Abstract
The clinicopathological value and exploration of the potential molecular mechanism of microRNA-183-5p (miR-183-5p) have been investigated in various cancers; however, to the best of the author's knowledge, no similar research has been reported for bladder cancer. In the present study, it was revealed that the expression level of miR-183-5p was notably increased in bladder cancer tissues compared with adjacent non-cancerous tissues (P=0.001) and was markedly increased in the tissue samples of papillary, pathological T stage (T0-T2) and pathological stage (I–II) compared with tissue samples of their counterparts (P=0.05), according to data from The Cancer Genome Atlas. Receiver operating characteristic analysis revealed the robust diagnostic value of miR-183-5p for distinguishing bladder cancer from non-cancerous bladder tissues (area under curve=0.948; 95% confidence interval: 0.919–0.977). Amplification and deep deletion of miR-183-5p were indicated by cBioPortal, accounting for 1% (4/412) of bladder cancer cases. Data from YM500v3 demonstrated that compared with other cancers, bladder cancer exhibited high expression levels of miR-183-5p, and miR-183-5p expression in primary solid tumors was much higher compared with solid normal tissues. A meta-analysis indicated that miR-183-5p was more highly expressed in bladder cancer samples compared with normal counterparts. A total of 88 potential target genes of miR-183-5p were identified, 13 of which were discerned as hub genes by protein-protein interaction. The epithelial-to-mesenchymal transition pathway was the most significantly enriched pathway by FunRich (P=0.0001). In summary, miR-183-5p may participate in the tumorigenesis and development of bladder cancer via certain signaling pathways, particularly the epithelial-to-mesenchymal transition pathway. However, the exact molecular mechanism of miR-183-5p in bladder cancer must be validated by in vitro and in vivo experiments.
Keywords: bladder cancer, miR-183-5p, amplification, diagnosis, hub genes
Introduction
Due to the high mortality and recurrence, bladder cancer (BC) remains one of the most frequently appearing cancer of the urinary system (1). In America, ~79,030 new patients with BC will be diagnosed in 2017, including 60,490 males and 18,540 females (2). Although there has been huge progress in therapeutic methods, such as cystectomy and adjuvant treatments, BC occupies one of the most common cancers with a high mortality and recurrence rate worldwide (1). Among the patients with recurrent BC, more than one-tenth continue to develop into higher phases, such as muscle invasion and metastasis (3–5). The discovery of cancer at an advanced stage could lead to a poor prognosis. It is a prioritized event to explore the exact mechanism of BC to improve the sensitivity and specificity of early diagnosis. In recent years, evidence accumulation in the knowledge of molecular biology and application of bioinformatics has provided good opportunities to understand BC comprehensively via bioinformatic analysis, such as the application of microRNAs (miRNAs) (6–8).
miRNAs, a type of short non-coding RNA ~22 nucleotides in length, facilitate mRNA degradation by sequence-specific combination with mRNA, providing new strategies on diagnosis and treatment of cancers (9–12). miRNAs have been found to participate in the expression regulation of hub genes related to the development and progression of tumors, including BC (13–16). The abnormally expressed and genetically altered miRNAs have been reported to be involved in the biological process of BC, including miR-199a-5p, miR-1-3p, miR-9, miR-182 and miR-200b (17–19).
miR-183-5p, located on chromosome 7q32.2, has been studied in various types of cancers, such as human breast cancer (20), esophageal squamous cell carcinoma (21), esophageal squamous cell carcinoma (22), gastric cancer (23), human pancreatic adenocarcinoma (24) and melanoma (25). The expression of miR-183 in BC patients was higher in tissues (26–28) and serum (29) and showed a moderate value of the BC diagnosis (27). However, no functional study of miR-183-5p in BC has been reported previously. Most of the published studies on miR-183-5p tended to focus on the higher expression of miR-183-5p in samples from patients with BC, including tissues, urine and serum, than in samples from normal individuals, or the diagnostic efficiency of miR-183-5p. However, the clinical significance, as well as the promising target genes of miR-183-5p in BC, needs to be explored (26–29). In addition, the studies mentioned above were based on a small scale of BC patient cohorts, and big data analysis was needed to uncover the real role of miR-183-5p in BC.
Therefore, in the present study, we first attempted to investigate the clinical significance of the expression level and genetic alteration of miR-183-5p in BC based on the data from The Cancer Genome Atlas (TCGA; https://cancergenome.nih.gov/), cBioPortal for Cancer Genomics (http://www.cbioportal.org/), Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/), and YM500v3 (http://driverdb.tms.cmu.edu.tw/ym500v3/). In addition, we identified potential target genes of miR-183-5p via differential expressed genes calculated by RNA-seq data from TCGA, predicting platforms and gene profiling post miR-183-5p overexpression in vitro. Further bioinformatic analyses, including the enrichment of functional annotation and biological pathway analyses, were performed to explore the possible roles of miR-183-5p in the tumorigenesis and progression of BC.
Materials and methods
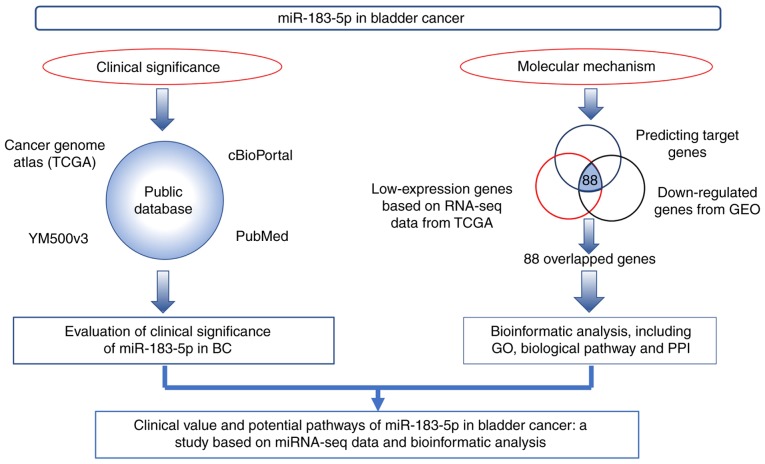
The work flow of the present study is shown in Fig. 1. Firstly, we evaluated the clinical significance of miR-183-5p in BC based on data from TCGA, cBioPortal, YM500v3 and PubMed. Secondly, the potential molecular mechanism of miR-183-5p in BC was explored via bioinformatic analysis with potential target genes overlapped with predicting target genes, low-expression genes based on RNA-seq data from TCGA and downregulated genes from Gene Expression Omnibus (GEO).
Figure 1.
Work flow of the clinical value evaluation and bioinformatical analysis for miR-183-5p in bladder cancer.
Data acquisition and analysis
The miRNA sequencing data of miR-183 and clinical information of patients with BC were obtained from TCGA and contained 412 cases and 19 para-carcinoma tissues of counterparts as controls (30,31). Only 409 cases possessed the miRNA sequencing data and clinical information. The clinical information and follow-up data of these cases, including sex, body mass index (BMI), primary therapy outcome, pack number of cigarettes smoked per year, primary therapy outcome, diagnosis subtype, lymphovascular invasion, pathologic T stage, pathologic N stage, pathologic M stage, pathologic stage and new tumor events after initial treatment were also downloaded to analyze the correlation between miR-183 expression and clinical parameters (Table I). Receiver operator characteristic curve (ROC) and Kaplan-Meier (K-M) analyses were used to assess the diagnostic and prognostic roles of miR-183.
Table I.
