Abstract
Expression of immune checkpoint molecules, including programmed cell death protein-1 (PD-1), has been reported on T cells in various types of cancer. However, the expression status of these molecules in the tumor microenvironment of epithelial ovarian cancer (EOC) has not yet been studied. A total of 54 cases of malignant ascites from patients with EOC were analyzed in the present study. The expression of PD-1, lymphocyte-activation gene-3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) and B and T lymphocyte attenuator (BTLA) on cluster of differentiation (CD)4+ and CD8+ T cells in malignant EOC ascites were investigated using multicolor flow cytometric analysis. The expression of PD-L1 in tumor cells, PD-L2 in HLA-DR-positive cells and galectin-9 in ascitic fluid was also analyzed. In addition, cytokine profiling of ascitic fluid was performed to understand the immune microenvironment of EOC. PD-1, LAG-3 TIM-3, and BTLA were expressed on 65.8, 10.6, 4.3 and 37.6% of CD4+ T cells, and on 57.7, 5.0, 4.9 and 15.7% of CD8+ T cells, respectively. Programmed cell death protein-1 (PD-1), LAG-3 and BTLA were more frequently expressed on CD4+ compared with CD8+ T cells. The co-expression of immune checkpoints was further investigated and results indicated that 39 (72.2%) and 37 patients (68.5%) expressed multiple immune checkpoints on CD4+ T cells and CD8+ T cells, respectively. In addition, lower levels of TNF-α and interleukin-6 in ascitic fluid were significantly associated with multiple immune checkpoint expression on CD8+ T cells. The present findings indicated that multiple immune checkpoint molecules were expressed on T cells in the EOC tumor microenvironment and the results may suggest the significance of simultaneous blockade of immune checkpoints to control EOC.
Keywords: ovarian cancer, ascites, programmed cell death protein-1, lymphocyte-activation gene-3, T-cell immunoglobulin and mucin-domain containing-3, B and T lymphocyte attenuator
Introduction
Epithelial ovarian cancer (EOC) is the most lethal disease among gynecological malignancies. Unlike other carcinomas, peritoneal dissemination is the most common mechanism of disease progression in ovarian cancer, and up to 70% of cases present with massive malignant ascites with peritoneal implants (1). Among patients with advanced ovarian cancer who undergo primary debulking surgery, those with no residual disease have a much better survival than women with any residual disease. Therefore, control of dissemination seems to be the most important strategy in the treatment of ovarian cancer (2). Despite cytoreductive surgery and platinum and taxane combination chemotherapy, most patients with advanced ovarian cancer experience relapse. The peritoneal cavity is the most frequent site of recurrence, and most patients with intraperitoneal recurrence eventually become chemoresistant and die from the disease (3). Thus, development of new treatment strategies for EOC is required (4,5).
Recent studies have shown that tumor cells acquire escape mechanisms to evade host immunity in the tumor microenvironment (6,7). To circumvent these mechanisms, extensive studies have been undertaken for regulatory T cells, immune checkpoints, myeloid-derived suppressor cells and M2 type macrophages (8–11). With the clinical success of immune checkpoint inhibitors such as ipilimumab and nivolumab for melanoma and lung cancer, immune checkpoints have received increased attention (12,13). Some of the early-phase clinical trials of immune checkpoint inhibitors for ovarian cancer, such as anti-programmed cell death protein 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) antibodies, have shown manageable safety profiles and demonstrated a durable anti-tumor response in a certain patient population (14). However, their response rates remain at 10 to 15% (15–17). Therefore, we need to explore predictive biomarkers for durable responders and to understand the underlying mechanism. Combination therapy with chemotherapy may be another way to enhance the value of immune checkpoint inhibitors for ovarian cancer (18). Since we observed relatively lower rates of clinical response in recurrent EOC patients in recent early-phase clinical trials for PD-1 blockade, we recently came to recognize not only PD-1 but also other immune checkpoint molecules, such as lymphocyte-activation gene-3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), B and T lymphocyte attenuator (BTLA), and VISTA, are expressed on T cells associated with cancer (19–21). A recent study showed that expression of PD-1 and LAG-3 on cluster of differentiation (CD)8+ T cells derived from tumor-infiltrating or tumor-associated lymphocytes can result in impaired IFN-γ and TNF-α production compared with CD8+ T cell subsets that express PD-1 alone (22). Dual blockade of PD-1 and LAG-3 pathways could potentially improve the therapeutic efficacy of cancer immunotherapy. Therefore, we sought to address the expression status of various immune checkpoints on T cells in the tumor microenvironment of EOC patients through the analysis of ascites cells.
Malignant ascites was thought to be an ideal source to assess the tumor immune microenvironment. Cells are basically in suspension in ascites, therefore it is easy to assess both immune and tumor cells by flow cytometric analysis. The expression of LAG-3, TIM-3, and BTLA on T cells in malignant ascites from EOC has not yet been assessed. Here, we evaluated the expression of immune checkpoint molecules on both CD4+ and CD8+ T cells in malignant ascites from EOC. In addition, expression of their potential ligands was addressed at the same time. Moreover, we measured levels of cytokines/chemokines in ascites fluid to understand the immunological background of the ovarian cancer tumor immune microenvironment.
