Abstract
The objective of the present study was to investigate the cytotoxic effects of sodium citrate on human gastric adenocarcinoma epithelia AGS cells. Numerous cytotoxicity-associated sodium citrate-induced effects were assessed, including cell viability and proliferation, cytokine expression and caspase activity. In vitro studies demonstrated that incubation with sodium citrate (>3.125 mM) inhibited AGS cell viability and proliferation in a dose-dependent manner. Incubation with sodium citrate for 24 h revealed that the levels of interleukin-1β (IL-1β), IL-8 and tumor necrosis factor increased with an increasing of dose of sodium citrate, whereas the IL-6 levels exhibited only a slight alteration. In addition, increases in caspase-3 and −9 activities were associated with increased duration of treatment and dosage of sodium citrate. Collectively, the results of the present study demonstrated that treatment with sodium citrate at higher concentrations or for longer durations exerts a cytotoxic effect on AGS cells via the induction of the intrinsic apoptosis pathway and the alteration in the levels of certain cytokines.
Keywords: sodium citrate, AGS cells, cytotoxic effect, proliferation, pro-inflammatory cytokine
Introduction
Sodium citrate is widely used in the fields of medicine (1,2) and food science as an adjuvant or additive (3). Citrate, an important intermediate in the tricarboxylic acid cycle, serves a notable role in cellular metabolism (4). Under normal physiological conditions, ATP production via oxidative phosphorylation in the mitochondria is an efficient and primary metabolic process, which produces far more ATP molecules from a given amount of glucose than does glycolysis (5). By contrast, the majority of cancer cells exhibit a high level of glycolysis for the generation of ATP to meet their energy requirements; the metabolism of cancer cells is often referred to as the ‘Warburg effect’ (6,7). Cancer cells primarily metabolize glucose via glycolysis, excreting large amounts of macromolecular precursors, including acetyl-CoA, for the production of fatty acids, non-essential amino acids and nucleotides (8). When glycolysis occurs in this manner, cancer cells undergo fermentation even when mitochondrial function is not impaired, in a process known as ‘aerobic glycolysis’ (9). Thus, any inhibition of glycolysis may restrict oncogenic proliferation or even halt it entirely, leading to cell death. During glycolysis, citrate acts as an inhibitor of phosphofructokinase and may obstruct the production of ATP or macromolecular precursors, causing the typical cytotoxicity in cancer cells, as has been confirmed in malignant pleural mesothelioma cells (10). Cells deficient in ATP frequently undergo apoptosis (11,12); the induction of cell apoptosis via citrate has been demonstrated in unicellular organisms as well as cancer cells (13,14).
Gastric cancer is one of the most common types of cancer in Asia (15,16). Over the last 4 decades its incidence has decreased globally; however, higher rates of mortality have been observed in East Asian countries than in other areas of the world (16). Genetic and environmental factors may serve a role in the etiology of gastric cancer (17,18), and efforts are being made to further understand and treat this disease.
Several micromolecular compounds have been previously investigated for cytotoxic activity in human gastric adenocarcinoma epithelia AGS cells, including sodium acetate (19), sodium nitrite, and magnesium sulfate (20,21). To varying degrees, the aforementioned compounds exhibited cytotoxic activity, either by altering the expression levels of pro-inflammatory cytokines in AGS cells or by reducing cell viability. In the present study, a range of tests were conducted to understand the anticancer activity exerted by sodium citrate on AGS cells. The antitumor effect of citrate, an anti-glycolytic agent inhibiting phosphofructokinase, was tested on BGC-823 and SGC7901 cell lines (22). Sodium citrate inhibited the growth and proliferation of MGC-803 cells by blocking the glycolytic pathway and regulating the Bcl-2 family genes to induce mitochondria-regulated apoptosis (23).
