitis (22.8%), posterior (16.2%), and intermediate uveitis (5.5%). Anterior herpetic uveitis (15.6%), Fuchs uveitis (9.7%), and HLA-B27 positive anterior uveitis (7.7%) were the most common specific diagnoses. Compared with the previous series, we observed an increased incidence of uveitis, and a different pattern of diagnoses. Rates of herpetic, HLA-B27 positive uveitis, and presumed ocular tuberculosis were higher, but Fuchs uveitis was less frequent.
Conclusions
The pattern of uveitis appears to be changing, very likely due to population-level increases in infectious diseases, to the availability of new diagnostic tests and to the interdisciplinary approach used in patient diagnosis.
Keywords: Italy, Epidemiology, Infection, Interdisciplinary approach, Systemic disease, Uveitis
Introduction
Uveitis represents a heterogeneous group of intraocular diseases, encompassing a range of inflammatory eye conditions affecting not only the uvea, but also the retina, the optic nerve, and the vitreous. Although a rare disease, its prevalence is rising [1–3]. Uveitis can vary substantially in terms of its clinical course, management, and prognosis. While certain types of uveitis require no treatment, have a self-limited course, and a favorable prognosis, other forms carry a considerable risk of vision loss. Uveitis can be limited to the eye or associated to systemic diseases. Conditions can be idiopathic, autoimmune, or caused by many different infectious agents. Since uveitis cases are often not clinically recognized, the diagnostic delay may increase the risk of irreversible sight-threatening complications. Also, as genetic, ethnic, geographic, socioeconomic, nutritional, and environmental factors influence the pathogenesis of uveitis, it is important to assess the patterns of the condition in different geographic regions over time [3, 4]. Drawing on a computerized register of all cases of uveitis at a tertiary-level referral center in Northern Italy, we analyzed the spectrum of uveitis patients from November 2013 to October 2015 and compared it to our previous published series [5].
Materials and methods
In November 2013, a database was developed to systematically collect data on uveitis patients referred to our Immunology Eye Unit in Reggio Emilia, Italy. We here review the database entries of patients with uveitis referred to our unit over 24 months. Human Immunodeficiency Virus (HIV) positive and masquerade uveitis patients were excluded. All patients gave written informed consent for analysis of their data, and the study was approved by the Arcispedale S. Maria Nuova-IRCCS Institutional Review Board.
Demographic data, medical history, ocular symptoms and signs, special investigations and therapy of patients were analyzed. At presentation, all patients underwent the following: best-corrected Snellen visual acuity, applanation tonometry, slit-lamp evaluation of the anterior segment, and fundus examination. When appropriate, fluorescein angiography, indocyanine green angiography, optical coherence tomography, ocular ultrasonography, and visual field testing were performed. Furthermore, when required, patients underwent complete diagnostic work-up, including laboratory tests and medical evaluation. The selection of diagnostic work-up was based on the positive predictive value of the laboratory tests. For example, as HLA-B27-positive anterior uveitis does not have a granulomatous component, in patients who are HLA-B27 positive and have anterior granulomatous uveitis, two distinct pathologies were considered (for example, herpetic uveitis with concurrent HLA-B27 positivity).
The anatomical classification and final diagnosis were based on the criteria of the International Uveitis Study Group (IUSG) and the Standardization of Uveitis Nomenclature (SUN) working group [6, 7]. Furthermore, granulomatous and non-granulomatous uveitis were categorized from a clinical (and not histological) point of view, a useful distinction in clinical practice, as some uveitis conditions are always non-granulomatous, such as HLA-B27 or Behçet-related uveitis. The clinical aspects of non-granulomatous uveitis are the presence of fine endothelial keratic precipitates (dots), in the absence of iris nodules and/or choroidal granulomas, and granulomatous if larger (“mutton fat”) keratic precipitates, Koeppe and/or Busacca nodules and/or choroidal granulomas [8]. Patients were referred to relevant specialists if systemic disease was suspected. Criteria for specific diagnosis included a defined etiology, typical clinical appearance, and history or classification based on pathological or diagnostic laboratory parameters.
Here, we briefly illustrate our approach for making specific diagnoses and, when necessary, interdisciplinary work-up.
HLA-B27-positive anterior uveitis
Patients with acute anterior uveitis, always clinically non-granulomatous, and typed for the presence of the HLA-B27, regardless of the presence of systemic disease.
Anterior herpetic uveitis
Intraocular inflammation that is usually acute, unilateral, and granulomatous with posterior synechiae and sectoral iris atrophy [5]. In selected cases, where clinical presentation is atypical and/or recurrent despite antiviral therapy, we performed both the serological profile of the suspected microorganism, Herpes Virus-1 (HSV-1), Herpes Virus-2 (HSV-2), Varicella Zoster Virus (VZV), and Real-Time Polymerase Chain Reaction (RT-PCR) on the aqueous tap [9, 10]. In case of clinical suspicion, but negative result of RT-PCR, antibody index coefficient (AIC) (Improved Goldmann–Witmer index) assessment is performed [9, 11, 12].
Anterior cytomegalovirus (CMV) uveitis
In the case of granulomatous anterior uveitis, unilateral, recurrent episodic uveitis, without sectorial iris atrophy, with raised intraocular pressure, open angle, immunocompetent patients, after the assessment of seropositivity for CMV infection, we confirm the diagnosis with an aqueous tap for RT-PCR analysis, and Antibody Index testing in case of negative results of RT-PCR [11–13].
Fuchs uveitis
It is mostly unilateral granulomatous uveitis involving the anterior segment and the vitreous body. The main clinical signs are characteristic sparsely distributed stellate granulomatous keratic precipitates, iris stroma atrophic changes, the absence of synechiae, anterior vitritis, and the absence of cystoid macular oedema [5].
