Abstract
Kaposi’s sarcoma-associated herpesvirus (KSHV) is the etiological agent of three human malignancies Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. To persist and replicate within host cells, KSHV encodes proteins that modulate different signaling pathways. Manipulation of cell survival and proliferative networks by KSHV can promote the development of KSHV-associated malignancies. In this review, we discuss recent updates on KSHV pathogenesis and the viral life cycle. We focus on proteins encoded by KSHV that modulate the phosphatidylinositol-4,5-bisphosphate 3 kinase and extracellular signal-regulated kinases 1/2 pathways to create an environment favorable for viral replication and the development of KSHV malignancies.
Keywords: kinase, MAPK/ERK, PI3K/Akt/mTOR, viral life cycle
KSHV Associated Diseases
Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV8), is the etiological agent of three cancers: Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman’s disease (MCD) (Goncalves et al., 2017). KS is the most common cancer associated with KSHV, and is the most common malignancy in men in some Sub-Saharan African countries. KS tumors are of endothelial origin and endothelial cells within the tumor are infected with KSHV (Ye et al., 2011). There are four different types of KS that are characterized by the patients who present the disease (Goncalves et al., 2017). Classical KS occurs in elderly men of Mediterranean and eastern European descent, while African endemic KS presents itself in children and middle-aged adults from sub-Saharan Africa. The other two classes, AIDS-KS and iatrogenic/post-transplant KS, occur in the context of immunosuppression from either viral infection (HIV) or drugs (to prevent allograft rejection). The two other KSHV-associated cancers, PEL and MCD, are of B-cell origin. PEL is a clonal lymphomatous effusion generally present in the body cavities, while MCD is a polyclonal B-cell hyperplasia characterized by systemic inflammatory symptoms (Uldrick et al., 2011; Goncalves et al., 2017). PEL and MCD often exhibit rapid disease progression, and PEL patients have a median survival time of six months post-diagnosis (Bhatt et al., 2010). A recently characterized KSHV-linked disease is Kaposi’s sarcoma-associated herpesvirus inflammatory cytokine syndrome (KICS), which has symptoms similar to MCD (Goncalves et al., 2017). Patients have systemic inflammation characterized by high viral titers, viral interleukin 6 (vIL-6), human IL-6, and human IL-10, but lack the lymphadenopathy seen in MCD patients. KSHV-associated diseases generally occur in the context of immune deficiency such as in AIDS, transplant, or elderly patients. Demonstrating the role the immune system plays in KSHV malignancies, immune reconstitution due to antiretroviral therapy (ART) in AIDS patients often result in clinical regression of AIDS-KS (Letang et al., 2013; Goncalves et al., 2017). However, paradoxically and in rare cases, the pro-inflammatory environment generated following ART initiation known as immune reconstitution inflammatory syndrome (IRIS) can lead to the development of KS, termed IRIS-KS (Letang et al., 2013). Current treatment regimens of all three diseases consist of chemotherapy, radiation, and immunotherapy.
Viral Entry
Like all herpesviruses, infection with KSHV is life-long. Transmission of the virus often occurs through bodily fluids such as saliva and blood (Goncalves et al., 2017). KSHV infects a plethora of cell types ranging from monocytes, fibroblasts, endothelial, epithelial, dendritic, and B-cells (Chandran et al., 2009; Hahn et al., 2012). Viral entry is mediated by binding of different KSHV glycoproteins to a variety of host receptors. The ubiquitous cell surface molecule heparan sulfate aids in the binding of KSHV to multiple cell types (Chandran et al., 2009). There are several entry receptors that KSHV uses and these differ based on cell type. In endothelial cells, the ephrin receptor tyrosine kinase A2, the transmembrane cysteine/glutamine exchange transporter protein xCT, and several integrins (α3β1, αVβ3, αVβ5) are important for KSHV entry (Hahn et al., 2012). In macrophages, dendritic, and B-cells, KSHV binds the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) instead of heparan sulfate to mediate entry (Chandran et al., 2009). Upon binding, the virus predominantly enters the cell by endocytosis (Chandran et al., 2009; Hahn et al., 2012). The envelope of the virion fuses with the endocytic vesicle to release the capsid and tegument layer. The viral capsid is then transported along microtubules and is delivered into the nucleus. Once in the nucleus, KSHV can enter into either the lytic or latent lifecycle. KSHV infection is primarily latent with spontaneous bouts of lytic replication. In KS tumor biopsies, ~99% of KSHV positive tumor cells remain latent (Goncalves et al., 2017). In contrast, MCD biopsies display the highest degree of lytic replication.
