Abstract
Background
Hereditary myoclonus dystonia is often due to changes in the SGCE gene. Dystonia (DYT)-SGCE has a variable phenotype that can involve focal or generalized myoclonus and various forms of task-specific, segmental, or generalized dystonia. Psychiatric comorbidities are common.
Case Report
We report a case of a young woman with generalized myoclonus, dystonia, and intellectual disability. She was found to have a novel SGCE splice site variant.
Discussion
This novel variant is very likely pathogenic by in silico analysis and has not been previously reported. Additionally, her intellectual disability may constitute a novel phenotype for patients with SGCE variants.
Keywords: DYT11, intellectual disability, myoclonus dystonia, SGCE, sarcoglycan
Introduction
Hereditary myoclonus dystonia associated with SGCE variants was first reported in 2001.1 The phenotypic spectrum of this condition is broad, but most commonly features myoclonus of the upper trunk and arms, along with cervical or brachial dystonia.2 Isolated lower limb dystonia with myoclonus emerging years later has also been described.3 Classically, handwriting will exacerbate arm dystonia, and arm and cervical myoclonus.4 In many cases the myoclonus is alcohol responsive. Variants in the SGCE gene are estimated to be responsible for 30–50% of myoclonus dystonia syndromes,1 but variants in RELN,5 ANO3,6 TOR1A,7 and the locus for DYT158 have been reported to have similar phenotypes. DYT-SGCE has been reported with psychiatric comorbidities such as anxiety and obsessive compulsive disorder.9–11 The condition can be managed medically with a variety of agents including valproate, leviteracetam,12 clonazepam, tetrabenazine,13 and sodium oxybate.14 Pallidal deep brain stimulation has been shown to provide benefit for some patients as well.15–19 Cognitive profiles of patients with DYT-SGCE are varied among reports. Some describe no abnormalities in cognition.9,10,20 Others have indicated above-average verbal intellectual functioning with impairments in free recall and executive functioning.21,22 Frank intellectual disability has not been previously described in these patients. Here we report a patient with a novel SGCE variant and a history of intellectual disability.
Case report
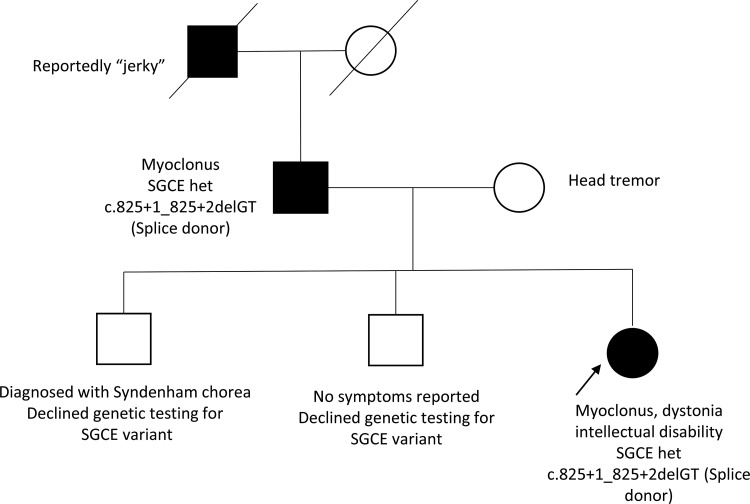
A 21-year-old female presented to our clinic with a history of generalized myoclonus since childhood with developmental delay and intellectual disability. She was the product of an uneventful pregnancy and was delivered by C-section due to fetal distress. She had Apgar scores of 8 and 9 at 5 and 10 minutes of life, respectively, an unremarkable postnatal course, and was able to be discharged home on her third day of life. Whole-body occasional jerking with preservation of consciousness was noted at about 1 year. Her jerking seemed to worsen with activity. She had delayed gross and fine motor milestones (sitting at 9 months, walking at 19 months, difficulty running). She was originally assessed by two local child neurologists; a definitive diagnosis was not reached after a work-up that included a normal magnetic resonance imaging (MRI) brain scan and normal electroencephalogram although a provisional diagnosis of cerebral palsy was entertained. At age 10, she was administered the Wechsler Intelligence Scale for children, fourth edition.23 Performance on that assessment showed a full-scale intelligence quotient of 74 with deficiencies in perceptual reasoning, working memory, and processing speed (see Supplementary Figure 1). She graduated from a high school special education program and enrolled in a few community college courses with special accommodations. Over the intervening years, her myoclonus worsened in severity and began to interfere with handwriting and other daily activities, and she would occasionally fall. It was not known if the jerking lessened with alcohol consumption. There were reports of abnormal arm posturing during writing but no other complaints of neck or leg cramping, stiffness, or posturing. She also had a history of Restless Leg Syndrome (RLS) and generalized anxiety. Previous treatment with topiramate and clonazepam had been ineffective at controlling movement. Family history was significant for jerky movements in her father and paternal grandfather, who were both intellectually normal. The proband’s brother had been previously diagnosed with Sydenham’s chorea (see Figure 1). Clinical examination was notable for global hypotonia, moderate generalized spontaneous myoclonus that worsened with activity, mild cervical dystonia, and writer’s cramp (see Video 1). The Unified Myoclonus Rating Scale24 was administered with a total score of 99 (see Supplementary Figure 2). Her father was also noted to have milder generalized myoclonus during her initial office visit.
