Abstract
Background
Impedance is an integral property of neuromodulation devices that determines the current delivered to brain tissue. Long-term variability in therapeutic impedance following deep brain stimulation (DBS) has not been extensively investigated across different brain targets. The aim was to evaluate DBS impedance drift and variability over an extended postoperative period across common DBS targets.
Methods
Retrospective data from 1,764 electrode leads were included and drawn from 866 DBS patients enrolled in the University of Florida Institutional Review Board-approved INFORM database and analyzed up to 84 months post implantation. An exploratory analysis was conducted to identify trends in impedances using a Mann–Kendall test of trend.
Results
There were 866 patients and 1,764 leads available for analysis. The majority of subjects had Parkinson’s disease (60.7%). The mean age at implantation was 58.7 years old and the mean follow-up time was 36.8 months. There were significant fluctuations in the mean impedance of all electrodes analyzed that largely stabilized by 6 months except for the subthalamic nucleus (STN) target, in which fluctuations persisted throughout the duration of follow-up with a continued downward trend (p < 0.001).
Discussion
The drift in impedance observed primarily within the first 6 months is in keeping with prior studies and is likely due to surgical micro-lesioning effects and brain parenchyma remodeling at the electrode–tissue interface, typically at values approximating 1,000 Ω. The differences in impedance trends over time in the various DBS targets may be due to underlying differences in structure and tissue composition.
Keywords: Deep brain stimulation, impedance, Parkinson’s disease, subthalamic nucleus, globus pallidus internus
Introduction
Deep brain stimulation (DBS) is an invasive neuromodulatory therapy that has been utilized for the treatment of selected patients with movement and psychiatric disorders.1 DBS is currently Food and Drug Administration approved for use in Parkinson’s disease (PD), dystonia, essential tremor (ET), and obsessive–compulsive disorder.2–5 It is under investigation for several other conditions including Tourette syndrome, depression and obesity.6–8 Although the therapeutic benefit from DBS is well established, the biological response and the mechanisms of action of DBS, as well as its long-term stability, remain unknown.9,10 Recent studies continue to focus on the electrical stability of DBS devices and the evaluation of device–tissue interactions across multiple brain targets and disorders.11–13
The simplest measure of device–tissue interaction is impedance, which is the resistance to electrical charge flow.14 With respect to DBS, impedance depends on electrode composition and the tissue–electrode interface. Large deviations in impedance can reflect issues in device functionality such as short circuits, migration of cerebrospinal fluid, and open circuits. Values less than 50 Ω likely represent a short circuit, and values greater than 2,000 Ω usually represent a broken lead.15 Studies measuring impedance in epileptic foci reported an initial period of instability, followed by a general convergence of impedances across patients.11 However, within the context of DBS, impedance studies are usually limited to within-subject analyses.16 Investigating impedance drift over time across subjects requires large sample sizes due to the wide range of factors including the choice of anatomical target, clinical diagnosis, and stimulation settings.
In this study, we present the long-term (up to 7 years) review of impedance measurements that were drawn from over 800 patients implanted with DBS in the subthalamic nucleus (STN), the globus pallidus internus (GPi), or the nucleus ventralis intermedius (VIM). We also examined impedance measurements from a few less-common brain targets and report available information from these targets. We discuss potential sources for deviations in impedance measurements, particularly between DBS targets.
Methods
Study subjects
Data were retrospectively collected following Institutional Review Board approval to access the University of Florida (UF) INFORM (Center for Movement Disorders & Neurorestoration) database. Patient data from 866 patients with 1,764 DBS leads were extracted from the database. The UF INFORM is a longitudinal clinical research database, which provides information on patient demographics, and clinical, surgical, and functional characteristics of patients. Currently, the database has approximately 10,000 patients, including all DBS patients implanted at the University of Florida. Pertinent recorded data reviewed included indication for surgery, stimulator model, implantation site, implantation date, laterality of implant, therapeutic impedance measurement (measured at each follow-up visit), stimulation settings (measured at each follow-up visit), age, and gender. All patients in this study had either a Medtronic Soletra, Medtronic Inc., Minneapolis, MN, USA or Kinetra implantable pulse generator (IPG) and used a Medtronic 3387 lead. All DBS systems in this study utilized constant voltage stimulation settings.
