Abstract
Oral immunogenicity of recombinant hepatitis B surface antigen (HBsAg) derived from yeast (purified product) or in transgenic potatoes (uncooked unprocessed sample) was compared. An oral adjuvant, cholera toxin, was used to increase immune responses. Transgenic plant material containing HBsAg was the superior means of both inducing a primary immune response and priming the mice to respond to a subsequent parenteral injection of HBsAg. Electron microscopy of transgenic plant samples revealed evidence that the HBsAg accumulated intracellularly; we conclude that natural bioencapsulation of the antigen may provide protection from degradation in the digestive tract until plant cell degradation occurs near an immune effector site in the gut. The correlate of protection from hepatitis B virus infection is serum antibody titers induced by vaccination; the protective level in humans is 10 milliunits/ml or greater. Mice fed HBsAg-transgenic potatoes produced HBsAg-specific serum antibodies that exceeded the protective level and, on parenteral boosting, generated a strong long-lasting secondary antibody response. We have also shown the effectiveness of oral delivery by using a parenteral prime-oral boost immunization schedule. The demonstrated success of oral immunization for hepatitis B virus with an “edible vaccine” provides a strategy for contributing a means to achieve global immunization for hepatitis B prevention and eradication.
A highly effective and safe hepatitis B vaccine was licensed in the U.S. in 1986. It has been a great commercial success, with sales in the industrialized world exceeding $1 billion/year. With this outstanding product on the market, it is valid to ask why we have pursued an alternative vaccine to prevent hepatitis B virus (HBV) infections. As will be detailed below, the answer lies in finding an equivalent or improved vaccine that will serve poorer countries of the world, where the HBV disease burden remains at epidemic levels, and vaccine costs preclude the use of the currently available product.
The existing vaccine to prevent HBV infection is a biotechnology product that falls in the category of “subunit vaccines.” The gene encoding the hepatitis B surface antigen (HBsAg) is expressed in yeast cells grown by fermentation; the cells are broken, the protein is collected, and the HBsAg is caused to refold by chemical treatment to yield virus-like particles that can be formulated for injection (1). The resulting vaccine is the epitome of modern macromolecular pharmaceuticals derived from recombinant DNA technology; it is a superior product that contains only a “subunit” of the disease-causing agent. However, it is technology-intensive and therefore comparatively expensive as a health-care product for developing countries.
Although the licensed HBV vaccines developed to date have been for parenteral delivery, there is no a priori reason to exclude oral delivery. Oral vaccines are highly desirable from several standpoints. They can serve multiple immunization priorities, including simplicity of use (i.e., delivery of multiple immunogens), increase in compliance (as a result of increased ease/comfort of delivery), enhanced immune responses at mucosal sites, and stimulation of humoral immunity. For pathogens that cause enteric, respiratory, or sexually transmitted diseases, mucosal immune responses will provide an essential first line of defense. A highly successful oral vaccine that is globally available today contains attenuated poliovirus. However, to the present time, subunit vaccines have not been licensed for oral delivery, in part because of the lack of cost-effective production technology.
We have previously shown the expression of HBsAg in tobacco plants (2) and have demonstrated that the plant-derived antigen was immunogenic when administered parenterally to mice (3). We have now chosen to pursue a new strategy for HBsAg production in edible plants and to test a previously unused route of immunization (oral) for HBsAg delivery. The justification for the use of plants is 2-fold. First, transgenic edible crops can be produced at low cost. Furthermore, the technology development cost for creating the vaccine-producing plant can be fully borne at the outset of the project (such as by investments and/or philanthropy in the developed world), but then “in-country” production of the product by using indigenous agriculture and food processing technology would be used for product manufacture. In this way, sustainability of immunization, and independence from foreign supply, can become national priorities, and long-term public health can become both a source of national pride and local health-care company development.
In this paper, we evaluate the oral immunogenicity in mice of HBsAg produced in raw transgenic potatoes and delivered as food with minimal processing (peeling and cubing). We further compare this strategy with oral delivery of yeast-derived HBsAg that is presently used in injectable vaccines. We find that the potato vaccine is a more potent oral immunogen, perhaps because of the sequestration of the antigen within plant cells.
Methods
Generation of HBsAg Transgenic Tubers.