Materials and methods
The AGS cell line (Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) was originally cultured from stomach adenocarcinoma cells obtained prior to any anticancer treatment (24). Sodium citrate was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Quick Cell Proliferation Assay and Annexin V-Phycoerythrin Apoptosis Detection kits were purchased from Medical and Biological Laboratories Co., Ltd. (Aichi, Japan). The Lactate Dehydrogenase (LDH)-Cytotoxicity Assay kit was purchased from Biovision, Inc., (Milpitas, CA, USA) and the Apo Alert DNA Fragmentation Assay kit was purchased from BD Biosciences (Franklin Lakes, NJ, USA). Caspase 3 Activity Assay kit (cat. no. C1116), Caspase 6 Activity Assay kit (cat. no. C1136), Caspase 8 Activity Assay kit (cat. no. C1152) and Caspase 9 Activity Assay kit (cat. no. C1158) were all purchased from Beyotime Institute of Biotechnology (Haimen, China). Ham's F12 medium, 10% fetal bovine serum, penicillin, streptomycin, L-glutamine and phosphate buffered saline [PBS, (pH 7.4)] were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The 24-well and 96-well plates were purchased from Corning Inc. (Corning, NY, USA). Optical density was measured at 450 nm with a microplate reader (Ceres UV 900 HD; Bio-Tek Instruments, Inc., Winooski, VT, USA). Total RNA was extracted with Isogen Isolation Reagent kit from Nippon Gene (Tokyo, Japan). Interleukin (IL)-1β (Human) ELISA kit (cat. no. K4794), IL-6 (Human) ELISA kit (cat. no., K4143), IL-8 (Human) ELISA kit (cat. no. K4169) and TNF-α (Human) ELISA kit (cat. no. K4779) were purchased from BioVison, Inc. (Milpitas, CA, USA). Moloney murine leukemia virus reverse transcriptase was purchased from Invitrogen (M-MLV RT; Thermo Fisher Scientific, Inc.). The 5X First-Strand Buffer, DTT, dNTPs and random primers (6-mer) pd (N) 6 was purchased from Takara Bio, Inc. (Otsu, Japan). All solvents, chemicals, and reagents were analytical grade and were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Chemical agents and cells
Experiments were performed using the human AGS cell line, cultured in Ham's F12 nutrient mixture with L-glutamine, supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), and 10% fetal bovine serum (FBS). The culture plate was incubated at 37°C in a humidified atmosphere of 5% CO2 in ambient air. Sodium citrate was used as a stimulant.
Assessment of AGS cell viability
AGS cells were seeded into each a 24-well plate at a density of 8×103 cells/well and incubated for 3 h. The Ham's F12 medium was used to dilute sodium citrate to the desired concentration (0, 6.25, 12.5, 25, 50 and 100 mM), and the corresponding sodium citrate-containing medium was added to each well. The cells were then incubated at 37°C in a 5% CO2 incubator for an additional 24, 48 and 72 h. Control cells were inoculated without sodium citrate. At the end of each incubation, cell suspensions in PBS were treated with 0.04% Trypan blue at 37°C for 2 min, and the dye was thereafter rinsed off with culture medium. Under a light microscope (STZ10, at magnification, ×400) with a CCD camera (DP70) (both from Olympus Corporation, Tokyo, Japan), the numbers of stained (dead cells) and unstained cells (live cells) were counted in 8 randomly chosen microscope fields under a light microscope (magnification, ×400) using a hemocytometer to determine cell viability.
Cell proliferation assay
Cells were seeded in 96-well plates at a density of 4×103 cells/well and incubated at 37°C in a 5% CO2. Simultaneously, cells were treated in triplicate with sodium citrate, with the final sodium citrate concentrations ranging from 0 to 50 mM (0, 1.58, 3.13, 6.25, 12.5, 25 and 50 mM). Unstimulated controls were treated with phosphate buffered saline. Cell proliferation was determined at 24, 48, and 72 h using the Quick Cell Proliferation Assay kit, according to the manufacturer's instructions.
Lactate dehydrogenase release assay
Cell cytotoxicity was measured based on the release of LDH from cells. Briefly, LDH levels in the supernatant of cells pre-treated with 3.125, 6.25, 12.5, 25, or 50 mM sodium citrate for 1, 8, or 24 h were quantified using the LDH-cytotoxicity assay kit II (BioVision, Inc.). LDH oxidizes lactate to pyruvate, which forms a red formazan product with iodotetrazolium chloride. Dimethyl sulfoxide was added to dissolve the formazan crystals. The amount of formazan present in the supernatant is directly correlated with the number of lysed cells. The optical density was then measured 492 nm using a spectrophotometer. Triton X-100 (1%)-treated cells were used as the positive control. The cytotoxicity induced by each dose of citrate was expressed as a percentage of LDH released by treated cells of that released by cells treated with 1% Triton X-100.
Caspase activity assay
Caspase-3, −6, −8 and −9 activities were determined using a caspase colorimetric assay. Briefly, following treatment with 0, 1.25, 2.5, 5.0, 10.0 or 20.0 mM sodium citrate for 1, 3 or 6 h, AGS cells were lysed in caspase lysis buffer (Beyotime Institute of Biotechnology) for 15 min, followed by centrifugation at 16,000 × g at 4°C for 15 min. Then, 50 µl extracts were incubated with 10 µl 2 mM enzyme substrate (Ac-DEVD-pNA for caspase-3-like proteinase, Ac-VEID-pNA for caspase-3-like proteinase, Ac-IETD-pNA for caspase-8-like proteinase, and Ac-LEHD-pNA for caspase-9-like proteinase, Beyotime Institute of Biotechnology) in caspase activity assay buffer (40 µl; Beyotime Institute of Biotechnology) in a 100-µl reaction mixture in 96-well plates at 37°C for 4 h. The absorbance of the mixture was then measured at a wavelength of 405 nm using a microplate reader. The same volume lysis buffer replaced with sample extracts was used in the control group, and other components unchanged.