Following implantation, patients attended clinical follow-up visits for DBS programming. A trained clinical programmer assessed the therapeutic effectiveness of stimulation settings at each follow-up visit until a clinically defined best therapeutic setting was achieved (frequency, voltage, pulse width, active DBS contact(s), and lead location). The therapeutic impedance was measured with stimulation parameters thought to provide the best clinical benefit with no side effects. Data were derived from each programming visit, typically monthly for the first 6 months and then once or twice a year thereafter, as determined by individualized patient care. Impedance values in this study refer to the therapeutic impedance measurement at the clinically defined best setting from the active DBS contact. The therapeutic impedance was measured at the end of each clinic visit after all programming changes were made.
Database processing
The database was parsed for patient demographics, implantation indication, site, and laterality. Implantation sites with fewer than 10 total leads documented in the database were excluded from this query. Once this initial query was completed, a second iteration was performed to incorporate stimulation settings including the voltage, frequency, pulse width and the therapeutic impedance. Group stimulation settings were determined by calculating the mean and standard deviation of the patients in the dataset. Time was defined as the number of months since DBS implantation. As clinical parameters dictated patient follow-up, visits were more variable and irregular with increasing time from the date of implantation. To accommodate for this variability, time ranges rather than dates were used to encompass as many patient visits as possible and this method allowed for various issues including scheduling conflicts, transportation issues, and other barriers to scheduled clinic visits.
We examined programming settings across subjects for patient demographics, anatomical targets, implantation laterality, and diagnoses. Values were drawn from the first visit occurring between 18 and 30 months post implantation (i.e., 24 ± 6 months).
The therapeutic impedance measurements were also compared against time. Time points for each impedance measurement were calculated by determining the number of months between the date of the clinic visit and the date of implantation. The impedances of the three most frequently selected target sites (GPi, STN, and VIM) were plotted against time up to a maximum of 84 months’ follow-up. For each month from 0 to 84, the mean impedance and standard error of the mean were calculated from all recorded visits for each respective target site. A composite calculation was also performed to determine the mean impedance and standard error of the mean from all electrodes for each month. Impedance measurements of “NULL” or zero were excluded from this analysis as they were deemed to be erroneous measurements (n = 1178). Measurements at 2,000 Ω and 4,000 Ω were excluded as they likely represented a device or recording malfunction causing a reading greater than the maximum device threshold (n = 376). These values were chosen based on the technical specifications described in the Medtronic application manual for the Soletra and Kinetra IPGs that listed the maximum device impedance as 2,000 and 4,000 Ω respectively. Additionally, impedance measurements over 9,000 Ω were excluded (n = 6) as they were considered to be either erroneous or likely associated with mechanical failure.15 Impedance measurements from clinic visits that preceded the surgery date were also excluded as they were thought to be erroneously recorded or represented patients who underwent DBS surgery at an outside facility and were referred to our clinic for evaluation of DBS failure with subsequent lead revision (n = 164). A total of 8,322 time points remained for analysis. After the mean impedances were determined, a Mann–Kendall test of trend was performed to evaluate for evidence of upward or downward trend in impedance over time. A Wilcoxon signed rank test was also performed on the mean impedances of all electrodes from months 0 to 12. Both the Mann–Kendall test and the Wilcoxon signed rank test were chosen as we did not assume the impedance measures to be normally distributed. An alpha level was set at 0.05 (i.e., a confidence level of 95%). All the statistical analyses and calculations were performed using MathWorks MATLAB R2015b and IBM SPSS Statistics 24.
Results
Patient records from 866 patients with 1,764 leads were collected for analysis. A total of 8,322 lead measurements were utilized in the study after the exclusion criteria were applied. Forty-five percent of patients received unilateral DBS; all others (n = 389) received bilateral DBS. A summary of implantation sites and indications for DBS is shown in Table 1. The majority of the subjects included in this study had PD (60.7%). The mean age at implantation was 58.7 years. Of patients, 35.6% were female and 54.5% of leads were placed in the left hemisphere. The mean follow-up time was 36.8 months and the maximum follow-up time was 143 months. A visualization of the relationship between the patient’s primary diagnosis and implantation site is summarized in Table 2. A comprehensive breakdown of indication, lead location, gender, average stimulation settings, and average impedance is available in Table 3.