Toxoplasmosis “typical retinochoroiditis”
The diagnosis is based on a compatible fundus lesion and positive serology for toxoplasma antibodies. A solitary inflammatory focus near an old pigmented scar is typical [5]. If the diagnosis is uncertain in patients seropositive for toxoplasmosis, an aqueous sample is used to confirm the diagnosis with RT-PCR [7, 12].
Acute retinal necrosis (ARN)
The diagnosis was based on the criteria of American Uveitis Society [8].
Sarcoidosis
The presumed diagnosis is made on consistent clinical findings with two of the following three diagnostic criteria: elevated angiotensin converting enzyme or lysozyme, cutaneous anergy, and/or with positive chest tomography [14]. We prefer to obtain diagnostic confirmation directly by a positive mediastinoscopic lymphonodular biopsy rather than by interlocutory tests (such as bronchoalveolar lavage) or trans-bronchiolar biopsy (sometimes difficult to obtain).
Pars planitis
It commonly indicates a subset of intermediate uveitis where there is snowbank or snowball formation occurring in the absence of an associated infection or systemic disease (that is, “idiopathic”) [7].
Behçet’s disease (BD)
We used the International Study Group (ISG) criteria for BD [15], which includes the presence of recurrent oral ulcers plus two of the following: (a) recurrent genital ulcers, (b) eye lesions, (c) skin lesions, and (d) positive pathergy test.
Presumed tuberculous (TB) uveitis
We define any kind of uveitis compatible with a tuberculous etiology with Quantiferon Gold TB positivity [16] or Mantoux tuberculin skin test (2U) >15 mm diameter of induration at 48–72 h, with or without abnormalities on chest X-ray, exclusion of other possible causes of uveitis, and the response to anti-tuberculosis treatment [5].
Primary Inflammatory Choriocapillaropathies (PICCP)
The common denominator in PICCP is choriocapillaris non-perfusion and secondary ischaemia of the outer retina. Autofluorescence (FAF), Fluoroangiography (FA), and Indocyanine green angiography (ICG) are fundamental diagnostic tests for detecting choriocapillaris non-perfusion [17]. The clinical differences between the PICCP could possibly be explained by the level and the severity of the inflammation of the choriocapillaris circulation. Before the diagnosis of PICCP is made, an infectious cause, a neoplastic process, or a systemic vasculitis must be ruled out. The classification of PICCP into any of the known and well-defined entities is useful in predicting evolution and defining therapy [5, 18]: multiple evanescent white dot syndrome (MEWDS), acute posterior multifocal placoid pigment epitheliopathy (APMPPE), multifocal choroiditis, and serpiginous choroiditis.
Vogt–Koyanagi–Harada (VKH) disease
It is a multisystem disease. Chronic, bilateral, granulomatous panuveitis are associated with the central nervous system, auditory and integumentary manifestations. Its hallmark is bilateral multifocal exudative retinal detachments, hyperemia, and edema of the optic disk. We include lumbar puncture when necessary at the onset of the disease, especially in patients without systemic steroid therapy showing cerebrospinal fluid (CSF) pleocytosis [19, 20].
The term idiopathic is used whenever the intraocular inflammation cannot be attributed to a specific diagnosis.
Descriptive data are presented as mean and standard deviation (SD), or median and interquartile range (IQR), where appropriate. Categorical variables are expressed as frequency and percentages. To assess differences among groups, Student’s t test and ANOVA were used for continuous data, and Chi–square tests were conducted for categorical variables. Statistical analysis was performed using SPSS (IBM SPSS statistics v.22).
Results
Nine-hundred and ninety consecutive patients with uveitis (1481 eyes) diagnosed from November 1, 2013 to October 31, 2015 were included. The majority were female (n = 587, 59%; Table 1). There were 120 (12%) cases among children (≤18 years), while 713 (72%) predominated in those aged 19–64, and 157 (16%) in those aged above 65 (p < 0.001). Age at onset of uveitis was a median 39 (IQR 24-54), while age at presentation at our clinic was a median 44 years (IQR 29–57). The majority was of Italian ethnicity (884, 89%). Of these, 57% were resident in Emilia Romagna region, 79% in other parts of Northern Italy, and 21% in Central–Southern Italy.
Table 1.
Demographic and clinical characteristics of patients, by time period and anatomical site
| Variable | Cases 2002–2008 (N = 1064) | Cases 2013–2015 | P | ||||
|---|---|---|---|---|---|---|---|
| Total cases N = 990 | Anterior N = 530 | Intermediate N = 74 | Panuveitis N = 226 | Posterior N = 160 | |||
| Median age at presentation years (IQR) | 40 (26–55) | 44 (29–57)* | 45 (32–59) | 26 (15–46) | 45 (30–58) | 42 (29–55) | <0.001 |
| Gender | 0.300 | ||||||
| Male | 481 (45) | 403 (41)* | 204 (38) | 36 (49) | 93 (41) | 70 (44) | |
| Female | 583 (55) | 587 (59) | 326 (62) | 38 (51) | 133 (59) | 90 (56) | |
| Site | <0.001 | ||||||
| Unilateral | 825 (78) | 499 (50)* | 364 (69) | 13 (18) | 57 (25) | 65 (41) | |
| Bilateral | 239 (22) | 491 (50) | 166 (31) | 61 (82) | 169 (75) | 95 (59) | |
| Granulomatous | <0.001 | ||||||
| Yes | 553 (52) | 545 (55) | 312 (59) | 13 (18) | 147 (65) | 73 (46) | |
| No | 511 (48) | 445 (45) | 218 (41) | 61 (82) | 79 (35) | 87 (54) | |
| Non-Italian ethnicity | 31 (3) | 106 (11)* | 43 (8) | 6 (8) | 44 (19) | 13 (8) | <0.001 |
| Has a specific diagnosis | 787 (74) | 759 (77) | 404 (76) | 45 (61) | 177 (78) | 133 (84) | 0.002 |
| Infectious etiology | 257 (24) | 301 (30)* | 173 (43) | 3 (7) | 68 (38) | 57 (43) | <0.001 |
| Systemic disease | na | 259 (26) | 114 (21) | 12 (16) | 110 (49) | 23 (14) | <0.001 |
* Indicates P value <0.05 for comparison between previous cases and current. Data are reported as median (interquartile range) or n (%)
Anterior uveitis was the most frequent (53.5% of cases), followed by panuveitis (22.8%), posterior (16.2%), and intermediate uveitis (7.5%). There were considerable differences in disease location of uveitis in terms of age, but not of gender (Table 1). Unilateral involvement was significantly higher in anterior uveitis (69%) than in other anatomical sites. Granulomatous forms were especially prevalent in anterior and panuveitis (59 and 65%, respectively). Panuveitis was the more frequent among patients of non-Italian ethnicity.