Analysis of cytokine protein levels
The levels of IL-1β, IL-6, IL-8 and tumor necrosis factor-α (TNF-α) present in the supernatants of AGS cells exposed to sodium citrate were detected via sandwich ELISA assay. Supernatants were collected and stored at −20°C until the time of assay, at which point RNA was extracted using TRIzol reagent (Life Technologies; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. To the standard wells, sample wells and control wells, standard, sample and standard diluents [(pH 7.2), 0.01 mol/l PBS], respectively, was added (50 µl in each well). A total of 100 µl of biotin-conjugated anti-Human antibody work solution (IL-1β, IL-6, IL-8, TNF-α) was added into the above wells (standard, sample and control wells). The plates were sealed with an adhesive strip and incubated for 60 min at 37°C, then washed 5 times with washing buffer [(pH 7.2) 0.01 mol/l PBS and 0.05% Tween-20; Beyotime Institute of Biotechnology; code no. PI305-7]. After the final wash, inverted plate, and clapped the plate on absorbent filter papers. 100 µl HRP-conjugated secondary antibodies (cat. no. PI305-6; Beyotime Institute of Biotechnology) was added to each well, covered with an adhesive strip and incubated in dark for 60 min at 37°C, then washed 4 times with washing buffer as used above. After the final wash, the plate was inverted and clapped on absorbent filter papers. TMB substrate (100 µl) (cat. no. PI305-8; Beyotime Institute of Biotechnology) was added into each well, the plate was covered and incubated at 37°C in the dark for 20 min. Then, 50 µl Stop solution (2 mol/l sulfuric acid) was added to each well. Then, gentle mixing was performed followed by incubated in the dark for 15 min at 37°C. The optical density was read at 450 nm using a microplate reader within 15 min. Origin 9.0 Software (Microcal Software Inc., Northampton, MA, USA) was used to make a standard curve (linear regression) and calculate the concentration of cytokine in the samples. The intensity of the color change in the ELISA was measured at 450 nm. Results from all experiments were included in the analysis. The minimum detectable dose for these assays were 0.3 pg/ml for IL-1β, 5 pg/ml for IL-6, 8 pg/ml for IL-8 and 30 pg/ml for TNF-α.
Analysis of cytokine mRNA levels via reverse transcription-polymerase chain reaction (RT-PCR)
AGS cells in culture medium were incubated for 24 h at 37°C in the presence of sodium citrate (6.25, 12.5 and 25 mM). Total RNA was then extracted with Isogen. Aliquots (2.5 µg) of total RNA were incubated at 70°C for 5 min, chilled on ice, and reverse-transcribed in a final volume of 10 µl composed of the following components: Moloney murine leukemia virus reverse transcriptase (cat. no. 2640A; Takara Bio, Inc.); 5X First-Strand Buffer; 0.1 mM DTT; 2.5 mmol dNTPs; and random primers (6-mer) pd (N)6. Reactions were performed under the following conditions: 22°C for 10 min; 37°C for 60 min; and 80°C for 5 min. The resulting cDNAs were stored at −20°C until use. Each cDNA (1 µl) was added to 29 µl reactions containing 3 µl 10X PCR reaction buffers (Takara Bio, Inc.), 1 µl, 4 nmol of each primer, 0.1 µl 5 U/µl Taq DNA polymerase, and H2O. The oligonucleotide primers (19) are summarized in Table I. PCR was performed with an automatic thermal cycler, Promgram Temp control system PC-701 (Biometra GmbH, Gottingen, Germany).
Table I.
Sequences of the 5′ and 3′ primers of the 4 target genes.
| mRNA | Direction | Primer sequence | PCR fragment size, bp |
|---|---|---|---|
| GAPDH | Sense | 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ | 985 |
| Antisense | 5′-CATGTGGGCCATGAGGTCCACCAC-3′ | ||
| IL-1β | Sense | 5′-ATAAGCCCACTCTACAGCT-3′ | 443 |
| Antisense | 5′-ATTGGCCCTGAAAGGAGAGA-3′ | ||
| IL-6 | Sense | 5′-GTACCCCCAGGAGAAGATTC-3′ | 819 |
| Antisense | 5′-CAAACTGCATAGCCACTTTC-3′ | ||
| IL-8 | Sense | 5′-GGCACAGTGGAACAAGGACT-3′ | 585 |
| Antisense | 5′-GGCACAGTGGAACAAGGACT-3′ | ||
| TNF-α | Sense | 5′-TCGGGCCAATGCCCTCCTGGCCAA-3′ | 468 |
| Antisense | 5′-GTAGACCTGCCCAGACTCGGCAAA-3′ |
IL-1β, Interleukin 1β; IL-6, interleukin-6; IL-8, interleukin-8; TNF-α, tumor necrosis factor- α.
