Abstract
We tested the ability of avicins, a family of triterpenoid saponins obtained from Acacia victoriae (Bentham) (Leguminosae: Mimosoideae), to inhibit chemically induced mouse skin carcinogenesis. Varying doses of avicins were applied to shaved dorsal skin of SENCAR mice 15 min before application of 100 nmol of 7,12-dimethylbenz[a]anthracene (DMBA) twice a week for 4 weeks (complete carcinogenesis model). The dorsal skin of a second group of mice was treated with one dose of 10 nmol of DMBA. Avicins were then applied 15 min before repetitive doses of 2 μg of phorbol 12-tetradecanoate 13-acetate (TPA) twice a week for 8 weeks (initiation/promotion model). At 12 weeks, avicins produced a 70% decrease in the number of mice with papillomas and a greater than 90% reduction in the number of papillomas per mouse in both protocols. We also observed a 62% and 74% reduction by avicins in H-ras mutations at codon 61 in the DMBA and DMBA/TPA models, respectively, as well as a significant inhibition of the modified DNA base formation (8-OH-dG) in both protocols. Marked suppression of aneuploidy occurred with treatment at 16 weeks in the initiation/promotion experiment. These findings, when combined with the proapoptotic property of these compounds and their ability to inhibit hydrogen peroxide (H2O2) generation, nuclear factor-κB (NF-κB) activation, and inducible nitric oxide synthase (iNOS) induction reported elsewhere, suggest that avicins could prove exciting in reducing oxidative and nitrosative stress and thereby suppressing the development of human skin cancer and other epithelial malignancies.
Triterpenoid saponins, which are present in some plants and marine animals, have been suggested to have anticarcinogenic properties (1). We have recently reported the characterization of an extracted mixture of novel triterpenoid saponins (designated Fraction 35; F035) from above-ground plant parts of Acacia victoriae (Bentham) (Leguminosae: Mimosoideae; ref. 2). We have also extracted a seedpod sample of A. victoriae to obtain a comparable triterpenoid saponin mixture (F094). Both extracts (F035 and F094) contain multiple individual triterpene glycoside molecular species. Pure compounds were then fractionated from F094 and designated avicins D and G (3). All data comparing the in vitro activity of F035 and F094 with avicins D and G demonstrate consistently that they share similar properties in cancer cell lines. HPLC analysis indicated similar chemical profiles for both F035 and F094, but relative amounts of triterpenes varied. For example, avicin G (lipophilic region) was more abundant in F035, and avicin D (polar region) was more abundant in F094.
In the present study, we use the murine skin carcinogenesis model, an ideal system for identifying critical target genes for the action of chemical or physical carcinogens. Skin carcinogenesis in mice can be accomplished by using either complete carcinogenesis or multistage protocols (2). Complete carcinogenesis protocols involve the administration of a single dose or repeated applications of smaller doses of a carcinogen to an experimental animal (2). The multistage model involves the processes defined operationally and mechanistically as initiation and promotion/progression (4). Because the mutagenic effects of tumor initiation are irreversible, it is essential for the prevention of neoplasia to identify agents that are effective against the initially reversible propagation phase of tumorigenesis. The tumor initiation/promotion (multistage model) of carcinogenesis in mouse skin provides a means to distinguish between the anti-initiating and the antitumor-promoting effects of the anticancer compounds tested. In turn, these experiments provide valuable clues regarding the mechanism of action of the compounds (5).
We have shown that triterpene saponins and purified avicins are growth inhibitory (6) and induce apoptosis in vitro by directly perturbing the mitochondria in a Jurkat cell line (human T-cell leukemia) (3) while decreasing generation of hydrogen peroxide (H2O2). The present study extends the analysis to in vivo measurements of inhibition of the initiation and promotion of skin tumors in mice.
Materials and Methods
Purification of Triterpenoid Saponins.
Plant tissue was harvested from A. victoriae trees grown in research fields in Tucson, Arizona. The material was air-dried, ground to 3-mm particle size in a Wiley mill, and extracted by percolation with dichloromethane:methanol (1:1) followed by methanol. The solvent was removed under vacuum. Fractionation of the first extract by a combination of solvent extractions and repetitive column chromatography yielded a series of mixtures. The most biologically active fraction tested for growth inhibitory activity against a panel of cancer cell lines in vitro (2) was a mixture of triterpenoid saponins designated F035. A biologically inactive fraction isolated during the purification steps and designated F060 was used as a negative control.
Chemicals.
7,12-Dimethylbenz[a]anthracene (DMBA) was obtained from Eastman Kodak. Phorbol 12-tetradecanoate 13-acetate (TPA) was obtained from LC Laboratories (Woburn, MA). The extracts from A. victoriae (F035 and F060) were prepared by J. Hoffman, University of Arizona, Office of Arid Land Studies (Tucson, AZ). All solutions of DMBA, TPA, F035, and F060 were prepared immediately before use with spectral grade acetone.
Treatment of Mice.
Five-week-old female SENCAR mice were obtained from the National Cancer Institute (Frederick, MD). At 7–9 weeks of age, the dorsal area of the mice was shaved. After a 2-day period, those mice in the resting phase of the hair growth cycle were selected for testing. Treatment solutions were topically applied to the dorsal surface of the mice skin in a total volume of 0.2 ml of spectral grade acetone. Unless specified otherwise, each experimental group contained 25 mice, housed 5 per cage, and fed a rodent chow diet. Food and water were provided ad libitum. Experiments were carried out under protocols approved by the American Medical Center Cancer Research Center and M. D. Anderson Cancer Center Animal Care Committees.