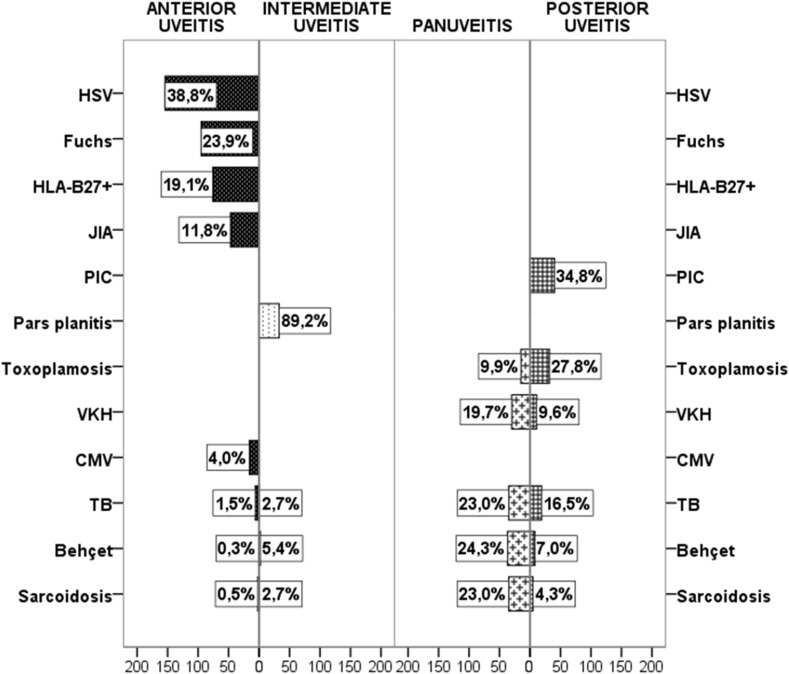
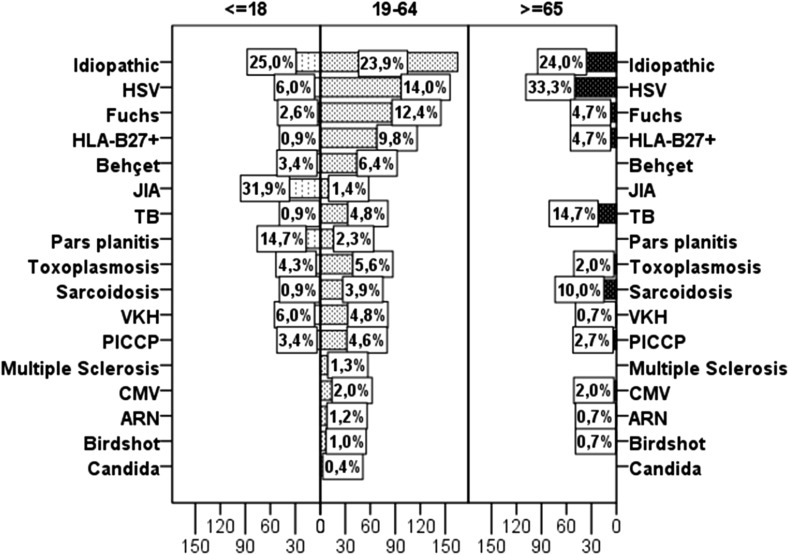
An etiological diagnosis of uveitis was established for 77% of patients, with the remainder classified as having idiopathic disease (Table 2). Among specific diagnoses, the most frequent were anterior herpetic uveitis (15.6%), followed by Fuchs’ uveitis (9.7%), HLA-B27-positive anterior uveitis (7.8%), and presumed tuberculous uveitis (5.7%, Fig. 1) The most frequent specific diagnoses in those aged below 18 years were Juvenile Idiopathic Arthritis (JIA) (31.9%), pars planitis (14.7%), followed by VKH and anterior herpetic uveitis (6.0%). Among adults (19–64 years), the most common specific diagnosis was anterior herpetic uveitis (14.0%), followed by Fuchs uveitis (12.3%) and HLA B27-positive anterior uveitis (9.8%). In older subjects (≥65 years), the most prevalent entities were anterior herpetic uveitis (33.3%), presumed tuberculous uveitis (14.7%), and presumed sarcoidosis (9.8%) (Fig. 2).
Table 2.