Materials and methods
Patients and ascites
This study was reviewed and approved by the Institutional Review Board of Saitama Medical University International Medical Center (no. 13-092). Eighty-nine patients who were clinically suspected to have EOC before surgery at Saitama Medical University International Medical Center (Hidaka-shi, Japan) were enrolled in this study from December 2010 to November 2014. Eighty-two patients were pathologically diagnosed with malignant tumors, while two had borderline and five had benign ovarian tumors. Of 82 malignant ovarian tumors, 80 were diagnosed as EOC. One patient was diagnosed with ovarian metastasis of a primary colorectal cancer and one with a germ cell tumor. Twenty-six cases were excluded because of insufficient levels of ascites cells for analysis. Thus, ascites cells from the remaining 54 patients were analyzed. The median age of the patients was 63.5 years with a range of 30–80 years. The EOC cases consisted of 4 (7.4%) stage I, 4 (7.4%) stage II, 35 (64.8%) stage III and 11 (20.4%) stage IV according to the International Federation of Gynecology and Obstetrics (FIGO) system. There were 31 (57.4%) serous, 8 (14.8%) clear cell and 6 (11.1%) endometrioid carcinoma. Furthermore there were 13 (24.1%) type I and 41 (75.9%) type II. Informed written consent was obtained from all patients in this study.
Flow cytometry analysis
The following monoclonal antibodies (mAbs) were used for flow cytometry: FITC-labeled anti-human CD4 antibody (BD Biosciences Pharmingen, San Diego, CA, USA), PE-labeled anti-human CD273 (B7-DC, PD-L2; BioLegend, Inc., San Diego, CA, USA), anti-human CD274 (PD-L1, B7-H1; BioLegend, Inc.), anti-human CD279 (PD-1; BioLegend, Inc.), anti-human CD366 (TIM-3; (BioLegend, Inc.), anti-human CD272 (BTLA; BioLegend, Inc.), anti-human LAG3 (R&D Systems Inc., Minneapolis, MN, USA) and mouse IgG1 isotype (BioLegend, Inc.) antibodies, PC5-labeled anti-CD3 (BioLegend, Inc.) antibody, APC-labeled anti-CD326 (EpCAM), anti-CD45 (Miltenyi Biotec, Bergisch Gladbach, Germany), anti-HLA-DR (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and mouse IgG1 isotype (eBioscience, San Diego, CA, USA) antibodies, and Pacific Blue-labeled anti-CD45 (BioLegend, Inc.) and anti-CD8a (BioLegend, Inc.) antibodies. Fixable Viability Dye eFluor 780 (eBioscience) was used to exclude dead cells. Ascites cells were harvested by centrifugation, stained with the mAbs described above and analyzed on a Gallios (Beckman Coulter, San Diego, CA, USA). The data were processed using Kaluza software (Beckman Coulter).
Cytokine measurement
Cytokines, including interleukin (IL)-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, bFGF, eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1 (MCAF), MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, and VEGF in ascites fluid were measured using Bio-Plex Pro Human Cytokine 27-plex Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The assay was performed according to the manufacturer's instructions. Briefly, ascites was incubated with microbeads labeled with specific antibodies to one of the aforementioned cytokines for 60 min. Following a washing step, the beads were incubated with the detection antibody cocktail with each antibody specific to a single cytokine for 30 min. After another washing step, the beads were incubated with streptavidin-phycoerythrin for 10 min, washed again and then the concentration of each cytokine was determined using the array reader. Cytokines of which standard deviation values were larger than 20 were subsequently analyzed.
Measurements of galectin-9
Galectin-9 in ascites fluid was measured using a Human Galectin-9 DuoSet ELISA development kit (R&D Systems Inc.) according to the manufacturer's instructions.
Statistical analysis
Differences between the groups of patients were assessed by one-way ANOVA, Student's t-test and Chi-square test. Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). All reported P-values were two-sided, and P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of immune checkpoint molecules on T cells in ascites from EOC patients
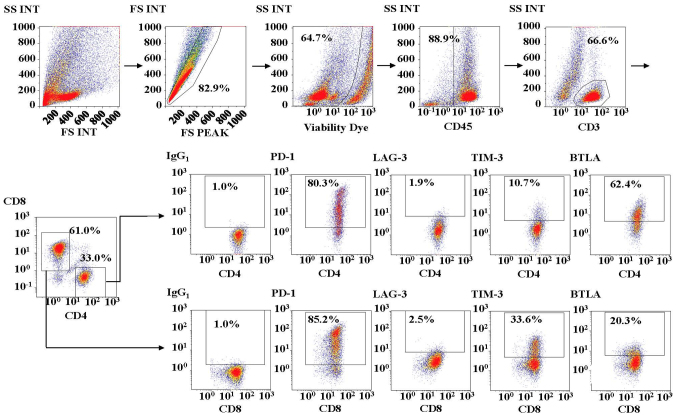
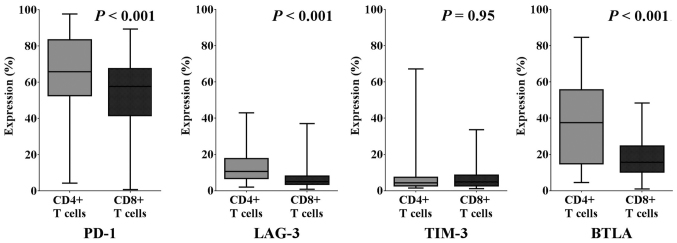
First, we investigated the expression of various immune checkpoint molecules on T cells in malignant ascites. Fig. 1 shows the representative analysis pipeline for the immune checkpoint molecules on T cells in malignant ascites. We observed that each immune checkpoint molecule was expressed at various levels on both CD4+ and CD8+ T cells in ascites from EOC. As shown in Fig. 2, 65.8% (range, 4.4–97.6%), 10.6% (1.9–43.0%), 4.3% (1.4–67.2%) and 37.6% (4.5–84.6%) of CD4+ T cells expressed PD-1, LAG-3, TIM-3, and BTLA, respectively. We also found that 57.7% (range, 0.7–89.4%), 5.0% (0.8–37.0%), 4.9% (1.2–33.6%) and 15.7% (1.0–48.4%) of CD8+ T cells expressed PD-1, LAG-3, TIM-3, and BTLA, respectively. We observed higher expression rates of PD-1, LAG-3, and BTLA on CD4+ T cells than on CD8+ T cells in ascites from EOC patients (P<0.001).