The amplification cycle consisted of an initial denaturation of the template DNA at 95°C for 5 min, and then denaturation at 94°C for 1 min, annealing at 60°C for 1 min and an extension step at 72°C for 1 min. The final cycle included an extension step for 7 min at 72°C to ensure full extension of the product. Aliquots (10 µl) of each PCR product were analyzed by electrophoresis through 1.5% agarose S (Wako Pure Chemical Industries, Ltd.) gels containing ethidium bromide. The GAPDH gene was used as an internal control for normalization (19). The GAPDH expression was additionally used to establish the degree of expression of each cytokine by dividing the cytokine mRNA expression by the level of the GAPDH mRNA expression, which represented the average expression rate of the cytokines.
Statistical analysis
Statistical analysis was performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). Data from three parallel experiments are expressed as the means ± standard deviation (SD). Student's t-test was used to perform comparisons between two groups. Multi-group comparisons of the means were performed by one-way analysis of variance with post hoc Student-Newman-Keuls test. The correlation coefficient (R) was calculated for cytokine levels and LDH release by Spearman's rank correlation test. P<0.05 was considered to indicate a statistically significant difference.
Results
Inhibitory effects of sodium citrate on cell viability of AGS cells
To evaluate the effect of sodium citrate on cell viability, the viability of AGS cells was observed using a trypan blue exclusion assay following treatment with various concentrations of sodium citrate for 24, 48 and 72 h. Cell viability was expressed as a percentage of the control. As presented in Fig. 1, the viability of AGS cells decreased in a dose- and time-dependent manner following treatment with sodium citrate. Compared with the control conditions, high concentrations of sodium citrate, excluding 12.5 mM, significantly decreased the viability of AGS cells. In addition, a decrease in cell viability was associated with longer durations of sodium citrate administration. These results were confirmed by the cell proliferation assay, which assessed the effect of various concentrations of sodium citrate on AGS cell proliferation. The rate of AGS cell proliferation increased in a dose-dependent manner at the low concentration (0–3.125 mM); however, the proliferation rate decreased when the concentration exceeded 3.125 mM for each incubation time interval (24, 48 and 72 h; Fig. 2). Cell proliferation was significantly inhibited at the two higher concentrations (25 and 50 mM). Compared with controls, the proliferation rate decreased >60% (25 and 50 mM). In addition, various durations of administration revealed a similar effect to that of variations in dosage; low concentrations of sodium citrate (<3.125 mM) promoted AGS cell proliferation; however, concentrations >3.125 mM inhibited AGS cell proliferation (Fig. 2). These data revealed that the toxicity of sodium citrate for AGS cells was time- and dose-dependent from 3.125 to 50 mM for 24, 48 and 72 h. The cytotoxicity of sodium citrate was analyzed with an LDH release assay following the incubation of AGS cells with various concentrations of sodium citrate (Fig. 3). Administration times of 1 or 4 h had no effect with 3.125–50.0 mM sodium citrate. However, with an administration time of 24 h, LDH release increased in association with the increasing concentration of sodium citrate (3.125–50.0 mM; Fig. 3).
Figure 1.
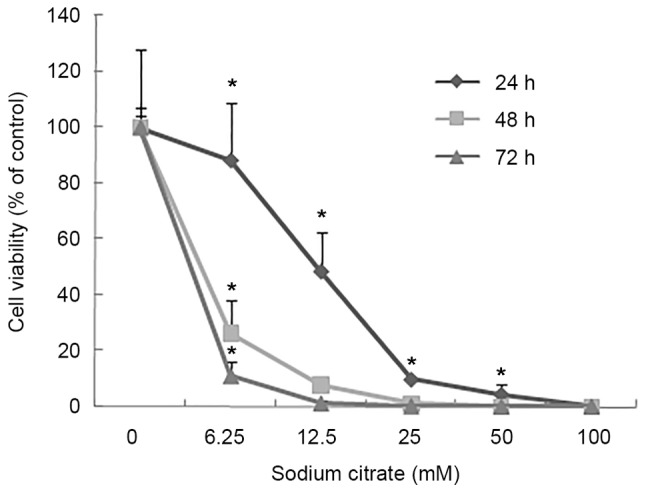
Percentage of viable AGS cells after 24, 48, or 72 h of various concentrations of sodium citrate treatment. AGS cell viability was reduced in a dose-dependent manner. Longer durations of exposure to sodium citrate, with increased dosages, elicited a marked decrease in AGS cell viability. Data are represented as the mean ± standard deviation. *P<0.05 vs. control. Statistical significance indicated by the asterisks.
Figure 2.
