Abstract
The endoplasmic reticulum (ER) is the principal organelle responsible for the synthesis, initial post-translational modification, folding, export and secretion of proteins. It is also responsible for the maintenance of cellular homeostasis. In response to cellular stress conditions including glucose deprivation, hypoxia and changes in calcium homeostasis, ER stress machinery is activated and triggers the unfolded protein response, resulting in the restoration of homeostasis or activation of cell death. Glucose-regulated protein 78 (GRP78), a molecular chaperone, may be induced by ER stress at the transcriptional and translational level. A number of studies have demonstrated that GRP78 serves an important role in tumor cell proliferation, metastasis, angiogenesis and drug-resistance. The present review systematically describes the association between GRP78 expression and gastric cancer pathogenesis, and emphasizes that GRP78 is a novel diagnostic and therapeutic biomarker of gastric cancer.
Keywords: endoplasmic reticulum stress, unfolded protein response, glucose-regulated protein 78, gastric cancer
1. Introduction
Gastric cancer (GC) ranks fourth in incidence and second as a cause of mortality among all types of cancer worldwide (despite the decreased incidence in certain regions) (1). Surgical resection, chemotherapy and radiation remain the most common therapeutic modalities. Surgical resection is currently the only curative treatment for early-stage GC. However, the majority of patients are diagnosed at advanced disease stages or relapse following curative surgical treatment (1).
Despite advances in the detection, surgical resection and adjuvant therapy for GC, the 5-year survival rates of these patients remain <30% (2). The aggressive nature of human GC is associated with a variety of intracellular events including activation of various oncogenes, inactivation of tumor suppressor genes and abnormal expression of growth factors and their receptors (3,4). These perturbations result in a marked growth advantage for GC cells. Thus, to improve the low survival outcomes and assist in earlier diagnosis of patients with GC, identification and validation of new prognostic and therapeutic tumor markers is urgently required. These improved biomarkers may in turn provide new approaches for the early detection and effective treatment of GC.
2. Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR)
The ER is an organelle responsible for the synthesis, initial post-translational modification, folding, export and secretion of proteins (5). Disturbances in the ER environment by cellular stress conditions, including nutrient deprivation, alterations in glycosylation status, hypoxia, pH changes, poor vascularization, changes in calcium homeostasis and treatment with a variety of agents, may lead to ER stress and subsequent accumulation of unfolded or misfolded proteins in the ER (6,7). To overcome perturbations in ER function and ER stress to improve survival, the ER has evolved specific signaling pathways, which are collectively termed the UPR (8). The UPR is initiated in concerted action through the signaling of three prototypical ER-localized stress sensors: RNA-dependent protein kinase-like ER kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring enzyme 1 (IRE1) (6,9,10). Upon ER stress, ER-resident chaperones [e.g., glucose-regulated protein (GRP) 78] bind to misfolded proteins, activating IRE1, ATF6 and PERK. PERK is also activated by dimerization and autophosphorylation, subsequently phosphorylates eukaryotic initiation factor 2α (eIF2α). Phosphorylated eIF2α inhibits protein synthesis and activates the transcription of ATF4, inducing the transcription of its downstream genes (11–13). IRE1 assists in protein folding and degradation, and produces a spliced form of X box-binding protein-1 due to its RNase activity. ATF6 translocates from the ER to the Golgi apparatus where it is cleaved by protease activity, forming active nuclear ATF6, a regulator of gene expression (14). Collectively, ER stress is alleviated by the downstream effects of the UPR. However, if ER stress is severe or prolonged, distinct death signals may be transduced during the UPR, leading to cellular apoptosis (15,16). These signals include CCAAT/enhancer-binding protein homologous protein (CHOP) transcription factor, p53 unregulated modulator of apoptosis (PUMA), c-Jun N-terminal kinase (JNK), nicotinamide adenine dinucleotide phosphate oxidase activator (NOXA), B-cell lymphoma 2 (Bcl-2)-like 11 and Bcl-2 homology (BH) 3-only proteins and caspases (16–22).
3. ER stress and tumors
Tumor cells proliferate continuously and require effective high-energy-producing systems due to their high proliferation characteristics compared with non-tumorigenic cells. Solid tumors typically grow faster than their blood supply is able to nurture, creating specific growth conditions characterized by hypoxia, glucose deprivation and lactic acidosis, which trigger ER stress (23). The interactions between cancerous cells and this tumor microenvironment during the course of multistep tumorigenesis are reported to serve a critical role in the modulation of tumor growth, metabolism and metastasis (24–26).