Frequency of specific diagnoses (n, %)
| Specific diagnosis | N | % |
|---|---|---|
| Anterior herpetic uveitis | 154 | 15.6 |
| Fuchs uveitis | 96 | 9.7 |
| HLA-B27+anterior uveitis | 76 | 7.7 |
| Presumed ocular tuberculosis | 56 | 5.7 |
| Behçet | 48 | 4.8 |
| JIA | 47 | 4.7 |
| Toxoplasmosis | 47 | 4.7 |
| Presumed sarcoidosis | 43 | 4.3 |
| Vogt–Koyanagi–Harada | 41 | 4.1 |
| PICCP | 40 | 4.0 |
| Pars planitis | 33 | 3.3 |
| Anterior Cytomegalovirus uveitis | 17 | 1.7 |
| ARN | 9 | 0.9 |
| Multiple sclerosis | 9 | 0.9 |
| Birdshot retinochoroiditis | 8 | 0.8 |
| Syphilis | 8 | 0.8 |
| Serpiginous tuberculosis | 5 | 0.5 |
| Sympathetic ophthalmia | 5 | 0.5 |
| Tinu | 5 | 0.5 |
| Candida | 3 | 0.3 |
| Others | 10 | 0.2 |
Fig. 1.
Distribution of the most frequent specific diagnoses according to anatomical classification
Fig. 2.
Age distribution of most frequent diagnoses
Women were considerably more likely to have had a specific diagnosis (p = 0.004), except for Behçet disease (56% males vs 44% females), CMV (71% males vs 29% females), and syphilis (75% males vs 35% females).
Infectious uveitis was diagnosed in 301 subjects (30% of total cases), most due to HSV (51%), followed by tuberculosis (19%), and toxoplasmosis (16%) (Table 1). Infectious uveitis was more frequent in males (34%) than females (28%) and in subjects over age 65 years (54%), compared to 12% in children and 28% in middle-age subjects (p < 0.001). In 150 (50%) patients with presumed infectious uveitis, ocular fluid biopsy was performed in order to confirm the clinical suspicion. Forty-seven samples (31.3%) tested positive for the following infectious agents: 18 CMV, 12 HSV, 11 Toxoplasma, 5 VZV, and 1 Listeria. Sixteen patients positive for CMV and 9 positive for HSV-1 presented with hypertensive anterior granulomatous uveitis.
In a quarter of cases, uveitis was associated with systemic diseases (259/990), with levels higher among females (29%) than males (22%, p = 0.016) and in the younger age subjects (44%) than those aged above 18 years (25%, p < 0.0001). According to anatomical classification, in those with systemic disease, panuveitis was the most prevalent (49%), followed by anterior (21%), intermediate (19%), and posterior uveitis (15%, p < 0.0001). The most common specific diagnoses in patients with systemic diseases were JIA and Behçet’s (n = 47, 18.5%), followed by presumed sarcoidosis (n = 43, 17%), VKH, and HLA-B27-positive anterior uveitis (n = 41, 16.1%).
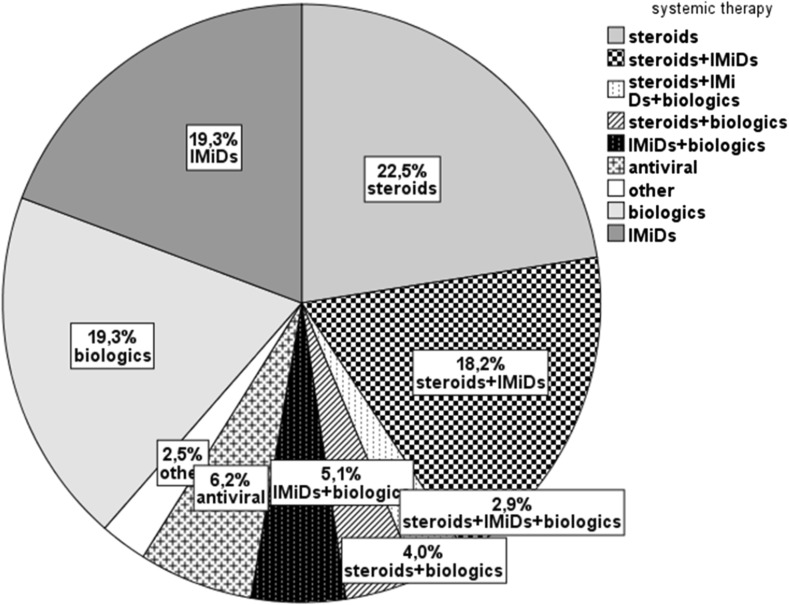
With regard to treatment, 277 (28%) patients were on systemic therapy, with steroids alone (22.5%), or steroids and either immunomodulatory agents (IMiDs) (18.2%) or biologics (4.0%), or with all three of these drug groups (2.9%). Fifty-three patients (19.3%) had been treated with biologics and immunomodulatory agents alone (Fig. 3). Overall, 549 (56%) patients developed at least one complication. Cataract was the most common, occurring in 19% of cases, followed by posterior synechiae (14%), macular edema (13%), and glaucoma (11%).
Fig. 3.
Ongoing systemic therapy (n = 277 cases)
Discussion
This is only the fourth epidemiological study on uveitis in Italy [5, 21, 22], with the most recent one covering the period 2002–2008 [5]. In the same territory (533,000 inhabitants) during 24 months (2013–2015), we observed 164 new cases of uveitis, giving an estimated incidence of 15.4 cases per year per 100,000 inhabitants, higher than the value in our previous analysis in 2008 (14.6/year/100,000 people).
The distribution of cases showed the predominance of anterior uveitis, consistent with the previous studies [23–57], including our own [5]. In the last series from our site, age at presentation was significantly higher with a significant female prevalence (see Table 1). As compared to the previous series, we observed a similar frequency of anterior and intermediate uveitis (54 and 51%, and 7 and 6%, respectively) and a significant prevalence of panuveitis (in the latter study) respect to posterior uveitis (23 vs 20%, p < 0.001).