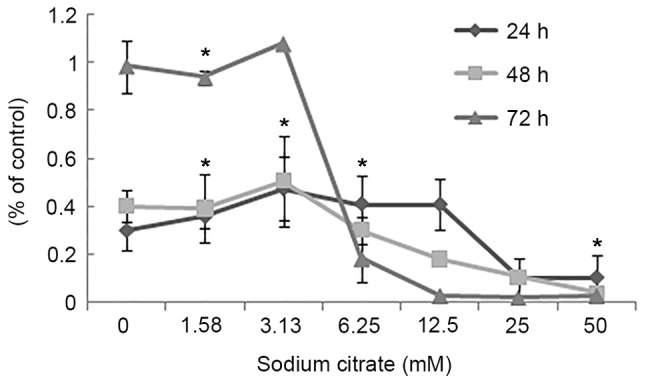
Effects of sodium citrate on AGS cell proliferation. As treatment time increased, the reduction in proliferation was more marked. When the treatment time >48 h, and the concentrations >3.125 mM, the reduction of proliferation was in an almost dose-dependent manner. Data are represented as the mean ± standard deviation. *P<0.05 vs. control. Statistical significance indicated by the asterisks.
Figure 3.
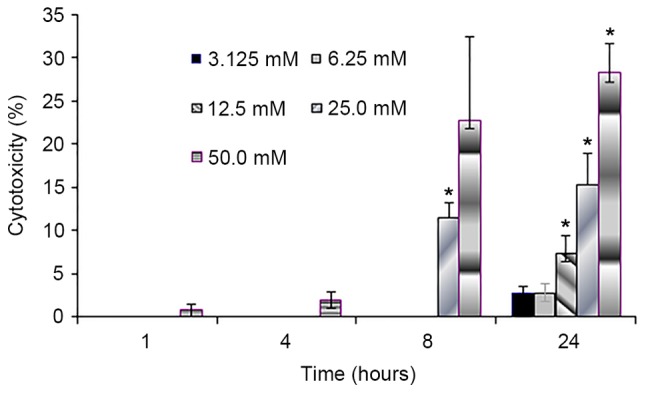
Lactate dehydrogenase release from the AGS cells following pre-treatment with various sodium citrate concentrations for a range of durations. Treatments for 8 and 24 h revealed a positive correlation between the degree of LDH release and concentration of sodium citrate (r=0.9697 for 8 h, *P<0.05; r=0.9906 for 24 h, P<0.05). Data represent mean values of determinations ± standard deviation. Statistical significance indicated by the asterisks. *P<0.05 vs. control.
AGS cells cultured in the presence of 3.125 and 6.25 mM sodium citrate exhibited LDH release values of <3%. Following a 24 h exposure to 3.125, 6.25, 12.5, 25 and 50 mM sodium citrate, the values of LDH release were 2.72, 2.83, 7.50, 15.40 and 28.30%, respectively. The results of the present study revealed that 1 and 4 h exposure to 3.125–50.0 mM sodium citrate had no effect on LDH release within AGS cells; however, longer durations of sodium citrate exposure time were associated with gradual increases in LDH release. Treatments for 8 and 24 h revealed a positive correlation between the increased degree of LDH release and concentration of sodium citrate (correlation coefficient was 0.9697 for 8 h, P<0.05; correlation coefficient 0.9906 for 24 h; P<0.05). The result was consistent with the aforementioned cell viability and proliferation experiment.
Effects of sodium citrate on the expression levels of cytokines
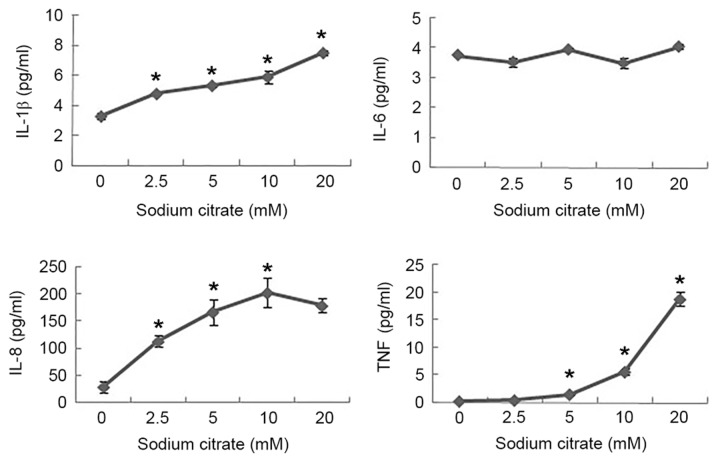
To investigate the effect of sodium citrate on the expression of cytokines, supernatants harvested at 24 h were determined by cytokine-specific ELISA, and the secretion of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6 and IL-8) from AGS cells stimulated with sodium citrate was analyzed. In the absence of sodium citrate, AGS cells released small amounts of IL-1β, IL-8 and TNF-α; however, in the presence of sodium citrate, the levels of IL-1β secretion increased in a dose-dependent manner. The highest levels of IL-1β secretion were 2.3-fold greater compared with that in the control. The levels of IL-8 secretion increased significantly following treatment with 2.5 mM sodium citrate, and increased further with rising concentrations of sodium citrate. The highest level of IL-8 secretion was 7.2-fold greater than the control level in stimulated cells. In addition, the levels of TNF-α increased with 10.0 mM sodium citrate; however, treatment with 20.0 mM sodium citrate further increased the levels of TNF-α to 18.8 pg/ml. Compared with the control, significant alterations in IL-6 levels were not observed (Fig. 4).