Latency
No virions are produced during latency; instead KSHV persists within host cells utilizing viral proteins to pass its genome to daughter cells following cell division. Only a few viral genes are expressed during latency to prevent detection by the host immune system. The latency locus encodes the latent proteins, ORF71 to ORF73, the kaposins, and twelve microRNAs (miRNAs) (Ye et al., 2011). During latency, lytic gene expression is suppressed; however, several proteins highly expressed in the lytic cycle are present at low levels during latency such as K1 and vIL-6 (Bhatt et al., 2012; Giffin et al., 2015). The viral genome is tethered as an episome to the host chromosome by ORF73/LANA (latency-associated nuclear antigen) and is replicated in coordination with the host genome using host replication machinery (Ye et al., 2011). LANA also binds multiple lytic promoters, such as ORF50, to suppress lytic gene expression. Many of the latent proteins upregulate cell proliferation and survival to promote persistence of the infected cell. For instance, ORF71 can bind and activate the IκB kinase (IKK), thereby activating the nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) pathway to upregulate cell survival genes (Ye et al., 2011). Another latent gene, ORF72/vCyclin is a homolog of cellular cyclin D and can bind to cyclin-dependent kinase 6 (CDK6) to phosphorylate retinoblastoma (Rb) and other targets to drive cell cycle progression and proliferation (Ye et al., 2011). Many of the latent genes are expressed in KSHV malignancies. Indicative of the role latent genes have in KSHV tumorigenesis, expression of the latency locus in B-cells in C57BL/6 mice led to a significant increase of lymphomas and hyperplasia in mice (Sin et al., 2013).
Lytic Cycle
Although latency is the dominant program in the KSHV life cycle, spontaneous lytic reactivation does take place (Bhatt et al., 2012). While the physiological stimuli that reactivates KSHV from latency is not known, environmental stresses, histone deacetylase (HDAC) inhibitors, or phorbol esters can cause KSHV to reactivate in vitro (Ye et al., 2011; Goncalves et al., 2017). Expression of genes during the lytic phase is separated into three groups: the immediate-early (IE), delayed early (DE), and late (Ye et al., 2011). During the lytic phase, KSHV replicates its genome and produces infectious progeny to spread to other cells. After viral progeny are produced the infected cell subsequently dies (Goncalves et al., 2017). Unlike other oncogenic herpesviruses, proteins encoded during both the latent and lytic phases of the lifecycle are necessary for the development of KSHV-associated diseases. Highlighting the importance of lytic gene expression, use of a viral replication inhibitor, ganciclovir reduced the risk of KS by at least 75% in AIDS patients (Martin et al., 1999). In a group of KSHV-MCD patients, treatment with zidovudine and valganciclovir resulted in a greater than 80% clinical response (Uldrick et al., 2011). Zidovudine and ganciclovir (the active form of valganciclovir) are phosphorylated into their toxic moieties by the lytic kinases, ORF21 and ORF36, respectively. It is proposed that lytic cells can exhibit a paracrine effect on neighboring cells by secreting inflammatory or angiogenic factors that are highly prevalent in KSHV-associated diseases. For instance, endothelial cells treated with media harvested from viral G protein-coupled receptor (vGPCR)/ORF74 expressing cells had activated extracellular signal-regulated kinases 1/2 (ERK1/2), which is a kinase upregulated in KSHV malignancies (Bakken et al., 2010).
Cellular Signaling Pathways Activated by KSHV
KSHV modulates numerous host cellular pathways to facilitate viral persistence and replication. Pathways upregulated during KSHV infection include the NF-κB, phosphatidylinositol-4,5-bisphosphate 3 kinases (PI3K)/ protein kinase B (Akt)/mammalian target of rapamycin (mTOR), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), and mitogen-activated protein kinase (MAPK) pathways (Brinkmann et al., 2003; Xie et al., 2008; Bhatt et al., 2010; Giffin et al., 2015). These pathways rely on kinases to induce a signaling cascade by phosphorylating proteins to recruit and/or alter activity of target substrates. Therefore, kinase inhibitors have represented an attractive target in treating KSHV malignancies (Stallone et al., 2005; Koon et al., 2014). Currently there are clinical trials to test the efficacy of Selumetinib, an inhibitor of the dual specificity mitogen-activated protein kinase kinase 1/2 (MEK1/2) in KS patients, and Sirolimus (rapamycin), an mTOR complex 1 (mTORC1) inhibitor in HIV-positive MCD patients (Goncalves et al., 2017). MEK1/2 is part of the MAPK/ERK pathway and mTORC1 is part of the PI3K/Akt/mTOR pathway (Mendoza et al., 2011; Bhatt et al., 2012). Both these pathways are often dysregulated in cancers as they regulate cell proliferation and growth. In the following sections, we briefly review the signaling and cross-talk between the PI3K and MAPK/ERK pathways, and the viral proteins encoded by KSHV that modulate these pathways.