In this study, specific diagnoses were made in 77% of patients, higher than in other reports, [5, 23–57] which range from 42 to 75%. The increase of specific diagnoses is likely the result of both an interdisciplinary approach and the introduction of new diagnostic techniques. PCR and Antibody Index tests on eye fluids helped to confirm the diagnosis of different herpetic etiologies.
Also, the introduction of interferon gamma tests like Quantiferon helped to confirm the diagnosis of tuberculosis.
The prevalence of infectious uveitis (30%) was significantly higher (p = 0.002, Table 1) than in our previous series (24%): anterior herpetic uveitis, the most common specific diagnosis, increased from 9.1% in 2008 to 15.6%, while no difference was detected between the proportion with tuberculosis (4.4 in 2008 to 5.7% in current series, p = 0.19). Eye fluids analysis was able to confirm some specific infectious etiologies, e.g., HSV and CMV, while other diagnoses, such as Fuchs uveitis, decreased from 22.7 to 9.7% in the latter series. We observed also a lower prevalence of toxoplasmosis, from levels of 6.9% in the first analysis to 4.7% in the present analysis (Table 3).
Table 3.
Frequencies (percentages of total cases) of key uveitis diagnoses in comparison with previous studies
| Country | Author | N; Period | Fuchs | HLA-B27 | HSV | Behçet’s | Toxoplasmosis | Sarcoidosis | TB | VKH |
|---|---|---|---|---|---|---|---|---|---|---|
| Italy | Present data | 990; 2013–2015 | 9.7 | 7.7 | 15.6 | 4.8 | 4.7 | 4.3 | 5.7 | 4.1 |
| Cimino9 | 1064; 2002–2008 | 22.7 | 5.3 | 9.9 | 5.3 | 6.9 | 2.2 | 4.4 | 1.9 | |
| Mercanti24 | 655; 1986–1993 | 2.1 | na | 11.7 | 3.0 | 17.7 | 0.8 | 7.0 | 1.4 | |
| Pivetti-Pezzi25 | 1417; 1986–1993 | 8.3 | 3.4 | 5.3 | 1.3 | 6.6 | 0.2 | na | 2.0 | |
| UK | Jones26 | 3000; 1991–2013 | 11.5 | 4.5 | 1.9 | 2.7 | 6.9 | 9.7 | 3.3 | 0.8 |
| Austria | Barisani-Asenbauer27 | 2619; 1995–2009 | 4.6 | 19.5 | 7.1 | 1.9 | 6.6 | 3.2 | 0.8 | 0.4 |
| Spain | Llorenç28 | 1022; 2009–2012 | 1.0 | 13.0 | 12.0 | 5.0 | 7.0 | 3.0 | 5.0 | 1.0 |
| Germany | Grajewski29 | 476; 2012–2013 | 7.0 | 19.0 | 12.0 | 2.0 | 7.1 | 11.0 | na | 0.6 |
| Jacob30 | 1916; 2001–2006 | 6.9 | 7.1 | 6.1 | 1.8 | 4.2 | 4.5 | 1.1 | 0 | |
| Swiss | Tran31 | 558; 1990–1993 | 5.4 | 15.9 | 13.8 | 1.1 | 9.5 | 5.9 | 9.5 | 0.2 |
| France | Bodaghi32 | 927; 1991–1996 | 2.5 | 4.7 | 8.6 | 6.1 | 11.9 | 6.4 | 4.1 | 2.0 |
| Japan | Nakahara39 | 695; 2010–2012 | 2.1 | 3.0 | 6.0 | 5.6 | 0 | 9.4 | 1.5 | 7.9 |
| Saudi Arabia | Al Dhahri18 | 642; 1998–2013 | 11.3 | 9.0 | 10.0 | 8.4 | 6.9 | 4.5 | 17.8 | 19.6 |
| Tunisia | Khairallah47 | 472; 1992–2003 | 3.0 | 2.8 | 11.9 | 12.3 | 10.1 | 1.7 | 1.1 | 4.4 |
| Turkey | Kazokoglu49 | 761; 2004 | 5.1 | 2.4 | 2.8 | 32.1 | 4.7 | 0.9 | 0.3 | 1.1 |
| US | Rodriguez50 | 1237; 1982–1992 | 2.6 | 3.8 | 7.2 | 2.5 | 4.8 | 9.6 | 0.6 | 0.9 |
Tuberculosis and syphilis accounted for 20% and almost 3% of infectious etiologies, respectively, an increase in the recent years, possibly due to the rise in immigration in Italy. The percentage of non-Italian ethnicity among patients rose from 3% in the first series to 10.7% in this report (p < 0.0001, Table 1).
The relatively high percentage of uveitis associated with systemic diseases (JIA, sarcoidosis, VKH, HLA-B27) is consistent with what Barisani-Asenbauer et al. reported [24], underlining the need for an interdisciplinary approach to reduce the diagnostic delay, and thus ocular as well as systemic complications due to non-appropriate therapies. In our series, 44 patients presented acute anterior non-granulomatous uveitis (38/42 HLA-B27-positive anterior uveitis) associated with ankylosing spondylitis. In 35/42 of cases, the ocular diagnosis preceded the systemic one.
The leading complications observed in our study were cataract, glaucoma, and macular edema, in line with several previous reports [8]. Regarding medications, traditional immunomodulatory agents still seem to be preferred by ophthalmologists in autoimmune conditions, rather than biologics, except for HLA-B27-associated uveitis, where anti-TNFs are prescribed more frequently.