Figure 4.
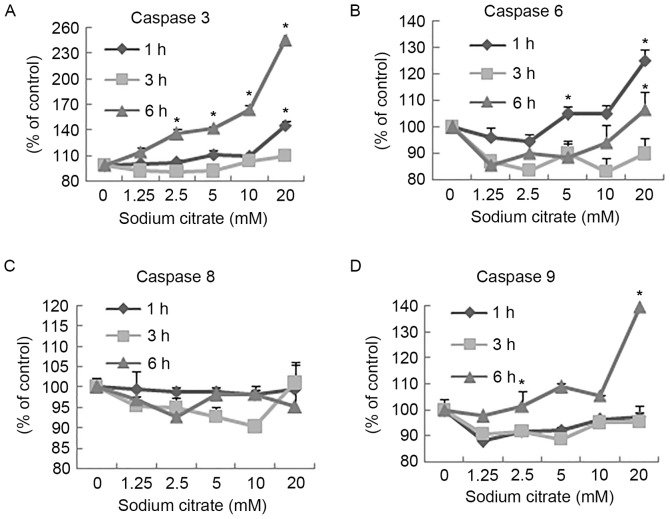
Activities of (A) caspase-3, (B) caspase-6, (C) caspase-8 and (D) caspase-9 within AGS cells treated with the indicated concentrations of sodium citrate for 1, 3 or 6 h. Data are represented as the mean ± standard deviation. *P<0.05 vs. control. Statistical significance indicated by the asterisks.
To verify the results of the present study, the levels of cytokine mRNAs for IL-1β, IL-6, IL-8 and TNF-α were assessed by RT-PCR. The highest levels of IL-1β mRNA was detected with 20 mM sodium citrate treatment. The levels of IL-8 and TNF-α mRNA expression increased with increasing doses of sodium citrate. IL-6 mRNA expression was not detected at any dose of sodium citrate (Fig. 5). The results of this assay were consistent with those of the previous ELISA assay results.
Figure 5.
Cytokines secretion profiles of cultured AGS cells in the presence or absence of sodium citrate. Cytokine protein levels are presented (ng/ml of culture supernatant) in the presence of sodium citrate for 24 h (6.25, 12.5 and 25 mM). Data are represented as the mean ± standard deviation. *P<0.05 vs. control. IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α. Statistical significance indicated by the asterisks.
Effects of sodium citrate on caspase activity
The activities of caspase-3 and −9 in AGS cells increased following 6 h of treatment with different concentrations of sodium citrate compared with the control (Fig. 4A and D). Treatment of cells with 20 mM sodium citrate for 1 h resulted in a marked increase in the activities of caspase-3 and −6 compared with cells treated with the control. The highest activities of all caspases were observed in the 20 mM treatment groups, with the exception of caspase-8, which was not altered with varying duration or concentration, compared with the control (Fig. 6).
Figure 6.
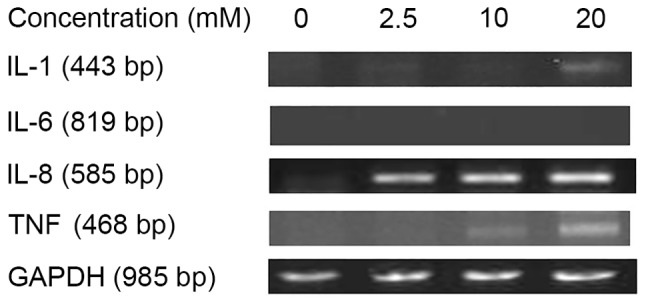
Cytokine mRNA profiles following in vitro treatment of AGS cells with the indicated concentrations of sodium citrate, GAPDH served as the normalization gene. IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α.
Discussion
A potential strategy to inhibit cancer cell growth may involve the targeting of glycolytic inhibitors (25). As high concentrations of citrate may inhibit the glycolytic pathway (26,27), the cytotoxic effects of citrate on AGS cells were investigated in the present study. Low concentrations of citrate (<3.125 mM) promoted AGS cell proliferation; however, higher doses were cytotoxic. This result may be caused by citrate affecting glucose metabolism within cancer cells (27). Furthermore, the cytotoxic effect of citrate within cancer cells has been reported to exert a synergistic effect with certain anticancer drugs (10). Additionally, as defined by the Warburg effect, LDH is an enzyme involved in obtaining energy following aerobic glycolysis in cancer cells. The positive correlation between the increased degree of LDH release and concentration of sodium citrate treatment indicated that the cytotoxic effect of sodium citrate observed in AGS cells increased as the concentration or time of treatment increased.