Complete Carcinogenesis Protocol.
Mice in the complete carcinogenesis protocol were treated with 100 nmol DMBA in 0.2 ml acetone applied twice weekly for 4 weeks. F035 and F060 solutions were applied to the shaved area of the experimental animals 15 min before DMBA application twice a week for 4 weeks. F035 and F060 were applied at doses of 0.5 mg and 1.0 mg per application in 0.2 ml acetone. Two days after the last application of test compounds, five mice per group were killed to evaluate for hyperplasia. The remaining mice did not receive further treatment. After an additional 4 weeks, five more mice from each group were killed to evaluate for hyperplasia. The rest of the mice were kept for a total of 16 weeks to evaluate tumor burden.
Tumor Initiation/Promotion Protocol.
In the tumor initiation/promotion protocol, tumors were initiated in mice by a single application of 10 nmol of DMBA in 0.2 ml of acetone. Beginning 1 week later, mice were treated twice a week with 2 μg TPA in 0.2 ml of acetone for the duration of the experiment. Promotion was stopped after 16 weeks. F035 and F060 (0.5 mg and 1.0 mg per application, in 0.2 ml of acetone) were applied to the shaved dorsal area of the experimental animals 15 min before TPA application throughout the experiment. Control mice were treated with 0.2 ml of acetone. Five mice per group were killed after 8 weeks of treatment. The remaining mice were kept for 8 additional weeks to evaluate tumor burden. Tumor incidence and multiplicity were recorded weekly for each individual mouse.
Histological Evaluation.
When mice were killed, all skin samples were routinely fixed in formalin and processed for histological analysis. Tissues for histological evaluation were prepared by using conventional paraffin sections and hematoxylin-eosin staining. Approximately 1 cm2 of each skin was preserved in formalin for slide preparation; the remainder was rapidly frozen in liquid nitrogen for isolation of DNA. Epithelial thickness was determined from at least 20 randomly selected sites in formalin-fixed skin samples. Dermal thickness, from the basement membrane to the adipose tissue, was also determined at a minimum of 20 randomly selected sites per animal. At 2 days after cessation of dosing, the relative proportion of inflammatory cells (polymorphonuclear leukocytes and lymphocytes, macrophages, fibroblasts, and mast cells) of the dermis was determined by measuring the number of cells per square μm in 10 high-magnification fields (×1000) per animal. This number is defined for experimental purposes as total dermal cellularity.
In addition, tumors obtained at 12 and 16 weeks underwent chromosomal analysis by using a direct cytogenetic technique (7). The papillomas were homogenized and incubated in an enzymatic solution containing 0.02 μg/ml colcemid (GIBCO). The dispersed cells were washed twice and resuspended in hypotonic 0.075 M KCl solution for 10 min at 37°C. Cells were pelleted, fixed in methanol-acetic acid (3:1), and stained with Giemsa. The chromosomes were counted by the LAI Automated Finding System (Westminster, MD) as described (7).
Detection of Oxidative Damage.
Two days after the last dosing, DNA was isolated from freshly frozen tissue from five individual mice per group, following phenol/chloroform extraction and ethanol precipitation (8). Approximately 100 μg of isolated DNA were digested to nucleosides with nuclease P1 and alkaline phosphatase. Analysis of modified DNA base 8-hydroxy-2′-deoxyguanosine (8-OH-dG) was accomplished by HPLC (LC-600/SPD-6A-CR4A HPLC System, Shimadzu) connected with an electrochemical detection unit (ECD; ESA, Chelmsford, MA). Normal bases were quantitated by HPLC with UV detection. Data were expressed as the ratio of 8-OH-dG/105 dG (pmol/pmol). All analyses were performed in triplicate, with appropriate standard curves to correlate area units or peak height with concentration. Skins from mice treated with DMBA (100 nmol, twice a week for 4 weeks; or 10 nmol administered once and followed by 2 μg of TPA, twice a week, for 8 weeks) served as a positive control. Skins from solvent-treated and untreated animals served as negative controls.
Analysis of H-ras Mutations.
PCR amplification was carried out by using DNA obtained from paraffin-embedded tissues as described (9). DNA for H-ras analysis was obtained from paraffin-embedded sections cut at 8 μm. Twenty-five sections from each paraffin block were placed in microfuge tubes. The paraffin was removed with xylene and ethanol, and the sections were centrifuged and resuspended in 5% Chelex 100 with proteinase K. The procedure used for the codon 61 assay was derived from Nelson et al. (10). The 3MSP61 mutant-specific reverse primer was designed such that its 3′ end nucleotide (A) pairs with the middle nucleotide (underlined) of a CAA→CTA transversion in codon 61, and selectively amplifies mutated DNA under the conditions described below. The assay was based on the fact that Taq polymerase lacks 3′ exonuclease activity and thus cannot repair a mismatch at the 3′ end of the annealed primer. The following primers were used in this assay: 5MSP61 (23 mer), 5′-CTA AGC CTG TTG TTT TGC AGG AC-3′; 3MSP61Mt (20 mer), 5′-CAT GGC ACT ATA CTC TTC TA-3′; and 3MSP61Wt (20 mer), 5′-CAT GGC ACT ATA CTC TTC TT-3′. By using the same forward primer, one reaction was run with the reverse mutant (Mt) primer (3MSP61Mt), and another reaction was run with a reverse wild-type (Wt) primer (3MSP61Wt) for each sample. Amplification conditions were identical for the wild- and mutant-type PCR reactions, thereby allowing for a direct comparison of the products. In both reactions the amplified fragment size was 110 bp. This protocol detects only the CAA→CTA transversions (mutations that are the most prevalent in DMBA-induced H-ras codon 61; ref. 11). The reactions containing the mismatch products were run on a 2% low melting point agarose gel for subsequent analyses. The ratio of the amount of wild-type DNA to mutated DNA was determined by quantifying the 32P label. The DNA from a skin cancer cell line (CA3/7) tumor containing H-ras mutations at codon 61 was used as a positive control for the assay (2, 12). Signal analysis was performed with the Millipore Visage 60 BioImage workstation (BioImage, Madison, WI), running the bioimage software and its associated camera for digitizing autoradiograms.