Figure 1.
Analysis of immune checkpoint molecules PD-1, LAG-3, TIM-3, and BTLA on CD4+ and CD8+ T cells in malignant ascites from ovarian cancer by multicolor flow cytometry. Various immune checkpoint molecules were expressed on both CD4+ and CD8+ T cells in ascites from EOC. FS, forward scatter; SS, side scatter; INT, integral; PD-1, programmed cell death protein-1; LAG-3, lymphocyte-activation gene-3; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; BTLA, B and T lymphocyte attenuator.
Figure 2.
The median, quartile and range of expression rates of PD-1, LAG-3, TIM-3, and BTLA on CD4+ and CD8+ T cells in ovarian cancer ascites. PD-1, LAG-3, and BTLA exhibited higher expression levels on CD4+ T cells than on CD8+ T cells in ascites of EOC patients (P<0.001). PD-1, programmed cell death protein-1; LAG-3, lymphocyte-activation gene-3; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; BTLA, B and T lymphocyte attenuator.
Clinicopathological features and immune checkpoint molecule expression in patients with EOC
Tables I and II summarize the relationship between clinicopathological features and the expression of immune checkpoint molecules on CD4+ and CD8+ T cells in malignant ascites from EOC. We found higher rates of PD-1 expression on CD4+ T cells in ascites from type I EOC patients than that from type II EOC patients (76.9% vs. 41.5%, P=0.03). Likewise, high rates of TIM-3 expression were observed on CD8+ T cells in ascites from type II EOC than that from type I (58.5 vs. 23.1%, P=0.03). No correlation was found between the expression of immune checkpoint molecules on T cells and other clinical variables.
Table I.
Expression of immune checkpoint molecules on CD4+ T cells in malignant ascites from ovarian carcinoma.
| Characteristcs | High PD-1/total (%) | P-value | High LAG-3/total (%) | P-value | High TIM-3/total (%) | P-value | High BTLA/total (%) | P-value |
|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||
| ≥65 | 12/25 (48.0) | 0.78 | 11/25 (44.0) | 0.41 | 10/25 (40.0) | 0.17 | 13/25 (52.0) | 0.78 |
| ≤64 | 15/29 (51.7) | 16/29 (55.2) | 17/29 (58.6) | 14/29 (48.3) | ||||
| FIGO stage | ||||||||
| I + II | 5/8 (62.5) | 0.44 | 5/8 (62.5) | 0.44 | 3/8 (37.5) | 0.44 | 3/8 (37.5) | 0.44 |
| III + IV | 22/46 (47.8) | 22/46 (47.8) | 24/46 (52.2) | 24/46 (52.2) | ||||
| Histology | ||||||||
| Serous | 14/31 (45.2) | 0.67 | 12/31 (38.7) | 0.29 | 16/31 (51.6) | 0.73 | 19/31 (61.3) | 0.18 |
| Clear cell | 5/8 (62.5) | 5/8 (62.5) | 5/8 (62.5) | 3/8 (37.5) | ||||
| Endometrioid | 4/6 (66.7) | 4/6 (66.7) | 2/6 (33.3) | 3/6 (50.0) | ||||
| Others | 4/9 (44.4) | 6/9 (66.7) | 4/9 (44.4) | 2/9 (22.2) | ||||
| Type | ||||||||
| I | 10/13 (76.9) | 0.03a | 9/13 (69.2) | 0.11 | 7/13 (53.8) | 0.75 | 4/13 (30.8) | 0.11 |
| II | 17/41 (41.5) | 18/41 (43.9) | 20/41 (48.8) | 23/41 (56.1) |
Low grade serous carcinoma, low grade endometrioid carcinoma, clear cell carcinoma and mucinous carcinoma were included in type I EOC. High grade serous carcinoma, high grade endometrioid carcinoma and carcinosarcoma were included in type II EOC. PD-1, programmed cell death protein-1; LAG-3, lymphocyte-activation gene-3; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; BTLA, B and T lymphocyte attenuator; FIGO, International Federation of Gynecology and Obstetrics.
Table II.
Expression of immune checkpoint molecules on CD8+ T cells in malignant ascites from ovarian carcinoma.
| Characteristcs | High PD-1/total (%) | P-value | High LAG-3/total (%) | P-value | High TIM-3/total (%) | P-value | High BTLA/total (%) | P-value |
|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||
| ≥65 | 13/25 (52.0) | 0.78 | 13/25 (52.0) | 0.78 | 13/25 (52.0) | 0.78 | 12/25 (48.0) | 0.78 |
| ≤64 | 14/29 (48.3) | 14/29 (48.3) | 14/29 (48.3) | 15/29 (51.7) | ||||
| FIGO stage | ||||||||
| I + II | 6/8 (75.0) | 0.13 | 5/8 (62.5) | 0.44 | 3/8 (37.5) | 0.44 | 3/8 (37.5) | 0.44 |
| III + IV | 21/46 (45.7) | 22/46 (47.8) | 24/46 (52.2) | 24/46 (52.2) | ||||
| Histology | ||||||||
| Serous | 18/31 (58.1) | 0.51 | 15/31 (48.4) | 0.85 | 19/31 (61.3) | 0.29 | 19/31 (61.3) | 0.18 |
| Clear cell | 3/8 (37.5) | 4/8 (50.0) | 3/8 (37.5) | 3/8 (37.5) | ||||
| Endometrioid | 3/6 (50.0) | 4/6 (66.7) | 2/6 (33.3) | 3/6 (50.0) | ||||
| Others | 3/9 (33.3) | 4/9 (44.4) | 3/9 (33.3) | 2/9 (22.2) | ||||
| Type | ||||||||
| I | 7/13 (53.8) | 0.75 | 7/13 (53.8) | 0.75 | 3/13 (23.1) | 0.03a | 4/13 (30.8) | 0.11 |
| II | 20/41 (48.8) | 20/41 (48.8) | 24/41 (58.5) | 23/41 (56.1) |
PD-1, programmed cell death protein-1; LAG-3, lymphocyte-activation gene-3; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; BTLA, B and T lymphocyte attenuator; FIGO, International Federation of Gynecology and Obstetrics.