The HBsAg gene from a pMT-SA clone of a Chinese adr isolate of HBV (2) was inserted into a transformation plasmid vector pHB114 (4) that was mobilized into Agrobacterium tumefaciens (LBA4404), which was then used to transform Solanum tuberosum L. cv. FL1607. The transformed FL1607 was cured of the A. tumefaciens and clonally propagated, and the FL1607 HB114–16 line was selected for its high level of HBsAg expression. This line was then clonally propagated to multiply the number of plants and potted in soil to produce the potato tubers. Extracts of the FL1607 HB114–16 line expressed HBsAg detected by ELISA (AUSZYME, Abbott). The tissue from the untransformed FL1607 tubers did not express any proteins that were reactive with antibodies to HBsAg.
Transmission Electron Microscopy (TEM).
Transgenic potato plants of line HB114–16 and nontransgenic control FL1607 plants were grown in tissue-culture boxes on standard plant inorganic nutrient agar. Young growing leaves were excised, cut into 1-mm squares with a razor blade, and fixed in 2% paraformaldehyde + 2% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 6.8, for 0.5 h at 23°C followed by 1 h at 4°C. The tissue was postfixed in 2% osmium tetroxide at 23°C for 1 h, washed, and dehydrated in a graduated ethanol series at 4°C, 1:1 ethanol/acetone, and 100% acetone. The tissue was infiltrated with epon/araldite resin at 23°C and cured at 60°C overnight. Sections of about 70 nm in thickness were obtained with a Reichert Ultracut E microtome. The sections were viewed on a Philips 201 TEM and a Philips Tecnai 12 Biotwin (Philips, Eindhoven, The Netherlands).
Oral Immunization with Yeast-Derived Recombinant (r)HBsAg.
Before oral immunization, groups of mice were deprived of food for 2 h, followed by intragastric administration of 0.2 ml of an isotonic bicarbonate solution (8 parts Hanks' balanced salt solution and 2 parts of 7.5% sodium bicarbonate) to neutralize stomach acidity. Mice were then immunized with 150 μg of rHBsAg mixed with 10 μg of cholera toxin (CT) in a final volume of 0.2 ml. Oral dosing was done by using a 1-ml syringe fitted with an oral feeding needle.
Feeding Mice Transgenic Potatoes.
Tubers of transgenic potato line FL1607 HB114–16 expressing HBsAg at 8.35 μg/gm of fresh tuber weight were used to feed BALB/c mice that were fasted overnight before being fed peeled cubed raw tuber. Nontransformed line FL1607 was used as the control tuber. For the boiled potato experiment, potatoes were cooked in boiling water ≈10 min until soft. For each experiment, 10 mice were fed either 5 gm each of FL1607 or FL1607 HB 114–16 + 10 μg of CT (Sigma) once per week for 3 consecutive weeks. Each mouse was housed separately for the feedings and the amount of tuber weighed before and after feeding to monitor the amount ingested by individual mice. The potato was left in the cage until all of it was consumed or 24 h afterwards, whichever was earlier. For the duration of the experiment, animals were fed regular animal chow. At the times indicated in the legend to Fig. 1, mice were injected with 0.5 μg of yeast-derived alum-adsorbed rHBsAg (Merck Sharpe and Dohme).
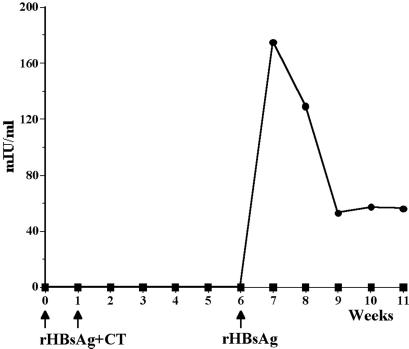
Figure 1.
Serum anti-HBsAg response after delivery of yeast-derived rHBsAg with a mucosal adjuvant CT by gavage. Mice were gavaged with either 150 μg of rHBsAg + CT or PBS + CT at weeks 0 and 1, and all mice were boosted with 0.5 μg of alum-adsorbed rHBsAg at week 6. Circles, rHBsAg + CT; squares = PBS + CT.
Measurement of Anti-HBsAg Antibodies.
Antibodies to HBsAg were detected by using the AUSAB EIA (Abbott). Polystyrene beads coated with HBsAg were incubated with the serum from either experimental or control animals for 18 ± 2 h at room temperature. At the end of this incubation period, beads were washed and HBsAg tagged with biotin, and rabbit antibiotin conjugated with horseradish peroxidase was incubated at 40 ± 1°C for 2 h with the antigen–antibody complex bound to the beads. Unbound biotin–antibiotin complex was removed, and the beads were washed. Next, o-phenylenediamine solution containing hydrogen peroxide was added to the bead and the reaction allowed to proceed for 30 min at room temperature. The reaction was stopped by the addition of 0.5 M sulfuric acid. The yellow color that develops is proportional to the amount of anti-HBsAg bound to the beads. The absorbance is measured at 492 nm in a Quantum II (Durham, NC) spectrophotometer. The AUSAB quantitative standard panel is included with each assay performed and permits the conversion of OD values to milli-International units (mIU)/ml.