ER stress has a dual effect on tumors. First, it has an adaptive effect, enhancing tumor growth. ER stress may restore homeostasis and make the adjacent environment hospitable for tumor survival, growth and expansion (27). Baird et al (28) demonstrated that the UPR was induced and promoted the neoplastic transformation of Helicobacter-infected gastric mucosa in the milieu of Helicobacter-induced chronic inflammation and mucous metaplasia. Pike et al (29) revealed that under severely hypoxia conditions, ATF4 transcriptionally upregulated unc-51-like autophagy-activating kinase 1 (ULK1), which is required for autophagy contributing to human breast cancer cell survival. Hypoxia also induces breast cancer cell migration via the PERK/ATF4/lysosome-associated membrane protein 3 (LAMP3) signaling pathway (30). On the other hand, ER stress contributes cytotoxic effects, inducing apoptosis. Signal transducer and activator of transcription 6 (STAT6) silencing elicited ER stress-mediated apoptosis in lung cancer cells through CHOP induction, alteration of BH3 protein expression and reactive oxygen species (ROS) production (31). Resveratrol (3,5,4′-trihydroxy-trans-stilbene) exerted its cytotoxic role in cancer cells exposed to palmitate, a saturated fatty acid, triggering a lipid-mediated cell death, which is promoted by ER stress through a CHOP-mediated apoptotic process (32). Lactacystin (LAC) treatment increases the expression of protein disulfide-isomerase (PDI), GRP78, CHOP, cleaved caspase-4 and cleaved caspase-3 induced by cisplatin in HeLa cells, suggesting that LAC may enhance cisplatin cytotoxicity by increasing ER stress-associated apoptosis (33).
4. GRP78 and its signaling pathway
GRP78, also known as the immunoglobulin heavy chain-binding protein, and other GRPs are ER chaperones which belong to the heat-shock protein family (34,35). In the late 1970s, upon rapid depletion of glucose from the culture medium of chick embryo fibroblasts, the amount of an unknown protein with a molecular mass of 78 kDa was identified to be significantly increased and was subsequently termed GRP78 (36). GRP78 was also revealed to be present in the plasma membrane, cytoplasm, mitochondria, nucleus and the cellular secretions of tumor cells (37). The GRP78 promoter region contains a highly conserved region (consisting of CCAAT-like sequences flanked by GC-rich motifs) termed the cis-acting endoplasmic reticulum stress-response element (ERSE), reportedly required for transcriptional activation in response to ER stress (38). The promoter also contains other important motifs including a cyclic adenosine 3′,5′-monophosphate response element (CRE) and 12-O-tetradecanoylphorbol-13-acetate (TPA) DNA-response element (TRE) motif (39,40). As an ER stress-associated protein, GRP78 is involved in protein folding and assembly, proteasomal degradation of misfolded proteins, ER Ca2+ binding and cell survival during damaging conditions (41).
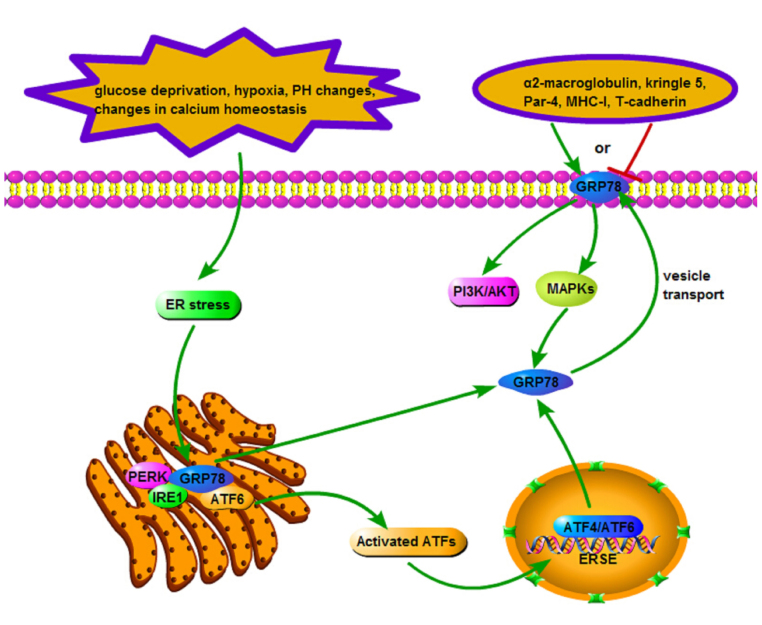
Under non-stress conditions, GRP78 binds to three UPR sensors (PERK, IRE1 and ATF6) rendering them inactive. In response to ER stress, GRP78 preferentially associates with the unfolded proteins instead of the typical sensors (42), following which the UPR becomes activated. The UPR sensors are important as they elicit damage control pathways synergistically, partially due to the activation by ATFs (ATF4 and ATF6). Nuclear form ATFs act on the ERSE, increasing the expression of GRP78. Overexpression of GRP78 is hypothesized to be redistributed to the cell surface by means of vesicle transport (43). It recognizes extracellular ligands including α2-macroglobulin, kringle 5, prostate apoptosis response-4 (Par-4), major histocompatibility complex I (MHC-I) and T-cadherin, transducing corresponding signals. Ligands binding to the N-terminal domain of GRP78 induce a proproliferative and antiapoptotic response, whereas binding to the C-terminal domain inhibits cell proliferation and triggers apoptosis (44). This biological effect is associated with the activation of the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) and mitogen-activated protein kinase (MAPK) signaling pathways (45,46). The activation of the MAPK signaling pathways leads to a rapid induction of GRP78 (47), presenting GRP78-associated signal transduction in the form of a feedback loop (Fig. 1) (48).