Multiple immune checkpoint molecule expression on T cells in ascites from EOC
Next, we asked whether there were any overlapping immune checkpoint inhibitory pathways on T cells from patients with malignant ascites. We therefore further investigated the multiple expression of immune checkpoint molecules on T cells in malignant ascites. We considered a higher percentage above the median values as higher immune checkpoint expression. We found that 39 (72.2%) patients and 37 (68.5%) patients exhibited expression of multiple immune checkpoint molecules on CD4+ and CD8+ T cells, respectively. We also examined the relationship between multiple immune checkpoint expression and clinicopathological factors but did not find any correlation (Table III).
Table III.
Multiple immune checkpoint molecules expression on CD4+ and CD8+ T cells in malignant ascites from ovarian carcinoma.
| CD4+ T cells | CD8+ T cells | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristcs | Multiple/total (%) | Single/total (%) | None/total (%) | P-value | Multiple/total (%) | Single/total (%) | None/total (%) | P-value |
| Age (years) | ||||||||
| ≥65 | 16/25 (64.0) | 7/25 (28.0) | 2/25 (8.0) | 0.21 | 16/25 (64.0) | 5/25 (20.0) | 4/25 (16.0) | 0.51 |
| ≤64 | 23/29 (79.3) | 4/29 (13.8) | 2/29 (6.9) | 21/29 (72.4) | 5/29 (17.2) | 3/29 (10.3) | ||
| FIGO stage | ||||||||
| I + II | 6/8 (75.0) | 1/8 (12.5) | 1/8 (12.5) | 0.85 | 6/8 (75.0) | 0 | 2/8 (25.0) | 0.67 |
| III + IV | 33/46 (71.7) | 10/46 (21.7) | 3/46 (6.5) | 31/46 (67.4) | 10/46 (21.7) | 5/46 (10.9) | ||
| Histology | ||||||||
| Serous | 22/31 (71.0) | 7/31 (22.6) | 2/31 (6.5) | 0.46 | 24/31 (77.4) | 5/31 (16.1) | 2/31 (6.5) | 0.07 |
| Clear cell | 7/8 (87.5) | 0 | 1/8 (12.5) | 5/8 (62.5) | 1/8 (12.5) | 2/8 (25.0) | ||
| Endometrioid | 5/6 (83.3) | 1/6 (16.7) | 0 | 5/6 (83.3) | 0 | 1/6 (16.7) | ||
| Others | 5/9 (55.6) | 3/9 (33.3) | 1/9 (11.1) | 3/9 (33.3) | 4/9 (44.4) | 2/9 (22.2) | ||
| Type | ||||||||
| I | 12/13 (92.3) | 0 | 1/13 (7.7) | 0.06 | 9/13 (69.2) | 1/13 (7.7) | 3/13 (23.1) | 0.95 |
| II | 27/41 (65.9) | 11/41 (26.8) | 3/41 (7.3) | 28/41 (68.3) | 9/41 (22.0) | 4/41 (9.8) | ||
CD, cluster of differentiation; FIGO, International Federation of Gynecology and Obstetrics.
PD-L1 and PD-L2 expression on ascites cells from EOC patients
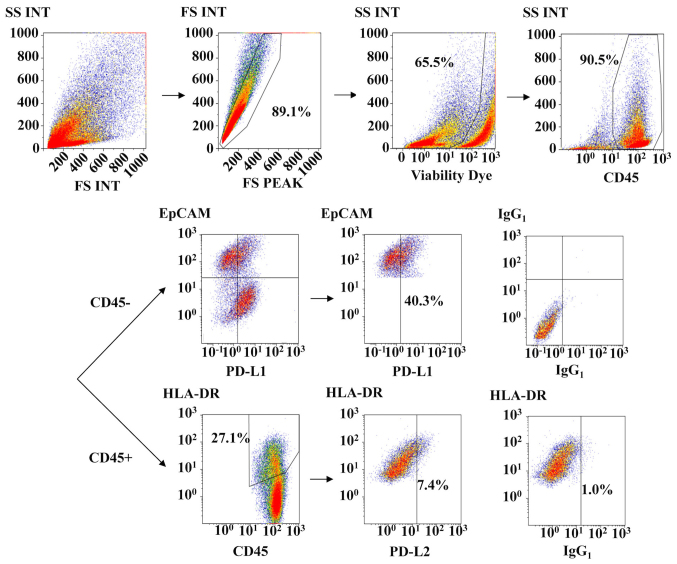
We next assessed the expression of PD-1 ligands, such as PD-L1 and PD-L2, on tumor cells and antigen-presenting cells in malignant ascites. Of the 54 EOC patients, 30 cases could be analyzed for PD-L1 and PD-L2. Fig. 3 shows the representative analyses of PD-L1 and PD-L2 expression on EpCAM-positive cells and HLA class II-positive lymphocytes in malignant ascites, respectively. We investigated PD-L1 and PD-L2 expression based on the PD-1 expression status of T cells from the same patient. We defined above the median values of percent PD-1 expression as high PD-1 expression. As shown in Fig. 4A, PD-L1 expression was found in 43.9% (3.5–91.7%) of tumor cells in patients who had high PD-1-expressing CD4+ T cells, but only 27.3% (8.5–60.0%) of tumor cells in patients who had low PD-1-expressing CD4+ T cells (P=0.02). However, no difference in PD-L1 expression was observed between patients with high and low PD-1 expression on CD8+ T cells, at 34.1% (3.5–91.7%) and 27.3% (8.5–68.0%), respectively. As shown in Fig. 4B, PD-L2 expression was 2.4% (0.8–8.7%) in patients who had high PD-1 on CD4+ T cells and 3.4% (1.2–10.7%) in patients who had low PD-1 on CD4+ T cells (P=0.63), and was 2.3% (0.8–10.7%) in patients who had high PD-1 on CD8+ T cells and 3.2% (1.6–10.7%) in patients who had low PD-1 on CD8+ T cells (P=0.99). No correlation was found between PD-L1/2 expression and clinical variables (Table IV). Moreover, we did not observe any association between PD-L1/2 expression and clinical outcomes (data not shown).