Results
We tested the immunogenicity of recombinant HBsAg derived from yeast (purified soluble product) administered to mice by oral gavage. Mice were immunized with 150 μg of rHBsAg mixed with 10 μg of CT by oral gavage once per week for 2 consecutive weeks. A group of control mice were gavaged with PBS plus 10 μg of CT. No serum HBsAg-specific antibodies were detectable in mice of either group at up to 6 weeks, at which time all animals were boosted with a subimmunogenic dose of alum-adsorbed yeast rHBsAg. Only mice that had been orally primed with rHBsAg made an immediate secondary antibody response, with a peak response of 175 mIU/ml at week 7 (Fig. 1). No HBsAg-specific antibodies were detectable in the control mice.
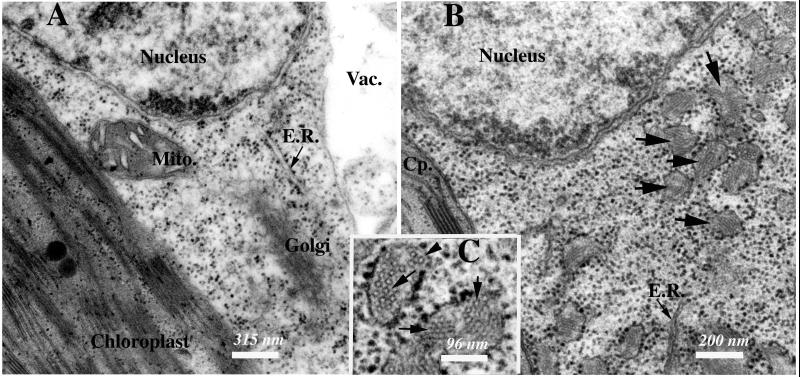
To determine whether expression of HBsAg in a plant presented any special advantage (protection from rapid degradation in the gut, slow release of antigen), we created transgenic potato plants that express HBsAg in tubers (4). For this study, we used line FL1607 HB114–16, which was transformed with pHB114 (Fig. 2). The HBsAg gene is unmodified from its native form and therefore is expected to be targeted to the endoplasmic reticulum (ER), as observed in yeast recombinant expression. We examined cells of HB114–16 and nontransformed FL1607 plants by TEM to determine whether HBsAg accumulates as virus-like particles (VLP). HB114–16 cells harbored unusual membrane-bound vesicular bodies (indicated by arrows in Fig. 3B). These vesicles, which may be derived from ER, contained circular structures that are ≈17 nm in diameter (indicated by arrows in Fig. 3C). This image provides, to our knowledge, the first evidence in transgenic HBsAg-expressing plants that the antigen is retained within vesicular structures. We have shown in a detailed study that the material within the vesicles stained specifically with antibody against HBsAg (M. Smith, L.R., C.J.A., M. Shuler, and H.S.M., et al., unpublished work). These circular structures are very similar to the VLP found in serum samples of HBV-infected patients (5), and the distended ER vesicles are similar to those produced in yeast expressing rHBsAg (6) or Chinese hamster ovary cells transfected with the HBsAg gene (7). Microscopic examination of cells of nontransformed FL1607 leaves (Fig. 3A) did not reveal any subcellular vesicles containing circular structures. We thus conclude that transgenic plant cells accumulate rHBsAg inside membrane vesicles, and that the recombinant antigen assembles in structural units.
Figure 2.
Structure of the T-DNA region of pHB114, the vector used for expression of HBsAg. The small HBsAg gene is inserted into an expression cassette from which transcription is driven by the cauliflower mosaic virus 35S promoter. The tobacco etch virus 5′-UTR provides enhancement of translation, and the Pin2 3′ element mediates polyadenylation. The selectable marker for plant transformation is the Npt2 gene, which confers resistance to kanamycin. LB and RB indicate the left and right borders of the T-DNA region, which, during Agrobacterium-mediated DNA delivery, become stably integrated into nuclear chromosomal DNA.
Figure 3.