Figure 1. Pedigree. The proband has 2 male siblings, one of which was diagnosed with Syndenham chorea but has declined genetic testing. The proband’s father has mild generalized myoclonus and was found to have the same mutation. His father was reportedly ‘jerky’ but is now deceased.
Video 1. Video of Neurological Exam. Mild cervical dystonia with left turn. Myoclonus affecting the neck and trunk. Myoclonus is elicited by auditory and tactile stimuli and worsened in the trunk and arms with posture and intention. The gait is slightly wide based and appears hypotonic. Handwriting induces worsening of cervical dystonia and neck and truncal myoclonus. Myoclonus affects handwriting and water pouring.
MRI of the brain with a 1.5-T Siemens scanner was normal. A dystonia comprehensive sequencing panel was carried out by Invitae Laboratories and showed a novel, likely pathogenic variant in SGCE, c.825+1_825+2delGT (NM_003919.2). This test was performed using next-generation sequencing, and deletion/duplication analysis was performed on the same assay utilizing an in-house algorithm that determines the copy number at each target. The variants identified through next-generation sequencing were subsequently confirmed via appropriate methods, including, in this case, Sanger sequencing. This 2-base pair deletion at the consensus donor splice site is expected to disrupt RNA splicing and likely results in an absent or disrupted protein product with an 'Human Splicing Finder (HSF) score of 3.0 and Combined Annotation Dependent Depletion (CADD) prediction score of 27.6.25,26 This variant is absent from population databases (gnomAD27) and has not been previously reported in individuals with SGCE-related disease. Testing of her father revealed the same variant. The family does not have contact with the father’s extended family. Her mother and siblings have not consented to genetic testing as yet. Owing to the presence of intellectual disability, a whole-genome array comparative genomic hybridization with single-nucleotide polymorphism (SNP) analysis from GeneDx™ was performed and failed to show any abnormalities. This test is performed on a custom-designed oligonucleotide microarray (GenomeDx v5) and the design is based on human genome build GRCh37/UCSChg19 and contains approximately 118,000 probes that provide copy number data and 66,000 probes that generate genotype information through analysis of SNPs. A repeat neuropsychological evaluation was performed and showed deficits, including abstract reasoning, working memory, receptive and expressive language, and executive functioning in the setting of anxiety with intact short-term memory, and delayed visual recall (see Supplementary Figure 3). She was initially treated with higher doses of clonazepam but was limited by somnolence. Leviteracetam was also trialed but failed to control her myoclonus adequately. She has undergone bilateral pallidal deep brain stimulation and, at her last follow up two months postoperatively, has demonstrated reduction in myoclonus.
Discussion
Hereditary myoclonus dystonia associated with SGCE variants was first reported in 2001.1 There are a wide variety of clinical presentations and genetic changes that have been reported in association with the phenotype.1,5,6,8,28 Most variants have no reported abnormalities in cognition.9,10 Others have indicated above-average verbal intellectual functioning with impairments in free recall and executive functioning.20,22 This family illustrates the well-documented variability in penetrance and phenotypic expression that is thought to be due to maternal imprinting.1,29 The proband illustrates a novel phenotype that includes intellectual disability. We do not think it likely that her birth history contributed to her intellectual disability given her Apgar scores, reported lack of encephalopathy after birth, and normal brain MRI. With these items she would not meet criteria for hypoxic ischemic encephalopathy or cerebral palsy. We attempted to rule out other genetic causes of intellectual disability with a SNP array panel but acknowledge the limitation of this approach. The significance of this variant is predicted to by highly pathogenic given its location by in silico programs; however, we did not attempt to determine the effect of this variant on the RNA level.
In conclusion, we describe a case of DYT-SGCE due to a novel SGCE variant. In light of the novel phenotype herein decribed, clinicians should not discount the possibility of an SGCE variant in a patient who otherwise exhibits compatible signs. Such patients may benefit from similar medical management or pallidal deep brain stimulation,15–18 and continued attention to psychiatric comorbidities that have been described in patients with other SGCE variant.
Footnotes
Funding: None.