Figure 1.
Signaling pathway of GRP78. Upon ER stress, GRP78 separates from UPR sensors (PERK, IRE1 and ATF6) and preferentially associates with unfolded proteins, upon which UPR is activated. These sensors elicit damage control pathways synergistically, activated partially by ATFs (ATF4 and ATF6). The nuclear form ATFs subsequently act on ERSE, increasing the expression of GRP78. Overexpressed GRP78 may be redistributed to the cell surface by means of vesicle transport, recognizing extracellular ligands and activating PI3K/AKT and MAPK signaling pathways. The activation of PI3K/AKT and MAPK signaling pathways may also lead to the rapid induction of GRP78. GRP, glucose-regulated protein; ER, endoplasmic reticulum; UPR, unfolded protein response; PERK, protein kinase-like ER kinase; IRE1, inositol-requiring enzyme 1; ATF, activating transcription factor; ERSE, endoplasmic reticulum stress-response element; PI3 K, phosphoinositide 3-kinase; AKT, protein kinase B; MAPK, mitogen-activated protein kinase; MHC, major histocompatibility complex; Par-4, prostate apoptosis response-4.
5. GRP78 and gastric cancer
Numerous studies on GRP78 have been focused on tumor development and progression. Overexpression of GRP78 has been identified in a variety of tumors including digestive, urinary, cerebral, mammary and respiratory system tumors. In general, GRP78 expression is positively associated with tumor malignancy. For example, GRP78 expression increased with the progression from early to advanced colorectal cancer stages (49). Furthermore, a significant association was identified between GRP78 expression and response to chemotherapy (49). Guan et al (50) reported that GRP78 and melanoma differentiation-associated gene-9 (MDA-9) were expressed in lymph node metastases at increased levels. Furthermore, exosomes from serum samples of patients with metastatic melanoma contained increased levels of MDA-9 and GRP78 compared with patients without metastases, indicating the potential of MDA-9 and GRP78 as biomarkers for the early detection of metastasis. Caspases cause poly (ADP-ribose) polymerase (PARP) cleavage and inactivation during apoptosis. Jiang et al (51) demonstrated that knockdown of GRP78 by small interfering (si)RNA enhanced PARP cleavage in human pancreatic cancer cell lines. Conversely, induction of GRP78 on the cell surface by doxorubicin and tunicamycin has been previously associated with CHOP/growth arrest- and DNA damage-inducible gene 153 (GADD153) upregulation and increased apoptosis in triple-negative breast cancer tumor cells (52). Overexpression of GRP78 is associated with early clinical stage and improved survival in patients with neuroblastoma (53). The following subsections systematically and comprehensively summarize the studies which have elucidated the role of GRP78 in GC.
GRP78 expression and its clinical characteristics in GC
Numerous studies have identified that GRP78 is detected in the sera of patients with GC along with its autoantibody (54), and it is significantly upregulated in GC cells as well as in surgical specimens of gastric tumors (55–58). Other studies have demonstrated that GRP78 is positively associated with tumor size, depth of invasion, poor differentiation, tumor-node-metastasis stage, lymphatic and venous invasion, lymph node metastasis, short time to recurrence and chemoresistance, although it is not associated with sex or age (55–58). In the light of this evidence, GRP78 is considered an objective and effective marker for predicting the aggressive behavior and poor prognosis of patients with GC.
GRP78, cell proliferation and apoptosis in GC
In a gastric tumor, cancer cells are able to adapt to a variety of ER stressors by inducing GRP78, which promotes cancer cell proliferation and inhibits apoptosis. For example, in flow cytometry analysis, it had previously been revealed that the downregulation of GRP78 markedly inhibited the proliferation of GC cells at the G1 phase, whereas GRP78 overexpression promoted cell cycle progression (55). These results suggest that GRP78 promotes GC cell proliferation. Under hypoxia stress, a protein kinase Cε/Raf-1/MAPK-extracellular-signal-regulated kinase (ERK) kinase (MEK)/ERK/activator protein 1 (AP1) signaling cascade induced GRP78 expression in human GC cells by acting on a TPA-response element-like element of the GRP78 promoter (47). In the presence of the MEK inhibitor U0126, activation of caspase-3 and cleavage of its substrate PARP by the ER stress inducer tunicamycin or thapsigargin was enhanced in GC cells, although overexpression of Bcl-2 inhibited this apoptosis (59). Therefore, Zhang et al (59) concluded that the inhibition of MEK blocked the ER stress-mediated upregulation of GRP78 and enhanced ER stress-induced apoptosis through a caspase- and mitochondria-mediated mechanism. This activation of the MEK/ERK signaling pathway by ER stress is hypothesized to be necessary for the induction of GRP78, which protects GC cells against apoptosis (59).