Diagnostic delays are often due to difficulties in establishing the primary diagnosis of uveitis. Comparisons among surveys conducted in uveitis clinics elsewhere in the world often present great differences in the prevalence of diagnostic categories (Table 3). Such differences are probably related to genetic, geographical, social, and environmental factors. They may also be attributable to heterogeneity in the diagnostic criteria and definitions, together with the availability of different investigation techniques, as well as different interpretations of the anatomical categorization of intraocular inflammation.
In conclusion, the pattern of uveitis appears to be changing, very likely due to the spreading of infectious diseases, to the availability of new diagnostic tests, such as aqueous analysis by PCR and AIC, and to more refined classification (such as the PICCP grouped together on the basis of similar etiology and particular clinical and imaging aspects). Moreover, an interdisciplinary approach that includes ophthalmologists, rheumatologists, pediatricians, and infectious disease specialists with uveitis experience may help to raise the standards of treatment, and improve prognosis and clinical course of the disease, thereby reducing the risk of complications.
Acknowledgements
We thank Dr. Paola Masini, Servizio Tecnologie Informatiche e Telematiche (STIT) ASMN-IRCCS, for technical support in the database.
Compliance with ethical standards
Conflicts of interest
All authors have no conflict of interest to declare.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
An erratum to this article is available online at https://doi.org/10.1007/s10792-017-0466-x.
Contributor Information
Cimino Luca, Email: cimino.luca@asmn.re.it, Email: l.cimino64@gmail.com.
Aldigeri Raffaella, Email: raffaella.aldigeri@unipr.it.
Savoldi Luisa, Email: savoldi.luisa@asmn.re.it.
De Fanti Alessandro, Email: defanti.alessandro@asmn.re.it.
Parmeggiani Maria, Email: parmeggiani.maria@asmn.re.it.
Chersich Matthew, Email: MChersich@wrhi.ac.za.
Salvarani Carlo, Email: salvarani.carlo@asmn.re.it.
Fontana Luigi, Email: fontana.luigi@asmn.re.it.
References
- 1.Henderly DE, Genstler AJ, Smith RE, Rao NA. Changing patterns of uveitis. Am J Ophthalmol. 1987;103:131–136. doi: 10.1016/S0002-9394(14)74217-5. [DOI] [PubMed] [Google Scholar]
- 2.Rathinam SR, Namperumalsamy P. Global variation and pattern changes in epidemiology of uveitis. Indian J Ophthalmol. 2007;55:173–183. doi: 10.4103/0301-4738.31936. [DOI] [PubMed] [Google Scholar]
- 3.Wakefield D, Chang JH. Epidemiology of uveitis. Int Ophthalmol Clin. 2005;45:1–13. doi: 10.1097/01.iio.0000155938.83083.94. [DOI] [PubMed] [Google Scholar]
- 4.McCannel CA, Holland GN, Helm CJ, et al. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol. 1996;121:35–46. doi: 10.1016/S0002-9394(14)70532-X. [DOI] [PubMed] [Google Scholar]
- 5.Cimino L, Aldigeri R, Salvarani C, et al. The causes of uveitis in a referral centre of Northern Italy. Int Ophthalmol. 2010;30:521–529. doi: 10.1007/s10792-010-9359-y. [DOI] [PubMed] [Google Scholar]
- 6.Bloch-Michel E, Nussenblatt RB. International uveitis study group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–235. doi: 10.1016/S0002-9394(14)74235-7. [DOI] [PubMed] [Google Scholar]
- 7.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Dhahri H, Al Rubaie K, Hemachandran S, et al. Patterns of uveitis in a university-based tertiary referral center in Riyadh, Saudi Arabia. Ocul Immunol Inflamm. 2014;24:1–9. doi: 10.3109/09273948.2014.939197. [DOI] [PubMed] [Google Scholar]
- 9.De Groot-Mijnes JD, Rothova A, Van Loon AM, et al. Polymerase chain reaction and Goldmann–Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am J Ophthalmol. 2006;141:313–318. doi: 10.1016/j.ajo.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Van der Lelij A, Ooijman FM, Kijlstra A, Rothova A. Anterior uveitis with sectoral iris atrophy in the absence of keratitis: a distinct clinical entity among herpetic eye diseases. Ophthalmology. 2000;107:1164–1170. doi: 10.1016/S0161-6420(00)00115-9. [DOI] [PubMed] [Google Scholar]
- 11.Quentin CD, Reiber H. Fuchs heterochromic cyclitis: Rubella virus antibodies and genome in aqueous humor. Am J Ophthalmol. 2004;138:46–54. doi: 10.1016/j.ajo.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 12.Cimino L, Aldigeri R, Parmeggiani M, et al. Searching for viral antibodies and genome in intraocular fluids of patients with Fuchs uveitis and non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2013;251:1607–1612. doi: 10.1007/s00417-013-2287-6. [DOI] [PubMed] [Google Scholar]