TEM of nontransformed and HBsAg-expressing potato cells. (A) Thin-section TEM image of a nontransformed FL1607 potato cell. (Bar = 315 nm.) (B) Thin-section TEM image of a transgenic HB114–16 transgenic potato cell. Arrows indicate membrane-bound vesicles containing 17-nm putative HBsAg particles. (Bar = 200 nm.) (C) Enlarged image of membrane-bound vesicles containing 17 nm putative HBsAg particulate structures indicated by arrows. (Bar = 96 nm.) Cp, chloroplast; E.R., endoplasmic reticulum; Mito., mitochondrion; Vac., vacuole.
For animal feeding experiments, plants were grown to maturity in soil and harvested to collect tubers, which contained on average 8.35 μg of HBsAg per gram of fresh tuber. To evaluate the oral immunogenicity of HBsAg expressed in potato tubers, we fed peeled potato pieces to mice once per week for 3 consecutive weeks. Each mouse received 5 g of tuber (containing an average of 42 μg of HBsAg per dose) per feed. CT was used as a mucosal adjuvant, and 10 μg of CT was placed on the potato pieces and consumed by the animals in conjunction with the antigen. Nontransformed potatoes FL1607 plus CT were fed to groups of control mice. Serum samples of all mice were analyzed for the presence of anti-HBsAg antibodies, and the results are expressed as mIU of HBsAg-specific antibody per milliliter of serum.
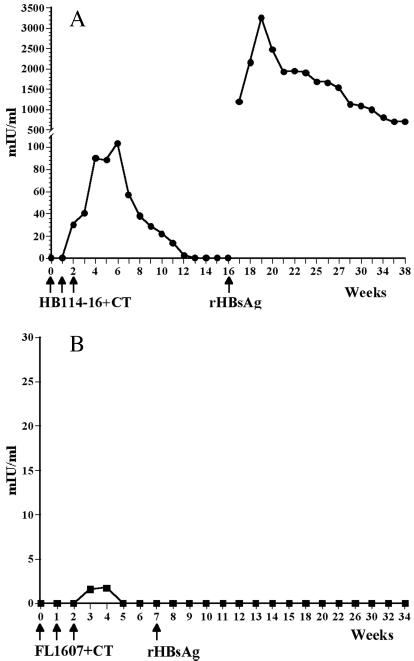
Multiple long-term immunogenicity and efficacy trials of the current licensed hepatitis vaccine have defined a protective anti-HBsAg level as >10 mIU/ml (8–11). In mice fed HBsAg-transgenic potatoes, anti-HBsAg antibodies were first detected 1 week after two doses; the response was increased to a peak value of 103 mIU/ml 4 weeks after the third dose (Fig. 4A) and was >10mIU/ml at week 11. To determine whether memory immune cells had been established as a result of oral immunization, all animals received a parenteral boost of a subimmunogenic dose of 0.5 μg of alum-adsorbed yeast-derived rHBsAg vaccine after the primary response antibody levels had returned to baseline. An immediate and strong secondary antibody response was obtained only in mice that had been previously fed HBsAg-transgenic potatoes. A peak antibody response of 3,300 mIU/ml was measured 3 weeks after the booster injection, and the response was maintained at high levels (700 mIU/ml) 5 months later, when the experiment was terminated. Mice fed control nontransformed potatoes following a schedule identical to that for the experimental group showed no primary antibody response, and no response could be elicited in these mice after boosting with the same parenteral dose of rHBsAg, which elicited a strong secondary response in the experimental mice (Fig. 4B). Collectively, the data allow us to conclude that plant-derived HBsAg, delivered as food, is orally immunogenic in mice and will elicit a primary antibody response. Furthermore, the strong secondary response seen after boosting with rHBsAg represents a true memory response, generated as a result of the mice being fed HBsAg transgenic potatoes; it was not merely a primary response caused by parenteral injection of rHBsAg.
Figure 4.
Serum anti-HBsAg antibodies detected in mice fed HBsAg transgenic but not in mice fed nontransformed potatoes. (A) Mice were fed HB114–16 potatoes + CT at weeks 0, 1, and 2; a primary antibody response was elicited, and a strong memory response was obtained when the mice were boosted with a subimmunogenic dose of yeast-derived alum-adsorbed rHBsAg. (B) Mice were fed nontransformed FL1607 potatoes + CT at weeks 0, 1, and 2, then boosted with a subimmunogenic dose of yeast-derived alum-adsorbed rHBsAg. No antibody response was elicited.