Conversely, if the stress exceeds the threshold that GC cells can afford, apoptosis may be initiated. For instance, following treatment with 10 µg/ml tunicamycin for 24 h, GRP78 and CHOP were upregulated whereas Bcl-2 was downregulated in the GC cell line BGC823, ultimately leading to apoptosis (60). Vitamin E succinate (RRR-α-tocopheryl succinate; VES) causes cytological changes typical of apoptosis by increasing ER dilation and cytosolic Ca2+ concentration. Upon treatment with VES at a concentration of 20 µg/ml, GRP78 was demonstrated to be transcriptionally and translationally induced in a time-dependent manner while the induction of CHOP, caspase-4 and JNK were observed (61). Huang et al (62) additionally revealed that, in response to α-tocopheryl succinate (α-TOS), induction of GRP78 and CHOP and activation of caspase-4 were also observed which are cytological changes typical of apoptosis.
GRP78 variants and promoter polymorphisms in GC
Rauschert et al (63) isolated a human monoclonal IgM antibody, SAM-6, from a patient with GC. The antibody was revealed to bind a previously unknown variant of GRP78 with a molecular mass of 82 kDa, a variant eventually known as GRP78SAM-6. The epitope is an O-linked carbohydrate moiety which is only expressed on malignant cell membranes. This variant qualifies as a target for immune surveillance and antibody responses, making it an ideal target for novel therapeutic approaches of patients with GC. Winder et al (64) have additionally reported that patients with GC with the combined GRP78 rs391957 C/T and T/T genotype exhibit an increased risk of tumor recurrence and mortality than those with C/C. These data suggest that GRP78 rs391957 polymorphism may be capable of predicting clinical outcomes in patients with localized GC (64).
GRP78 and GC cell invasion and metastasis
Previous studies have identified that the expression of GRP78 has a clear association with the invasion and metastasis of GC cells (55,65). Zhang et al (65) revealed that overexpression of GRP78, induced by the transcription factor specificity protein 1 (Sp1), increased lymph node metastasis in patients with GC. Knocking down GRP78 expression inhibited GC cell invasion in vitro and cellular proliferation and metastasis in a xenograft nude mouse model. Yang et al (55) similarly reported that GRP78 expression was increased in tumors from GC patients with deep tumor infiltration and lymph node metastasis compared with tumors from patients without these features. These results suggest that GRP78 may promote invasion and metastasis of GC cells and that the dysregulated expression of GRP78 may contribute to the development and progression of GC.
GRP78 and chemoresistance of GC cells
Over the last decades, standard multimodal treatment strategies have failed to cure a large proportion of patients with GC, particularly those with advanced and metastatic disease, ultimately contributing to poor survival rates (1). Certain investigators have suggested that this is possibly due to a chemoresistance phenomenon occurring during treatment (66). For example, an adenosine 5′-triphosphate tumor chemosensitivity assay has demonstrated that increased GRP78 expression is associated with the chemoresistance of GC cells to chemotherapeutic agents, whereas negative GRP78 expression was associated with increased sensitivity to drugs and regimens (56). However, the underlying molecular mechanisms of this observed outcome remain to be further clarified and are urgently required for more effective clinical intervention and improved patient management.
Celecoxib, a non-steroidal anti-inflammatory drug, induces apoptosis in cancer cells. In human GC cells, overexpression of GRP78 induced by celecoxib partially suppresses the induction of CHOP and protects cancer cells from celecoxib-induced apoptosis (67). Additionally, suppression of GRP78 expression by siRNA markedly stimulates the expression of CHOP and cellular apoptosis in the presence of celecoxib. These results suggest that upregulation of ER chaperones by celecoxib decreases the potential antitumor activity of celecoxib.