Correlation between miR-183 expression and clinical parameters.
| miR-183 expression | ||||
|---|---|---|---|---|
| Clinical parameters | n | Mean ± SD | t | P-value |
| Tissue | −7.489 | <0.001 | ||
| Adjacent | 19 | 8.975±2.409 | ||
| Tumor | 403 | 13.155±1.562 | ||
| Sex | −0.151 | 0.880 | ||
| Male | 298 | 13.148±1.561 | ||
| Female | 105 | 13.175±1.572 | ||
| BMI | 0.591 | 0.555 | ||
| ≤25 | 146 | 13.188±1.381 | ||
| >25 | 209 | 13.088±1.683 | ||
| Primary therapy outcome | 0.899 | 0.370 | ||
| CR+PR+SD | 187 | 13.175±1.533 | ||
| PD | 42 | 12.938±1.613 | ||
| Pack number of cigarettes smoked per year | 1.513 | 0.132 | ||
| <39.003 | 132 | 13.225±1.486 | ||
| ≥39.003 | 88 | 12.886±1.824 | ||
| Primary therapy outcome | 0.899 | 0.370 | ||
| CRR+PRR+SD | 187 | 13.175±1.533 | ||
| PD | 42 | 12.938±1.613 | ||
| Diagnosis subtype | −3.353 | 0.001 | ||
| Non-papillary | 270 | 12.993±1.700 | ||
| Papillary | 129 | 13.486±1.186 | ||
| Lymphovascular invasion | −1.051 | 0.294 | ||
| No | 130 | 13.054±1.668 | ||
| Yes | 147 | 13.253±1.488 | ||
| Pathologic T stage | 2.106 | 0.036 | ||
| T0-T2 | 123 | 13.333±1.502 | ||
| T3-T4 | 247 | 12.966±1.614 | ||
| Pathologic N stage | 1.369 | 0.172 | ||
| N0-N1 | 278 | 13.208±1.553 | ||
| N2-N3 | 84 | 12.948±1.430 | ||
| Pathologic M stage | −0.925 | 0.356 | ||
| M0 | 193 | 13.515±1.317 | ||
| M1 | 10 | 13.921±1.975 | ||
| Pathologic stage | 2.533 | 0.012 | ||
| Stage I–II | 132 | 13.420±1.445 | ||
| Stage III–IV | 269 | 13.017±1.604 | ||
| New tumor event after initial treatment | 0.864 | 0.388 | ||
| No | 224 | 13.168±1.488 | ||
| Yes | 69 | 12.987±1.619 | ||
BMI, body mass index; CRR, complete remission/response; PRR, partial remission/response; SD, stable disease; PD, progressive disease.
Furthermore, the expression data and prognostic analysis of miR-183-5p were provided from database of YM500v3 (http://driverdb.tms.cmu.edu.tw/ym500v3/). The genetic alteration of miR-183 was validated from the cBioPortal database (http://www.cbioportal.org/).
Meta-analysis of miR-183 expression based on the data from TCGA, GEO and the literature
A resourceful GEO database could provide strong support in mining the expression data of miRNAs in human cancers. Therefore, we also searched the GEO database to mine the miR-183-5p expression level in BC. The following terms were used for searching: (bladder OR urothelial OR urinary OR urogenital) AND (cancer OR carcinoma OR tumor OR neoplasm* OR malignant*). The miR-183-5p expression data were extracted from BC and relevant controls.
A comprehensive search in several main literary databases worldwide, including PubMed, Chinese VIP, CNKI, WanFang database, SinoMed, Embase, Web of science, Science Direct and Wiley Online Library, was performed for the validation of the miR-183 expression level, up to July 1, 2017. The following terms were used for searching: (bladder OR urothelial OR urinary OR urogenital) AND (cancer OR carcinoma OR tumor OR neoplasm* OR malignant*) AND (MicroRNA183 OR miRNA183 OR miR183 OR miR-183 OR miRNA-183 OR microRNA-183 OR ‘microRNA183’ OR ‘miRNA1’ OR ‘miR183’ OR miR-183-5p OR miRNA-183-5p OR microRNA-183-5p).
Prediction of the prospective target genes of miR-183-5p
The prediction of miR-183-5p target genes was conducted with different bioinformatics tools, including miRWalk, MicroT4, miRanda, miRBridge, miRDB, miRMap, miRNAMap, PICTAR2, PITA, RNA22, RNAhybrid, TargetScan. Only 7,421 genes appearing for over 4 times among 12 platforms were regarded as potential target genes of miR-183-5p.
Potentially related genes of miR-183-5p in BC cells as detected by microarray
According to the information provided by GSE24782 microarray, a few human cancer cell lines, including BOY, T24, A498, PC3, DU145, FaDu, SAS, HSC3 and IMC3, were transfected with different miRNAs (miR-183-5p, miR-218, miR-145, miR-1 and miR-874). Based on the functional mechanism of miRNA, low-expression genes with a fold change (FC) <0.85 after transfected with miR-183-5p in BOY and T24 cells were regarded as potential target genes of miR-183-5p. A total of 3,163 genes were selected for further analysis.
Selection of low-expression genes based on RNA-seq dataset from TCGA
The RNA-seq dataset from TCGA contained 60,483 mRNAs, which were then used for the analysis of the differential expression with the R package of edgeR (32,33). Differentially expressed genes with the expression data missing in >10% samples were excluded. Due to the high expression of miR-183 in tumor tissues, low-expression genes were selected as the candidates of target genes of miR-183-5p. Therefore, 2,918 low-expression genes with log2 FC<-1 were included.
Protein-protein interaction (PPI) analysis, Gene ontology (GO) and biological pathway
PPI was conducted to identify the hub genes by STRING: Functional protein association networks (https://string-db.org/). Furthermore, to study the prospective biological effects of miR-183-5p in BC, the potential target genes of miR-183-5p were sent for GO and biological pathway analyses, which was performed via FunRich: Functional Enrichment analysis tool (34,35) (http://www.funrich.org/).
Moreover, to validate the reliability of the potential target genes of miR-183-5p, correlation analysis between the mRNA expression data of the prospective target genes of miR-183-5p enriched in the first pathway of the biological pathway and miR-183-5p expression data were performed. The comparisons of potential target genes between BC and non-cancerous tissues were also carried out.
Statistical analysis
The miR-183-5p expression data are displayed as the means ± standard deviation (SD). Student's t-test for independent samples was performed to evaluate the relationship between miR-183 and clinical parameters. Pearson correlation analysis and Student's t-test were performed for the verification of genes significantly enriched in biological pathways. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical significance of miR-183
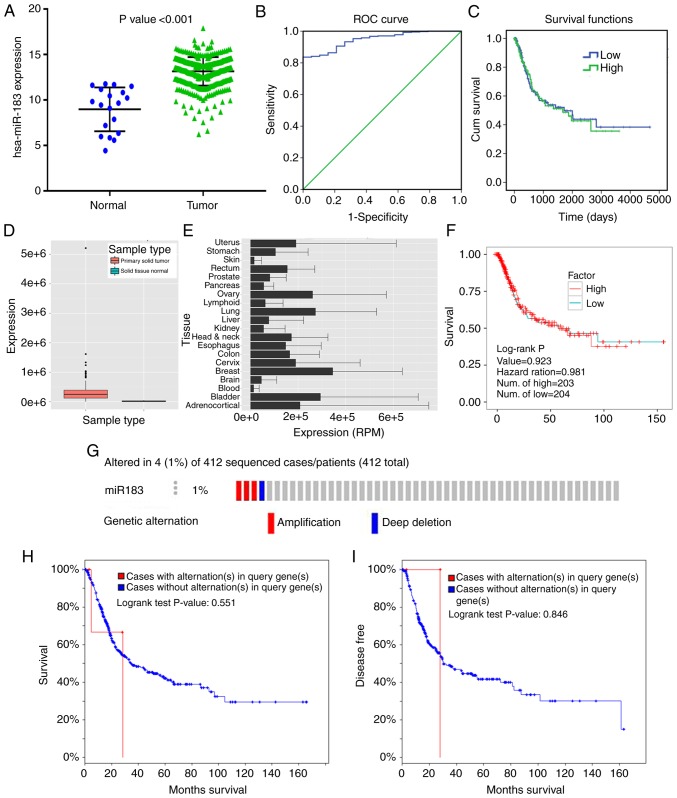
Due to the lack of expression data of mature miR-183-5p in the dataset of TCGA, the comparison of the stem-loop mir-183 expression level between BC and adjacent non-cancerous tissues was provided. The miR-183 expression level in BC tissues was notably higher than that in adjacent tissues (Fig. 2A; Table I). High expression of miR-183 was notably associated with the papillary subtype (t=−3.353, P=0.001), low pathologic T stage (t=2.106, P=0.036), and early pathologic stage (t=2.533, P=0.012). However, no remarkable differences were found between miR-183 expression and sex, BMI, primary therapy outcome, pack number of cigarettes smoked per year (mean), primary therapy outcome, lymphovascular invasion present, pathologic N stage, pathologic M stage or new tumor event after initial treatment (Table I).