Financial Disclosures: T.B. is a consultant for Genome Medical Inc. A.D. has attended advisory boards for Teva pharmaceuticals and Adamas pharmaceuticals in the past year.
Conflict of Interests: The authors report no conflict of interest.
Ethics Statement: All patients that appear on video have provided written informed consent; authorization for the videotaping and for publication of the videotape was provided.
References
- 1.Zimprich A, Grabowski M, Asmus F, Naumann M, Berg D, Bertram M, et al. Mutations in the gene encoding [varepsilon]-sarcoglycan cause myoclonus-dystonia syndrome. Nat Genet. 2001;29:66. doi: 10.1038/ng709. doi: 10.1038/ng709. [DOI] [PubMed] [Google Scholar]
- 2.Koukouni V, Valente EM, Cordivari C, Bhatia KP, Quinn NP. Unusual familial presentation of epsilon‐sarcoglycan gene mutation with falls and writer's cramp. Mov Disord. 2008;23:1913–1915. doi: 10.1002/mds.21935. doi: 10.1002/mds.21935. [DOI] [PubMed] [Google Scholar]
- 3.Peall KJ, Kurian MA, Wardle M, Waite AJ, Hedderly T, Lin JP, et al. SGCE and myoclonus dystonia: motor characteristics, diagnostic criteria and clinical predictors of genotype. J Neurolo. 2014;261:2296–2304. doi: 10.1007/s00415-014-7488-3. doi: 10.1007/s00415-014-7488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinugawa K, Vidailhet M, Clot F, Apartis E, Grabli D, Roze E. Myoclonus‐dystonia: an update. Mov Disord. 2009;24:479–489. doi: 10.1002/mds.22425. doi: 10.1002/mds.22425. [DOI] [PubMed] [Google Scholar]
- 5.Groen JL, Ritz K, Jalalzadeh H, van der Salm SMA, Jongejan A, Mook OR, et al. RELN rare variants in myoclonus‐dystonia. Mov Disord. 2015;30:415–419. doi: 10.1002/mds.26070. doi: 10.1002/mds.26070. [DOI] [PubMed] [Google Scholar]
- 6.Stamelou M, Charlesworth G, Cordivari C, Schneider SA, Kägi G, Sheerin UM, et al. The phenotypic spectrum of DYT24 due to ANO3 mutations. Mov Disord. 2014;29:928–934. doi: 10.1002/mds.25802. doi: 10.1002/mds.25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabakci K, Hedrich K, Leung JC, Mitterer M, Vieregge P, Lencer R, et al. Mutations in DYT1. Extension of the phenotypic and mutational spectrum. Neurology. 2004;62:395–400. doi: 10.1212/01.wnl.0000113024.84178.f7. doi: 10.1212/01.WNL.0000113024.84178.F7. [DOI] [PubMed] [Google Scholar]
- 8.Han F, Racacho L, Lang AE, Bulman DE, Grimes DA. Refinement of the DYT15 locus in myoclonus dystonia. Mov Disord. 2007;22:888–892. doi: 10.1002/mds.21400. doi: 10.1002/mds.21400. [DOI] [PubMed] [Google Scholar]
- 9.van Tricht MJ, Dreissen YE, Cath D. Cognition and psychopathology in myoclonus-dystonia. J Neurol Neurosurg Psychiatry. 2012;83:814–820. doi: 10.1136/jnnp-2011-301386. doi: 10.1136/jnnp-2011-301386. [DOI] [PubMed] [Google Scholar]
- 10.Foncke EM, Cath D, Zwinderman K, Smit J, Schmand B, Tijssen M. Is psychopathology part of the phenotypic spectrum of myoclonus-dystonia? a study of a large Dutch MD family. Cogn Behav Neurol. 2009;22:127–133. doi: 10.1097/WNN.0b013e3181a7228f. doi: 10.1097/WNN.0b013e3181a7228f. [DOI] [PubMed] [Google Scholar]
- 11.Saunders–Pullman R, Shriberg J, Heiman G, Raymond D, Wendt K, Kramer P, et al. Myoclonus dystonia: possible association with obsessive–compulsive disorder and alcohol dependence. Neurology. 2002;58:242–245. doi: 10.1212/wnl.58.2.242. doi: 10.1212/WNL.58.2.242. [DOI] [PubMed] [Google Scholar]
- 12.Striano P, Manganelli F, Boccella P, Perretti A, Striano S. Levetiracetam in patients with cortical myoclonus: a clinical and electrophysiological study. Mov Disord. 2005;20:1610–1614. doi: 10.1002/mds.20530. doi: 10.1002/mds.20530. [DOI] [PubMed] [Google Scholar]