GRP78, diagnosis and targeted therapy of GC
Since no specific symptoms have been characterized in patients with early GC, there is a lack of convenient means of census screening, consequently contributing to the currently low detection rate of patients with early GC. Radiation or chemotherapy of patients with GC is often accompanied by enormous biocytotoxicity and side effects, often resulting in poor patient outcomes. Molecular targeting therapy has been proposed to eradicate tumors through the targeting of specific tumor markers. Uncovering reliable biomarkers of GC associated with tumorigenesis and progression is crucial for effective diagnosis and successful treatment, leading to improved therapeutic outcomes and patient quality of life (68,69). Interestingly, the radioactive intensity measured in animals with GC xenografts administered with GRP78-binding peptide-guided 111In-labeled micelles is statistically increased compared with animals administered with 111In-labeled micelles alone (70). These results suggest that GRP78 is an effective probing target in the application of nuclear imaging for GC diagnosis. Kang et al (71) identified that GC multidrug resistance (MDR) cell-specific binding peptide GMBP1 may specifically bind to GRP78 on the surface of GC MDR cells, resensitizing GC MDR cells to a variety of chemotherapeutic agents by downregulating GRP78 expression and inhibiting MDR1 expression. These results provide new insight into the management of MDR in GC cells.
Furthermore, versipelostatin (VST), a novel macrocyclic compound, may inhibit transcription from the GRP78 promoter. VST alone and in combination with cisplatin significantly inhibits tumor growth of GC cell MKN74 xenografts compared with untreated controls (72). Cheng et al (73) designed a GRP78-binding peptide which may selectively recognize and bind to GC MKN45 cells in vitro. Additionally, the overexpression of GRP78 has been used as a targeted protein to guide drugs to GC cells, leading to a more effective treatment for GC xenografts (73). This study demonstrated that a GRP78-mediated drug targeting system may deliver chemotherapeutic drugs with increased targeting precision to GC cells, leading to minimized side effects in patients during chemotherapy (73).
Finally, the thymidine kinase gene of herpes simplex virus (HSV-tk) is a suicide gene when administrated with the prodrug ganciclovir (GCV). HSV-tk may phosphorylate GCV to become GCV triphosphate which is incorporated into cellular DNA, resulting in termination of DNA synthesis and cell death (74). In an experimental system, the HSV-tk gene may be controlled by the GRP78 promoter or the long terminal repeat (LTR) promoter. Under stress conditions in a fast-growing solid tumor, the LTR promoter was suppressed and thus unable to sustain the foreign gene expression (75); however, the stress-inducible protein GRP78 was markedly induced (76). This constitutes an ideal gene therapy system to selectively kill tumor cells without affecting normal tissues. Azatian et al (77) have demonstrated that, compared with LTR-tk/GCV, GRP78-tk/GCV treatment resulted in complete tumor elimination in GC cells with no p53 mutations in vitro and in vivo (Table I).
Table I.
Anti-GRP78 drugs and their effects and molecular mechanisms in GC.
| Anti-GRP78 drug | Effect | Mechanism |
|---|---|---|
| GMBP1 | Resensitizes GC MDR cells to chemotherapeutic agents | Downregulates GRP78 and MDR1 expression |
| Versipelostatin | Inhibits tumor growth of GC cell | Inhibits transcription from the promoter of GRP78 |
| GRP78BP | A targeted protein to guide drugs to GC cells | Selectively recognizes and binds to GC cells |
| GRP78-tk/GCV | A suicide gene when administrated with the prodrug GCV | The HSV-tk gene may be controlled by GRP78 promoter |
GRP, glucose-regulated protein; GC, gastric cancer; MDR, multidrug resistance; BP, binding protein; tk, thymidine kinase; GCV, ganciclovir; HSV, herpes simplex virus.
6. Conclusions and perspective
The ER stress-associated protein GRP78 is overexpressed in GC and promotes proliferation and inhibition of apoptosis of GC cells. GRP78 may serve a critical role in GC cell invasion and metastasis as well as the development of chemotherapeutic resistance. Clinically, GRP78 expression has a clear association with the prognosis of patients with GC. As a biomarker of GC, GRP78 may improve the efficiency of early diagnosis for patients with GC. As a therapeutic target, GRP78-targeting therapy may improve therapeutic outcomes and quality of life in patients with GC. However, the causal role of GRP78 in GC pathogenesis and eventual translation into the clinic warrants further study, particularly the aspects of the underlying molecular mechanisms of action. Other functions of GRP78 in GC, including angiogenesis, should also be explored in depth. Further understanding of the roles of GRP78 in GC may provide physicians broader prospects for the effective treatment of GC patients.
Acknowledgements
The preparation of the present review was supported by the National Natural Science Foundation of China (grant no. 81401025) and the Youth Science and Research Foundation of Nantong Municipal Planning Commission (grant no. WQ2016058).