PI3K/Akt/mTOR and MAPK/ERK Pathway
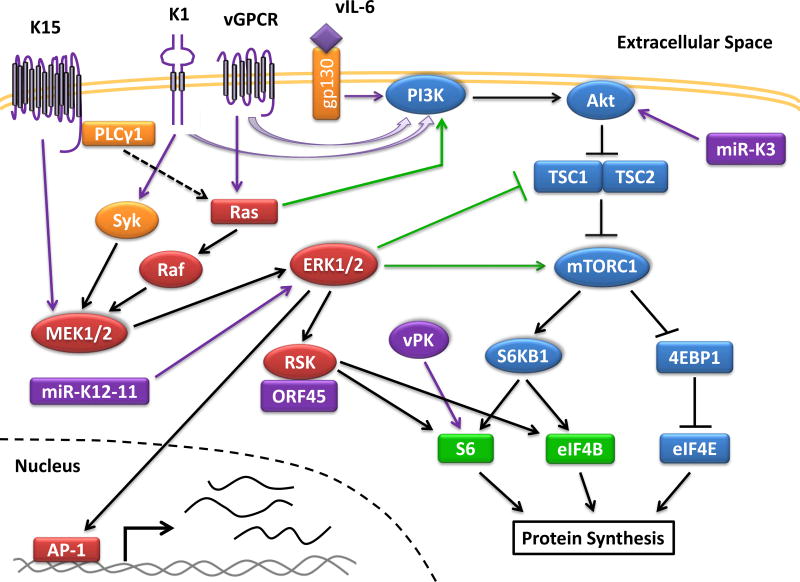
There are four different classes of PI3K kinases (Bhatt et al., 2012). The class IA and 1B PI3K kinases generate phosphatidylinositol-3,4,5-trisphosphate (PIP3), which recruits phosphoinositide dependent kinase 1 (PDK1) and Akt to the membrane, where PDK1 phosphorylates and activates Akt (Figure 1). Akt then inhibits tuberous sclerosis complex 2 (TSC2), leading to the activation of mTORC1. The activation of mTORC1 promotes protein synthesis by activating several translation initiation factors. The phosphorylation of the eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) by mTORC1 releases the eukaryotic translation initiation factor 4E (eIF4E) leading to cap-dependent mRNA translation. The phosphorylation of p70 S6 kinase (S6KB1) by mTORC1 also leads to activation of the ribosomal protein S6 and the eukaryotic translation initiation factor 4B (eIF4B) (Mendoza et al., 2011; Bhatt et al., 2016). Overall the activation of mTORC1 leads to the upregulation of anabolic processes to promote cell growth. Normally, energy deficiencies and stress signals feedback into mTORC1 thereby inhibiting cell proliferation. In non-viral cancers many genes within the PI3K/Akt/mTOR pathway are mutated to prevent the shutdown of this pathway, while in viral cancers oncogenic viral proteins activate this pathway at the protein level (Mendoza et al., 2011; Bhatt et al., 2012). The production of new virions requires energy generation in the cell. Since mTORC1 is the master regulator of energy metabolism, KSHV modulates this pathway to keep key biosynthesis pathways activated during the viral life cycle.
Figure 1. Activation of the PI3K/Akt/mTOR and MAPK/ERK Pathway in KSHV Infection.
KSHV activates the PI3K and ERK1/2 pathways during infection, which are heavily reliant on kinases (drawn as circles) to propagate the signaling cascade. Multiple viral proteins (colored in purple) activate the PI3K/Akt/mTOR pathway (in blue) to promote protein translation by activating subunits of the translation initiation complex. The ERK1/2 pathway (in red) is similarly activated by viral proteins to promote protein translation through RSK. Some cross-talk (shown in green) occurs between both pathways. Activation of ERK1/2 also leads to the transcription of genes regulated by AP-1. Genes under the control of AP-1 include cytokines, which after being transcribed and translated are secreted out of the cell.