Table 1. Patient Demographics and Implantation Information.
| Characteristics | N (%) |
|---|---|
| Gender, mean (SD) | |
| Male | 836 (64.3) |
| Female | 463 (35.6) |
| Not specified | 465 |
| Age, mean (SD) | |
| <40 | 106 (12.4) |
| 40–60 | 254 (29.6) |
| >60 | 498 (58.0) |
| Not specified | 906 |
| Primary diagnosis, mean (SD) | |
| Parkinson’s disease | 789 (60.7) |
| Essential tremor | 192 (14.8) |
| Primary dystonia | 143 (11.0) |
| Tremor (not otherwise specified) | 80 (6.2) |
| Secondary dystonia | 36 (2.8) |
| Obsessive compulsive disorder | 13 (1.0) |
| Tourette syndrome | 9 (0.7) |
| Other | 37 (2.8) |
| Not specified | 465 |
| Implantation site, mean (SD) | |
| Subthalamic nucleus | 688 (45.1) |
| Globus pallidus internus | 444 (29.1) |
| Ventralis intermedius of thalamus | 332 (21.8) |
| Ventralis oralis posterior/anterior of thalamus | 39 (2.6) |
| Anterior limb of internal capsule | 13 (0.9) |
| Centromedian nucleus of thalamus | 10 (0.7) |
| Not specified | 238 |
| Implantation laterality, mean (SD) | |
| Left | 962 (54.5) |
| Right | 802 (45.5) |
| Total number of leads | 1,764 |
Abbreviation: SD, Standard Deviation.
The number of implanted leads within various targets and for different disorders and the summary statistics of the patient population from which these results were derived are shown. In the supplementary methods is a more complete summary of the dataset with additional information.
Table 2. Patient Diagnoses and Targets.
| Diagnosis | STN | GPi | VIM | Voa/Vop | ALIC | CM |
|---|---|---|---|---|---|---|
| Primary diagnosis, N | ||||||
| Parkinson’s disease | 403 | 223 | 23 | 3 | 0 | 0 |
| Essential tremor | 5 | 2 | 168 | 4 | 0 | 0 |
| Primary dystonia | 19 | 108 | 7 | 0 | 0 | 0 |
| Tremor (not otherwise specified) | 4 | 0 | 31 | 27 | 0 | 0 |
| Secondary dystonia | 10 | 19 | 2 | 1 | 0 | 0 |
| Obsessive compulsive disorder | 0 | 0 | 0 | 0 | 12 | 0 |
| Tourette syndrome | 0 | 2 | 2 | 0 | 0 | 4 |
| Other | 8 | 18 | 4 | 1 | 0 | 0 |
| Not specified | 239 | 72 | 95 | 3 | 1 | 6 |
Abbreviations: ALIC, Anterior Limb of Internal Capsule; CM, Centromedian Nucleus of the Thalamus; GPi, Globus Pallidus Internus; STN, Subthalamic Nucleus; VIM, Ventralis Intermedius of Thalamus; Voa/Vop, Ventralis Oralis Posterior/Anterior of Thalamus.
The table shows a summary of the various DBS implantation target sites with respect to the primary patient diagnosis.
Table 3. Summary Statistics.
