Abstract
Background:
Dengue is a kind of infectious disease that was distributed in the tropical and sub-tropical areas. To date, there is no clinically approved dengue vaccine or antiviral for humans, even though there have been great efforts towards this end. Therefore, finding the effective compound against dengue virus (DENV) replication is very important. Among the complex compounds, copper(II)-imidazole derivatives are of interest because of their biological and medicinal benefits.
Materials and Methods:
In the present study, antiviral activity of [Cu(2,4,5-triphenylimidazole)2]n, was evaluated against different stages of dengue virus type 2 (DENV-2) replication in Vero cell using focus forming unit reduction assay and quantitative ELISA.
Results:
[Cu(2,4,5-triphenylimidazole)2]n inhibited DENV-2 replication in Vero cells with IC50 = 2.3 μg/ml and SI= 19.42 when cells were treated 2 days after virus infection, whereas its CC50 for cytotoxicity to Vero cells was 44.174 μg/ml.
Conclusion:
The compound has high anti-DENV2 activity, less toxicity, and a high possibility to be considered a drug candidate.
Keywords: DENV-2; Antiviral activity; Vero cell; [Cu(2,4,5-triphenylimidazole)2]n
Introduction
Dengue is a kind of infectious disease that is distributed in the tropical and sub-tropical areas (Bhatt et al., 2013; Franco et al., 2010; Guzman et al., 2010). Dengue is transmitted to human by Aedes aegypti. More than 250,000-500,000 cases dengue infection occurred in the world every years (Bharaj et al., 2008; Reiter, 2001). Four distinct serotypes were reported, DENV-1, DENV-2, DENV-3, and DENV-4 (Balmaseda et al., 2006; Holmes and Twiddy, 2003). Indonesia is one of the largest countries in the dengue-endemic region worldwide (Fahri et al., 2013; Kotaki et al., 2014; Kotaki et al., 2016; Setiati et al., 2006). In Indonesia, dengue occurred for the first time as an outbreak in Jakarta and Surabaya, in 1968 (Sumarmo, 1987). To date, there is no clinically approved dengue vaccine or antiviral for humans, even though there have been great efforts towards this end.
2,3,5-triphenylimidazole, is a derivate of imidazole. The strong therapeutic properties of imidazole-containing drugs have encouraged medicinal chemists to synthesize a large number of novel chemotherapeutic agents comprising this entity. N5-(4-fluorophenyl)-N4-(2-(pyridin-4-yl)benzyl)-1H-imidazole-4,5-dicarboxamide, a derivate of imidazole, was reported anti-DENV activity (Saudi et al., 2014).
The complex compound forms as a result of metal and organic compound reaction. It can be used as an anti-inflammatory (Agotegaray et al., 2012), antimicrobial (Carcelli et al., 1995), antifungal, antibacterial (Arjmand et al., 2005), antivirus (Ranford et al., 1993). For example, Pt-acesulfame compound showeds a good inhibition of dengue virus replication mainly at 200 μM, when compared to the vehicle-treated cells (Cavicchioli et al., 2010). Therefore, in this research we sought to screen Cu(II)-Imidazole for dengue type 2 inhibition.
Materials and Methods
Materials
Chemical reagents used in this research include copper(II)chloride dihydrate (CuCl2.2H2O) (Merck 99.0%), N,N-dimethyl formamide (DMF) (Merck 99.8%), 2,4,5-triphenylimidazole ligand (Sigma-Aldrich 90%), dimethyl sulfoxide (DMSO) (Sigma-Aldrich 99.8%) and ethanol (Sigma-Aldrich 96%).
Vero cells used for screening antiviral activity were incubated at 37 °C in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum. Dengue virus type 2 (DENV-2) strains from Surabaya, Indonesia and cell monolayers were examined for the presence of viral antigen by immunostaining with a flavivirus-specific monoclonal antibody (D1-4G2; American Type Culture Collection, Manassas, VA).
Methods
Synthesis of [Cu(2,4,5-triphenylimidazole)2]n
The compound was synthesized using solvothermal method with heating time of 3 hours at temperature of 120°C (Han et al., 2012).