- 13.de Schryver I, Rozenberg F, Cassoux N, et al. Diagnosis and treatment of cytomegalovirus iridocyclitis without retinal necrosis. Br J Ophthalmol. 2006;90:852–855. doi: 10.1136/bjo.2005.086546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbort CP, Mochizuki M, Rao NA, and the members of the Scientific Committee of the First International Workshop on Ocular Sarcoidosis IWOS International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop on Ocular Sarcoidosis (IWOS) Ocul Immunol Inflamm. 2009;17:160–169. doi: 10.1080/09273940902818861. [DOI] [PubMed] [Google Scholar]
- 15.International Study Group for Behçet’s Disease Evaluation of diagnostic ‘‘classification’’ criteria in Behcet’s disease: toward internationally agreed criteria. Lancet. 1990;335:1078–1080. [Google Scholar]
- 16.Gupta V, Gupta A, Rao NA. Intraocular tuberculosis–an update. Surv Ophthalmol. 2007;52:561–587. doi: 10.1016/j.survophthal.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A, Giani A, Herbort CP, Staurenghi G. Interpretation of fundus autofluorescence changes in choriocapillaritis: a multi-modality imaging study. Graefes Arch Clin Exp Ophthalmol. 2016;254:1473–1479. doi: 10.1007/s00417-015-3205-x. [DOI] [PubMed] [Google Scholar]
- 18.Chang J, Wakefield D. Uveitis a global perspective. Ocul Immunol Inflamm. 2002;10:263–279. doi: 10.1076/ocii.10.4.263.15592. [DOI] [PubMed] [Google Scholar]
- 19.Moorthy RS, Inomata H, Rao NA. Complications and prognostic factors in Vogt-Koyanagi-Harada disease. Surv Ophthalmol. 1995;39:265–292. doi: 10.1016/S0039-6257(05)80105-5. [DOI] [PubMed] [Google Scholar]
- 20.Read RW, Rechodouni A, Butani N, et al. Complications and prognostic factors in Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2001;131:599–606. doi: 10.1016/S0002-9394(01)00937-0. [DOI] [PubMed] [Google Scholar]
- 21.Mercanti A, Parolini B, Bonora A, et al. Epidemiology of endogenous uveitis in northeastern Italy. Analysis of 655 new cases. Acta Ophthalmol Scand. 2001;79:64–68. doi: 10.1034/j.1600-0420.2001.079001064.x. [DOI] [PubMed] [Google Scholar]
- 22.Pivetti-Pezzi P, Accorinti M, La Cava M, et al. Endogenous uveitis: an analysis of 1,417 cases. Ophthalmologica. 1996;10:234–238. doi: 10.1159/000310715. [DOI] [PubMed] [Google Scholar]
- 23.Jones NP. The manchester uveitis clinic: the first 3000 patients—epidemiology and casemix. Ocul Immunol Inflamm. 2015;23:118–126. doi: 10.3109/09273948.2013.855799. [DOI] [PubMed] [Google Scholar]
- 24.Barisani-Asenbauer T, Maca SM, Mejdoubi L, et al. Uveitis—a rare disease often associated with systemic diseases and infections: a systemic review of 2619 patients. Orphanet J Rare Dis. 2012;7:57–64. doi: 10.1186/1750-1172-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llorenç V, Mesquida M, Sainz de la Maza M, et al. Epidemiology of uveitis in a Western urban multiethnic population. The challenge of globalization. Acta Ophthalmol. 2015;93:561–567. doi: 10.1111/aos.12675. [DOI] [PubMed] [Google Scholar]
- 26.Grajewski RS, Caramoy A, Frank KF, et al. Spectrum of uveitis in a German tertiary center: review of 474 consecutive patients. Ocul Immunol Inflamm. 2015;23:346–352. doi: 10.3109/09273948.2014.1002567. [DOI] [PubMed] [Google Scholar]
- 27.Jakob E, Reuland MS, Mackensen F, et al. Uveitis subtypes in a German interdisciplinary uveitis center—analysis of 1916 patients. J Rheumatol. 2009;36:127–136. doi: 10.3899/jrheum.080102. [DOI] [PubMed] [Google Scholar]
- 28.Tran VT, Auer C, Gueex-Crosier Y, et al. Epidemiology of uveitis in Switzerland. Ocul Immunol Inflamm. 1994;2:169–176. doi: 10.3109/09273949409057073. [DOI] [PubMed] [Google Scholar]
- 29.Bodaghi B, Cassoux N, Wechsler B, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltim) 2001;80:263–270. doi: 10.1097/00005792-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Liberman P, Gauro F, Berger O, Urzua CA. Causes of uveitis in a tertiary center in Chile: a cross-sectional retrospective review. Ocul Immunol Inflamm. 2015;23:339–345. doi: 10.3109/09273948.2014.981548. [DOI] [PubMed] [Google Scholar]
- 31.Singh R, Gupta V, Gupta A. Pattern of uveitis in a referral eye clinic in north India. Indian J Ophthalmol. 2004;52:121–125. [PubMed] [Google Scholar]
- 32.Soheilian M, Heidari K, Yazdani S, et al. Patterns of uveitis in a tertiary eye care center in Iran. Ocul Immunol Inflamm. 2004;12:297–310. doi: 10.1080/092739490500174. [DOI] [PubMed] [Google Scholar]
- 33.Kianersi F, Mohammadi Z, Ghanbari H, et al. Clinical patterns of uveitis in an Iranian tertiary eye-care center. Ocul Immunol Inflamm. 2015;23:278–282. doi: 10.3109/09273948.2014.902474. [DOI] [PubMed] [Google Scholar]
- 34.Kitamei H, Kitaichi N, Namba K, et al. Clinical features of intraocular inflammation in Hokkaido, Japan. Acta Ophthalmol. 2009;87:424–428. doi: 10.1111/j.1755-3768.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 35.Keino H, Nakashima C, Watanabe T, et al. Frequency and clinical features of intraocular inflammation in Tokyo. Clin Experiment Ophthalmol. 2009;37:595–601. doi: 10.1111/j.1442-9071.2009.02102.x. [DOI] [PubMed] [Google Scholar]