| Characteristics | N (%) | Voltage (V) | Frequency (Hz) | Pulse Width (µs) | Impedance (Ohms) |
|---|---|---|---|---|---|
| Gender, mean (SD) | |||||
| Male | 206 (68.9) | 2.7 (0.6) | 147.9 (31.3) | 112.3 (60.1) | 948.9 (315.1) |
| Female | 93 (31.1) | 2.7 (0.7) | 140.1 (40.2) | 126.4 (81.0) | 960.7 (312.4) |
| Age, mean (SD) | |||||
| <40 | 36 (12.0) | 2.9 (0.6) | 126.0 (46.3) | 186.9 (113.0) | 1003.5 (433.4) |
| 40–60 | 101 (33.8) | 2.6 (0.7) | 144.0 (33.3) | 114.7 (61.2) | 975.2 (305.1) |
| >60 | 162 (54.2) | 2.7 (0.7) | 151.0 (30.3) | 102.2 (44.8) | 927.1 (286.4) |
| Primary diagnosis, mean (SD) | |||||
| Parkinson’s disease | 183 (61.2) | 2.7 (0.6) | 148.6 (28.4) | 94.7 (23.5) | 955.2 (320.6) |
| Essential tremor | 48 (16.0) | 2.7 (0.7) | 155.5 (23.6) | 103.3 (31.6) | 931.8 (287.7) |
| Primary dystonia | 36 (12.0) | 2.8 (0.8) | 117.5 (50.6) | 215.6 (115.4) | 994.8 (251.1) |
| Implantation site, mean (SD) | |||||
| Globus pallidus internus | 86 (28.8) | 2.8 (0.7) | 136.2 (43.6) | 156.5 (106.3) | 1004.3 (296.4) |
| Subthalamic nucleus | 141 (47.2) | 2.6 (0.6) | 146.9 (28.9) | 95.4 (23.3) | 932.9 (311.5) |
| Ventralis intermedius of thalamus | 59 (19.7) | 2.7 (0.7) | 156.5 (25.1) | 105.9 (39.2) | 952.1 (300.7) |
| Ventralis oralis posterior/anterior | 8 (2.7) | 2.7 (0.6) | 169.4 (21.9) | 112.5 (13.9) | 941.8 (523.9) |
| Anterior limb of internal capsule | 4 (1.3) | 2.9 (0.6) | 116.3 (37.5) | 172.5 (56.8) | 515.3 (132.8) |
| Implantation laterality, mean (SD) | |||||
| Left | 188 (62.9) | 2.7 (0.6) | 147.9 (33.1) | 113.9 (60.5) | 945.0 (286.3) |
| Right | 111 (37.1) | 2.7 (0.7) | 141.7 (36.3) | 121.3 (77.9) | 965.3 (356.6) |
Abbreviation: SD, Standard Deviation.
The table shows a comprehensive breakdown of average stimulation settings and impedance seen at approximately 24 months post implantation grouped by gender, age, indication and lead location.
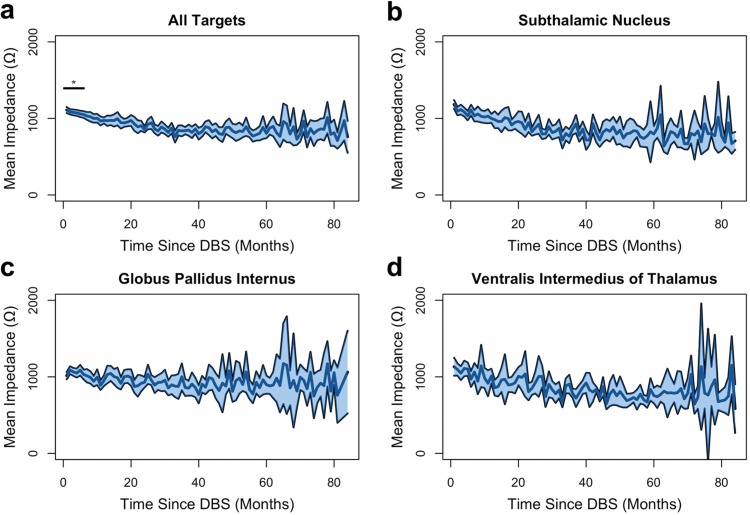
Impedance measurements were analyzed as a function of time post implantation. The results are shown in Figure 1. There were significant fluctuations in the mean impedance of all electrodes, but these changes stabilized by the sixth month for all lead locations except for the STN, where fluctuations persisted throughout the duration of follow-up. For all recorded electrodes, Wilcoxon signed rank tests were performed individually between the impedance at 12 months and month 0 to month 11. We found that the mean at months 0–5 were significantly different from the mean at 12 months (p < 0.001). There was no significant difference in the mean impedances from month 6–11 versus month 12 by the Wilcoxon signed rank test. This finding suggested that impedance tended to fluctuate most within the first 6 months.
Figure 1. Average Impedance of Deep Brain Stimulation Electrode Leads Versus Time. The solid center blue line represents the average impedance of all electrode measurements of the specified target site: (A) for all targets, (B) for subthalamic nucleus, (C) for globus pallidus internus, and (D) for ventralis intermedius of thalamus at 1-month intervals from 0 to 84 months. The shaded region above and below the line represents two standard errors of the mean. Asterisk with bar indicates p < 0.001 difference from average impedance at month 12.