Cytotoxicity assay
Using WST-1 cell proliferation reagent (Roche Applied Science, Mannheim, Germany) (Chew et al., 2015). Vero cells (1 x 105 cells/ml) were seeded in 96-well plate at 37 °C in 5% CO2 overnight. Following serial dilution 100 µl of the compound was incubated with Vero cells for 24 h. 10 µl of Cell Proliferation Reagent WST-1 was added into each well, incubated for 1 hour at 37 °C. The plate was read at 450 nm (main filter) and 655 nm (reference filter) using an ELISA reader (iMark™ Microplate Absorbance Reader).
In vitro study
Vero cells (1 x 105 cells/ml) were also seeded in 96-well plate at 37 °C in 5% CO2 overnight. DENV-2 virus solution (MOI of 2) was prepared in MEM containing 10% FBS. Another serially diluted compound was added and incubated 37 °C for 1 hour. The supernatant was discarded and the pellet was washed with sterilized PBS three times, then MEM containing 10% FBS was added, followed by serially diluted test compound to the Vero cells incubated at 37 °C for 2 days. The supernatant was used for ELISA at 450 nm (Wang et al., 2009) using an ELISA reader (iMark™ Microplate Absorbance Reader).
Results and Discussions
Cytotoxicity of [Cu(2,4,5-triphenylimidazole)2]n to DENV-2-infected Vero cells
The result of the cell proliferation assay at 1 hour is shown in Figure 1. The compound, when added to Vero cells exhibited cytotoxic effects at CC50 = 44.74 µg/ml. One precent DMSO (negative control) did not show any cytotoxic effects against Vero cells.
Figure 1.
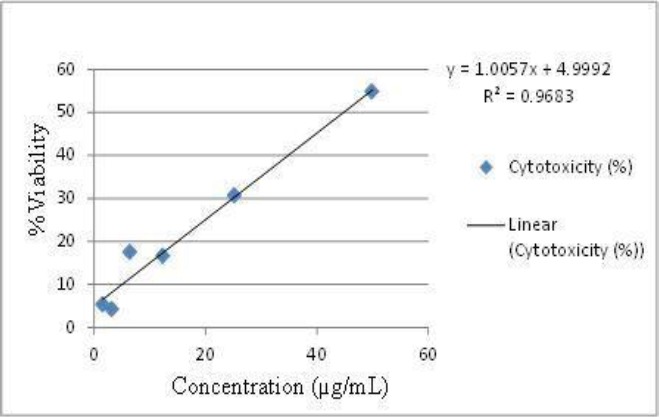
Linear curve for cytotoxicity of [Cu(2,4,5-triphenylimidazole)2]n
CC50 is cytotoxicity level of [Cu(2,4,5-triphenylimidazole)2]n (compound) to cause death to 50% of Vero cells. IC50 was calculated from regression linier curve; y = 1.0057x + 4.9992 with the axis (x) is concentration of compound and ordinate (y) is %viability.
Inhibition of DENV-2 Infection by [Cu(2,4,5-triphenylimidazole)2]n in vitro
The inhibitory ability of the compound against DENV-2 infection was determined via ELISA method. The compound was incubated with DENV-2 for 1 hour prior addition to Vero cells. The compound exhibited adsorption inhibitory activity against DENV-2 at IC50 = 2.3 µg/ml (SI value of 19.42). Percentage inhibition increased with increasing concentrations of compound (Figure 2). This indicates that dengue virus replication was inhibited. The inhibition at IC50 was not significantly high (p<0.005) compared to that of the metal-free imidazole (IC50 = 0.13 µg/ml). But, the metal-free imidazole more toxic for Vero cells (CC50 = 5.03 µg/ml) (Sucipto et al., 2017). However, studies on the compound of imidazole-4,5- showed higher antiviral potency against yellow fever virus (YFV) than dengue virus (DENV). This bioactivity may be within the imidazole series of a ‘para-‘attachment of a heterocycle to its ‘C’ (Saudi et al., 2014).
Figure 2.