Figure 3.
Analysis of PD-L1 and PD-L2 expression on EpCAM-positive cells and HLA class II-positive lymphocytes in malignant ascites by multicolor flow cytometry. PD-L1, programmed cell death-ligand 1; FS, forward scatter; SS, side scatter; INT, integral.
Figure 4.
(A) Expression of PD-L1 on EpCAM-positive cells in high or low PD-1 expression groups of the CD4+ and CD8+ T cells in ascites from EOC. (B) Expression of PD-L2 on HLADR-positive cells in high or low PD-1 expression groups of the CD4+ and CD8+ T cells in ascites from EOC. No correlation between PD-L2 and PD-1 on CD4+ and CD8+ T cells was identified (P=N.S.). (C) Evaluation of galectin-9 in ascites fluid classified by high or low TIM-3 expression on CD4+ and CD8+ T cells.
Table IV.
Expression of PD-L1 on EpCAM-positive cells and PD-L2 on HLA-DR-positive cells.
| Characteristcs | High PD-L1/total (%) | P-value | High PD-L2/total (%) | P-value |
|---|---|---|---|---|
| Age (years) | 0.46 | 0.46 | ||
| ≥65 | 8/14 (57.1) | 8/14 (57.1) | ||
| ≤64 | 7/16 (43.8) | 7/16 (43.8) | ||
| FIGO stage | 0.28 | 1 | ||
| I + II | 1/4 (25.0) | 2/4 (50.0) | ||
| III + IV | 14/26 (53.8) | 13/26 (50.0) | ||
| Histology | 0.29 | 0.25 | ||
| Serous | 10/21 (47.6) | 9/21 (42.9) | ||
| Clear cell | 1/3 (33.3) | 1/3 (33.3) | ||
| Endometrioid | 1/3 (33.3) | 3/3 (100.0) | ||
| Others | 3/3 (100.0) | 2/3 (66.7) | ||
| Type | 1 | 0.36 | ||
| I | 3/6 (50.0) | 4/6 (66.7) | ||
| II | 12/24 (50.0) | 11/24 (45.8) |
PD-L1, programmed cell death-ligand 1; PD-L2, programmed cell death-ligand 2; FIGO, International Federation of Gynecology and Obstetrics.
We also investigated the levels of galectin-9, a ligand of TIM-3, in ascites fluids from EOC patients. We observed higher levels of galectin-9 in patients who had high TIM-3 on CD8+ T cells compared with those who had low TIM-3 (6,004 pg/ml [3,584.6–9,562.6 pg/ml]) vs. 4,067.0 pg/ml [667.5–9,428.6 pg/ml]) (P=0.04).
Relationship between immune checkpoint expression and ascites cytokine profile
To further investigate the local immune inhibitory environment, we determined the cytokine and chemokine profile of ascitic fluids by suspension arrays. We assessed the relationship between immune checkpoint molecule expression and ascites cytokine profile (Table V). We observed that lower TNF-α and IL-6 levels in ascitic fluids were significantly associated with multiple immune checkpoint expression on CD8+ T cells (P=0.03 and P=0.02, respectively). Higher VEGF and lower G-CSF levels were also associated with multiple immune checkpoint expression with borderline significance (P=0.06).
Table V.
Cytokines in multiple immune checkpoint molecule expression on CD4+ and CD8+ T cells in malignant ascites from ovarian carcinoma.