Statistical Analysis.
To determine which treatment groups differed from the control groups (untreated, acetone-treated), a one-way ANOVA was performed on each measured parameter (i.e., epidermal thickness, inflammation, and 8-OH-dG). If the treatment means were determined to be significantly different (P < 0.05), they were compared by using a Tukey honestly significant difference (HSD) ranked mean comparison test (13), a standard method used to compare all means. We established approximate thresholds for deviation from the control levels seen in this study. Tests for significant differences in mean tumor multiplicity (i.e., papillomas per mouse) were performed by the ANOVA and by the Kruskal-Wallis test. Analysis of tumor incidence and percent conversion data were performed by the χ2 test.
Results
Body Weight and Food Consumption.
The mean weights of the animals in all treatment groups were analyzed by one-way ANOVA. There were no significant differences in body weight, body weight gains, or food consumption between animals treated with the test compounds, or acetone-treated or untreated animals.
Inhibition of Epidermal Hyperplasia.
The results from the skin tumorigenesis experiments are shown in Fig. 1 and summarized in Table 1. The overall means of normal epidermal thickness for acetone-treated groups were in the range of 13.61 ± 1.26 μm and did not differ significantly from other controls treated with test compounds alone. The overall means of epidermal thickness for DMBA-treated animals were in the range of 66.15 ± 6.62 μm. Triterpenoid saponins F035 in the complete carcinogenesis protocol produced a good dose-response relationship for the two dose levels tested, inhibiting epidermal hyperplasia in animals treated with a high dose of DMBA by more than 60%. Similar results were achieved in the tumor initiation/promotion protocol. F060, used as negative control, did not inhibit hyperplasia.
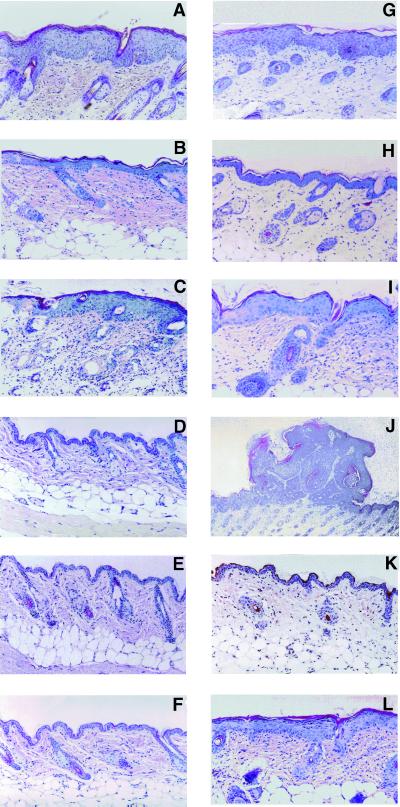
Figure 1.
(Left) Representative microphotographs of SENCAR mice skin sections obtained from experimental and control mice in the complete carcinogenesis experiment after a 4-week treatment with DMBA (A, 100 nmol of DMBA, twice per week), F035/DMBA (B, 1.0 mg of F035 followed by 100 nmol of DMBA, twice per week), and F060/DMBA (C, 1.0 mg of F060 followed by 100 nmol of DMBA, twice per week). (D, E, and F) Skin sections from control animals. (D) Acetone treatment only. (E) F035 treatment only (1.0 mg, twice per wk). (F) F060 treatment only (1.0 mg, twice per wk). Note the strong preventative effect of triterpenoid saponins F035 on epidermal hyperplasia in B. Hematoxylin/eosin (H&E) ×200. (Right) Representative microphotographs of SENCAR mice skin sections obtained from experimental mice in the complete carcinogenesis experiment after an 8-week treatment with DMBA alone (G, 100 nmol of DMBA, twice per wk), F035/DMBA (H, 1.0 mg of F035 followed by 100 nmol of DMBA, twice per week), and F060/DMBA (I, 1.0 mg of F060 followed by 100 nmol of DMBA, twice per week). (J–L) Skin sections obtained at 8 weeks from experimental mice in the initiation/promotion experiment. (J) Papilloma-like structure appearing at 8 weeks after a single application of DMBA (10 nmol) followed by twice per week TPA; (K) 10 nmol of DMBA once followed by twice per week F035/TPA. (L) Ten nanomoles once followed by twice per week F060/TPA. Note the strong preventative effect of triterpenoid saponins F035 on hyperplasia in H. H&E ×200.
Table 1.