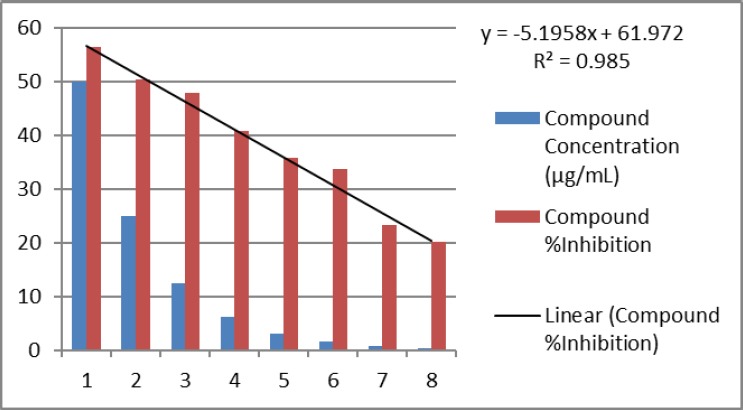
Inhibition curve of [Cu(2,4,5-triphenylimidazole)2]n to DENV-2
IC50 (maximal inhibitory concentration) is measure of the effectiveness of a substance in inhibiting a specific DENV-2. In this curve was used 8 concentration; 50 µg/ml, 25 µg/ml, 12.5 µg/ml, 6.25 µg/ml, 3.13 µg/ml, 1.56 µg/ml, 0.78 µg/ml and 0.39 µg/ml. IC50 was calculated from regression linier curve; y = -5.1958x + 61.972.
Acknowledgments
This work was supported by the joined program of the Japan Initiative for Global Research Network on Infectious Disease (J-GRID); Research Grant Mandat Universitas Airlangga (HRMUA); Institute of Tropical Disease (ITD) the Center of Excellence (COE) program by the Ministry of Research and Technology (RISTEK) Indonesia.
Footnotes
Conflict of Interest: Authors declare that they have no competing interest.
References
- 1.Agotegaray MA, Dennehy M, Boeris MA, Grela MA, Burrow RA, Quinzani OV. Therapeutic properties, SOD and catecholase mimetic activities of novel te, ary copper(II) complexes of the anti-inflammatory drug fenoprofen with imidazole and caffeine. Polyhedron. 2012;34:74–83. [Google Scholar]
- 2.Arjmand F, Mohani B, Ahmad S. Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu(II) complex. Europ. J. Med. Chem. 2005;40:1103–1110. doi: 10.1016/j.ejmech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Balmaseda A, Hammond S.N, Pérez L, Tellez Y, Saborío SI, Mercado JC, Cuadra R, Rocha J, Pérez MA, Silva S, Rocha C, Harris E. Serotype-specific differences in clinical manifestations of dengue. Am. J. Trop. Med. Hyg. 2006;74:440–456. [PubMed] [Google Scholar]
- 4.Bharaj P, Chahar HS, Pandey A, Diddi K, Dar L, Guleria R, Kabra SK, Broor S. Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol. J. 2008;5:1–5. doi: 10.1186/1743-422X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carcelli M, Mazza P, Pelizzi C, Pelizzi G, Zani F. Antimicrobial and genotoxic activity of 2,6-diacetylpyridine bis(Acylhydrazones) and their complexes with some first transition series metal ions. X-ray crystal structure of dinuclear copper(II) complex. J. Inorg. Biochem. 1995;57:43–62. doi: 10.1016/0162-0134(94)00004-t. [DOI] [PubMed] [Google Scholar]
- 7.Cavicchioli M, Massabni AC, Heinrich TA, Costa-Neto CM, Abrão EP, Fonseca BAL, Castellano EE, Corbi PP, Lustri WR, Leite C.Q.F. Pt(II) and Ag(I) complexes with acesulfame: Crystal structure and a study of their antitumor, antimicrobial and antiviral activities. J. Inorg. Biochem. 2010;104:533–540. doi: 10.1016/j.jinorgbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Chew MF, Tham HW, Rajik M, Sharifah SH. Anti-dengue virus serotype 2 activity and mode of action of a novel peptide. J. App. Microbio. 2015;119:1170–1180. doi: 10.1111/jam.12921. [DOI] [PubMed] [Google Scholar]
- 9.Fahri S, Yohan B, Trimarsanto H, Sayono S, Hadisaputro S, Dharma E, Syafruddin D, Sasmono RT. Molecular surveillance of dengue in Semarang, Indonesia revealed the circulation of an old genotype of dengue virus serotype-1. Plos Neg. Trop. Dis. 2013;7:1–12. doi: 10.1371/journal.pntd.0002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco L, Caro AD, Carietti F, Vapalahti O, Renaudat C, Zeller H, Tenorio A. Recent expansion of dengue virus serotype 3 in West. Eur. J. Inf. Dis. Epid. Prev. Con. 2010:73–76. [PubMed] [Google Scholar]