Caspases are central components of the apoptotic process, which function as initiators or executors in programmed cell death (28). Caspase-8 and −9 have been reported to act as initiators of apoptosis, and caspase-3 and −6 as executors (29). In the present study, the activities of caspase-3, −6, −8 and −9 were investigated. After 6 h of treatment with various concentrations of sodium citrate, the activities of caspase-3 and caspase-9 increased. These results are consistent with those of a previous study (30), which demonstrated the induction of caspase-3 and −8 cascade activation, resulting in cancer cell apoptosis. The experiment also demonstrated that, when AGS cells were exposed to 20 mM citrate for 1 h, caspase-6 was likely to be involved in regulating this apoptosis, as its activity level decreased shortly afterwards. However, the activity of caspase-8 was not affected by any concentration of sodium citrate (31). Therefore, treatment with sodium citrate induced AGS cell-apoptosis via the intrinsic, and not the extrinsic apoptotic pathway (32).
Cytokines are mediators involved in gastric physiology, as well as pathophysiology, and may serve important roles in the etiology of gastric cancer (33). In addition, numerous caspases serve as critical mediators in the integration of apoptotic and inflammatory pathways (32). However, certain pro-inflammatory cytokines, such as IL-1β, may be activated by caspase-8; the results of the present study revealed that IL-1β and IL-8 levels were increased, although an increase in caspase-8 activity was not observed within AGS cells. This indicated that the levels of IL-1β and IL-8 may be regulated by other caspases, such as caspase-1 or −11, or by various mechanisms. Although TNF-α was not detected in the ELISA assay, it was detected by RT-PCR. The discrepancy between the ELISA and RT-PCR results may be due to sodium citrate affecting only the expression of TNF-α mRNA. In addition, the levels of IL-6 were possibly too low for detection in the present study.
As observed in the extrinsic apoptosis pathway, moderate levels of IL-6 and IL-8 cytokines are detected in Fas-mediated apoptosis (33). Fas receptor stimulation has also been demonstrated to induce phagocyte migration in vivo (33), indicating that it activates the apoptotic and pro-inflammatory pathways, thus facilitating the elimination of dying cells. Therefore, the apoptosis of AGS cells induced by sodium citrate via the intrinsic pathway is likely to occur via the same mechanism. This cytotoxic mechanism probably operates synergistically, inducing apoptosis via the intrinsic pathway and altering the cytokine expression profile.
In the present study, exposing AGS cells to higher concentrations of sodium citrate or longer durations of treatment may elicit a cytocidal effect, which was observed via a reduction in cell viability and proliferation, an increase in LDH release, an induction of the intrinsic pathway of apoptosis and alterations in the expression levels of certain cytokines.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81473017 and 31460249).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Leumann E, Hoppe B, Neuhaus T, Blau N. Efficacy of oral citrate administration in primary hyperoxaluria. Nephrol Dial Transpl. 1995;10(Suppl 8):S14–S16. doi: 10.1093/ndt/10.supp8.14. [DOI] [PubMed] [Google Scholar]
- 2.Phillips R, Hanchanale VS, Myatt A, Somani B, Nabi G, Biyani CS. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst Rev: CD010057. 2015 doi: 10.1002/14651858.CD010057.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastorino J, Hansen CL, McMahon DJ. Effect of sodium citrate on structure-function relationships of Cheddar cheese. J Dairy Sci. 2003;86:3113–3121. doi: 10.3168/jds.S0022-0302(03)73912-5. [DOI] [PubMed] [Google Scholar]
- 4.Westergaard N, Waagepetersen HS, Belhage B, Schousboe A. Citrate, a ubiquitous key metabolite with regulatory function in the CNS. Neurochem Res. 2017;42:1583–1588. doi: 10.1007/s11064-016-2159-7. [DOI] [PubMed] [Google Scholar]
- 5.Wang JY, Zhu SG, Xu CF. Essential Biochemistry. 1st. Vol. 20. Higher Education Press; Beijing: 2010. pp. 340–342. [Google Scholar]
- 6.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 9.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Varin E, Allouche S, Lu Y, Poulain L, Icard P. Effect of citrate on malignant pleural mesothelioma cells: A synergistic effect with cisplatin. Anticancer Res. 2009;29:1249–1254. [PubMed] [Google Scholar]