There are four canonical MAPKs which are the p38 MAPK, c-jun N-terminal kinase (JNK), ERK1/2, and ERK5 kinases (Sharma-Walia et al., 2005; Pan et al., 2006). The canonical MAPK pathways work in a similar fashion to transduce a signal. A stimulus leads to the activation of a MAPKK kinase (MAPKKK) (Mendoza et al., 2011). The activated MAPKKK then phosphorylates and activates a MAPK kinase (MAPKK), which subsequently phosphorylates and activates its MAPK. In the ERK1/2 (also known as the MAPK/ERK) pathway, there are three MAPKKKs from the Raf family, two MAPKKs, MEK1 and MEK2, and two MAPKs, ERK1 and ERK2 which propagate the signaling cascade (Figure 1). The activation of the MAPK/ERK pathway promotes cell growth and proliferation. ERK1/2 achieves this by activating the p90 S6 kinase (RSK) leading to the phosphorylation of S6 and eIF4B, or by promoting the activation of the transcription factor activator protein 1 (AP-1) (Mendoza et al., 2011; Avey et al., 2015). The pathway is activated by ligands such as growth factors and chemokines binding to their respective receptor. Some receptors activate Ras, which then recruits Raf to the cell membrane to initiate the signaling cascade. Similar to the PI3K/Akt/mTOR pathway, the MAPK/ERK pathway is frequently activated in cancers.
Some cross-talk exists between the PI3K/Akt/mTOR and MAPK/ERK pathways. RSK and S6KB1 share many common target substrates. There is some cross-regulation between both pathways, which is described in more detail in another review (Mendoza et al., 2011). ERK1/2 can activate mTORC1 by phosphorylating inhibitory residues on TSC2 or activating residues on Raptor, a subunit of mTORC1. Cross-inhibition can also occur within the two pathways as Akt can phosphorylate inactivating residues on Raf. Suggestive of cross-talk and/or overlapping roles between the two pathways, use of dual inhibitors to the PI3K/Akt and MAPK/ERK pathways resulted in no synergistic inhibition of PEL growth in vitro (Anders et al., 2015).
The PI3K/Akt/mTOR pathway is highly upregulated and is important for cell survival in KSHV-infected cells. Rapamycin treatment of PEL cells inhibited cell growth in vitro and significantly attenuated tumor growth in mice (Sin et al., 2007). Low doses of NVP-BEZ235, a dual inhibitor that targets both PI3K and mTOR resulted in apoptosis in PEL cells (Bhatt et al., 2010). Inhibition of the PI3K/Akt/mTOR pathway attenuated cytokine secretion from PEL cells as well (Sin et al., 2007; Bhatt et al., 2010). Interestingly, addition of IL-6 or IL-10 restored cell growth in a dose-dependent manner despite rapamycin treatment, indicating the secretion of autocrine and paracrine growth factors is a significant factor driving PEL proliferation (Sin et al., 2007). In renal-transplant patients, KS biopsies at the time of diagnosis showed elevated levels of activated Akt and S6KB1, and increased vascular endothelial growth factor (VEGF) expression (Stallone et al., 2005). Treatment of these patients with Sirolimus (rapamycin) resulted in remission of KS in all patients by six months. Similarly, the MAPK/ERK pathway is upregulated in KSHV-infected cells and is important for controlling KSHV life cycle progression. While there is less clinical work performed showing the efficacy of MAPK/ERK inhibitors in treating KSHV diseases, the use of MAPK inhibitors in vitro attenuates de novo infection of endothelial cells and proper reactivation from latency in PEL cells (Sharma-Walia et al., 2005; Pan et al., 2006; Xie et al., 2008).
Some KSHV proteins that regulate these two pathways include vIL-6, vGPCR, K1, K15, ORF45, and ORF36 (Brinkmann et al., 2003; Bhatt et al., 2012; Avey et al., 2015; Bhatt et al., 2016). Interestingly, these proteins are expressed at high levels only during the lytic cycle; while K1, K15, and vIL-6 are expressed at low levels during latency. As mentioned before, paracrine signaling from lytic cells is thought to help drive KSHV pathogenesis by stimulating neighboring latent cells in a paracrine fashion. By upregulating mTORC1 activity (which controls protein synthesis), and the MAPK pathways (which control transcription of some cytokines and growth factors) KSHV may utilize both pathways in conjunction to produce secretory molecules that promote KSHV pathogenesis. A latent miRNA, miR-K3 also regulates the PI3K/Akt pathway, while another latent miRNA, miR-K12-11 upregulates MAPK signaling (Qin et al., 2013; Hu et al., 2015). Below, we have described the viral proteins or microRNAs and their effects on the PI3K/Akt and/or MAPK pathway.