References
- 1.Piazuelo MB, Correa P. Gastric cáncer: Overview. Colomb Med (Cali) 2013;44:192–201. [PMC free article] [PubMed] [Google Scholar]
- 2.Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156–169. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: Friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 4.Lee AS. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/S0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz A, Herzog V. Endoplasmic reticulum-associated degradation: Exceptions to the rule. Eur J Cell Biol. 2004;83:501–509. doi: 10.1078/0171-9335-00412. [DOI] [PubMed] [Google Scholar]
- 6.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 7.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI0216886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell. 2001;107:827–830. doi: 10.1016/S0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman RJ, Scheuner D, Schröder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/MCB.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 13.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/S1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 14.Schindler AJ, Schekman R. In vitro reconstitution of ER-stress induced ATF6 transport in COPII vesicles; Proc Natl Acad Sci USA; 2009; pp. 17775–17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 16.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 18.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: A matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 19.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 20.Rutkowski DT, Kaufman RJ. Atripto the ER: Coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-Q. [DOI] [PubMed] [Google Scholar]
- 22.Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation ofcaspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 23.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 24.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 25.Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 26.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 27.Martinon F. Targeting endoplasmic reticulum signaling pathways in cancer. Acta Oncol. 2012;51:822–830. doi: 10.3109/0284186X.2012.689113. [DOI] [PubMed] [Google Scholar]
- 28.Baird M, Woon Ang P, Clark I, Bishop D, Oshima M, Cook MC, Hemmings C, Takeishi S, Worthley D, Boussioutas A, et al. The unfolded protein response is activated in Helicobacter-induced gastric carcinogenesis in a non-cell autonomous manner. Lab Invest. 2013;93:112–122. doi: 10.1038/labinvest.2012.131. [DOI] [PubMed] [Google Scholar]
- 29.Pike LR, Singleton DC, Buffa F, Abramczyk O, Phadwal K, Li JL, Simon AK, Murray JT, Harris AL. Transcriptional up-regulation of ULK1 by ATF4 contributes to cancer cell survival. Biochem J. 2013;449:389–400. doi: 10.1042/BJ20120972. [DOI] [PubMed] [Google Scholar]
- 30.Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC, Span PN. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2. doi: 10.1186/bcr3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubey R, Saini N. STAT6 silencing up-regulates cholesterol synthesis via miR-197/FOXJ2 axis and induces ER stress-mediated apoptosis in lung cancer cells. Biochim Biophys Acta. 2015;1849:32–43. doi: 10.1016/j.bbagrm.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Rojas C, Pan-Castillo B, Valls C, Pujadas G, Garcia-Vallve S, Arola L, Mulero M. Resveratrol enhances palmitate-induced ER stress and apoptosis in cancer cells. PLoS One. 2014;9:e113929. doi: 10.1371/journal.pone.0113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Li D, Zeng L, Wang C, Zhang L, Wang Y, Yu Y, Liu S, Li Z. Proteasome inhibitor lactacystin enhances cisplatin cytotoxicity by increasing endoplasmic reticulum stress-associated apoptosis in HeLa cells. Mol Med Rep. 2015;11:189–195. doi: 10.3892/mmr.2014.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS. The glucose-regulated proteins (GRP78 and GRP94): Functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr. 1994;4:1–18. doi: 10.1615/CritRevEukarGeneExpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- 35.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 36.Pouysségur J, Shiu RP, Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977;11:941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki CK, Bonifacino JS, Lin AY, Davis MM, Klausner RD. Regulating the retention of T-cell receptor alpha chain variants within the endoplasmic reticulum: Ca(2+)-dependent association with BiP. J Cell Biol. 1991;114:189–205. doi: 10.1083/jcb.114.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- 39.Lee AS. Mammalian stress response: Induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992;4:267–273. doi: 10.1016/0955-0674(92)90042-B. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Zhang K, Li Z. Unfolded protein response in cancer: The physician's perspective. J Hematol Oncol. 2011;4:8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: Signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- 43.Misra UK, Gonzalez-Gronow M, Gawdi G, Pizzo SV. The role of MTJ-1 in cell surface translocation of GRP78, a receptor for alpha 2-macroglobulin-dependent signaling. J Immunol. 2005;174:2092–2097. doi: 10.4049/jimmunol.174.4.2092. [DOI] [PubMed] [Google Scholar]