Effect of F035 and F060 control on DMBA-induced hyperplasia and inflammatory response in SENCAR mouse skin
| Treatment | Dose(s) per application | Protocol
and duration of treatment
|
Epidermal hyperplasia, μm | Total dermal cellularity* | |
|---|---|---|---|---|---|
| Complete carcinogenesis | Initiation/ promotion | ||||
| Acetone | 0.2 ml | 4 wk | 13.61 ± 1.26b | 5.23 ± 1.02a | |
| DMBA | 100 nmol | 4 wk | 66.15 ± 6.62a | 27.6 ± 2.18b | |
| F035 | 0.5 mg | 4 wk | 11.74 ± 1.54b | 6.56 ± 0.89a | |
| F035 | 1.0 mg | 4 wk | 12.46 ± 2.17b | 5.92 ± 0.77a | |
| F060 | 0.5 mg | 4 wk | 11.80 ± 1.27b | 6.15 ± 1.34a | |
| F060 | 1.0 mg | 4 wk | 12.10 ± 1.38b | 6.08 ± 0.99a | |
| F035/DMBA | 0.5 mg/100 nmol | 4 wk | 24.87 ± 5.35c | 8.16 ± 1.28a | |
| F035/DMBA | 1.0 mg/100 nmol | 4 wk | 22.75 ± 4.61c | 7.81 ± 0.79a | |
| F060/DMBA | 0.5 mg/100 nmol | 4 wk | 65.04 ± 8.43a | 22.4 ± 1.89b | |
| F060/DMBA | 1.0 mg/100 nmol | 4 wk | 60.58 ± 12.2a | 23.6 ± 2.26b | |
| DMBA/TPA | 10 nmol 1 ×/2 μg | 8 wk | 67.22 ± 2.23a | 26.1 ± 3.08b | |
| DMBA/F035/TPA | 10 nmol 1 × 0.5 mg/2 μg | 8 wk | 33.56 ± 7.20c | 9.34 ± 0.68a | |
| DMBA/F035/TPA | 10 nmol 1 × 1.0 mg/2 μg | 8 wk | 28.08 ± 3.41c | 5.34 ± 0.68a | |
| DMBA/F060/TPA | 10 nmol 1 × 0.5 mg/2 μg | 8 wk | 66.69 ± 2.93a | 28.1 ± 1.88b | |
| DMBA/F060/TPA | 10 nmol 1 × 1.0 mg/2 μg | 8 wk | 65.65 ± 4.52a | 29.6 ± 2.08b | |
| Acetone | 0.2 ml | 8 wk | 10.47 ± 0.53b | 5.12 ± 0.56a | |
Values marked with the same letter are not significantly different from each other. DMBA (10 nmol, once) did not cause hyperplasia or inflammation; TPA alone gave a response similar to that of DMBA/TPA (data not shown).
Total dermal cellularity is a measure of inflammatory response.
Inhibition of Cellular Inflammation.
Total dermal cellularity, which is an experimental measure of tissue inflammation, is presented in Table 1. F035 significantly inhibited inflammation in the dermal portion of the skin in the DMBA and DMBA/TPA experiments. In contrast, total dermal cellularity was not affected by the F060 control in the complete carcinogenesis and initiation/promotion experiments.
Reduction of Papilloma Development.
The complete carcinogenesis protocol in mice gives rise to a low number of papillomas, accompanied by a high incidence of squamous cell carcinomas if the experiment is prolonged up to 50 weeks (2, 4, 5). In comparison, the initiation-promotion protocol gives rise to a large number of papillomas followed by a high incidence of squamous cell carcinomas (4, 5). Skin papillomas were induced in SENCAR mice by using either a high dose of DMBA in the complete carcinogenesis protocol or a low dose of DMBA followed by multiple applications of TPA in the initiation/promotion protocol. Table 2 shows the status of papilloma development in SENCAR mice in both carcinogenic protocols with and without triterpenoid saponins. F035 markedly reduced both tumor incidence and multiplicity, whereas the F060 control had no effect.
Table 2.
F035 inhibition of papilloma development in complete carcinogenesis and initiation/promotion experiments in SENCAR mice
| Treatment | Mice with papillomas at 12 wk, % | No. of papillomas per mouse at 12 wk | Mice with papillomas at 16 wk, % | No. of papillomas per mouse at 16 wk |
|---|---|---|---|---|
| Complete carcinogenesis | ||||
| DMBA* | 100 | 8.6 (0%) | 100 | 10.6 (10%) |
| F035†/DMBA* | 33 | 0.7 (0%) | 42 | 2.4 (0%) |
| F060‡/DMBA* | 100 | 8.2 (0%) | 100 | 9.3 (10%) |
| Initiation/promotion | ||||
| DMBA§/TPA | 100 | 12.6 (30%) | 100 | 20.6 (50%) |
| DMBA§/F035¶/TPA‖ | 39 | 1.45 (0%) | 58 | 2.9 (0%) |
| DMBA§/F060**/TPA‖ | 100 | 18.2 (30%) | 100 | 22.3 (51%) |
The concentrations of the test compounds:
, 100 nmol (2×/wk for 4 wk);
, 1.0 mg (2×/wk for 4 wk);
, 1.0 mg (2×/wk for 4 wk);
, 10 nmol (1×);
, 1.0 mg (2×/wk for 8 wk);
, 2 μg (2×/wk for 8 wk);
, 1.0 mg (2×/wk for 8 wk). Shown in parentheses are % papillomas with aneuploidy.
Suppression of Aneuploidy.