Figure 2.
Clinical value of miR-183 in bladder cancer (BC) with different databases. (A) Expression data of miR-183-5p in normal and tumor tissues from TCGA (the y-axis represents log2 scaled). (B) Receiver operating characteristic (ROC) curve analysis of miR-183 to identify BC tissues from non-tumor bladder tissues based on TCGA dataset. (C) Kaplan-Meier survival curve of miR-183 for patients with BC. (D) Expression level of miR-183-5p in primary solid tumor and solid normal tissue from YM500v3 (http://driverdb.tms.cmu.edu.tw/ym500v3/). (E) Expression level of miR-183-5p in different cancers from YM500v3. (F) Relationship between miR-183-5p expression and overall survival by K-M curve analysis from YM500v3. (G) Genetic alterations of miR-183 in BC from cBioPortal (http://www.cbioportal.org). (H) Relationship between the alteration of miR-183 and overall survival from cBioPortal. (I) Disease-free survival from cBioPortal.
ROC curve analysis showed that the area under the curve (AUC) was 0.948 (95% CI: 0.919–0.977) with 83.6% sensitivity and 100% specificity (Fig. 2B), indicating that a high expression level of miR-183 may be an ideal marker for BC diagnosis. Furthermore, the K-M curve uncovered that no significant difference in the survival time was noted between patients in the low and high miR-183 expression groups (P=0.861) (Fig. 2C).
The expression of miR-183-5p in primary solid tumors were much higher than that in solid normal tissues (Fig. 2D), which was consistent with the result from TCGA (Fig. 2A). The miR-183-5p expression data in various cancers were provided in YM500v3 (Fig. 2E). Compared with other cancers, BC exhibited higher expression levels of miR-183-5p. This finding again confirmed that there was no significant prognostic value of miR-183-5p in patients with BC (Fig. 2F).
The data from cBioPortal revealed that miR-183 contained two kinds of genetic alterations, including amplification and deep deletion, accounting for 1% (4/412) of patients with BC (Fig. 2G). In addition, no prominent correlation was found between miR-183 alteration and the outcome of BC patients, including overall survival or disease-free survival (Fig. 2H and I).
miR-183-5p expression level from GEO and literatures
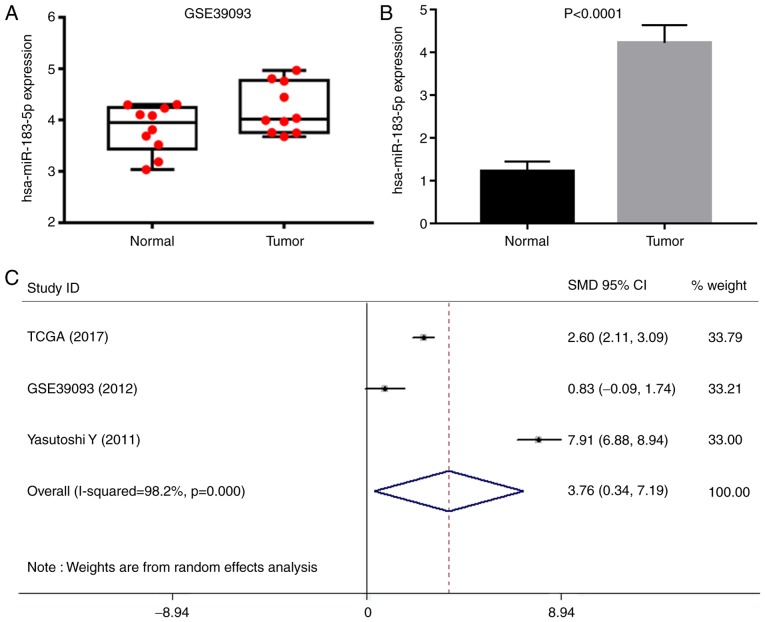
A total of 16 microarrays were obtained from GEO, including GSE20414, GSE20418, GSE2564, GSE31616, GSE31617, GSE36121, GSE39067, GSE39093, GSE40355, GSE48008, GSE50894, GSE59483, GSE81201, GSE83586, GSE84525 and GSE86411. Eventually, only GSE39093 was eligible, and the data were used for recalculation. The expression level of miR-183-5p (4.216±0.485) in 10 BC tissue samples was moderately higher than that in the 10 normal counterparts (3.825±0.460, P>0.05) (Fig. 3A). A comprehensive search was performed in several literature databases; however, only one study provided normalized expression data. In detail, the expression of miR-183-5p in 104 urothelial carcinomas (4.225±0.414) was significantly higher than that in 31 normal bladder epitheliums (1.224±0.224, P<0.0001) (Fig. 3B) (27). All of the eligible expression data of miR-183-5p extracted from TCGA, one GEO microarray and one literature research provided an overall SMD of 3.76 (0.34–7.19) as analyzed by STATA software (Fig. 3C), suggesting that miR-183-5p was more highly expressed in samples from BC than in those from normal counterparts. The source of patients with BC and different detection methods may lead to strong heterogeneity.
Figure 3.
Meta-analysis of miR-183-5p. (A) Expression level of miR-183-5p from GSE39093. (B) miR-183-5p expression reported by Yamada et al (27). (C) Forest plot of the meta-analysis.
Identification of the potential target genes of miR-183-5p
In the present study, the overlapped genes from 3 subsets of genes, including predicted target genes, related potential target genes of miR-183-5p from GEO and low-expression genes from TCGA, were regarded as the potential target genes of miR-183-5p. Finally, 88 overlapped genes were obtained (Table II).
Table II.
A total of 88 potential target genes of miR-183-5p.