To investigate impedance fluctuations further, we assessed for trends using a Mann–Kendall test of trend across electrodes and separated by DBS brain targets (Figure 1B–D). Although the Mann–Kendall test is primarily used for monotonic trends, it has been extensively used in the environmental sciences literature to evaluate cyclic trends such as in annual rain fall and water quality surveillance.17–19 In this scenario, the Mann–Kendall test evaluates the global trend but is unable to interpret any single cycle trends. With respect to the impedance data shown in Figure 1, we can analyze the degree of trend over time but are unable to comment on month-to-month changes. In the composite dataset of all electrodes, there was evidence of significant downward trending from months 0 to 23 (p < 0.043) but downward trending from months 24 to 84 was not significant (p = 0.058). The Tau b coefficients (the degree of trending measured by the Mann–Kendall test) were –0.612 and –0.166 respectively. In the STN target, there was evidence of significant downward trending including all 84 months of follow-up (p = 1.00 × 10–13). The Tau b coefficient was –0.552. In the GPi target, there was no statistical evidence of upward or downward trending for all 84 months (p = 0.195). The Kendall Tau b correlation coefficient was –0.0958. In the VIM target, there was evidence of downward trending from months 0 to 27 (p < 0.036); however, from months 28 to 84 there was no evidence of trending (p = 0.079). The Tau b coefficients were –0.305 and –0.159 respectively.
Discussion
Our study found that impedance drift was most prominent within the first 6 months following DBS implantation. Following this initial period, changes in impedance were more subtle but overall trended downward with time in all targets except in the GPi. The stability of the GPi and downward trend of other targets were consistent with observations from previously performed smaller studies.12,14,20,21 These changes in impedance have been attributed to cerebral edema and inflammation secondary to surgical micro-lesioning effects and due to foreign body responses as the brain parenchyma remodels to accommodate the electrode–tissue interface; however, this explanation remains unconfirmed in the human brain.12,13,15,22 In this study, the therapeutic impedance from all implantation sites equalized toward the range of approximately 1000 Ω, presumably as the electrode–tissue interface stabilized. Downward trends in impedance weakened with continued follow-up over time, supporting the idea of stabilization of the electrode–tissue interface. One limitation of this retrospective analysis is the significant decrease in clinic visits after 60 months of follow-up. As such, the variability of impedance in all targets after 60 months is much higher than prior to 60 months. This is likely due to the decrease in sample size rather than changes to the electrode–tissue interface.
While impedance in the GPI target was relatively stable throughout the duration of follow-up, impedance in the STN continued to trend downward throughout the entire 84 months of clinical follow-up. One main component of impedance variability in DBS is the degree of tissue encapsulation as a foreign body reaction to the DBS electrode and the conductivity of that encapsulation.15 It is thought that electrical stimulation can modify the tissue architecture of the immediate vicinity, causing an increase in conductivity and decrease in impedance. The long-term findings of this study may suggest that the encapsulation response in the STN is not entirely identical to that of the GPi. This may also be related to the underlying structural differences between the two regions as well as due to the chemical composition of the tissue and neurotransmitters that primarily constitute affected neural structures.23 Researchers have proposed that differences in the gray to white matter ratio and corresponding distribution of peri-electrode CSF could play a role in the conductance of the overall system.8 It is also thought that these factors may be tied to the underlying encapsulation response, although no studies have definitively described this relationship yet.