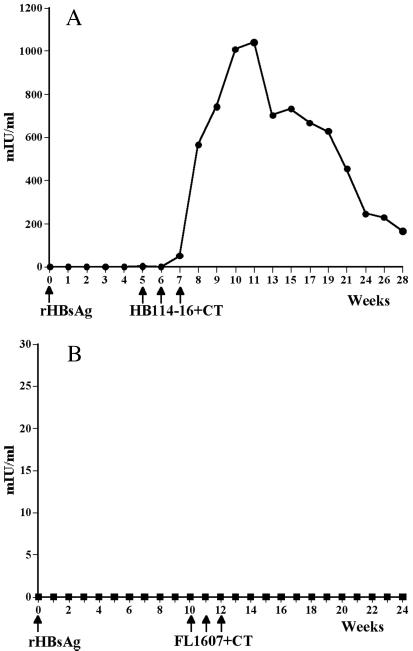
In converse experiments, we tested the ability of the HBsAg transgenic tubers to boost mice that had been primed with a single injection of the yeast-derived recombinant HBsAg. We first immunized mice with a single subimmunogenic parenteral dose of rHBsAg, which resulted in no detectable increase in anti-HBsAg antibodies over 5 weeks. When we then boosted them with three weekly feedings of HBsAg transgenic potatoes, a rapid increase in anti-HBsAg antibodies occurred after ingestion of the second dose of HBsAg-transgenic potatoes, and a peak antibody titer of >1,000 mIU/ml was measured 3 weeks after the third feeding (Fig. 5A). When mice were fed control nontransformed potatoes after a single priming dose of yeast rHBsAg, no antibodies were generated at any time point (Fig. 5B), thus confirming the specificity of the response measured in the sera from experimental mice.
Figure 5.
Booster response seen by feeding HBsAg potatoes to mice. (A) Serum anti-HBsAg antibodies were measured in mice fed HB114–16 potatoes + CT at weeks 5, 6, and 7 after priming with a subimmunogenic dose of 0.5 μg of yeast-derived alum-adsorbed rHBsAg at week 0. (B) Nontransformed FL1607 potatoes + CT were fed to mice after priming at week 0 with 0.5 μg of yeast-derived alum-adsorbed rHBsAg. No antibodies were elicited.
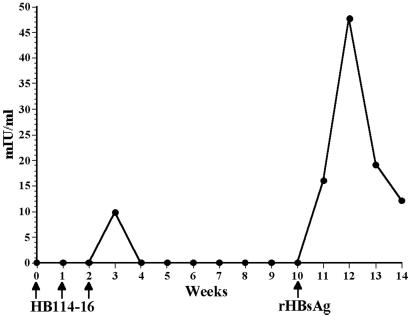
We further examined the ability of transgenic potato tubers to stimulate a primary anti-HBsAg immune response in the absence of an oral adjuvant. Feeding of three weekly doses of HB114–16 tubers without CT resulted in a very limited and transient primary response, which was boosted to a maximum of 48 mIU/ml after challenge with an injection of rHBsAg (Fig. 6). We conclude that CT exerted a substantial adjuvant effect, because feeding of HBsAg-tubers with CT stimulated a stronger antibody response (Fig. 4A).
Figure 6.
Immunogenicity of unadjuvanted potatoes. Mice were fed HB114–16 transgenic potatoes alone without CT at weeks 0, 1, and 2, followed by a boost with a single injection of a subimmunogenic dose of yeast-derived alum-adsorbed rHBsAg at week 10. A very weak and transient primary response and a low level of secondary antibody response were generated in the absence of CT.
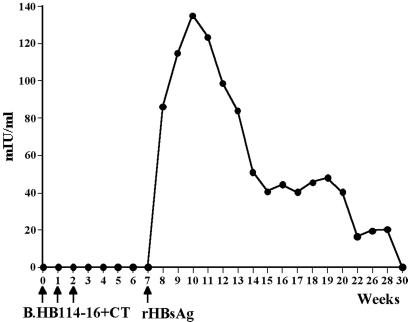
To determine the effect of heat on the immunogenicity of HB114–16 tubers, a sample of transgenic potatoes was boiled before feeding. When mice were fed the boiled tubers plus the adjuvant CT, following a schedule identical to that used when mice were fed fresh uncooked HB114–16 tubers, no primary antibody response was detected. Further, when these mice were boosted with the subimmunogenic dose of rHBsAg, anti-HBsAg antibody levels of 135 mIU/ml were elicited (Fig. 7) in contrast to the peak secondary antibody response of 3,300 mIU/ml in animals fed uncooked tubers (Fig. 4A). We therefore conclude that heat adversely affected but did not eliminate the immunogenicity of the HBsAg-transgenic plant derived vaccine, at least at this duration and level of heating.
Figure 7.