| Ensemble ID | Gene name | Ensemble ID | Gene name | Ensemble ID | Gene name |
|---|---|---|---|---|---|
| ENSG00000163431 | LMOD1 | ENSG00000138685 | FGF2 | ENSG00000140450 | ARRDC4 |
| ENSG00000118496 | FBXO30 | ENSG00000136842 | TMOD1 | ENSG00000135269 | TES |
| ENSG00000102271 | KLHL4 | ENSG00000184985 | SORCS2 | ENSG00000163661 | PTX3 |
| ENSG00000113196 | HAND1 | ENSG00000169554 | ZEB2 | ENSG00000108797 | CNTNAP1 |
| ENSG00000100784 | RPS6KA5 | ENSG00000144655 | CSRNP1 | ENSG00000163083 | INHBB |
| ENSG00000104447 | TRPS1 | ENSG00000167483 | FAM129C | ENSG00000105835 | NAMPT |
| ENSG00000140090 | SLC24A4 | ENSG00000064309 | CDON | ENSG00000083067 | TRPM3 |
| ENSG00000017427 | IGF1 | ENSG00000159167 | STC1 | ENSG00000152217 | SETBP1 |
| ENSG00000183454 | GRIN2A | ENSG00000152102 | FAM168B | ENSG00000104313 | EYA1 |
| ENSG00000143878 | RHOB | ENSG00000134531 | EMP1 | ENSG00000151929 | BAG3 |
| ENSG00000105974 | CAV1 | ENSG00000166974 | MAPRE2 | ENSG00000007312 | CD79B |
| ENSG00000173334 | TRIB1 | ENSG00000105784 | RUNDC3B | ENSG00000138944 | KIAA1644 |
| ENSG00000188385 | JAKMIP3 | ENSG00000173068 | BNC2 | ENSG00000182168 | UNC5C |
| ENSG00000095794 | CREM | ENSG00000188803 | SHISA6 | ENSG00000169083 | AR |
| ENSG00000106829 | TLE4 | ENSG00000169504 | CLIC4 | ENSG00000163788 | SNRK |
| ENSG00000187098 | MITF | ENSG00000058272 | PPP1R12A | ENSG00000004799 | PDK4 |
| ENSG00000113448 | PDE4D | ENSG00000172399 | MYOZ2 | ENSG00000145861 | C1QTNF2 |
| ENSG00000126351 | THRA | ENSG00000115252 | PDE1A | ENSG00000151892 | GFRA1 |
| ENSG00000163328 | GPR155 | ENSG00000164741 | DLC1 | ENSG00000169946 | ZFPM2 |
| ENSG00000178662 | CSRNP3 | ENSG00000066382 | MPPED2 | ENSG00000101333 | PLCB4 |
| ENSG00000198961 | PJA2 | ENSG00000181773 | GPR3 | ENSG00000163171 | CDC42EP3 |
| ENSG00000018408 | WWTR1 | ENSG00000140416 | TPM1 | ENSG00000107968 | MAP3K8 |
| ENSG00000058668 | ATP2B4 | ENSG00000073910 | FRY | ENSG00000136267 | DGKB |
| ENSG00000018625 | ATP1A2 | ENSG00000107562 | CXCL12 | ENSG00000126524 | SBDS |
| ENSG00000136158 | SPRY2 | ENSG00000112320 | SOBP | ENSG00000091831 | ESR1 |
| ENSG00000134201 | GSTM5 | ENSG00000142627 | EPHA2 | ENSG00000079308 | TNS1 |
| ENSG00000118922 | KLF12 | ENSG00000058866 | DGKG | ENSG00000131016 | AKAP12 |
| ENSG00000186354 | C9orf47 | ENSG00000162616 | DNAJB4 | ENSG00000131018 | SYNE1 |
| ENSG00000078687 | TNRC6C | ENSG00000157368 | IL34 | ENSG00000146151 | HMGCLL1 |
| ENSG00000067900 | ROCK1 |
Bioinformatic analyses of the target genes of miR-183-5p
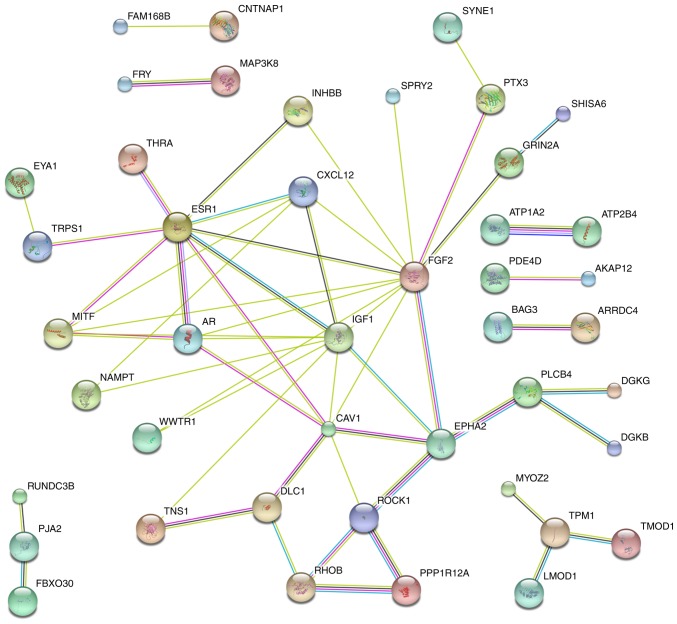
As shown in Fig. 4, hub genes, which contained >3 connected lines, were identified, including estrogen receptor 1 (ESR1), melanogenesis-associated transcription factor (MITF), androgen receptor (AR), C-X-C motif chemokine ligand 12 (CXCL12), insulin-like growth factor 1 (IGF1), caveolin 1 (CAV1), DLC1 Rho GTPase activating protein (DLC1), fibroblast growth factor 2 (FGF2), EPH receptor A2 (EPHA2), Rho associated coiled-coil containing protein kinase 1 (ROCK1), Ras homolog family member B (RHOB), phospholipase C beta 4 (PLCB4) and tropomyosin 1 (TPM1) (single genes without connections are not shown) (Fig. 4).
Figure 4.
Protein-protein interaction (PPI) of the probable target genes of miR-183-5p in bladder cancer. The PPI network is pictured with the candidates target genes of miR-183-5p by STRING (http://string-db.org). Small nodes represent protein of unknown 3D structure; large nodes represent some 3D structure is known or predicted; colored nodes represent query proteins and first shell of interactors; white nodes represent second shell of interactors.
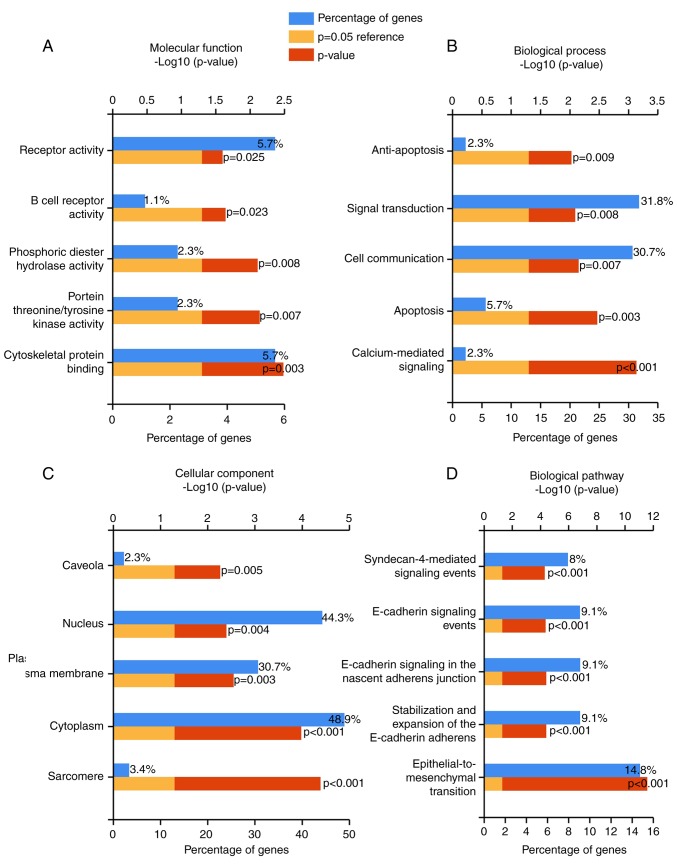
The overlapped genes were also categorized in GO and biological pathway analyses using the software of FunRich: Functional Enrichment analysis tool. The top 5 pathways in molecular functions (MFs), such as cytoskeletal protein binding, protein threonine/tyrosine kinase activity, were displayed in Fig. 5A and Table III. In addition, the top 5 pathways of biological processes (BPs) (Fig. 5B) and cellular component (CC) were also noted (P-value <0.05) (Fig. 5C; Table III). Via the biological pathway analysis, we discovered that the epithelial-to-mesenchymal transition pathway was the most significantly enriched (P-value <0.00001) (Fig. 5D; Table III).
Figure 5.
The top 5 enriched (A) molecular function (MF) pathways, (B) biological process (BP) pathways, (C) cellular component (CC) pathways and (D) biological pathways of candidate target genes of miR-183-5p in bladder cancer from GO analysis.
Table III.