- 13.Luciano AY, Jinnah H, Pfeiffer RF, Truong DD, Nance MA, LeDoux MS. Treatment of myoclonus-dystonia syndrome with tetrabenazine. Parkinsonism Relat Disord. 2014;20:1423–1426. doi: 10.1016/j.parkreldis.2014.09.029. doi: 10.1016/j.parkreldis.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frucht SJ, Bordelon Y, Houghton WH, Reardan D. A pilot tolerability and efficacy trial of sodium oxybate in ethanol‐responsive movement disorders. Mov Disord. 2005;20:1330–1337. doi: 10.1002/mds.20605. doi: 10.1002/mds.20605. [DOI] [PubMed] [Google Scholar]
- 15.Kimura Y, Mihara M, Kawarai T, Kishima H, Sakai N, Takahashi MP, et al. Efficacy of deep brain stimulation in an adolescent patient with DYT11 myoclonus‐dystonia. Neurol Clin Neurosci. 2014;2:57–59. doi: 10.1111/ncn3.75. [Google Scholar]
- 16.FitzGerald J, Rosendal F, De Pennington N, Joint C, Forrow B, Fletcher C, et al. Long-term outcome of deep brain stimulation in generalised dystonia: a series of 60 cases. J Neurol Neurosurg Psychiatry. 2014;85:1371–1376. doi: 10.1136/jnnp-2013-306833. doi: 10.1136/jnnp-2013-306833. [DOI] [PubMed] [Google Scholar]
- 17.Azoulay-Zyss J, Roze E, Welter M-L, Navarro S, Yelnik J, Clot F, et al. Bilateral deep brain stimulation of the pallidum for myoclonus-dystonia due to ε-sarcoglycan mutations: a pilot study. Arch Neurol. 2011;68:94–98. doi: 10.1001/archneurol.2010.338. doi: 10.1001/archneurol.2010.338. [DOI] [PubMed] [Google Scholar]
- 18.Gruber D, Kühn AA, Schoenecker T, Kivi A, Trottenberg T, Hoffmann KT, et al. Pallidal and thalamic deep brain stimulation in myoclonus‐dystonia. Mov Disord. 2010;25:1733–1743. doi: 10.1002/mds.23312. doi: 10.1002/mds.23312. [DOI] [PubMed] [Google Scholar]
- 19.Rughani AI, Lozano AM. Surgical treatment of myoclonus dystonia syndrome. Mov Disord. 2013;28:282–287. doi: 10.1002/mds.25326. doi: 10.1002/mds.25326. [DOI] [PubMed] [Google Scholar]
- 20.Doheny D, Brin M, Morrison C, Smith CJ, Walker RH, Abbasi S, et al. Phenotypic features of myoclonus-dystonia in three kindreds. Neurology. 2002;59:1187–1196. doi: 10.1212/wnl.59.8.1187. doi: 10.1212/WNL.59.8.1187. [DOI] [PubMed] [Google Scholar]
- 21.Doheny D, Danisi F, Smith C, Morrison C, Velickovic M, de Leon D, et al. Clinical findings of a myoclonus-dystonia family with two distinct mutations. Neurology. 2002;59:1244–1246. doi: 10.1212/wnl.59.8.1244. doi: 10.1212/WNL.59.8.1244. [DOI] [PubMed] [Google Scholar]
- 22.Ben‐Pazi H, Jaworowski S, Shalev RS. Cognitive and psychiatric phenotypes of movement disorders in children: a systematic review. Dev Med Child Neurol. 2011;53:1077–1084. doi: 10.1111/j.1469-8749.2011.04134.x. doi: 10.1111/j.1469-8749.2011.04134.x. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D. Wechsler intelligence scale for children—WISC-IV. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 24.Frucht SJ, Leurgans SE, Hallett M, Fahn S. The unified myoclonus rating scale. Adv Neurol. 2002;89:361–376. [PubMed] [Google Scholar]
- 25.Desmet F-O, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kircher M, Witten DM, Jain P, O'roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310. doi: 10.1038/ng.2892. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285. doi: 10.1038/nature19057. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gatto EM, Pardal MM, Micheli FE. Unusual phenotypic expression of the DYT1 mutation. Parkinsonism Relat Disord. 2003;9:277–279. doi: 10.1016/s1353-8020(02)00128-1. doi: 10.1016/S1353-8020(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 29.Müller B, Hedrich K, Kock N, Dragasevic N, Svetel M, Garrels J, et al. Evidence that paternal expression of the ε-sarcoglycan gene accounts for reduced penetrance in myoclonus-dystonia. Am J Hum Genet. 2002;71:1303–1311. doi: 10.1086/344531. doi: 10.1086/344531. [DOI] [PMC free article] [PubMed] [Google Scholar]