Cooking HBsAg potatoes reduces immunogenicity. HB114–16 potatoes were boiled before being fed to mice along with CT at weeks 0, 1, and 2, followed by a boost with a single subimmunogenic injection of yeast-derived alum-adsorbed rHBsAg at week 7. Cooking the potatoes substantially reduced levels of anti-HBsAg antibodies that were elicited but did not eliminate priming, as shown by the modest boosting effect.
Discussion
We have previously produced HBsAg in tobacco plants and administered it to mice as a parenteral vaccine. The plant-derived sample elicited B- and T-cell responses similar to those obtained against the commercial vaccine (3). Because toxic alkaloids in tobacco preclude its use in feeding experiments, we optimized the expression of HBsAg in potatoes (4). The present study was designed to test the potential of potato tubers expressing HBsAg to provoke immune responses when mice were fed this edible vaccine. The maximum dose of HBsAg in the present studies was imposed by the animals themselves, because it was found that they would reliably eat up to 5 g of raw tuber overnight, once per week (or about 42 μg HBsAg/dose).
Primary and Booster Immune Responses.
Three feedings of transgenic potatoes with CT gave a primary serum antibody response that rose above 100 mIU/ml but declined to less than 10 mIU/ml by week 12. The increase in serum antibody could be detected after two doses of potatoes, whereas two doses of purified yeast-derived HBsAg gave no detectable serum antibody increase, even though the dosage of yeast-derived material was 4-fold higher.
Establishment of a memory response is critical in studies pertaining to vaccine design and implementation. Our studies show that feeding fresh raw HBsAg potatoes to mice stimulated a primary immune response, and that several weeks after the decline of specific antibodies to background levels, a single parenteral injection of rHBsAg provoked a rapid and robust recall response that persisted for at least 5 months. No responses to the parenteral boost were obtained in mice that were fed nontransgenic potatoes, showing that the boosting response elicited in animals fed HBsAg tubers was indeed the result of priming and establishment of immune memory to HBsAg presented in the gut. These data indicate that HBsAg, produced and delivered in transgenic potato, is an effective oral immunogen. The peak levels of antibody 3,300 mIU/ml, obtained after parenteral boosting, were impressive in view of the rather modest level of ≈42 μg of HBsAg delivered per dose of potato vaccine.
We have compared the oral immunogenicity of purified rHBsAg (from yeast) to that delivered in unprocessed potatoes. No primary immune response was detected after two doses of yeast-derived HBsAg (containing a total of 300 μg of HBsAg), whereas a primary immune response began after two feedings of the transgenic potatoes (which contained only a total of 85 μg of HBsAg). This apparent contradiction can be explained in light of the intracellular localization of the rHBsAg in potato. If rHBsAg is subject to degradation in the gut, the protein compartmentalized within plant cells would be protected by a natural “bioencapsulation.” It is relevant to note that mastication of food breaks it into smaller pieces, but the majority of plant cell degradation occurs in the intestines as a result of digestive or bacterial enzyme actions. We propose that the release of at least some rHBsAg from plant cells occurs near the Peyer's patches that function as mucosal immune effector sites. Further, we conclude that the particulate forms of rHBsAg that are evident in the plant cells (Fig. 3) may be especially immunogenic when released near these effector sites. Thus, “bioencapsulation” of the antigen may facilitate a more robust immune response. It is also possible that proteins or secondary metabolites (e.g., polyphenolics) may associate with HBsAg and account for its protection in the gastrointestinal tract, although we have no evidence to support this assertion. Further, proteinase inhibitors contained in potato tubers (12) could slow proteolytic cleavage in the gut.
We also showed that HBsAg transgenic potatoes fed to mice could be used to boost an immune response. Mice primed with a single subimmunogenic parenteral dose of rHBsAg and fed potatoes containing HBsAg developed immediate antibody responses that peaked at >1,000 mIU/ml and were maintained at levels >200 mIU/ml for 5 months, when the experiment was terminated. In a practical context, these results provide a basis for a new immunization strategy, whereby only a single injection would be needed, followed by additional doses of orally delivered vaccine. This strategy could be a particularly useful approach to large-scale HBV immunization in developing countries where the logistics of multiple delivery doses of parenteral vaccine have hampered global HBV immunization efforts.