- 44.Misra UK, Pizzo SV. Modulation of the unfolded protein response in prostate cancer cells by antibody-directed against the carboxyl-terminal domain of GRP78. Apoptosis. 2010;15:173–182. doi: 10.1007/s10495-009-0430-y. [DOI] [PubMed] [Google Scholar]
- 45.Fu R, Yang P, Wu HL, Li ZW, Li ZY. GRP78 secreted by colon cancer cells facilitates cell proliferation via PI3K/Akt signaling. Asian Pac J Cancer Prev. 2014;15:7245–7249. doi: 10.7314/APJCP.2014.15.17.7245. [DOI] [PubMed] [Google Scholar]
- 46.Lu MC, Lai NS, Yin WY, Yu HC, Huang HB, Tung CH, Huang KY, Yu CL. Anti-citrullinated protein antibodies activated ERK1/2 and JNK mitogen-activated protein kinases via binding to surface-expressed citrullinated GRP78 on mononuclear cells. J Clin Immunol. 2013;33:558–566. doi: 10.1007/s10875-012-9841-6. [DOI] [PubMed] [Google Scholar]
- 47.Song MS, Park YK, Lee JH, Park K. Induction of glucose-regulated protein 78 by chronic hypoxia in human gastric tumor cells through a protein kinase C-epsilon/ERK/AP-1 signaling cascade. Cancer Res. 2001;61:8322–8330. [PubMed] [Google Scholar]
- 48.Zhang LH, Zhang X. Roles of GRP78 in physiology and cancer. J Cell Biochem. 2010;110:1299–1305. doi: 10.1002/jcb.22679. [DOI] [PubMed] [Google Scholar]
- 49.Mhaidat NM, Alzoubi KH, Almomani N, Khabour OF. Expression of glucose regulated protein 78 (GRP78) determines colorectal cancer response to chemotherapy. Cancer Biomark. 2015;15:197–203. doi: 10.3233/CBM-140454. [DOI] [PubMed] [Google Scholar]
- 50.Guan M, Chen X, Ma Y, Tang L, Guan L, Ren X, Yu B, Zhang W, Su B. MDA-9 and GRP78 as potential diagnostic biomarkers for early detection of melanoma metastasis. Tumour Biol. 2015;36:2973–2982. doi: 10.1007/s13277-014-2930-9. [DOI] [PubMed] [Google Scholar]
- 51.Jiang X, Kanda T, Nakamoto S, Haga Y, Sasaki R, Nakamura M, Wu S, Mikata R, Yokosuka O. Knockdown of glucose-regulated protein 78 enhances poly (ADP-ribose) polymerase cleavage in human pancreatic cancer cells exposed to endoplasmic reticulum stress. Oncol Rep. 2014;32:2343–1248. doi: 10.3892/or.2014.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raiter A, Yerushalmi R, Hardy B. Pharmacological induction of cell surface GRP78 contributes to apoptosis in triple negative breast cancer cells. Oncotarget. 2014;5:11452–11463. doi: 10.18632/oncotarget.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinreb I, Goldstein D, Irish J, Perez-Ordonez B. Expression patterns of Trk-A, Trk-B, GRP78, and p75NRT in olfactory neuroblastoma. Hum Pathol. 2009;40:1330–1335. doi: 10.1016/j.humpath.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Tsunemi S, Nakanishi T, Fujita Y, Bouras G, Miyamoto Y, Miyamoto A, Nomura E, Takubo T, Tanigawa N. Proteomics-based identification of a tumor-associated antigen and its corresponding autoantibody in gastric cancer. Oncol Rep. 2010;23:949–956. doi: 10.3892/or_00000719. [DOI] [PubMed] [Google Scholar]
- 55.Yang L, Yang S, Liu J, Wang X, Ji J, Cao Y, Lu K, Wang J, Gao Y. Expression of GRP78 predicts taxane-based therapeutic resistance and recurrence of human gastric cancer. Exp Mol Pathol. 2014;96:235–241. doi: 10.1016/j.yexmp.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Yang L, Yang SY, Ji JM, Cao YF, Ji CF, Ji JF, Xu WW, Wang JH. GRP78 expression in gastric cancer and its clinical significance. Zhonghua Zhong Liu Za Zhi. 2013;35:837–842. (In Chinese) [PubMed] [Google Scholar]
- 57.Wu JY, Cheng CC, Wang JY, Wu DC, Hsieh JS, Lee SC, Wang WM. Discovery of tumor markers for gastric cancer by proteomics. PLoS One. 2014;9:e84158. doi: 10.1371/journal.pone.0084158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, Takano Y. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Zhang LJ, Chen S, Wu P, Hu CS, Thorne RF, Luo CM, Hersey P, Zhang XD. Inhibition of MEK blocks GRP78 up-regulation and enhances apoptosis induced by ER stress in gastric cancer cells. Cancer Lett. 2009;274:40–46. doi: 10.1016/j.canlet.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 60.Xu YY, You YW, Ren XH, Ding Y, Cao J, Zang WD, Feng R, Zhang QX. Endoplasmic reticulum stress-mediated signaling pathway of gastric cancer apoptosis. Hepatogastroenterology. 2012;59:2377–2384. doi: 10.5754/hge12369. [DOI] [PubMed] [Google Scholar]