- 11.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martines E, Nathan MB, Pelegrio JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nature. 2010:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han S, Lough AJ, Kim J. C. Synthesis, Crystal Structures and Properties of Macrocyclic Copper(II) Compexes Containing Imidazole Pendants. Bull. Korean Chem. Soc. 2012;33:2381–2384. [Google Scholar]
- 13.Holmes EC, Twiddy SS. The origin, emergence and evolutionary genetics of dengue virus. Inf. Gen. Evol. 2003;3:19–28. doi: 10.1016/s1567-1348(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 14.Kotaki T, Yamanaka A, Mulyatno KC, Churrotin S, Labiqah A, Sucipto TH, Soegijanto S, Kameoka M, Konishi E. Continuous dengue type 1 virus genotype shifts followed by co-circulation, clade shifts and subsequent disappearance in Surabaya, Indonesia, 2008-2013. Inf. Gen. Evol. 2014;28:48–54. doi: 10.1016/j.meegid.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Kotaki T, Yamanaka A, Mulyatno KC, Churrotin S, Sucipto TH, Labiqah A, Ahwanah N.L.F, Soegijanto S, Kameoka M, Konishi E. Divergence of the dengue virus type 2 cosmopolitan genotype associated with two predominant serotype shifts between 1 and 2 in Surabaya, Indonesia, 2008-2014. Inf. Gen. Eval. 2016;37:88–93. doi: 10.1016/j.meegid.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Ranford JD, Sadler PJ, Tocher DA. Cytotoxicity and antiviral activity of transition-metal salicylate complexes and crystal structure of bis(diisopropylsalicylato)(1,10-phenanthroline) copper(II) J. Chem. Soc. 1993;3:3393–3399. [Google Scholar]
- 17.Reiter P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001;109:141–161. doi: 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saudi M, Zmurko J, Kaptein S, Rozenski J, Neyts J, Aerschot AV. Synthesis and evaluation of imidazole-4,5- and pyrazine-2,3- dicarboxamides targeting dengue and yellow fever virus. Europ. J. Med. Chem. 2014;87:529–539. doi: 10.1016/j.ejmech.2014.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setiati TE, Wagenaar J.F.P, Kruil MD, Mairuhu ATA, Gorp ECM, Soemantri A. Changing epidemiology of dengue haemorrhagic fever in Indonesia. Dengue Bull. 2006;30:1–14. [Google Scholar]
- 20.Sucipto TH, Churrotin S, Setyawati H, Kotaki T, Martak F, Soegijanto S. Antiviral activity of copper(II)chloride dehydrate against dengue virus type-2 in vero cell. Indonesian J. Trop. Inf. Dis. 2017;6:90–93. [Google Scholar]
- 21.Sumarmo. Dengue haemorrhagic fever in Indonesia. Southeast Asian J. Trop. Med. Pub. Health. 1987;18:269–274. [PubMed] [Google Scholar]
- 22.Wang QY, Patel S.J, Vangrevelinghe E, Xu HY, Rao R, Jaber D, Schul W, Gu F, Heudi O, Ma NL, Poh MK, Phong WY, Keller TH, Jacoby E, Vasudevan SG. A Small-Molecule Dengue Virus Entry Inhibitory. Antimicrob. Agents Chemother. 2009;53:1823–1831. doi: 10.1128/AAC.01148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