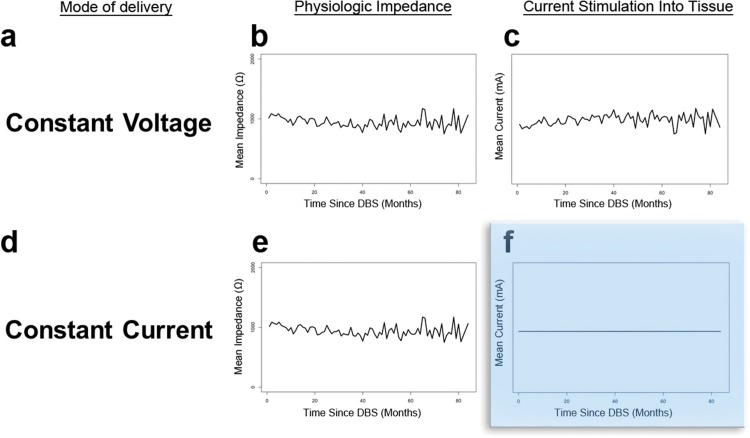
Impedance at the electrode–tissue interface is an important factor that can influence the current delivered by DBS. In traditional constant voltage DBS devices, it may be particularly important since electrode impedance directly correlates to the amount of electrical current delivered to the brain tissue (Ohm’s law: V = IR). In constant current devices, the impedance plays a lesser role since the output voltage is regulated to maintain a specified current independent of the local impedance. The electrophysiologic differences between these two types of devices are demonstrated with hypothetical data in Figure 2. Though some degree of impedance drift is an expected phenomenon that clinicians should be aware of, the overall stability of impedance demonstrated in this large database cohort study may explain why clinicians have not observed dramatic differences in outcomes between constant current and constant voltage.24 To date, there have been no large studies that have directly compared constant current DBS devices against constant voltage devices. Smaller studies have revealed constant current devices to be similar in safety and efficacy when compared to constant voltage devices.25,26 Additionally, there have been subtle differences documented in impedance among the various brain implantation sites and this may be related to inherent tissue properties and composition. Future studies should investigate potential correlations between age, stimulation settings, therapeutic impedance, disease states, and clinical outcomes and these studies should provide a more structured follow-up.
Figure 2. Constant Voltage vs. Constant Current. The characteristics of a constant-voltage device can be seen in A–C and constant current device in D–F. The blue box highlights a key feature of constant-current devices: regardless of physiologic impedance, the amount of current stimulation delivered to the target tissue will always be the same.
Conclusions
The large database cohort assessed in our study provided a unique opportunity to evaluate the impedances of empirically determined stimulation settings for a large number of brain targets and regions. Impedance drift occurs primarily in the first 6 months post DBS implantation, and downward trends (when present) weaken over time. Fluctuations in impedance are most prominent and downward trends most persistent in the STN target, which may be due to differences in structure and tissue composition when compared with GPI DBS. These results suggest that constant current devices could be more effective in maintaining impedance stability. Further investigations of the relationship between impedance and other DBS parameters could possibly streamline the process of initial DBS programming after implantation and may aid in the optimization of DBS programming settings.
Acknowledgments
We thank the Parkinson’s Foundation for their support through the center of excellence program and we thank the support of the UF INFORM database.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interests: The authors report no conflict of interest.
Ethics Statement: This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. The authors’ institutional ethics committee has approved this study and all patients have provided written informed consent.
References
- 1.Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223–229. doi: 10.1016/S1474-4422(07)70035-2. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R, Lozano AM, Sime E, Lang AE. Long-term follow-up of thalamic deep brain stimulation for essential and parkinsonian tremor. Neurology. 2003;61:1601–1604. doi: 10.1212/01.wnl.0000096012.07360.1c. doi: 10.1212/01.WNL.0000096012.07360.1C. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 6.Shute JB, Okun MS, Opri E, Molina R, Ross PJ, Martinez-Ramirez D, et al. Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. Neuroimage Clin. 2016;12:165–172. doi: 10.1016/j.nicl.2016.06.015. doi: 10.1016/j.nicl.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204–1212. doi: 10.1016/j.biopsych.2013.01.034. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Whiting DM, Tomycz ND, Bailes J, de Jonge L, Lecoultre V, Wilent B, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg. 2013;119:56–63. doi: 10.3171/2013.2.JNS12903. doi: 10.3171/2013.2.JNS12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, Luciano MS, Galifianakis NB, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat Neurosci. 2015;18:779–786. doi: 10.1038/nn.3997. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abosch A, Lanctin D, Onaran I, Eberly L, Spaniol M, Ince NF. Long-term recordings of local field potentials from implanted deep brain stimulation electrodes. Neurosurgery. 2012;71:804–814. doi: 10.1227/NEU.0b013e3182676b91. doi: 10.1227/NEU.0b013e3182676b91. [DOI] [PubMed] [Google Scholar]