- 36.Nakahara H, Kaburaki T, Takamoto M, et al. Statistical analyses of endogenous uveitis patients (2007–2009) in central Tokyo area and comparison with previous studies (1963–2006) Ocul Immunol Inflamm. 2015;23:291–296. doi: 10.3109/09273948.2014.920036. [DOI] [PubMed] [Google Scholar]
- 37.Abdulaal M, Antonios R, Barikian A, et al. Etiology and clinical features of ocular inflammatory diseases in a tertiary center in Lebanon. Ocul Immunol Inflamm. 2015;23:271–277. doi: 10.3109/09273948.2014.902077. [DOI] [PubMed] [Google Scholar]
- 38.Hamade IH, Elkum N, Tabbara KF. Causes of uveitis at a referral center in Saudi Arabia. Ocul Immunol Inflamm. 2009;17:11–16. doi: 10.1080/09273940802491850. [DOI] [PubMed] [Google Scholar]
- 39.Al-Mezaine HS, Kangave D, Abu El-Asrar AM. Patterns of uveitis in patients admitted to a university hospital in Riyadh, Saudi Arabia. Ocul Immunol Inflamm. 2010;18:424–431. doi: 10.3109/09273948.2010.502284. [DOI] [PubMed] [Google Scholar]
- 40.Mi H, Ho SL, Lim WK, Wong EP, Teoh SC. Trends in patterns of posterior uveitis and panuveitis in a tertiary institution in Singapore. Ocul Immunol Inflamm. 2015;23:329–338. doi: 10.3109/09273948.2014.946148. [DOI] [PubMed] [Google Scholar]
- 41.Pathanapitoon K, Kunavisarut P, Ausayakhun S, et al. Uveitis in a tertiary ophthalmology centre in Thailand. Br J Ophthalmol. 2008;92:474–478. doi: 10.1136/bjo.2007.132175. [DOI] [PubMed] [Google Scholar]
- 42.Sittivarakul W, Bhurayanontachai P, Ratanasukon M. Pattern of uveitis in a university-based referral center in southern Thailand. Ocul Immunol Inflamm. 2013;21:53–60. doi: 10.3109/09273948.2012.730651. [DOI] [PubMed] [Google Scholar]
- 43.Silpa-Archa S, Noonpradej S, Amphornphruet A. Pattern of uveitis in a referral ophthalmology center in the central district of Thailand. Ocul Immunol Inflamm. 2015;23:320–328. doi: 10.3109/09273948.2014.943773. [DOI] [PubMed] [Google Scholar]
- 44.Khairallah M, Yahia SB, Ladjimi A, et al. Pattern of uveitis in a referral centre in Tunisia, North Africa. Eye (Lond) 2007;21:33–39. doi: 10.1038/sj.eye.6702111. [DOI] [PubMed] [Google Scholar]
- 45.Nalcacioglu-Yuksekkaya P, Ozdal PC, Yazici A, Tirhis H. Clinical and demographic characteristics of patients with uveitis starting later in life. Ocul Immunol Inflamm. 2015;23:304–310. doi: 10.3109/09273948.2014.938761. [DOI] [PubMed] [Google Scholar]
- 46.Kazokoglu H, Onal S, Tugal-Tutkun I, et al. Demographic and clinical features of uveitis in tertiary centers in Turkey. Ophthalmic Epidemiol. 2008;15:285–293. doi: 10.1080/09286580802262821. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez A, Calonge M, Pedroza-Seres M, et al. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol. 1996;114:593–599. doi: 10.1001/archopht.1996.01100130585016. [DOI] [PubMed] [Google Scholar]
- 48.Bajwa A, Osmanzada D, Osmanzada S, et al. Epidemiology of uveitis in the mid-Atlantic United States. Clin Ophthalmol. 2015;20:889–901. doi: 10.2147/OPTH.S80972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karaconji T, Maconochie Z, McCluskey P. Acute anterior uveitis in Sydney. Ocul Immunol Inflamm. 2013;21:108–114. doi: 10.3109/09273948.2012.745882. [DOI] [PubMed] [Google Scholar]
- 50.Wakefield D, Dunlop I, McCluskey PJ, Penny R. Uveitis: aetiology and disease associations in an Australian population. Aust N Z J Ophthalmol. 1986;14:181–187. doi: 10.1111/j.1442-9071.1986.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 51.Chao JR, Khurana RN, Fawzi AA, Reddy HS, Rao NA. Syphilis: reemergence of an old adversary. Ophthalmology. 2006;113:2074–2079. doi: 10.1016/j.ophtha.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 52.Sengun A, Karadag R, Karakurt A, et al. Causes of uveitis in a referral hospital in Ankara, Turkey. Ocul Immunol Inflamm. 2005;13:45–50. doi: 10.1080/09273940590909121. [DOI] [PubMed] [Google Scholar]
- 53.Miyanaga M, Shimizu K, Kawaguchi T, et al. A clinical survey of uveitis in HTLV-1 endemic region. Ocul Immunol Inflamm. 2009;17:335–341. doi: 10.3109/09273940903137667. [DOI] [PubMed] [Google Scholar]
- 54.Bodaghi B, Wechsler B, Du-Boutin LT, et al. Chronic severe uveitis: classification, search for etiology and therapeutic approach. Rev Med Interne. 2003;24:794–802. doi: 10.1016/S0248-8663(03)00140-1. [DOI] [PubMed] [Google Scholar]
- 55.Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23:705–717. doi: 10.5301/ejo.5000278. [DOI] [PubMed] [Google Scholar]
- 56.Merrill PT, Kim J, Cox TA, et al. Uveitis in Southeastern United States. Curr Eye Res. 1997;16:865–874. doi: 10.1076/ceyr.16.9.865.5048. [DOI] [PubMed] [Google Scholar]
- 57.Oruc S, Kaplan AD, Galen M, Kaplan HJ. Uveitis referral pattern in a Midwest University Eye Center. Ocul Immunol Inflamm. 2003;11:287–298. doi: 10.1076/ocii.11.4.287.18270. [DOI] [PubMed] [Google Scholar]