Viral Transmembrane Proteins: KSHV vGPCR, K1, and K15, and their impact on PI3K/Akt and MAPK/ERK pathways
KSHV encodes several transmembrane proteins, including a chemokine receptor vGPCR, which is a cellular homologue of the interleukin 8 receptor (Bakken et al., 2010). KSHV also encodes two other transmembrane proteins K1, which mimics the B-cell receptor (BCR), and K15 (Steinbruck et al., 2015). K1 and K15 are partial functional homologues to the latent membrane protein 2A (LMP2A) encoded by a related gammaherpesvirus, Epstein-Barr virus (EBV). Cells infected with a mutant KSHV virus lacking K1 had attenuated activation of Akt following reactivation (Zhang et al., 2016). Moreover, it was recently shown that K1 also enhances survival of KSHV infected cells through AMPK (Anders et al., 2016). All three transmembrane proteins activate the MAPK/ERK pathway. Similar in manner to BCR signaling, K1 signals through spleen tyrosine kinase (Syk) to activate AP-1, while vGPCR activates ERK1/2 in a SHP-2 dependent manner (Lee et al., 2005; Bakken et al., 2010). Knockdown of SHP-2 in endothelial cells attenuated activation of ERK1/2 and vGPCR-mediated cell migration (Bakken et al., 2010). K15 activates the MAPK/ERK pathway, but through MEK1/2 in a TNF receptor-associated factor 2 (TRAF-2) dependent manner (Brinkmann et al., 2003). K15 was also reported to interact and activate phospholipase c, gamma 1 (PLCγ1) (Gramolelli et al., 2015).
Both K1 and vGPCR exhibit transformative properties when expressed in cells (Bhatt et al., 2012). The expression of vGPCR immortalizes endothelial cells which is dependent on PI3K/Akt activation as addition of a PI3K inhibitor results in apoptosis (Bais et al., 2003). Furthermore, nude mice present vascular tumors when injected with stably expressing vGPCR-NIH3T3 cells (Bais et al., 1998). Both vGPCR- and K1-expressing transgenic mice develop tumors (Guo et al., 2003; Bhatt et al., 2012). In vGPCR-transgenic mice, vascular KS-like tumors express CD31, an endothelial cell marker present in KS (Guo et al., 2003). However, many of the cells within the tumor did not express the vGPCR protein and only some tissues harvested from mice with tumors expressed ORF74 transcripts. Knockdown of vGPCR also severely attenuated tumor growth and secretion of VEGF in a mouse model where endothelial cells transfected with a bacterial artificial chromosome encoding KSHV were injected into nude mice (Mutlu et al., 2007). These observations would suggest paracrine signaling by vGPCR-expressing cells to neighboring cells play a role in the development of KSHV malignancies.
KSHV vIL-6 and its impact on PI3K/Akt and MAPK signaling
KSHV encodes a cellular homologue of IL-6 known as vIL-6; however unlike IL-6 which requires binding to the gp130 and IL-6 receptor, vIL-6 can transduce a signal by binding to gp130 alone (Bhatt et al., 2012). Similar to human IL-6, vIL-6 activates the JAK/STAT, MAPK, and PI3K/Akt pathways upon binding to gp130. In endothelial cells, vIL-6 activates gp130 leading to the upregulation of several lymphatic markers including podoplanin in a Akt- and STAT3-dependent manner (Bhatt et al., 2012). Unlike human IL-6, vIL-6 can upregulate the expression of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) to promote endothelial cell migration (Giffin et al., 2015). Stably-expressing vIL-6 NIH3T3 cells can also induce tumor formation in nude mice and transgenic mice expressing vIL-6 exhibit MCD-like disease (Bhatt et al., 2012; Zhu et al., 2014). However, expressing vIL-6 in IL-6 deficient mice resulted in no MCD-like disease suggesting that human IL-6 is important for MCD pathogenesis (Bhatt et al., 2012). Recently it was shown that the HIV-1 Nef protein synergistically augments the angiogenesis of vIL-6 in an Akt-dependent manner (Zhu et al., 2014). Endothelial cells stably expressing vIL-6 showed a significant increase in cell growth, angiogenesis, and levels of activated Akt upon addition of soluble Nef. Furthermore, nude mice injected with NIH3T3 cells co-expressing Nef and vIL-6 failed to develop any tumor growth upon treatment with a PI3K inhibitor. These results suggest a novel mechanism on how HIV-infection can play a role in the development of KSHV malignancies besides immune suppression.
KSHV ORF36/vPK and its impact on PI3K/Akt and MAPK signaling
KSHV ORF36 encodes a serine/threonine viral protein kinase (vPK) conserved among the herpesviruses and has been shown to activate JNK, a MAPK family member (Hamza et al., 2004; Avey et al., 2016; Bhatt et al., 2016). Though vPK is classified as a late lytic gene, it can be expressed in hypoxic conditions. Knock down of vPK negatively affects lytic replication and attenuates expression of several late genes (Avey et al., 2016). A ~3 to 10 fold reduction in viral progeny production and infection was observed in deficient or kinase-dead vPK viruses. We recently reported that vPK can mimic S6KB1 and residues lining the catalytic pocket of vPK are conserved to S6KB1 (Bhatt et al., 2016). Similar to S6KB1, vPK can phosphorylate S6, which was inhibited upon addition of a S6KB1 specific inhibitor, but not a JNK or Aurora kinase inhibitor. Overexpression of vPK in cells augmented anchorage-independent growth, angiogenesis, and cell proliferation as well.