In the complete carcinogenesis protocol, at 12 weeks the papillomas were well-differentiated hyperplastic lesions with little or no cellular atypia. The cells were diploid. At 16 weeks, 10% of the cells were dysplastic and hyperdiploid. None of the papillomas that developed in mice treated with the triterpenoid saponins were hyperdiploid. In the initiation/promotion experiment, at 12 weeks 30% of the control preneoplastic tumors were hyperdiploid. At 16 weeks, 50% of the tumors were hyperdiploid. Remarkably, no evidence of aneuploidy was observed in mice treated with the triterpenoid saponins.
Reduction of H-ras Mutations.
Mouse epidermal cells have frequently been used to study the role of the H-ras oncogene in transformation both in vivo and in vitro. After initiation with DMBA in vivo, the majority (95%) of the papillomas that eventually appear have an A to T mutation in the second position of codon 61 or the CAA→CTA mutation (14–17). This transversion is presumed an initiating event (14). The mutation is detected very early after initiating treatment; therefore quantitation of this mutation is warranted starting as early as 4–12 weeks after the carcinogenic insult. In this study, papillomas were not visible at the early time points, but the skins appeared to be hyperplastic (Fig. 1). Autoradiography results of H-ras oncogene codon 61 analysis by using the mismatch specific primers (MSP) assay for DNA samples from the complete carcinogenesis experiment are shown in Fig. 2. Both the wild type and mutant primer PCR analyses were conducted in parallel for the same sample. With amplification conditions identical and within the linear range of the amplification curve for both the mutant and wild-type PCR reactions, mutant and wild-type signals were directly compared. The mutant signal intensity is shown in Table 3 as a percentage of the wild-type signal intensity.
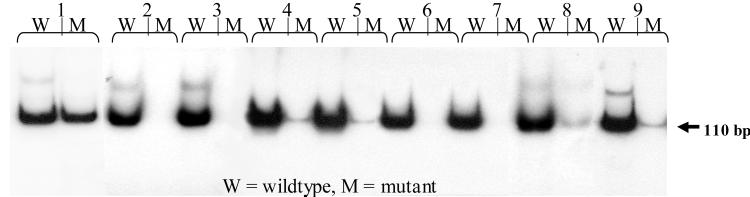
Figure 2.
Autoradiography results of the MSP assay for H-ras oncogene codon 61 mutations in DNA isolated at 8 weeks from experimental and control mice in a complete carcinogenesis assay. Shown for each DNA sample are the wild-type (W) and mutant (M) primer PCR MSP assay results: column 1, DNA positive for the codon 61 mutation, isolated from the CA3/7 mouse cancer cell line, which carries the H-ras mutation; columns 2 and 3, DNA isolated from skins treated with acetone only; columns 4 and 5, DNA isolated from DMBA-treated skins; columns 6 and 7, DNA isolated from F035/DMBA-treated skins; columns 8 and 9, DNA isolated from F060/DMBA-treated skins. Note the presence of mutant DNA in columns 4, 5, 8, and 9.
Table 3.
Analysis of mutations in codon 61 of H-ras oncogene in skin samples harvested from SENCAR mice after DMBA or DMBA/TPA treatment with and without pretreatment with F035 and F060 control
| Complete carcinogenesis (4
wk)
|
Initiation/promotion (8 wk)
|
||||
|---|---|---|---|---|---|
| Treatment | Animals positive for Mut H-ras, % | Mut/Wt, % | Treatment | Animals positive for Mut H-ras, % | Mut/Wt, % |
| DMBA* | 80.0 | 14.9 ± 3.8 | DMBA§/TPA‖ | 90.0 | 27.6 ± 10.9 |
| Acetone | 0.0 | 0.0 ± 0.0 | Acetone/TPA‖ | 0.0 | 0.0 ± 0.0 |
| F035†/DMBA* | 20.0 | 5.7 ± 2.3 | DMBA§/F035¶/TPA‖ | 25.0 | 7.2 ± 2.8 |
| F060‡/DMBA* | 100.0 | 13.9 ± 2.4 | DMBA§/F060**/TPA‖ | 80.0 | 26.1 ± 9.2 |
The concentrations of the test compounds:
, 100 nmol (2×/wk for 4 wk);
, 1.0 mg (2×/wk for 4 wk);
, 1.0 mg (2×/wk for 4 wk);
, 10 nmol (1×);
, 1.0 mg (2×/wk for 8 wk);
, 2 μg (2×/wk for 8 wk);
, 1.0 mg (2×/wk for 8 wk). The values in columns with % Mut/Wt are averages of the data of five animals per group, tested at least two times. Mut, mutant; Wt, wild-type H-ras.
In the two-step carcinogenesis protocol, DNA obtained from 10 animals killed after 8 weeks of treatment was used for H-ras mutation analyses (Table 3). Validation of the assay was conducted by using a known wild-type control (Wt) DNA, mutant control (Mut) DNA isolated from the CA3/7 cancer cell line (12), a negative control (H2O), and a 100-bp ladder. At the 4- and 8-week time point of the complete carcinogenesis protocol, 80% of DMBA-treated skin samples showed H-ras mutations, whereas 90% of the DNA samples obtained at week 8 in the DMBA/TPA experiment were positive for H-ras mutations in codon 61.