| CD4+ T cells | CD8+ T cells | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristcs | Multiple (n=39) | Single (n=11) | None (n=4) | P-value | Multiple (n=37) | Single (n=10) | None (n=7) | P-value |
| IFNγ (pg/ml) | 378.2 | 372.8 | 430.2 | 0.85 | 369.4 | 394.3 | 420.6 | 0.48 |
| TNFα (pg/ml) | 165.0 | 174.4 | 281.2 | 0.33 | 150.2 | 202.0 | 267.7 | 0.03a |
| IL1Ra (pg/ml) | 365.0 | 380.8 | 447.3 | 0.71 | 333.5 | 463.2 | 454.3 | 0.15 |
| IL1b (pg/ml) | 9.0 | 10.1 | 13.2 | 0.65 | 7.4 | 14.2 | 13.4 | 0.11 |
| IL2 (pg/ml) | 9.4 | 8.9 | 11.0 | 0.97 | 8.9 | 9.6 | 12.1 | 0.20 |
| IL4 (pg/ml) | 7.5 | 6.7 | 8.8 | 0.81 | 6.9 | 8.4 | 9.0 | 0.04a |
| IL5 (pg/ml) | 6.4 | 6.2 | 7.6 | 0.94 | 6.2 | 7.0 | 7.3 | 0.63 |
| IL6 (pg/ml) | 5,315.4 | 4,859.1 | 6,607.5 | 0.99 | 4,411.6 | 5,742.1 | 9,246.3 | 0.02a |
| IL7 (pg/ml) | 25.3 | 26.7 | 23.9 | 0.88 | 25.9 | 25.1 | 24.1 | 0.78 |
| IL8 (pg/ml) | 905.9 | 1,410.5 | 710.7 | 0.60 | 768.5 | 1,862.4 | 907.8 | 0.23 |
| IL9 (pg/ml) | 93.7 | 97.7 | 106.4 | 0.75 | 91.6 | 101.9 | 106.1 | 0.53 |
| IL10 (pg/ml) | 178.5 | 179.2 | 187.0 | 0.96 | 153.0 | 235.3 | 231.0 | 0.12 |
| IL12 bp70 (pg/ml) | 476.2 | 500.6 | 509.5 | 0.80 | 519.8 | 327.1 | 528.3 | 0.28 |
| IL13 (pg/ml) | 31.6 | 35.5 | 36.2 | 0.46 | 33.8 | 25.7 | 37.9 | 0.56 |
| IL15 (pg/ml) | 19.4 | 31.1 | 26.0 | 0.05a | 21.4 | 24.9 | 23.8 | 0.56 |
| IL17a (pg/ml) | 90.2 | 104.0 | 124.3 | 0.42 | 88.7 | 99.4 | 124.9 | 0.36 |
| CCL2 (MCP1) (pg/ml) | 752.7 | 1179.0 | 1052.9 | 0.21 | 1020.1 | 605.1 | 468.1 | 0.12 |
| CCL3 (MIP1a) (pg/ml) | 20.2 | 18.0 | 15.7 | 0.85 | 15.6 | 37.7 | 11.9 | 0.41 |
| CCL4 (MIP1b) (pg/ml) | 825.1 | 676.4 | 787.5 | 0.78 | 746.7 | 1031.0 | 667.4 | 0.75 |
| CCL5 (Rantes) (pg/ml) | 122.4 | 221.1 | 107.1 | 0.36 | 127.9 | 255.5 | 50.9 | 0.55 |
| CXCL10 (IP10) (pg/ml) | 164,748.8 | 2,316,341.3 | 80,073.0 | 0.02a | 813,639.9 | 72,861.6 | 312,573.7 | 0.28 |
| CCL11 (Eotaxin) (pg/ml) | 349.8 | 567.7 | 297.8 | 0.25 | 426.0 | 414.8 | 188.9 | 0.40 |
| GMCSF (pg/ml) | 95.4 | 68.6 | 89.4 | 0.15 | 84.7 | 84.3 | 116.2 | 0.38 |
| bFGF (pg/ml) | 71.9 | 79.7 | 73.9 | 0.64 | 77.4 | 61.1 | 74.9 | 0.41 |
| VEGF (pg/ml) | 7,117.9 | 11,619.7 | 5,046.1 | 0.56 | 10,638.1 | 1,843.0 | 2,943.0 | 0.06 |
| PDGFbb (pg/ml) | 153.8 | 145.1 | 82.8 | 0.76 | 178.0 | 90.0 | 69.9 | 0.22 |
| GCSF (pg/ml) | 105.5 | 91.5 | 110.2 | 0.73 | 87.8 | 107.1 | 171.9 | 0.06 |
Statistical significance. CD, cluster of differentiation; CCL, chemokine (C-C motif) ligand; CXCL, chemokine (C-X-C motif) ligand; GMCSF, granulocyte-macrophage colony-stimulating factor; bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor; PDGFbb, platelet derived growth factor-BB; GCSF, granulocyte colony stimulating factor; IL, interleukin; IL1Ra, interleukin-1 receptor antagonist; TNF, tumor necrosis factor; IFN, interferon.
Discussion
In this study, we focused on the expression of various immune checkpoint molecules on T cells in the tumor microenvironment of EOC through the analysis of ascites cells. PD-1 has been reported to be upregulated on T cells from patients with EOC. PD-1 expression on T cells isolated from peripheral blood mononuclear cells (PBMCs) and ascites from patients with malignant ovarian tumors was high compared with benign/borderline in ovarian tumors (23). However, the expression status of other immune checkpoint molecules such as LAG-3, TIM-3, or BTLA on T cells in EOC patients have not been addressed yet, with the exception of a report about TIM-3 on PBMCs of EOC patients (24). The co-expression status of immune checkpoint molecules on T cells in the tumor microenvironment of EOC is important to understand the complex immune inhibitory mechanism of EOC patients.
We investigated the expression of various immune checkpoint molecules on T cells in malignant ascites from EOC patients. Among them, PD-1 was the most frequently expressed, with median expression rates of 65.8 and 57.7% on CD4+ T cells and CD8+ T cells, respectively. Conversely, the median expression rates of LAG-3, TIM-3, and BTLA were 10.6, 4.3, and 37.6% of CD4+ T cells; and 5.0, 4.9, and 15.7% of CD8+ T cells, respectively. These data suggest the immune inhibitory environment caused by immune checkpoint molecules in ascites of EOC patients was PD-1/PD-L1-axis dominant, or might be because of varying sensitivity/specificity for each antibody to its molecule. This aspect should be carefully considered when comparing the expression levels and/or rates of different molecules by different antibodies. However, we at least found not only PD-1 but also LAG-3, TIM-3, and BTLA were expressed on T cells in the tumor microenvironment of EOC.