Both CT and Escherichia coli heat-labile enterotoxin (LT) are potent oral antigens and, in addition, provide adjuvant activity to antigens that are coadministered with them orally (13, 14). Because the responses to potato HBsAg were much lower in the absence of CT, it is possible that an effective mucosal adjuvant will be needed for orally delivered material in human subjects. Although fully active native CT or LT will not be used in humans because of the risk of toxicity, there is a great potential for use of mutant forms with reduced toxicity that retain adjuvant activity. These altered toxins include active-site mutants LT-K63 (15) and LT-R72 (16) and the hinge cleavage mutant LT-G192 (17). These previous reports show that LT can be an effective mucosal adjuvant when its toxic ADP-ribosyltransferase activity is greatly attenuated. It should be noted that use of CT as a mucosal adjuvant does not abrogate tolerance already established against an antigen (18), and thus there is little chance that use of mutant CT or LT would cause food allergy.
By cooking the potatoes (boiling) before feeding the mice, we substantially reduced the immunogenicity of the vaccine. Most humans find raw potatoes unpalatable, which makes this particular plant an inappropriate delivery vehicle for an edible vaccine for humans. However, tomatoes and bananas are eaten raw and are grown widely, including in the developing world. HBsAg-transgenic tomatoes are currently being evaluated in experiments similar to those described in this paper.
The Need for a More Cost-Effective HBV Vaccine.
Despite the availability of a subunit vaccine for over a decade, HBV is still responsible for significant morbidity and mortality in poor countries. Oral immunization, because of its great convenience and patient compliance, represents a mode of delivery that could permit implementation of large-scale vaccination programs. However, to be adopted in an immunization program targeted to the people who need the vaccine the most, cost must also be a major consideration.
In the first 10 years of its use, yeast-derived HBV vaccine availability has been largely limited to the wealthy industrialized world. During the last decade, manufacturing processes improved, costs of production declined, and the vaccine has been adopted in some industrializing countries. More recently, revolving funds for vaccine procurement were established by the combined efforts of the World Health Organization, UNICEF, and philanthropic organizations. This effort has been highly effective in industrializing nations. An excellent example is the purchasing power of members of the Pan American Health Organization; however, even with this purchasing power, the most recent listed price of a single dose of rHBsAg vaccine is $0.90 (19). This purchase price is more than the daily income of nearly one billion people, meaning that a further very significant cost reduction must be achieved if HBV immunization is to become sustainable without large contributions from donors. It is relevant to note that the Bill and Melinda Gates Foundation is making significant inroads to HBV delivery by philanthropic purchases of HBV vaccines. However, a truly sustainable strategy for global immunization will build on this philanthropic effort to create technology for indigenous production of vaccines, whenever possible, to ensure in-country vaccine production as national sustainable priorities.
In the current study, we have demonstrated that plant-based rHBsAg is an oral immunogen in mice. This finding must be verified in human trials, but it is of predictive value for anticipating how oral vaccines could be produced for HBV prevention. First, it is very likely that the quantity of HBsAg to be delivered orally must be higher than is used for parenteral delivery. Second, multiple doses will probably be needed. In combination, these requirements appear daunting if one were dealing with the current production costs for HBsAg produced by fermentation. However, the amount of HBsAg needed for one dose of an oral vaccine could be achieved in the contents of a single potato. In unpublished data (L.R., H.S.M., and C.J.A.), we have also found that high levels of HBsAg can be achieved in a single tomato, and candidate HBsAg-containing bananas are now in the seedling stage.
We are confident that plant-based “edible vaccines” can be developed (20, 21). Two successful demonstrations of a prototype “edible vaccine” for diarrheal disease were demonstrated in human clinical trials. When potatoes containing the binding subunit of the heat-labile enterotoxin of E. coli (LT-B) were fed uncooked to volunteers, serum and secretory antibodies specific for LT-B were induced (22). In separate clinical studies, volunteers who ate uncooked potatoes containing the capsid protein of Norwalk virus (causal agent of epidemic gastroenteritis) developed both serum and secretory antibodies specific to the capsid protein (23). It is critical to note that both of the above immunogens are components of enteric pathogens, and hence their protein antigens may be special cases of gut-stable vaccine subunits.
We have demonstrated here that HBsAg (a subunit antigen of a nonenteric pathogen) expressed in potato tubers and delivered to mice as an edible vaccine can stimulate serum antibodies specific for HBsAg at levels that very significantly exceed the protective level (in humans) of 10 mIU/ml. The unique features of bioencapsulation of the antigen within plant cells may be the actual reason why plant-based HBsAg is effective for oral immunization.
Acknowledgments
We thank Shannon Caldwell and the Cornell Integrated Microscopy Center for excellent technical assistance with the TEM study. We thank Cheryl Zuber for excellent help in manuscript preparation. This work was supported by National Institutes of Health Grant AI42836 and World Health Organization Vaccines and Other Biologicals Grant I5/181/416 (to Y.T., H.S.M., and C.J.A.).