Treatment with triterpenoid saponins F035 prevented mutations in codon 61 in 62% (DMBA) and 74% (DMBA/TPA) relative to DMBA and DMBA/TPA only treatment, respectively (Table 3). Analyses of H-ras mutations in codon 61 in the complete carcinogenesis protocol were conducted by using DNA samples obtained from five animals killed at 4 weeks and an additional five animals killed 4 weeks after the initial 4-week treatment ended. There were no differences in the percentage of mutations in DNA obtained from the 4- and 8-week samples in the complete carcinogenesis protocol.
Decrease in 8-OH-dG Formation.
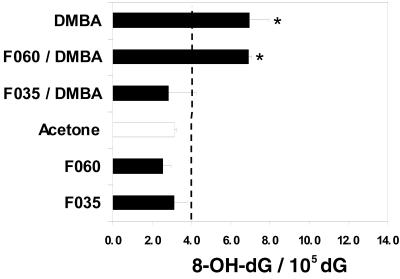
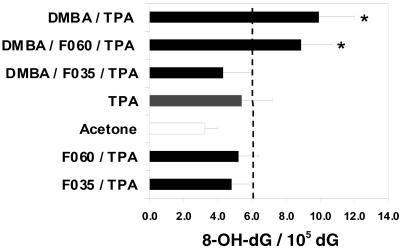
Reactive oxygen species (ROS) attack on DNA may lead to strand breakage and/or formation of modified bases like thymine glycol, hydroxymethyluracil, or 8-OH-dG (18). ROS damage to DNA may be repaired, depending on the type and extent of the damage, but little is known regarding the repair of 8-OH-dG in vivo (18–20). DMBA and DMBA/TPA protocols produced statistically significant increases in 8-OH-dG levels compared with control animals (untreated and acetone only), whereas F035 treatment (low and high concentrations) gave a statistically significant decrease in the 8-OH-dG-to-dG ratio in both the complete carcinogenesis and two-step carcinogenesis protocols (Figs. 3 and 4). As expected, 8-OH-dG levels were not elevated in control animals treated with any dose level of F035, F060, or acetone only. The overall means of normal 8-OH-dG/dG ratio values for untreated and acetone-treated groups did not differ significantly from each other. The mean values ±2 SD values served as a basis for calculating threshold levels (indicated with a dash line on Figs. 3 and 4). Values extending beyond the threshold levels were considered positive.
Figure 3.
Ratio of 8-OH-dG/105 dG (pmol/pmol) in DNA obtained from skins of experimental and control mice (complete carcinogenesis experiment) after a 4-week treatment with DMBA, triterpenoid saponins, or triterpenoid saponins and DMBA. Skins were harvested on day 2 after last dosing of the test compounds. Asterisks indicate groups that are statistically different from pooled controls.
Figure 4.
Ratio of 8-OH-dG/105 dG (pmol/pmol) in DNA obtained from skin of experimental and control mice after an 8-week treatment with either DMBA or DMBA/triterpenoid saponins/TPA. Skins were harvested on day 2 after last dosing of the test compounds in the multistage carcinogenesis experiment (DMBA/TPA). Asterisks indicate groups that are statistically different from pooled controls.
Discussion
Polycyclic aromatic hydrocarbons (PAH), widespread in the environment, likely play an important role in the development of many cancers. People are exposed to them through tobacco smoke, foods, and certain occupations (21, 22). As a representative PAH, DMBA is a well-known skin carcinogen and skin tumor initiator (2, 4, 5). When given a 100-nmol dose repetitively, DMBA induces skin cancer in mice. In this study, when a 100-nmol dose of DMBA was given twice a week for 4 weeks, DMBA induced epidermal hyperplasia, dermal inflammation, H-ras mutations, and DNA damage (8-OH-dG) as determined by the MSP assay. These parameters are early biomarkers used to determine the efficacy of chemoprevention treatment. A single, noncarcinogenic but tumor-initiating dose of DMBA (10 nmol) does not cause detectable changes in the above biomarkers (2, 4, 5). However, an initiating dose of DMBA followed by repetitive applications of TPA is able to induce detectable changes in these biomarkers (2, 4, 5). We have shown inhibition of epidermal hyperplasia, dermal inflammation, H-ras oncogene mutations, DNA damage (8-OH-dG), and aneuploidy when F035 was applied to mice skins in both protocols.
Mouse skin tumors initiated with DMBA demonstrate primarily mutations of the middle adenosine residue of H-ras, codon 61 (CAA; refs. 11 and 22). This mutation occurs early in tumor evolution at the time of benign papillomas. At the same time as base-DMBA adducts are formed, DMBA also induces oxidative stress, usually considered the hallmark of tumor promotion (23). These oxidized DNA base derivatives have been shown to be mutagenic (24). In addition, the base carcinogen adducts probably inhibit removal of the oxidized bases (23).
Aneuploidy, an imbalance of chromosomes that can lead to genomic instability, is a characteristic of most human solid cancers (25). Although genotoxic and nongenotoxic carcinogens alone can cause aneuploidy, tumor promoters such as described in this study are especially potent inducers of chromosomal damage, in part because of the release of ROS such as H2O2 (7, 25–27). Mechanisms to repair DNA damage are widely preserved throughout evolution. In addition, damaged cells can be removed by apoptosis (28). Debate continues whether somatic mutations precede aneuploidy or vice versa (29). However, genomic instability can result from activated oncogenes often associated with ROS (28, 30–32), abnormal chromosome complements, or mutations in mitotic apparatus (33, 34). Thus, the findings reported herein—that avicins suppress both H-ras mutations (which occurred before appearance of the aneuploid karyotype) and aneuploidy—is of great interest because H-ras mutations and aneuploidy are early events in the development of mouse skin papillomas and probably many human epithelial malignancies.