- 11.Sillay KA, Rutecki P, Cicora K, Worrell G, Drazkowski J, Shih JJ, et al. Long-term measurement of impedance in chronically implanted depth and subdural electrodes during responsive neurostimulation in humans. Brain Stimul. 2013;6:718–726. doi: 10.1016/j.brs.2013.02.001. doi: 10.1016/j.brs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Satzer D, Lanctin D, Eberly LE, Abosch A. Variation in deep brain stimulation electrode impedance over years following electrode implantation. Stereotact Funct Neurosurg. 2014;92:94–102. doi: 10.1159/000358014. doi: 10.1159/000358014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satzer D, Maurer EW, Lanctin D, Guan W, Abosch A. Anatomic correlates of deep brain stimulation electrode impedance. J Neurol Neurosurg Psychiatry. 2015;86:398–403. doi: 10.1136/jnnp-2013-307284. doi: 10.1136/jnnp-2013-307284. [DOI] [PubMed] [Google Scholar]
- 14.Lungu C, Malone P, Wu T, Ghosh P, McElroy B, Zaghloul K, et al. Temporal macrodynamics and microdynamics of the postoperative impedance at the tissue-electrode interface in deep brain stimulation patients. J Neurol Neurosurg Psychiatry. 2014;85:816–819. doi: 10.1136/jnnp-2013-306066. doi: 10.1136/jnnp-2013-306066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butson CR, Maks CB, McIntyre CC. Sources and effects of electrode impedance during deep brain stimulation. Clin Neurophysiol. 2006;117:447–454. doi: 10.1016/j.clinph.2005.10.007. doi: 10.1016/j.clinph.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sillay KA, Chen JC, Montgomery EB. Long-term measurement of therapeutic electrode impedance in deep brain stimulation. Neuromodulation. 2010;13:195–200. doi: 10.1111/j.1525-1403.2010.00275.x. doi: 10.1111/j.1525-1403.2010.00275.x. [DOI] [PubMed] [Google Scholar]
- 17.Hipel KW, McLeod AI. Time series modelling of water resources and environmental systems. Amsterdam: Elsevier Science; 1994. pp. 880–884. [Google Scholar]
- 18.Hirsch RM, Slack JR, Smith RA. Techniques of trend analysis for monthly water quality data. Water Resour Res. 1982;18:107–21. doi: 10.1029/WR018i001p00107. [Google Scholar]
- 19.Libiseller C, Grimvall A. Performance of partial Mann–Kendall tests for trend detection in the presence of covariates. Environmetrics. 2002;13:71–84. doi: 10.1002/env.507. [Google Scholar]
- 20.Wu C, Evans JJ, Skidmore C, Sperling MR, Sharan AD. Impedance variations over time for a closed-loop neurostimulation device: early experience with chronically implanted electrodes. Neuromodulation. 2013;16:46–50. doi: 10.1111/j.1525-1403.2012.00529.x. discussion 50. doi: 10.1111/j.1525-1403.2012.00529.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheung T, Nuño M, Hoffman M, Katz M, Kilbane C, Alterman R, et al. Longitudinal impedance variability in patients with chronically implanted DBS devices. Brain Stimul. 2013;6:746–751. doi: 10.1016/j.brs.2013.03.010. doi: 10.1016/j.brs.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Lempka SF, Miocinovic S, Johnson MD, Vitek JL, McIntyre CC. In vivo impedance spectroscopy of deep brain stimulation electrodes. J Neural Eng. 2009;6:046001. doi: 10.1088/1741-2560/6/4/046001. doi: 10.1088/1741-2560/6/4/046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing P, Miled MA, Sawan M. Glutamate, GABA and dopamine hydrochloride concentration effects on the conductivity and impedance of cerebrospinal fluid; 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER); San Diego, CA. IEEE; 2013:1037–1040. [Google Scholar]
- 24.Bronstein JM, Tagliati M, McIntyre C, MBBChir RC, Cheung T, Hargreaves EL, et al. The rationale driving the evolution of deep brain stimulation to constant-current devices. Neuromodulation. 2015;18:85–88. doi: 10.1111/ner.12227. discussion 88-89. doi: 10.1111/ner.12227. [DOI] [PubMed] [Google Scholar]
- 25.Okun MS, Gallo BV, Mandybur G, Jagid j, Foote KD, Revilla FJ, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol. 2012;11:140–149. doi: 10.1016/S1474-4422(11)70308-8. doi: 10.1016/S1474-4422(11)70308-8. [DOI] [PubMed] [Google Scholar]
- 26.Morrell MJ, Group RSiES Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]