Viral miRNAs: KSHV mIR-K3 and mIR-K12-11 and their impact on PI3K and MAPK signaling
The microRNAs (miRNA) encoded by KSHV are transcribed from the latent Kaposin/K12 promoter and are highly expressed in KSHV-associated malignancies (Hu et al., 2015). One miRNA, miR-K3 was shown to activate Akt by downregulating the G protein-coupled receptor kinase 2 (GRK2) which suppresses expression of an IL-8 receptor, C-X-C chemokine receptor type 2 (CXCR2) (Hu et al., 2015). Knockdown of CXCR2 led to decreased activation of Akt in endothelial cells similar to what was shown previously in skin keratinocytes. Furthermore, GRK2 can interact and inhibit Akt signaling in CXCR2-independent manner. The expression of miR-K3 resulted in enhanced cell migration and invasion of endothelial cells in an Akt-dependent manner.
Another miRNA encoded by KSHV, miR-K12-11 is known to upregulate MAPK signaling by indirectly suppressing the expression of dual specificity phosphatase 1 (DUSP1) (Qin et al., 2013). The expression of DUSP1 in endothelial cells inhibited ERK activity, the secretion of pro-migratory factors, and cell invasion during de novo KSHV infection. However, the expression of miR-K12-11 inhibited DUSP1 levels in an xCT-dependent manner, thereby reversing DUSP1 inhibition on ERK (Qin et al., 2013). Highlighting the importance of the MAPK/ERK pathway in de novo infection, DUSP1-mediated inhibition of ERK activity resulted in the attenuation of latent and lytic gene expression. Furthermore, expression of miR-K12-11 or a DUSP1 inhibitor activates p38 MAPK and JNK.
ORF45 and its impact on MAPK/ERK signaling
ORF45 is a lytic immediate early protein located in the cytoplasm and nucleus of infected cells (Avey et al., 2015; Avey et al., 2016). While ORF45 does not contain any intrinsic phosphorylation activity, ORF45 does mediate the activity of several kinases. ORF45 binds to RSK, thereby stabilizing RSK’s association with ERK. ORF45 associates with the RSK/ERK complex to sustain activation of the pathway by preventing the dephosphorylation of RSK and ERK (Avey et al., 2015). Suggestive of ORF45’s role in mediating protein synthesis, expression of ORF45 promotes the association of cellular and viral mRNAs with higher 5’ UTR complexity to polysomes. ORF45 also interacts and augments the activity of vPK in a poorly understood ORF45-RSK independent manner (Avey et al., 2016).
Concluding Remarks
The modulation of signaling pathways by KSHV proteins is critical to virus survival and persistence in the human host. Inhibition of these pathways by biochemical means or drug treatment often attenuates viral replication and/or induces cell death in KSHV-infected cells (Sin et al., 2007; Xie et al., 2008; Bhatt et al., 2010). Active drug discovery aimed at cellular kinases comprises a large proportion of pharmaceutical drug development. As reviewed above, KSHV manipulates kinase dependent pathways, and as such, novel specific kinase inhibitors identified by drug discovery programs may be viable treatment options for KSHV associated diseases. Already several kinase inhibitors such as Sirolimus and Imatinib have had partial success in treating KS patients (Stallone et al., 2005; Koon et al., 2014). To fully take advantage of current pharmacological advancement much work remains to understand the extent to which pathways affect KSHV pathogenesis.
Acknowledgments
We apologize for not citing many publications due to a strict reference limit per journal policy. We thank the members of the Damania lab for helpful discussions. Our work is supported by public health service grants CA019014, CA096500, CA163217, and DE023946. BD is a Leukemia and Lymphoma Society Scholar, and a Burroughs Wellcome Fund Investigator in Infectious Disease. JPW was supported in part by the National Institutes of Health training grant T32 AI007419.