The various properties of avicins reported in previous and current papers partially explain their potent anticarcinogenic properties. The inhibition of epidermal hyperplasia could be explained in part by avicins' growth inhibitory properties and their ability to inhibit phosphatidylinositol-3-kinase-signaling pathways and induce apoptosis of mutated cells (3, 6). Further, the potent antiinflammatory effects reported herein can be partially explained by the ability of avicins to inhibit nuclear factor-κB activation as well as its downstream targets of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX-2; ref. 35). Inflammation has been linked to oxidative stress (23, 36), and the generation of ROS provides a frequent source of endogenous genotoxins (37).
Oxidative damage to DNA contributes to a variety of diseases, including cancer (36). 8-OH-dG is a useful marker for such damage. A striking decrease in skin 8-OH-dG levels suggests that avicins may decrease generation of ROS. In fact, we have observed a decrease in the generation of H2O2 after in vitro treatment with avicins (ref. 3 and unpublished observations). Besides reduction of H2O2 generation (and likely the hydroxyl radical), the avicins inhibit iNOS, which can combine with superoxide to form a highly mutagenic substance, and peroxynitrate, which, like H2O2, can diffuse readily between cells (38). In addition, peroxynitrate can contribute to mutagenesis by inhibiting DNA repair (36). This ability of the avicins to inhibit iNOS induction as well as H2O2 generation could lead to suppression of both oxidative and nitrosative stress (39), thus reducing the mutagenesis and chromosomal instability described in the current study.
Thus, it appears that avicins, by reducing cell damage and compensatory hyperplasia, create a tissue microenvironment less favorable for tumor outgrowth because of less physiologic stress (40). The effects seem to inhibit both the environment for mutagenesis (41) and/or chromosomal damage as well as the selection process that occurs as a result of adaptation to stress (40, 42, 43) whereby advantageous alleles increase in frequency to promote tumor growth (44).
Most chemical carcinogens must undergo metabolic activation, forming electrophilic reactants (45). The chemoprotective efficacy of avicins may also be due to either inhibition of Phase 1 enzymes, or, more likely, the induction of phase 2 enzymes that neutralize reactive electrophiles and act as indirect antioxidants. Recently, Michael reaction acceptors have been shown to induce phase 2 enzymes, and their chemoprotective effects depend on their reactivity with sulfhydryl groups (46). Thus, the structure of avicins (3) may account for the inhibition of nuclear factor-κB–p65 binding to DNA as well as the induction of chemoprotective enzymes (see discussion in ref. 35). In conclusion, avicins could emerge as important preventative agents in the many clinical settings characterized by chronic inflammation, oxidative stress, and a high risk for neoplastic development (35).
Acknowledgments
Research support was provided by the Clayton Foundation for Research, the Foundation for Research, and the Biomedical Research Foundation.
Abbreviations
- F035
fraction 35 from triterpenoid saponin mixture
- F060
fraction 60 from triterpenoid saponin mixture
- DMBA
7,12-dimethylbez[a]anthracene
- TPA
phorbol 12-tetradecanoate 13-acetate
- 8-OH-dG
8-hydroxy-2′-deoxyguanosine
- MSP
mutation specific primers
- H2O2
hydrogen peroxide
- ROS
reactive oxygen species
Footnotes
See commentary on page 10986.
References
- 1.Hostettmann K, Marston A. Saponins. Cambridge, U.K.: Cambridge Univ. Press; 1995. pp. 1–548. [Google Scholar]
- 2.Slaga T J, O'Connell J, Rotstein J, Patskan G, Morris R, Aldaz C M, Conti C. Fundam Cancer Res. 1986;39:31–34. [PubMed] [Google Scholar]
- 3.Haridas V, Higuchi M, Jayatilake G, Bailey D, Mujoo K, Blake M E, Arntzen C J, Gutterman J U. Proc Natl Acad Sci USA. 2001;98:5821–5826. doi: 10.1073/pnas.101619098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slaga T J, Budunova I V, Gimenez-Conti I B, Aldaz C M. J Invest Dermatol Symp Proc. 1996;1:151–156. [PubMed] [Google Scholar]
- 5.Naito M, Naito Y, DiGiovanni J. Carcinogenesis. 1987;8:1807–1815. doi: 10.1093/carcin/8.12.1807. [DOI] [PubMed] [Google Scholar]
- 6.Mujoo K, Haridas V, Hoffman J J, Wachter G A, Hutter L K, Blake M E, Jayatilake G S, Bailey D, Mills G B, Gutterman J U. Cancer Res. 2001;61:5486–5490. [PubMed] [Google Scholar]
- 7.Conti C J, Aldaz C M, O'Connell J, Klein-Szanto A J P, Slaga T J. Carcinogenesis. 1986;7:1845–1848. doi: 10.1093/carcin/7.11.1845. [DOI] [PubMed] [Google Scholar]