Pathways of potential target genes of miR-183-5p in bladder cancer.
| Pathway | No. of genes in the dataset | Percentage of genes | Fold enrichment | P-value (Hypergeometric test) | Gene |
|---|---|---|---|---|---|
| MF: Cytoskeletal protein binding | 5 | 5.682 | 5.021 | 0.003 | TPM1; KLHL4; LMOD1; TMOD1; MAPRE2 |
| MF: Protein threonine/tyrosine kinase activity | 2 | 2.273 | 15.679 | 0.007 | MAP3K8; TRIB1 |
| MF: Phosphoric diester hydrolase activity | 2 | 2.273 | 15.139 | 0.008 | PDE1A; PDE4D |
| MF: B cell receptor activity | 1 | 1.136 | 44.049 | 0.023 | CD79B |
| MF: Receptor activity | 5 | 5.682 | 3.032 | 0.025 | CDON; GFRA1; UNC5C; CNTNAP1; SORCS2 |
| BP: Calcium-mediated signaling | 2 | 2.273 | 48.744 | 0.001 | PLCB4; TRPM3 |
| BP: Apoptosis | 5 | 5.682 | 4.976 | 0.003 | CSRNP1; CSRNP3; BAG3; SNRK; DLC1 |
| BP: Cell communication | 27 | 30.682 | 1.589 | 0.007 | STC1; CDC42EP3; GPR3; PLCB4; SPRY2; EPHA2; MAP3K8; RHOB; AR; CDON; CXCL12; DGKB; GRIN2A; PDE1A; PDE4D; TNS1; UNC5C; CNTNAP1; GPR155; IGF1; ROCK1; SORCS2; FGF2; INHBB; TRIB1; MYOZ2; RUNDC3B |
| BP: Signal transduction | 28 | 31.818 | 1.556 | 0.008 | STC1; CDC42EP3; GPR3; PLCB4; SPRY2; EPHA2; MAP3K8; RHOB; AR; CDON; CXCL12; DGKB; GFRA1; GRIN2A; PDE1A; PDE4D; TNS1; UNC5C; CNTNAP1; GPR155; IGF1; ROCK1; SORCS2; FGF2; INHBB; TRIB1; MYOZ2; RUNDC3B |
| BP: Anti-apoptosis | 2 | 2.273 | 13.720 | 0.009 | NAMPT; IGF1 |
| CC: Sarcomere | 3 | 3.409 | 43.816 | <0.001 | TPM1; SYNE1; MYOZ2 |
| CC: Cytoplasm | 43 | 48.864 | 1.653 | <0.001 | STC1; ATP2B4; CDC42EP3; SPRY2; THRA; AKAP12; ATP1A2; CLIC4; FBXO30; MAP3K8; TLE4; TPM1; AR; DGKB; DGKG; GFRA1; GRIN2A; KLHL4; LMOD1; MITF; NAMPT; PDE1A; PDE4D; PPP1R12A; SBDS; SETBP1; TMOD1; BAG3; HAND1; IGF1; MAPRE2; ROCK1; SYNE1; TRPM3; ESR1; EYA1; TRIB1; WWTR1; MYOZ2; CD79B; GSTM5; RPS6KA5; DLC1 |
| CC: Plasma membrane | 27 | 30.682 | 1.696 | 0.003 | ATP2B4; GPR3; SPRY2; AKAP12; ATP1A2; CLIC4; EPHA2; RHOB; AR; CAV1; CDON; DGKB; DGKG; GFRA1; GRIN2A; PPP1R12A; TNS1; UNC5C; CNTNAP1; GPR155; SORCS2; TRPM3; ESR1; FGF2; TES; SLC24A4; CD79B |
| CC: Nucleus | 39 | 44.318 | 1.458 | 0.004 | STC1; ZFPM2; ATP2B4; PLCB4; THRA; TRPS1; AKAP12; ATP1A2; CLIC4; CSRNP1; CSRNP3; TLE4; TPM1; ZEB2; AR; BNC2; CREM; GFRA1; MITF; NAMPT; PDE4D; PPP1R12A; SBDS; SETBP1; TMOD1; CNTNAP1; HAND1; MAPRE2; SYNE1; TRPM3; ESR1; EYA1; FGF2; TRIB1; KLF12; WWTR1; SNRK; RPS6KA5; DLC1 |
| CC: Caveola | 2 | 2.273 | 18.292 | 0.005 | CAV1; DLC1 |
| Biological pathway: Epithelial-to-mesenchymal transition | 13 | 14.773 | 15.365 | <0.001 | ZFPM2; AKAP12; CLIC4; ZEB2; BNC2; CAV1; CXCL12; SOBP; TNS1; IGF1; SYNE1; PTX3; WWTR1 |
| Biological pathway: Stabilization and expansion of the E-cadherin adherens junction | 8 | 9.091 | 6.364 | <0.001 | SPRY2; EPHA2; TLE4; AR; GFRA1; MITF; IGF1; ROCK1 |
| Biological pathway: E-cadherin signaling in the nascent adherens junction | 8 | 9.091 | 6.364 | <0.001 | SPRY2; EPHA2; TLE4; AR; GFRA1; MITF; IGF1; ROCK1 |
| Biological pathway: E-cadherin signaling events | 8 | 9.091 | 6.250 | <0.001 | SPRY2; EPHA2; TLE4; AR; GFRA1; MITF; IGF1; ROCK1 |
| Biological pathway: Syndecan-4-mediated signaling events | 7 | 7.955 | 7.328 | <0.001 | TLE4; AR; CAV1; CXCL12; MITF; ROCK1; FGF2 |
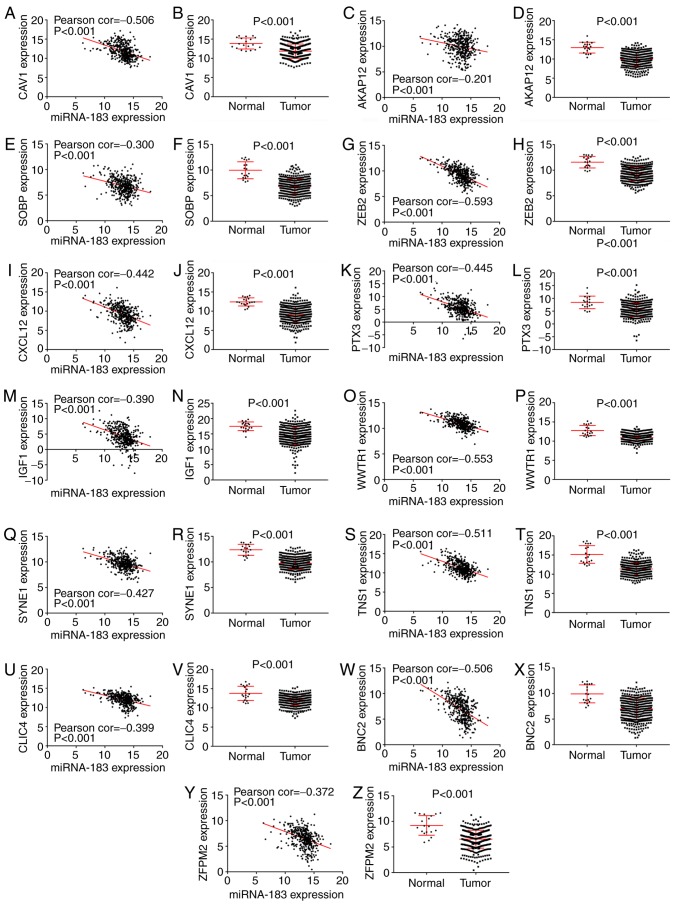
Validation of potential target genes enriched in the epithelial-to-mesenchymal transition pathway by mRNA expression data from TCGA. The mRNA expression data of potential target genes enriched in epithelial-to-mesenchymal transition pathway in BC and non-carcinomatous tissues, including zinc finger protein, FOG family member 2 (ZFPM2), A-kinase anchoring protein 12 (AKAP12), chloride intracellular channel 4 (CLIC4), zinc finger E-box binding homeobox 2 (ZEB2), basonuclin 2 (BNC2), caveolin 1 (CAV1), C-X-C motif chemokine ligand 12 (CXCL12), sine oculis binding protein homolog (SOBP), tensin 1 (TNS1), insulin-like growth factor 1 (IGF1), spectrin repeat containing nuclear envelope protein 1 (SYNE1), pentraxin 3 (PTX3), WW domain containing transcription regulator 1 (WWTR1), were extracted from TCGA. The correlation of the expression level between miR-183 and potential target genes in BC tissues was evaluated by Pearson correlation analysis. The results showed that miR-183 expression in BC tissues were all notably correlated with the expression levels of prospective target genes, respectively (P-value <0.001) (Fig. 6). Furthermore, Student' t test was executed to compare the expression levels of candidate genes between the BC and non-carcinomatous tissues. Consequently, candidate gene expression data were all remarkably higher in BC than in non-carcinomatous tissues (Fig. 6; Table IV).
Figure 6.