We did not observe a correlation between the expression of any of the immune checkpoint molecules examined and clinicopathological factors in our study. However, PD-1 expression was reported to be higher in advanced-stage breast (25), renal (26) and pancreatic cancers (27) than in the respective early-stage disease. Thus, the expression of immune checkpoint molecules in EOC seems to be independent from these factors, unlike in other cancer types. In other words, immune checkpoints were expressed even in early-stage EOC as well as in advanced-stage EOC. These results indicate that checkpoint blockade therapy can serve not only as second-line treatment for metastatic disease but as an adjuvant immunotherapy for early-stage EOC patients after initial surgery. With regard to patient survival, some of the previous studies reported that immune checkpoint molecule expression was associated with clinical outcomes (25,26,28–30). The presence of PD-1-expressing tumor-infiltrating lymphocytes correlates with poor prognosis in a number of cancer types, including lung (28), breast (25,29), renal (26), and nasopharyngeal cancer (30), and a low percentage of PD-1 expression on PBMCs was recently shown to be associated with improved progression free survival (PFS) and overall survival (OS) in ovarian cancer patients (31). We did not see a correlation between immune checkpoint expression and survival in EOC patients. This result might be because of a different source of T cells or the detection methods we used, or because of an insufficient number of events to determine it as a prognostic factor.
Since various immune checkpoint pathways have been reported in cancers (21), we further investigated the expression status of multiple immune checkpoint molecules on T cells in malignant ascites. We found that 72.2 and 68.5% patients had high multiple immune checkpoint molecule expression on CD4+ and CD8+ T cells, respectively. Data for multiple immune checkpoint molecules may be a reason for the relative low response rates of current PD-1/PD-L1 blockade therapy for recurrent EOC patients, which demonstrated response rates of 10 to 15% at most (15–17). Our findings may explain in part that single immune checkpoint inhibition alone may not be sufficient to control the growth of EOC. It is reasonable to consider combination therapy of immune checkpoint inhibitors for EOC patients. Several clinical trials for combination therapies of PD-1 inhibitor with other cancer immunotherapies are currently ongoing. In particular, a combination of nivolumab and ipilimumab for the treatment of melanoma increased PFS compared with either agent alone (32), and similar combination therapies are now being investigated in ovarian cancer (33). Double checkpoint blockade in which anti-PD-1 antibody is combined with immune modulators such as anti-LAG-3 antibody is currently under investigation for solid tumors as well (22). Based on our findings, combination therapy for the blockade of various immune checkpoint pathways would be effective as a multiple-targeting immunotherapy.
When we focused on the relationship between the expression of each immune checkpoint and its ligand, we observed expression of PD-1 on CD4+ and TIM-3 on CD8+ T cells was correlated with PD-L1 and galection-9 in ascites, respectively. We suggest that it may reflect an immune suppressive environment for EOC. Immune checkpoints and/or their ligand expression were considered as candidate biomarkers of EOC for immune checkpoint blockade therapy (34–37). Therefore, we postulate that EOC is a good target for blockade therapy of PD-1/PD-L1 and TIM-3/galectin-9 pathways. The relationship between PD-L1 and clinical outcomes is another issue because it remains controversial. Some reports have shown that PD-L1 expression is associated with poorer prognosis (34,35), but recent studies have shown better prognosis (36,37) in ovarian cancer. We demonstrated no correlation between PD-L1/L2 expression and clinical variables and outcome in this study, which might be because of the different antibodies, detection method, or different source of cancer cells (ascites or tumor) used.
To further evaluate the immune inhibitory environment in malignant ascites in patients with EOC, we assessed the relationship between immune checkpoint molecule expression and ascites cytokine/chemokine profiles. We observed lower TNF-α and IL-6 in ascitic fluids-indicative of impaired local inflammation-were significantly associated with multiple immune checkpoint expression on CD8+ T cells. This result could reflect a strong immunosuppressive tumor microenvironment in patients who had multiple immune checkpoint expression on their T cells.
The limitations of our study need to be addressed. First, our study was not a prospective study and the number of cases we assessed was slightly limited. Second, our immune checkpoint expression data were not based on single T cells. We do not know whether individual T cells express multiple immune checkpoint molecules or not.
In conclusion, we report in this study that expression of various immune checkpoint molecules was observed on both CD4+ and CD8+ T cells in ascites from EOC patients, and that this expression was independent of clinicopathological factors. There seemed to be a partial correlation between immune checkpoint expression and their respective ligands. In addition, we observed approximately 70% of the EOC patients exhibited multiple immune checkpoint expression, and those patients had suppressive levels of inflammatory cytokines in their tumor microenvironment. These data suggest the potential application of combination therapy for immune checkpoint blockade in high-risk stage I/II EOC patients as well as advanced-stage EOC patients.
Acknowledgements
The authors would like to thank Dr. A. Kurosaki and Dr. T. Hanaoka for their helpful support in sample collection during our study, and Ms. A. Miyara for her technical assistance.