- 8.Kasai H, Crain P F, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka S. Carcinogenesis. 1986;7:1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi A B, Navone N, Conti C J. Am J Pathol. 1991;138:279–284. [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson M A, Futscher B W, Kinsella T, Wymer J, Bowden G T. Proc Natl Acad Sci USA. 1992;89:6398–6402. doi: 10.1073/pnas.89.14.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross J A, Nesnow S. Mutat Res. 1999;424:155–166. doi: 10.1016/s0027-5107(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 12.Klann R C, Fitzgerald D J, Piccoli C, Slaga T J, Yamasaki H. Cancer Res. 1989;49:699–705. [PubMed] [Google Scholar]
- 13.Zar J H., Jr . Biostatistical Analysis. 3rd Ed. Englewood Cliffs, NJ: Prentice–Hall; 1996. [Google Scholar]
- 14.Slaga T J, Klein-Szanto A J P, Triplett L L, Yotti L P, Trosko J E. Science. 1981;213:1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- 15.Quintanilla M, Brown K, Ramsden M, Balmain A. Nature (London) 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 16.Brown K, Buchmann A, Balmain A. Proc Natl Acad Sci USA. 1990;87:538–542. doi: 10.1073/pnas.87.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finch J S, Albino H E, Bowden G T. Carcinogenesis. 1996;17:2551–2557. doi: 10.1093/carcin/17.12.2551. [DOI] [PubMed] [Google Scholar]
- 18.Floyd R A, West M S, Eneff K L, Schneider J E, Wong P K, Tingey D T, Hogsett W E. Anal Biochem. 1990;188:155–158. doi: 10.1016/0003-2697(90)90544-j. [DOI] [PubMed] [Google Scholar]
- 19.Malins D C. J Toxicol Environ Health. 1993;40:247–261. doi: 10.1080/15287399309531792. [DOI] [PubMed] [Google Scholar]
- 20.Ames B N, Shigenaga M K, Hagan T M. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips D H. Mutat Res. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 22.Balmain A, Harris C C. Carcinogenesis. 2000;21:371–377. doi: 10.1093/carcin/21.3.371. [DOI] [PubMed] [Google Scholar]
- 23.Frenkel K, Wei L, Wei H. Free Radical Biol Med. 1995;19:373–380. doi: 10.1016/0891-5849(95)00046-z. [DOI] [PubMed] [Google Scholar]
- 24.Frenkel K. Pharmacol Ther. 1992;53:127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]
- 25.Duesberg P, Li R L, Rasnick D, Rausch C, Willer A, Kraemer A, Yerganian G, Hehlmann R. Cancer Genet Cytogenet. 2000;119:83–93. doi: 10.1016/s0165-4608(99)00236-8. [DOI] [PubMed] [Google Scholar]
- 26.Cerutti P A. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 27.Dutton D R, Bowden G T. Carcinogenesis. 1985;6:1279–1284. doi: 10.1093/carcin/6.9.1279. [DOI] [PubMed] [Google Scholar]
- 28.Whal G, Vafa O. Cold Spring Harbor Symposia on Quantitative Biology LXV: Biological Responses to DNA Damage. Plainview, NY: Cold Spring Harbor Lab. Press; 2000. pp. 511–520. [Google Scholar]
- 29.Duesberg P, Rasnick D, Li R, Winters L, Rausch C, Hehlmann R. Anticancer Res. 1999;19:4887–4906. [PubMed] [Google Scholar]
- 30.Denko N C, Giaccia A J, Stringer J R, Stambrook P J. Proc Natl Acad Sci USA. 1994;91:5124–5128. doi: 10.1073/pnas.91.11.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felsher D W, Bishop J M. Proc Natl Acad Sci USA. 1999;96:3940–3944. doi: 10.1073/pnas.96.7.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hainaut P, Milner J. Cancer Res. 1993;53:4469–4473. [PubMed] [Google Scholar]
- 33.Cahill D P, Kinzler K W, Vogelstein B, Lengauer C. Trends Cell Biol. 1999;9:M57–M60. [PubMed] [Google Scholar]
- 34.Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 35.Haridas V, Arntzen C J, Gutterman J U. Proc Natl Acad Sci USA. 2001;98:11557–11562. doi: 10.1073/pnas.191363498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marnett L J. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 37.Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 38.Burney S, Caulfield J L, Niles J C, Wishnok J S, Tannenbaum S R. Mutat Res. 1999;424:37–49. doi: 10.1016/s0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 39.Marshall H E, Merchant K, Stamler J S. FASEB J. 2000;14:1889–1900. doi: 10.1096/fj.00.011rev. [DOI] [PubMed] [Google Scholar]
- 40.Rubin H. Cancer Res. 2001;61:799–807. [PubMed] [Google Scholar]
- 41.Loeb L A. Cancer Res. 2001;61:3230–3239. [PubMed] [Google Scholar]
- 42.Tomlinson I, Bodmer W. Nat Med. 1999;5:11–12. doi: 10.1038/4687. [DOI] [PubMed] [Google Scholar]
- 43.Hastings P J, Bull H J, Klump J R, Rosenberg S M. Cell. 2000;103:723–731. doi: 10.1016/s0092-8674(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 44.Rodin S N, Rodin A S. Proc Natl Acad Sci USA. 2000;97:2244–2249. . (First Published October 17, 2000; 10.1073/pnas.180320897) [Google Scholar]
- 45.Ramos-Gomez, M., Kwak, M.-K., Dolan, P. M., Itoh, K., Yamamoto, M., Talalay, P. & Kensler, T. W. Proc. Natl. Acad. Sci. USA 98, 3410–3415, 2001. [DOI] [PMC free article] [PubMed]
- 46.Dinkova-Kostova A T, Massiah M A, Bozak R E, Hicks R J, Talalay P. Proc Natl Acad Sci USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]