- 11.Izyumov DS, Avetisyan AV, Pletjushkina OY, Sakharov DV, Wirtz KW, Chernyak BV, Skulachev VP. ‘Wages of fear’: Transient threefold decrease in intracellular ATP level imposes apoptosis. Biochim Biophys Acta. 2004;1658:141–147. doi: 10.1016/j.bbabio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell. 1999;3:159–167. doi: 10.1016/S1097-2765(00)80307-X. [DOI] [PubMed] [Google Scholar]
- 13.Yousefi S, Owens JW, Cesario TC. Citrate shows specific, dose-dependent lympholytic activity in neoplastic cell lines. Leuk Lymphoma. 2004;45:1657–1665. doi: 10.1080/10428190310001603920. [DOI] [PubMed] [Google Scholar]
- 14.Wang YS, Wang ZY. Sodium citrate induces apoptosis in biocontrol yeast Cryptococcus laurentii. J Appl Microbiol. 2012;113:135–142. doi: 10.1111/j.1365-2672.2012.05312.x. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Misumi A, Shimaoka K, Aoki F, Esaki F. Stomach cancer-related mortality. Eur J Cancer Prev. 2001;10:61–67. doi: 10.1097/00008469-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol. 2014;20:13767–13774. doi: 10.3748/wjg.v20.i38.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XQ, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: Epidemiological and biological evidence. World J Gastroenterol. 2009;15:2204–2213. doi: 10.3748/wjg.15.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves GK, Pirie K, Green J, Bull D, Beral V, Million Women Study Collaborators Comparison of the effects of genetic and environmental risk factors on in situ and invasive ductal breast cancer. Int J Cancer. 2012;131:930–937. doi: 10.1002/ijc.26460. [DOI] [PubMed] [Google Scholar]
- 19.Sun J, Bi L, Chi Y, Aoki K, Misumi J. Effect of sodium acetate on cell proliferation and induction of proinflammatory cytokines: A preliminary evaluation. Food Chem Toxicol. 2005;43:1773–1780. doi: 10.1016/j.fct.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Aoki K, Wang W, Guo A, Misumi J. Sodium nitrite-induced cytotoxicity in cultured human gastric epithelial cells. Toxicol in Vitro. 2006;20:1133–1138. doi: 10.1016/j.tiv.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Bo A, Chi B, Xia Y, Su X, Sun J. Magnesium sulfate induced toxicity in vitro in AGS gastric adenocarcinoma cells and in vivo in mouse gastric mucosa. Asian Pac J Cancer Prev. 2015;16:71–76. doi: 10.7314/APJCP.2015.16.1.71. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Zhang X, Zhang H, Lan J, Huang G, Varin E, Lincet H, Poulain L, Icard P. Citrate induces apoptotic cell death: A promising way to treat gastric carcinoma? Anticancer Res. 2011;31:797–805. [PubMed] [Google Scholar]
- 23.Guo X, Zhang X, Wang T, Xian S, Lu Y. 3-Bromopyruvate and sodium citrate induce apoptosis in human gastric cancer cell line MGC-803 by inhibiting glycolysis and promoting mitochondria-regulated apoptosis pathway. Biochem Biophys Res Commun. 2016;475:37–43. doi: 10.1016/j.bbrc.2016.04.151. [DOI] [PubMed] [Google Scholar]
- 24.Barranco SC, Townsend CM, Jr, Casartelli JC, Macik BG, Burger NL, Boerwinkle WR, Gourley WK. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res. 1983;43:1703–1709. [PubMed] [Google Scholar]
- 25.Xintaropoulou C, Ward C, Wise A, Marston H, Turnbull A, Langdon SP. A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget. 2015;6:25677–25695. doi: 10.18632/oncotarget.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randle PJ, Denton RM, England PJ. Citrate as a metabolic regulator in muscle and adipose tissue. Biochem Soc Symp. 1968;27:87–103. [PubMed] [Google Scholar]
- 27.Icard P, Poulain L, Lincet H. Understanding the central role of citrate in the metabolism of cancer cells. Biochim Biophys Acta. 2012;1825:111–116. doi: 10.1016/j.bbcan.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Elinos-Báez CM, Maldonado V, Meléndezzajgla J. Caspases: Apoptosis inducing molecules. Gac Med Mex. 2003;139:493–499. (In Spanish) [PubMed] [Google Scholar]
- 29.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 30.Kruspig B, Nilchian A, Orrenius S, Zhivotovsky B, Gogvadze V. Citrate kills tumor cells through activation of apical caspases. Cell Mol Life Sci. 2012;69:4229–4237. doi: 10.1007/s00018-012-1166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim GY, Park SY, Jo A, Kim M, Leem SH, Jun WJ, Shim SI, Lee SC, Chung JW. Gecko proteins induce the apoptosis of bladder cancer 5637 cell by inhibiting Akt and activating intrinsic caspase cascade. BMB Rep. 2015;48:531–536. doi: 10.5483/BMBRep.2015.48.9.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creagh EM. Caspase crosstalk: Integration of apoptotic and innate immune signalling pathways. Trends Immunol. 2014;35:631–640. doi: 10.1016/j.it.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: A HuGE systematic review and meta-analyses. Am J Epidemiol. 2011;173:259–270. doi: 10.1093/aje/kwq370. [DOI] [PMC free article] [PubMed] [Google Scholar]