Validation of the potential target genes enriched in the epithelial-to-mesenchymal transition pathway by mRNA expression data from The Cancer Genome Atlas (TCGA), including (A and B) caveolin 1 (CAV1), (C and D) A-kinase anchoring protein 12 (AKAP12), (E and F) sine oculis binding protein homolog (SOBP), (G and H) zinc finger E-box binding homeobox 2 (ZEB2), (I and J) C-X-C motif chemokine ligand 12 (CXCL12), (K and L) pentraxin 3 (PTX3), (M and N) insulin-like growth factor 1 (IGF1), (O and P) WW domain containing transcription regulator 1 (WWTR1), (Q and R) spectrin repeat containing nuclear envelope protein 1 (SYNE1), (S and T) tensin 1 (TNS1), (U and V) chloride intracellular channel 4 (CLIC4), (W and X) basonuclin 2 (BNC2) and (Y and Z) zinc finger protein, FOG family member 2 (ZFPM2).
Table IV.
Comparison of the expression levels of potential target genes between normal bladder tissues and bladder cancer (BC) tissues.
| Gene | Group | N | Mean | Std. Deviation | P-value | Featurea |
|---|---|---|---|---|---|---|
| CAV1 | <0.001 | Down | ||||
| Normal | 19 | 13.87475 | 1.427754 | |||
| Tumor | 399 | 11.86889 | 1.857218 | |||
| AKAP12 | <0.001 | Down | ||||
| Normal | 19 | 12.96746 | 1.407823 | |||
| Tumor | 399 | 9.992088 | 1.793414 | |||
| SOBP | <0.001 | Down | ||||
| Normal | 19 | 9.964385 | 1.65852 | |||
| Tumor | 399 | 6.790578 | 1.474642 | |||
| ZEB2 | <0.001 | Down | ||||
| Normal | 19 | 11.57411 | 1.129419 | |||
| Tumor | 399 | 9.299417 | 1.35383 | |||
| CXCL12 | <0.001 | Down | ||||
| Normal | 19 | 12.41901 | 1.059525 | |||
| Tumor | 399 | 9.179279 | 2.088323 | |||
| PTX3 | <0.001 | Down | ||||
| Normal | 19 | 8.439694 | 2.440688 | |||
| Tumor | 399 | 5.515195 | 2.690033 | |||
| IGF1 | <0.001 | Down | ||||
| Normal | 19 | 7.488964 | 1.422601 | |||
| Tumor | 399 | 4.200803 | 2.727906 | |||
| WWTR1 | <0.001 | Down | ||||
| Normal | 19 | 12.77304 | 1.334257 | |||
| Tumor | 399 | 10.95718 | 0.966398 | |||
| SYNE1 | <0.001 | Down | ||||
| Normal | 19 | 12.37864 | 1.079004 | |||
| Tumor | 399 | 9.730599 | 1.186041 | |||
| TNS1 | <0.001 | Down | ||||
| Normal | 19 | 15.15798 | 2.307397 | |||
| Tumor | 399 | 11.36795 | 1.597026 | |||
| CLIC4 | <0.001 | Down | ||||
| Normal | 19 | 13.79678 | 1.851609 | |||
| Tumor | 399 | 12.06632 | 1.372628 | |||
| BNC2 | <0.001 | Down | ||||
| Normal | 19 | 9.935324 | 1.7555 | |||
| Tumor | 399 | 6.986046 | 2.130077 | |||
| ZFPM2 | <0.001 | Down | ||||
| Normal | 19 | 9.218381 | 1.91825 | |||
| Tumor | 399 | 6.539535 | 1.752474 |
Discussion
In the present study, the clinical role of miR-183-5p in BC was first evaluated according to the data from miRNA sequencing (TCGA, cBioPortal and YM5003v). miR-183-5p in BC contained two types of genetic alteration, gene amplification and deep deletion. Furthermore, pathway analyses uncovered that miR-183-5p could deeply affect multiple pathways and ‘epithelial-to-mesenchymal transition’ was the most remarkable one as defined by the FunRich: Functional Enrichment analysis tool, containing several genes ZFPM2, AKAP12, CLIC4, ZEB2, BNC2, CAV1, CXCL12, SOBP, TNS1, IGF1, SYNE1, PTX3 and WWTR1; among these genes, CAV1, CXCL12 and IGF1 were also identified as hub genes of miR-183-5p in BC by STRING: Functional protein association networks.
It has been fully demonstrated that miRNA expression levels could become reliable markers for the diagnosis and prognosis of cancers (36). miR-183-5p is one of the attractive cancer-relative miRNAs that has been reported to play important roles in various cancers with remarkably high or low expression levels compared with normal tissues. A remarkably high expression of miR-183-5p that could promote the proliferation and restrain apoptosis in cells through targeting PDCD4 was reported in human breast cancer tissues compared with that in the adjacent non-cancerous tissues (20). A similar phenomenon and function of miR-183-5p was also reported in esophageal squamous cell carcinoma and glioblastoma multiforme (21–23). Upregulation of oncogenic miR-183-5p was reported to enhance the ability of proliferation and metastasis in human pancreatic adenocarcinoma cells probably via targeting the SOCS-6 gene (24). A notable lower expression of miR-183-5p was found in nasopharyngeal carcinoma and cervical cancer tissues, where miR-183-5p could act as the tumor suppressor by targeting MTA1 and MMP-9, respectively (37,38). Reduced expression of miR-183-5p was discovered in pancreatic ductal adenocarcinoma tissues; and significantly associated with tumor grade, metastasis and TNM stage. Patients with low expression of miR-183-5p tended to have notably worse overall survival than those with high expression of miR-183-5p expression (38). Patients with low-expression of miR-183-5p tended to have a poor overall survival. miR-183-5p was involved in cell proliferation by regulating the expression of Bmi-1 (39). Low-expression miR-183-5p was found in melanoma tissues and cells and was correlated with poor overall survival, while high-expression of miR-183-5p caused remarkable inhibition of cell growth in vitro and in vivo (25). Thus, miR-183-5p could play different roles in various cancers.
To date, only several studies were reported concerning the research of miR-183-5p in BC. It was validated that miR-183-5p expression in samples from patients with BC, including tissues (26,40–42), urine (27,43) and serum (29), were all prominently higher than that those in normal counterparts. In the present study, we revealed the consistent results in tissue samples.
Eissa et al performed ROC analysis of miR-183-5p expression in urine samples. However, only the sensitivity (71.3%) and specificity (88.9%) were provided (43). Yamada et al showed the result of ROC analysis (AUC=0.817; 95% CI: 0.752–0.872) with 74.0% sensitivity and 77.3% specificity, indicating a moderate diagnostic efficiency of miR-183-5p expression in urine, and they also found a significant correlation between miR-183-5p expression and clinical parameters, including tumor grade and pathological stage (P-value <0.05) (27). In our study, ROC curve analysis showed a robust diagnostic efficiency of miR-183-5p in BC tissues (AUC=0.948; 95% CI: 0.919–0.977). The expression of miR-183-5p was markedly related to the diagnosis subtype, pathologic T stage and pathologic stage (P-value <0.05). However, K-M curve analysis showed no prognostic efficiency of miR-183-5p, based on the data from TCGA, YM500v3 and cBioPortal.
miR-183-5p was conformed to affect the biological behavior of cells by targeting different kind of proteins in various types of cancers, such as regulating cell proliferation by targeting PDCD4 in breast (44) and esophageal cancer (21), and esophageal squamous cell carcinoma (22), inhibiting tumorigenesis by targeting MTA1 in nasopharyngeal carcinoma (37), promoting cell proliferation by targeting NEFL in glioblastoma multiforme (45), and suppressing retinoblastoma cell growth by targeting LRP6 (46). However, no study has reported the possible molecular mechanisms of miR-183-5p in BC. In the present, the potential target genes of miR-183-5p were obtained by the overlap of genes from 3 datasets, including the available predicting online tools, low-expression genes from TCGA and low-expression genes from microarray data after miR-183-5p mimic transfection.