References
- 1.Anders P, Bhende PM, Foote M, Dittmer DP, Park SI, Damania B. Dual inhibition of phosphatidylinositol 3-kinase/mammalian target of rapamycin and mitogen activated protein kinase pathways in non-Hodgkin lymphoma. Leuk. Lymphoma. 2015;56:263–266. doi: 10.3109/10428194.2014.917639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders PM, Zhang Z, Bhende PM, Giffin L, Damania B. The KSHV K1 protein modulates AMPK function to enhance cell survival. PLoS Path. 2016;12:e1005985. doi: 10.1371/journal.ppat.1005985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avey D, Tepper S, Li W, Turpin Z, Zhu F. Phosphoproteomic analysis of KSHV-infected cells reveals roles of ORF45-activated RSK during lytic replication. PLoS Path. 2015;11:e1004993. doi: 10.1371/journal.ppat.1004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avey D, Tepper S, Pifer B, Bahga A, Williams H, Gillen J, Li W, Ogden S, Zhu F. Discovery of a coregulatory interaction between Kaposi's sarcoma-associated herpesvirus ORF45 and the viral protein kinase ORF36. J. Virol. 2016;90:5953–5964. doi: 10.1128/JVI.00516-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 6.Bais C, Van Geelen A, Eroles P, Mutlu A, Chiozzini C, Dias S, Silverstein RL, Rafii S, Mesri EA. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR. Cancer Cell. 2003;3:131–143. doi: 10.1016/s1535-6108(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 7.Bakken T, He M, Cannon ML. The phosphatase Shp2 is required for signaling by the Kaposi's sarcoma-associated herpesvirus viral GPCR in primary endothelial cells. Virology. 2010;397:379–388. doi: 10.1016/j.virol.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt AP, Bhende PM, Sin SH, Roy D, Dittmer DP, Damania B. Dual inhibition of PI3K and mTOR inhibits autocrine and paracrine proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood. 2010;115:4455–4463. doi: 10.1182/blood-2009-10-251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt AP, Damania B. AKTivation of PI3K/AKT/mTOR signaling pathway by KSHV. Front. Immunol. 2012;3:401. doi: 10.3389/fimmu.2012.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt AP, Wong JP, Weinberg MS, Host KM, Giffin LC, Buijnink J, van Dijk E, Izumiya Y, Kung HJ, Temple BR, et al. A viral kinase mimics S6 kinase to enhance cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 2016;113:7876–7881. doi: 10.1073/pnas.1600587113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkmann MM, Glenn M, Rainbow L, Kieser A, Henke-Gendo C, Schulz TF. Activation of mitogen-activated protein kinase and NF-kappaB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 2003;77:9346–9358. doi: 10.1128/JVI.77.17.9346-9358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandran B, Sharma-Walia N. KSHV entry and infection of target cells. In: Damania B, Pipas J, editors. DNA Tumor Viruses. New York, New York: Springer US; 2009. pp. 583–609. [Google Scholar]
- 13.Giffin L, West JA, Damania B. Kaposi's sarcoma-associated herpesvirus interleukin-6 modulates endothelial cell movement by upregulating cellular genes involved in migration. mBio. 2015;6:e01499–01415. doi: 10.1128/mBio.01499-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goncalves PH, Ziegelbauer J, Uldrick TS, Yarchoan R. Kaposi sarcoma herpesvirus-associated cancers and related diseases. Curr. Opin. HIV AIDS. 2017;12:47–56. doi: 10.1097/COH.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gramolelli S, Weidner-Glunde M, Abere B, Viejo-Borbolla A, Bala K, Ruckert J, Kremmer E, Schulz TF. Inhibiting the recruitment of PLCgamma1 to Kaposi's sarcoma herpesvirus K15 protein reduces the invasiveness and angiogenesis of infected endothelial cells. PLoS Path. 2015;11:e1005105. doi: 10.1371/journal.ppat.1005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo HG, Sadowska M, Reid W, Tschachler E, Hayward G, Reitz M. Kaposi's sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J. Virol. 2003;77:2631–2639. doi: 10.1128/JVI.77.4.2631-2639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn AS, Kaufmann JK, Wies E, Naschberger E, Panteleev-Ivlev J, Schmidt K, Holzer A, Schmidt M, Chen J, Konig S, et al. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi's sarcoma-associated herpesvirus. Nature Med. 2012;18:961–966. doi: 10.1038/nm.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamza MS, Reyes RA, Izumiya Y, Wisdom R, Kung HJ, Luciw PA. ORF36 protein kinase of Kaposi's sarcoma herpesvirus activates the c-Jun N-terminal kinase signaling pathway. J. Biol. Chem. 2004;279:38325–38330. doi: 10.1074/jbc.M400964200. [DOI] [PubMed] [Google Scholar]