Glossary
Abbreviations
- PD-1
programmed cell death protein-1
- LAG-3
lymphocyte-activation gene-3
- TIM-3
T-cell immunoglobulin and mucin-domain containing-3
- BTLA
B and T lymphocyte attenuator
- EOC
epithelial ovarian cancer
- PFS
progression free survival
- OS
overall survival
- PBMC
peripheral blood mononuclear cell
References
- 1.Roett MA, Evans P. Ovarian cancer: An overview. Am Fam Physician. 2009;80:609–616. [PubMed] [Google Scholar]
- 2.Rosen B, Laframboise S, Ferguson S, Dodge J, Bernardini M, Murphy J, Segev Y, Sun P, Narod SA. The impacts of neoadjuvant chemotherapy and debulking surgery on survival from advanced ovarian cancer. Gynecol Oncol. 2014;134:462–467. doi: 10.1016/j.ygyno.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–1382. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and clinical treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;10:211–224. doi: 10.1038/nrclinonc.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandalaft LE, Powell DJ, Jr, Singh N, Coukos G. Immunotherapy for ovarian cancer: What's next? J Clin Oncol. 2011;29:925–933. doi: 10.1200/JCO.2009.27.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schleinger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 7.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to human escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 8.Khong HT, Restifo NP. Natural selecyion of tumor variants in the generation of ‘tumor escape’ phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogen M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 10.Gordon IO, Freedman RS. Defective antitumor function of monocyte-derived macrophages from epithelial ovarian cancer patients. Clin Cancer Res. 2006;12:1515–1524. doi: 10.1158/1078-0432.CCR-05-2254. [DOI] [PubMed] [Google Scholar]
- 11.Tsai HF, Hsu PN. Cancer immunotherapy by targeting immune checkpoints: Mechanism of T cell dysfunction in cancer immunity and new therapeutic target. J Biomed Sci. 2017;24:35. doi: 10.1186/s12929-017-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittica G, Genta S, Aglietta M, Valabrega G. Immune checkpoint inhibitors: A new opportunity in the treatment of ovarian cancer? Int J Mol Sci. 2016;17(pii):E1169. doi: 10.3390/ijms17071169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33:4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 16.Disis ML, Patel MR, Pant S, Hamilton EP, Lockhart AC, Kelly K, Beck JT, Gordon MS, Weiss GJ, Taylor MH, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: Safety and clinical activity. J Clin Oncol. 2016;34(15 Suppl):S5533. [Google Scholar]
- 17.Varga A, Piha-Paul SA, Ott PA, Mehnert JM, Berton-Rigaud D, Johnson EA, Cheng JD, Yuan S, Rubin EH, Matei DE. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. J Clin Oncol. 2015;33(15 Suppl):S5510. [Google Scholar]
- 18.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Anti-PD-L1/PD-1 immune therapies in ovarian cancer: Basic mechanism and future clinical application. Int J Clin Oncol. 2016;21:456–461. doi: 10.1007/s10147-016-0968-y. [DOI] [PubMed] [Google Scholar]
- 19.Nirschl CJ, Drake CG. Molecular pathways: Coexpression of immune checkpoint molecules: Signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19:4917–4924. doi: 10.1158/1078-0432.CCR-12-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lines JL, Sempere LF, Broughton T, Wang L, Noelle R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res. 2014;2:510–517. doi: 10.1158/2326-6066.CIR-14-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collin M. Immune checkpoint inhibitors: A patent review (2010–2015) Expert Opin Ther Pat. 2016;26:555–564. doi: 10.1080/13543776.2016.1176150. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer; Proc Natl Acad Sci USA; 2010; pp. 7875–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maine CJ, Aziz NH, Chatterjee J, Hayford C, Brewig N, Whilding L, George AJ, Ghaem-Maghami S. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63:215–224. doi: 10.1007/s00262-013-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Liu C, Qian S, Hou H. The expression of Tim-3 in peripheral blood of ovarian cancer. DNA Cell Biol. 2013;32:648–653. doi: 10.1089/dna.2013.2116. [DOI] [PubMed] [Google Scholar]
- 25.Sun S, Fei X, Mao Y, Wang X, Garfield DH, Huang O, Wang J, Yuan F, Sun L, Yu Q, et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother. 2014;63:395–406. doi: 10.1007/s00262-014-1519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Lin J, Cui J, Han T, Jiao F, Meng Z, Wang L. Prognostic value and clinicopathological features of PD-1/PD-L1 expression with mismatch repair status and desmoplastic stroma in Chinese patients with pancreatic cancer. Oncotarget. 2017;8:9354–9365. doi: 10.18632/oncotarget.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lafuente-Sanchis A, Zúñiga Á, Estors M, Martínez-Hernández NJ, Cremades A, Cuenca M, Galbis JM. Association of PD-1, PD-L1, and CTLA-4 gene expression and clinicopathologic characteristics in patients with non-small-cell lung cancer. Clin Lung Cancer. 2017;18:e109–e116. doi: 10.1016/j.cllc.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ, Jin YT, Chang Y. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23:1393–1403. doi: 10.1038/modpathol.2010.130. [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee J, Dai W, Aziz NHA, Teo PY, Wahba J, Phelps DL, Maine CJ, Whilding L, Dina R, Trevisan G, et al. Clinical use of programmed cell death-1 (PD-1) and its ligand (PD-L1) expression as discriminatory and predictive markers in ovarian cancer. Clin Cancer Res: 2017;23:3453–3460. doi: 10.1158/1078-0432.CCR-16-2366. [DOI] [PubMed] [Google Scholar]
- 32.Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institunes of Health, corp-author. Nivolumab with or without ipilimumab in treating patients with persistent or recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. National Institunes of Health; Bethesda, Maryland: 2015. [Jul 15;2015 ]. [Google Scholar]
- 34.Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, Yamaguchi K, Murakami R, Yamamoto A, Kharma B, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19:1363–1374. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- 35.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Ta kakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer; Proc Natl Acad Sci USA; 2007; pp. 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol. 2016;141:293–302. doi: 10.1016/j.ygyno.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, Budczies J, Bockmayr M, Dietel M, Denkert C, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7:1486–1499. doi: 10.18632/oncotarget.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]