The potential molecular mechanism of miR-183-5p in BC was explored. In total, 7,421 genes were predicted using the online predicting tool of miRwalk2.0. The low-expression genes from the RNA-seq data of TCGA with log2 FC <-1 were regarded as potential target genes of miR-183-5p, and 2,918 genes were involved. The GSE24782 microarray, which contained the data of differential-expressed genes after the overexpression of miR-183-5p in BOY and T24 cells, was reassessed to identify the downregulated genes with a fold change <0.85; 3,163 eligible genes were included. Consequently, 88 overlapped genes were identified and were more prone to be the target genes of miR-183-5p in BC. Next, bioinformatics analyses discovered that these potential target genes were enriched in several pathways involved in the tumorigenesis and development of BC. Forty-three genes were enriched in the pathway of cytoplasm, which ranked the 2nd most significant pathways in the cellular component (CC). The most significant pathway indicated in molecular function (MF) was the pathway of cytoskeletal protein binding. Several significant pathways relative to signal transduction or cell communication were involved in biological process (BP), such as calcium-mediated signaling, cell communication and signal transduction. The pathway of epithelial-to-mesenchymal transition was the most enriched pathway in the biological pathway, playing a crucial role in tumor progression with genes of ZFPM2, AKAP12, CLIC4, ZEB2, BNC2, CAV1, CXCL12, SOBP, TNS1, IGF1, SYNE1, PTX3 and WWTR1. The expression of these 13 target genes was negatively correlated with the expression of miR-183-5p in BC tissues. miR-183-5p was conformed to target the tumor suppressor AKAP12 and plays an important role in human hepatocarcinogenesis (47). CAV1, CXCL12 and IGF1 were also identified as hub genes of miR-183-5p in BC which may deeply affect the generation and development of BC. However, rigorous experiments in vitro and in vivo are needed to confirm the potential molecular mechanism.
In summary, miR-183-5p may play critical roles in the tumorigenesis and development of BC; however, the clinical function of miR-183-5p in BC urgently requires exploration. Furthermore, several crucial pathways for miR-183-5p in BC were predicted by bioinformatics analysis. However, validation of the real molecular mechanisms of miR-183-5p in BC by well-designed and rigorous functional experiments is still needed.
Acknowledgements
The present study was supported by the Guangxi Natural Science Fund for Innovation Research Team (2013GXNSFFA019002, 2016GXNSFGA38006), the Guangxi Collaborative Innovation Center for genomic and personalized medicine (201319), the Promoting Project of Basic Capacity for Young and Middle-aged University Teachers in Guangxi (KY2016YB090), and the Innovation Project of Guangxi Graduate Education (YCBZ2017044).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ouyang H, Zhou Y, Zhang L, Shen G. Diagnostic value of microRNAs for urologic cancers: A systematic review and meta-analysis. Medicine. 2015;94:e1272. doi: 10.1097/MD.0000000000001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Bao Z, Zhang W, Dong D. A potential prognostic lncRNA signature for predicting survival in patients with bladder urothelial carcinoma. Oncotarget. 2017;8:10485–10497. doi: 10.18632/oncotarget.14441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrondo C, Flax J, Kucherov V, Siebert A, Osinski T, Rosenberg A, Fucile C, Richheimer S, Beckham CJ. Expression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS One. 2016;11:e0147236. doi: 10.1371/journal.pone.0147236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. Preoperative neutrophil-lymphocyte ratio can significantly predict mortality outcomes in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Oncotarget. 2017;8:12891–12901. doi: 10.18632/oncotarget.14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urquidi V, Netherton M, Gomes-Giacoia E, Serie DJ, Eckel-Passow J, Rosser CJ, Goodison S. A microRNA biomarker panel for the non-invasive detection of bladder cancer. Oncotarget. 2016;7:86290–86299. doi: 10.18632/oncotarget.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Wang X, Fu B, Meng L, Lang B. Differentially expressed genes and microRNAs in bladder carcinoma cell line 5637 and T24 detected by RNA sequencing. Int J Clin Exp Pathol. 2015;8:12678–12687. [PMC free article] [PubMed] [Google Scholar]
- 8.Falzone L, Candido S, Salemi R, Basile MS, Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M, Libra M. Computational identification of microRNAs associated to both epithelial to mesenchymal transition and NGAL/MMP-9 pathways in bladder cancer. Oncotarget. 2016;7:72758–72766. doi: 10.18632/oncotarget.11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y, Zhang X, Li P, Yang C, Tang J, Deng X, Yang X, Tao J, Lu Q, Li P. MiR-200c promotes bladder cancer cell migration and invasion by directly targeting RECK. Onco Targets Ther. 2016;9:5091–5099. doi: 10.2147/OTT.S101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egawa H, Jingushi K, Hirono T, Ueda Y, Kitae K, Nakata W, Fujita K, Uemura M, Nonomura N, Tsujikawa K. The miR-130 family promotes cell migration and invasion in bladder cancer through FAK and Akt phosphorylation by regulating PTEN. Sci Rep. 2016;6:20574. doi: 10.1038/srep20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding M, Li Y, Wang H, Lv Y, Liang J, Wang J, Li C. Diagnostic value of urinary microRNAs as non-invasive biomarkers for bladder cancer: A meta-analysis. Int J Clin Exp Med. 2015;8:15432–15440. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Zhang Y, Liu X, Fang A, Li P, Li Z, Liu T, Yang Y, Du L, Wang C. MicroRNA-203 is a prognostic indicator in bladder cancer and enhances chemosensitivity to cisplatin via apoptosis by targeting Bcl-w and Survivin. PLoS One. 2015;10:e0143441. doi: 10.1371/journal.pone.0143441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morais DR, Reis ST, Viana N, Piantino CB, Massoco C, Moura C, Dip N, Silva IA, Srougi M, Leite KR. The involvement of miR-100 in bladder urothelial carcinogenesis changing the expression levels of mRNA and proteins of genes related to cell proliferation, survival, apoptosis and chromosomal stability. Cancer Cell Int. 2014;14:119. doi: 10.1186/s12935-014-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu WB, Wang W, Du YH, Li H, Xia SJ, Liu HT. MicroRNA-3713 regulates bladder cell invasion via MMP9. Sci Rep. 2016;6:32374. doi: 10.1038/srep32374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng S, Liu J, Zhang Y, Lin Y, Liu Q, Li H, Huang J, Zhang P. Association detection between genetic variants in the microRNA binding sites of toll-like receptors signaling pathway genes and bladder cancer susceptibility. Int J Clin Exp Pathol. 2014;7:8118–8126. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Zhang W, Zuo Y, Ding M, Ke C, Yan R, Zhan H, Liu J, Wang J. miR-9 promotes cell proliferation and inhibits apoptosis by targeting LASS2 in bladder cancer. Tumour Biol. 2015;36:9631–9640. doi: 10.1007/s13277-015-3713-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhou M, Wang S, Hu L, Liu F, Zhang Q, Zhang D. miR-199a-5p suppresses human bladder cancer cell metastasis by targeting CCR7. BMC Urol. 2016;16:64. doi: 10.1186/s12894-016-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang A, Yang M, Shen F, Wang J, Wei J, Wang W, Lu W, Wang C, Wang C. MiR-1-3p suppresses the proliferation, invasion and migration of bladder cancer cells by up-regulating SFRP1 expression. Cell Physiol Biochem. 2017;41:1179–1188. doi: 10.1159/000464379. [DOI] [PubMed] [Google Scholar]