- 61.Huang X, Zhang Z, Jia L, Zhao Y, Zhang X, Wu K. Endoplasmic reticulum stress contributes to vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells. Cancer Lett. 2010;296:123–131. doi: 10.1016/j.canlet.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Huang X, Li L, Zhang L, Zhang Z, Wang X, Zhang X, Hou L, Wu K. Crosstalk between endoplasmic reticulum stress and oxidative stress in apoptosis induced by α-tocopheryl succinate in human gastric carcinoma cells. Br J Nutr. 2013;109:727–735. doi: 10.1017/S0007114512001882. [DOI] [PubMed] [Google Scholar]
- 63.Rauschert N, Brändlein S, Holzinger E, Hensel F, Müller-Hermelink HK, Vollmers HP. A new tumor-specific variant of GRP78 as target for antibody-based therapy. Lab Invest. 2008;88:375–386. doi: 10.1038/labinvest.2008.2. [DOI] [PubMed] [Google Scholar]
- 64.Winder T, Bohanes P, Zhang W, Yang D, Power DG, Ning Y, Gerger A, Wilson PM, Tang LH, Shah M, et al. GRP78 promoter polymorphism rs391957 as potential predictor for clinical outcome in gastric and colorectal cancer patients. Ann Oncol. 2011;22:2431–2439. doi: 10.1093/annonc/mdq771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Jiang Y, Jia Z, Li Q, Gong W, Wang L, Wei D, Yao J, Fang S, Xie K. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis. 2006;23:401–410. doi: 10.1007/s10585-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 66.Zhang D, Fan D. Multidrug resistance in gastric cancer: Recent research advances and ongoing therapeutic challenges. Expert Rev Anticancer Ther. 2007;7:1369–1378. doi: 10.1586/14737140.7.10.1369. [DOI] [PubMed] [Google Scholar]
- 67.Tsutsumi S, Namba T, Tanaka KI, Arai Y, Ishihara T, Aburaya M, Mima S, Hoshino T, Mizushima T. Celecoxib upregulates endoplasmic reticulum chaperones that inhibit celecoxib-induced apoptosis in human gastric cells. Oncogene. 2006;25:1018–1029. doi: 10.1038/sj.onc.1209139. [DOI] [PubMed] [Google Scholar]
- 68.Leja M, You W, Camargo MC, Saito H. Implementation of gastric cancer screening-the global experience. Best Pract Res Clin Gastroenterol. 2014;28:1093–1106. doi: 10.1016/j.bpg.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smyth EC, Cunningham D. Targeted therapy for gastric cancer. Curr Treat Options Oncol. 2012;13:377–389. doi: 10.1007/s11864-012-0192-6. [DOI] [PubMed] [Google Scholar]
- 70.Cheng CC, Huang CF, Ho AS, Peng CL, Chang CC, Mai FD, Chen LY, Luo TY, Chang J. Novel targeted nuclear imaging agent for gastric cancer diagnosis: Glucose-regulated protein 78 binding peptide-guided 111In-labeled polymeric micelles. Int J Nanomedicine. 2013;8:1385–1391. doi: 10.2147/IJN.S42003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang J, Zhao G, Lin T, Tang S, Xu G, Hu S, Bi Q, Guo C, Sun L, Han S, et al. A peptide derived from phage display library exhibits anti-tumor activity by targeting GRP78 in gastric cancer multidrug resistance cells. Cancer Lett. 2013;339:247–259. doi: 10.1016/j.canlet.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 72.Park HR, Tomida A, Sato S, Tsukumo Y, Yun J, Yamori T, Hayakawa Y, Tsuruo T, Shin-ya K. Effect on tumor cells of blocking survival response to glucose deprivation. J Natl Cancer Inst. 2004;96:1300–1310. doi: 10.1093/jnci/djh243. [DOI] [PubMed] [Google Scholar]
- 73.Cheng CC, Lu N, Peng CL, Chang CC, Mai FD, Chen LY, Liao MH, Wang WM, Chang J. Targeting to overexpressed glucose-regulated protein 78 in gastric cancer discovered by 2D DIGE improves the diagnostic and therapeutic efficacy of micelles-mediated system. Proteomics. 2012;12:2584–2597. doi: 10.1002/pmic.201100602. [DOI] [PubMed] [Google Scholar]
- 74.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 75.Dong D, Dubeau L, Bading J, Nguyen K, Luna M, Yu H, Gazit-Bornstein G, Gordon EM, Gomer C, Hall FL, et al. Spontaneous and controllable activation of suicide gene expression driven by the stress-inducible grp78 promote r resulting in eradication of sizable human tumors. Hum Gene Ther. 2004;15:553–561. doi: 10.1089/104303404323142006. [DOI] [PubMed] [Google Scholar]
- 76.Lee AS. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 77.Azatian A, Yu H, Dai W, Schneiders FI, Botelho NK, Lord RV. Effectiveness of HSV-tk suicide gene therapy driven by the Grp78 stress-inducible promoter in esophagogastric junction and gastric adenocarcinomas. J Gastrointest Surg. 2009;13:1044–1051. doi: 10.1007/s11605-009-0839-1. [DOI] [PubMed] [Google Scholar]