Abbreviations
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- rHBsAg
recombinant HBsAg
- CT
cholera toxin
- mIU
milli-International units
- TEM
transmission electron microscopy
- LT
heat-labile enterotoxin of E. coli
References
- 1.Sitrin R D, Wampler D E. In: Hepatitis B Vaccines in Clinical Practice. Ellis RW, editor. New York: Dekker; 1992. pp. 83–102. [Google Scholar]
- 2.Mason H, Lam D, Arntzen C J. Proc Natl Acad Sci USA. 1992;89:11745–11749. doi: 10.1073/pnas.89.24.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thanavala Y, Yang Y-F, Lyons P, Mason H S, Arntzen C. Proc Natl Acad Sci USA. 1995;92:3358–3361. doi: 10.1073/pnas.92.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter L J, Thanavala Y, Arntzen C J, Mason H S. Nat Biotechnol. 2000;18:1167–1171. doi: 10.1038/81153. [DOI] [PubMed] [Google Scholar]
- 5.Kann M, Gerlich W. In: Viral Hepatitis. Zuckerman J N, Harrison T J, Zuckerman A J, editors. London: Churchill Livingstone; 1998. pp. 77–105. [Google Scholar]
- 6.Biemans R, Thines D, Petre-Parent B, DeWilde M, Rutgers T, Cabezon T. DNA Cell Biol. 1992;11:621–626. doi: 10.1089/dna.1992.11.621. [DOI] [PubMed] [Google Scholar]
- 7.Patzer E J, Nakamura G R, Simonsen C C, Levinson A D, Brands R. J Virol. 1986;58:884–892. doi: 10.1128/jvi.58.3.884-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadler S C, Francis D P, Maynard J E, Thompson S E, Judson F N, Echenberg D F, Ostrow D G, O'Malley P M, Penley K A, Altman N L, et al. N Engl J Med. 1986;315:209–214. doi: 10.1056/NEJM198607243150401. [DOI] [PubMed] [Google Scholar]
- 9.Szmuness W, Stevens C E, Harley E J, Zang E A, Oleszko W R, William D C, Sadovsky R, Morrison J M, Kellner A. N Engl J Med. 1980;303:833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 10.Francis D P, Hadler S C, Thompson S E, Maynard J E, Ostrow D G, Altman N, Braff E H, O'Malley P, Hawkins D, Judson F N, et al. Ann Int Med. 1982;97:362–366. doi: 10.7326/0003-4819-97-3-362. [DOI] [PubMed] [Google Scholar]
- 11.Szmuness W, Stevens C E, Harley E J, Zang E A, Alter H J, Taylor P E, DeVera A, Chen G T, Kellner A. N Engl J Med. 1982;307:1481–1486. doi: 10.1056/NEJM198212093072403. [DOI] [PubMed] [Google Scholar]
- 12.Suh S G, Stiekma W J, Hannapel D J. Planta (Heidelberg) 1991;184:423–430. doi: 10.1007/BF00197888. [DOI] [PubMed] [Google Scholar]
- 13.Holmgren J, Lycke N, Czerkinsky C. Vaccine. 1993;11:1179–1184. doi: 10.1016/0264-410x(93)90039-z. [DOI] [PubMed] [Google Scholar]
- 14.Clements J D, Hartzog N M, Lyon F L. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 15.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris M T. Infect Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakobsen H, Schulz D, Pizza M, Rappuoli R, Jonsdottir I. Infect Immun. 1999;67:5892–5897. doi: 10.1128/iai.67.11.5892-5897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson B L, Clements J D. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedrud J, Sigmund N. Regional Immunol. 1991;3:217–222. [PubMed] [Google Scholar]
- 19.de Quadros C. EPI Newsletter: Expanded Program on Immunization in the Americas. XXII 2000. , No. 1, p. 8. [Google Scholar]
- 20.Walmsley A M, Mason H S, Arntzen C J. Mucosal Immunol Update. 1999;7:12–14. [Google Scholar]
- 21.Tacket C O, Mason H S. Microbes Infect. 1999;1:1–7. doi: 10.1016/s1286-4579(99)80080-x. [DOI] [PubMed] [Google Scholar]
- 22.Tacket C O, Mason H S, Losonsky G, Clements J D, Levine M M, Arntzen C J. Nat Med. 1998;4:607–609. doi: 10.1038/nm0598-607. [DOI] [PubMed] [Google Scholar]
- 23.Tacket C O, Mason H S, Losonsky G, Estes M K, Levine M M, Arntzen C J. J Infect Dis. 2000;182:302–305. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]