- 19.Pignot G, Cizeron-Clairac G, Vacher S, Susini A, Tozlu S, Vieillefond A, Zerbib M, Lidereau R, Debre B, Amsellem-Ouazana D, Bieche I. microRNA expression profile in a large series of bladder tumors: Identification of a 3-miRNA signature associated with aggressiveness of muscle-invasive bladder cancer. Int J Cancer. 2013;132:2479–2491. doi: 10.1002/ijc.27949. [DOI] [PubMed] [Google Scholar]
- 20.Gu W, Gao T, Shen J, Sun Y, Zheng X, Wang J, Ma J, Hu XY, Li J, Hu MJ. MicroRNA-183 inhibits apoptosis and promotes proliferation and invasion of gastric cancer cells by targeting PDCD4. Int J Clin Exp Med. 2014;7:2519–2529. [PMC free article] [PubMed] [Google Scholar]
- 21.Yang M, Liu R, Li X, Liao J, Pu Y, Pan E, Yin L, Wang Y. miRNA-183 suppresses apoptosis and promotes proliferation in esophageal cancer by targeting PDCD4. Mol Cells. 2014;37:873–880. doi: 10.14348/molcells.2014.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren LH, Chen WX, Li S, He XY, Zhang ZM, Li M, Cao RS, Hao B, Zhang HJ, Qiu HQ, Shi RH. MicroRNA-183 promotes proliferation and invasion in oesophageal squamous cell carcinoma by targeting programmed cell death 4. Br J Cancer. 2014;111:2003–2013. doi: 10.1038/bjc.2014.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Deng L, Zhi Q, Meng Q, Qian A, Sang H, Li X, Xia J. MicroRNA-183 functions as an oncogene by regulating PDCD4 in gastric cancer. Anticancer Agents Med Chem. 2016;16:447–455. doi: 10.2174/1871520615666150914114237. [DOI] [PubMed] [Google Scholar]
- 24.Miao F, Zhu J, Chen Y, Tang N, Wang X, Li X. MicroRNA-183-5p promotes the proliferation, invasion and metastasis of human pancreatic adenocarcinoma cells. Oncol Lett. 2016;11:134–140. doi: 10.3892/ol.2015.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Cheng H, Wang G, Yu G, Zhang D, Wang Y, Fan W, Yang W. Deregulation of miR-183 promotes melanoma development via lncRNA MALAT1 regulation and ITGB1 signal activation. Oncotarget. 2017;8:3509–3518. doi: 10.18632/oncotarget.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han Y, Chen J, Zhao X, Liang C, Wang Y, Sun L, Jiang Z, Zhang Z, Yang R, Chen J, et al. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS One. 2011;6:e18286. doi: 10.1371/journal.pone.0018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada Y, Enokida H, Kojima S, Kawakami K, Chiyomaru T, Tatarano S, Yoshino H, Kawahara K, Nishiyama K, Seki N, Nakagawa M. MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: Correlation with stage and grade, and comparison with urinary cytology. Cancer Sci. 2011;102:522–529. doi: 10.1111/j.1349-7006.2010.01816.x. [DOI] [PubMed] [Google Scholar]
- 28.Wei S, Bing Z, Yao Y, Master SR, Gupta P. Higher expression of miR-182 in cytology specimens of high-grade urothelial cell carcinoma: A potential diagnostic marker. Acta Cytol. 2015;59:109–112. doi: 10.1159/000371507. [DOI] [PubMed] [Google Scholar]
- 29.Scheffer AR, Holdenrieder S, Kristiansen G, von Ruecker A, Müller SC, Ellinger J. Circulating microRNAs in serum: Novel biomarkers for patients with bladder cancer? World J Urol. 2014;32:353–358. doi: 10.1007/s00345-012-1010-2. [DOI] [PubMed] [Google Scholar]
- 30.Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA, Akbani R, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171:540–556.e25. doi: 10.1016/j.cell.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li QQ, Hsu I, Sanford T, Railkar R, Balaji N, Sourbier C, Vocke C, Balaji KC, Agarwal PK. Protein kinase D inhibitor CRT0066101 suppresses bladder cancer growth in vitro and xenografts via blockade of the cell cycle at G2/M. Cell Mol Life Sci. 2017 Oct; doi: 10.1007/s00018-017-2681-z. (Epub ahead of print). doi: 10.1007/s00018-017-2681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng JH, Liang L, He RQ, Tang RX, Cai XY, Chen JQ, Luo DZ, Chen G. Comprehensive investigation of a novel differentially expressed lncRNA expression profile signature to assess the survival of patients with colorectal adenocarcinoma. Oncotarget. 2017;8:16811–16828. doi: 10.18632/oncotarget.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benito-Martin A, Peinado H. FunRich proteomics software analysis, let the fun begin. Proteomics. 2015;15:2555–2556. doi: 10.1002/pmic.201500260. [DOI] [PubMed] [Google Scholar]
- 35.Pathan M, Keerthikumar S, Ang CS, Gangoda L, Quek CY, Williamson NA, Mouradov D, Sieber OM, Simpson RJ, Salim A, et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics. 2015;15:2597–2601. doi: 10.1002/pmic.201400515. [DOI] [PubMed] [Google Scholar]
- 36.Xu F, Zhang H, Su Y, Kong J, Yu H, Qian B. Up-regulation of microRNA-183-3p is a potent prognostic marker for lung adenocarcinoma of female non-smokers. Clin Transl Oncol. 2014;16:980–985. doi: 10.1007/s12094-014-1183-9. [DOI] [PubMed] [Google Scholar]
- 37.Wang G, Wang S, Li C. MiR-183 overexpression inhibits tumorigenesis and enhances DDP-induced cytotoxicity by targeting MTA1 in nasopharyngeal carcinoma. Tumour Biol. 2017;39:1010428317703825. doi: 10.1177/1010428317703825. [DOI] [PubMed] [Google Scholar]
- 38.Fan D, Wang Y, Qi P, Chen Y, Xu P, Yang X, Jin X, Tian X. MicroRNA-183 functions as the tumor suppressor via inhibiting cellular invasion and metastasis by targeting MMP-9 in cervical cancer. Gynecol Oncol. 2016;141:166–174. doi: 10.1016/j.ygyno.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Zhang WG, Wang DS, Tao KS, Song WJ, Dou KF. MicroRNA-183 is involved in cell proliferation, survival and poor prognosis in pancreatic ductal adenocarcinoma by regulating Bmi-1. Oncol Rep. 2014;32:1734–1740. doi: 10.3892/or.2014.3374. [DOI] [PubMed] [Google Scholar]
- 40.Chen YH, Wang SQ, Wu XL, Shen M, Chen ZG, Chen XG, Liu YX, Zhu XL, Guo F, Duan XZ, et al. Characterization of microRNAs expression profiling in one group of Chinese urothelial cell carcinoma identified by Solexa sequencing. Urol Oncol. 2013;31:219–227. doi: 10.1016/j.urolonc.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Ichimi T, Enokida H, Okuno Y, Kunimoto R, Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama K, et al. Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int J Cancer. 2009;125:345–352. doi: 10.1002/ijc.24390. [DOI] [PubMed] [Google Scholar]
- 42.Friedman JM, Liang G, Liu CC, Wolff EM, Tsai YC, Ye W, Zhou X, Jones PA. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 43.Eissa S, Matboli M, Hegazy MG, Kotb YM, Essawy NO. Evaluation of urinary microRNA panel in bladder cancer diagnosis: Relation to bilharziasis. Transl Res. 2015;165:731–739. doi: 10.1016/j.trsl.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Cheng Y, Xiang G, Meng Y, Dong R. MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting the PDCD4. Reprod Biol. 2016;16:225–233. doi: 10.1016/j.repbio.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Wang ZY, Xiong J, Zhang SS, Wang JJ, Gong ZJ, Dai MH. Up-regulation of microRNA-183 promotes cell proliferation and invasion in glioma by directly targeting NEFL. Cell Mol Neurobiol. 2016;36:1303–1310. doi: 10.1007/s10571-016-0328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Wang X, Li Z, Liu H, Teng Y. MicroRNA-183 suppresses retinoblastoma cell growth, invasion and migration by targeting LRP6. FEBS J. 2014;281:1355–1365. doi: 10.1111/febs.12659. [DOI] [PubMed] [Google Scholar]
- 47.Goeppert B, Schmezer P, Dutruel C, Oakes C, Renner M, Breinig M, Warth A, Vogel MN, Mittelbronn M, Mehrabi A, et al. Down-regulation of tumor suppressor A kinase anchor protein 12 in human hepatocarcinogenesis by epigenetic mechanisms. Hepatology. 2010;52:2023–2033. doi: 10.1002/hep.23939. [DOI] [PubMed] [Google Scholar]