- 19.Hu M, Wang C, Li W, Lu W, Bai Z, Qin D, Yan Q, Zhu J, Krueger BJ, Renne R, et al. A KSHV microRNA directly targets G protein-coupled receptor kinase 2 to promote the migration and invasion of endothelial cells by inducing CXCR2 and activating AKT signaling. PLoS Path. 2015;11:e1005171. doi: 10.1371/journal.ppat.1005171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koon HB, Krown SE, Lee JY, Honda K, Rapisuwon S, Wang Z, Aboulafia D, Reid EG, Rudek MA, Dezube BJ, et al. Phase II trial of imatinib in AIDS-associated Kaposi's sarcoma: AIDS Malignancy Consortium Protocol 042. J. Clin. Oncol. 2014;32:402–408. doi: 10.1200/JCO.2012.48.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee BS, Lee SH, Feng P, Chang H, Cho NH, Jung JU. Characterization of the Kaposi's sarcoma-associated herpesvirus K1 signalosome. J. Virol. 2005;79:12173–12184. doi: 10.1128/JVI.79.19.12173-12184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letang E, Lewis JJ, Bower M, Mosam A, Borok M, Campbell TB, Naniche D, Newsom-Davis T, Shaik F, Fiorillo S, et al. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS. 2013;27:1603–1613. doi: 10.1097/QAD.0b013e328360a5a1. [DOI] [PubMed] [Google Scholar]
- 23.Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N. Engl. J. Med. 1999;340:1063–1070. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- 24.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem. Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutlu AD, Cavallin LE, Vincent L, Chiozzini C, Eroles P, Duran EM, Asgari Z, Hooper AT, La Perle KM, Hilsher C, et al. In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: a cell and animal model of virally induced Kaposi's sarcoma. Cancer Cell. 2007;11:245–258. doi: 10.1016/j.ccr.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan H, Xie J, Ye F, Gao SJ. Modulation of Kaposi's sarcoma-associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J. Virol. 2006;80:5371–5382. doi: 10.1128/JVI.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin Z, Dai L, Defee M, Findlay VJ, Watson DK, Toole BP, Cameron J, Peruzzi F, Kirkwood K, Parsons C. Kaposi's sarcoma-associated herpesvirus suppression of DUSP1 facilitates cellular pathogenesis following de novo infection. J. Virol. 2013;87:621–635. doi: 10.1128/JVI.01441-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, Chandran B. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 2005;79:10308–10329. doi: 10.1128/JVI.79.16.10308-10329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sin SH, Dittmer DP. Viral latency locus augments B-cell response in vivo to induce chronic marginal zone enlargement, plasma cell hyperplasia, and lymphoma. Blood. 2013;121:2952–2963. doi: 10.1182/blood-2012-03-415620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sin SH, Roy D, Wang L, Staudt MR, Fakhari FD, Patel DD, Henry D, Harrington WJ, Jr, Damania BA, Dittmer DP. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood. 2007;109:2165–2173. doi: 10.1182/blood-2006-06-028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N. Engl. J. Med. 2005;352:1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 32.Steinbruck L, Gustems M, Medele S, Schulz TF, Lutter D, Hammerschmidt W. K1 and K15 of Kaposi's sarcoma-associated herpesvirus are partial functional homologues of latent membrane protein 2A of Epstein-Barr virus. J. Virol. 2015;89:7248–7261. doi: 10.1128/JVI.00839-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uldrick TS, Polizzotto MN, Aleman K, O'Mahony D, Wyvill KM, Wang V, Marshall V, Pittaluga S, Steinberg SM, Tosato G, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood. 2011;117:6977–6986. doi: 10.1182/blood-2010-11-317610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie J, Ajibade AO, Ye F, Kuhne K, Gao SJ. Reactivation of Kaposi's sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways. Virology. 2008;371:139–154. doi: 10.1016/j.virol.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye F, Lei X, Gao SJ. Mechanisms of Kaposi's sarcoma-associated herpesvirus latency and reactivation. Adv. Virol. 2011;2011:193860. doi: 10.1155/2011/193860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Chen W, Sanders MK, Brulois KF, Dittmer DP, Damania B. The K1 Protein of Kaposi's sarcoma-associated herpesvirus augments viral lytic replication. J. Virol. 2016;90:7657–7666. doi: 10.1128/JVI.03102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu X, Guo Y, Yao S, Yan Q, Xue M, Hao T, Zhou F, Zhu J, Qin D, Lu C. Synergy between Kaposi's sarcoma-associated herpesvirus (KSHV) vIL-6 and HIV-1 Nef protein in promotion of angiogenesis and oncogenesis: role of the AKT signaling pathway. Oncogene. 2014;33:1986–1996. doi: 10.1038/onc.2013.136. [DOI] [PubMed] [Google Scholar]