Abstract
Backgrounds:
Justicia gendarussa Burm.f. has an anti-HIV activity. This study was conducted to evaluate the effects of incubation periods on the cytotoxicity and virucidal activities of the J. gendarussa leaves extract on MOLT-4 cells.
Materials and Methods:
The cytotoxicity assay was evaluated by using the WST-1 test with incubation periods of 3 days and 5 days. The virucidal activity test was determined by measuring the inhibitory activities on the syncytium formation.
Results:
The cytotoxicity assay showed the value of CC50 on MOLT-4 cell culture with the test material of 70% ethanol extract of J. gendarussa leaves as much as 3928.620 µg /mL and 3176.581 µg /mL (incubation day 3 and day 5, respectively); fractionated-70% ethanol extract = 81782.428 µg /mL and 12175.870 µg/mL; and water extract = 16372.689 µg/mL and 2946.117 µg/mL. The test results of the virucidal activities (inhibit ≥ 90% the formation of syncytium) of 70% ethanol extract of J. gendarussa leaves is at a concentration 250 µg/mL, 500 µg/mL and 1000 µg/mL (3-day incubation) and 250 µg/mL (5-day incubation); and fractionated-70% ethanol extract at a concentration 250 µg /mL, 500 µg/mL and 1000 µg/mL (3-day incubation) and 1000 µg/mL (5-day incubation).
Conclusion:
70% ethanol extract, fractionated-70% ethanol extract, and water extract of J. gendarussa leaves were relatively nontoxic toward MOLT-4 cells, and fractionated-70% ethanol extract had better potentials in virucidal activities.
Keywords: Justicia gendarussa Burm.f, MOLT-4 cells, cytotoxicity, virucidal, incubation periods
Introduction
Justicia gendarussa Burm.f. (Acanthaceae) is often used as traditional medicine. Parts of the plant that are commonly used are the root and leaves. J. gendarussa leaves have several benefits, one of which is an antiviral. The results of in vitro tests, 70% ethanol extract, fractionated-70% ethanol extract, and water extract of J. gendarussa leaves showed activities inhibiting HIV’s reverse transcriptase enzyme (Woradulayapinij et al., 2005; Prajogo et al., 2016).
J. gendarussa leaves contain substituted aromatic amine (Chakravarty et al., 1982), flavonoid glycosides such as gendarusin A and B (Prajogo et al., 2014), and alkaloids of justidrusamides A, B, C and D (Kiren et al., 2014). The main component in the 70% ethanol extract is apigenin flavonoid glycoside called Gendarusin A (Prajogo et al., 2009). Flavonoid compounds act as a natural source of anti-HIV therapy for patients with AIDS by inhibiting HIV’s reverse transcriptase (Veljkovic et al., 2007; Ko et al., 2009). Flavonoid compounds in high concentrations can be cytotoxic which causes an increase in the mitochondrial permeability, release of cytochrome c, activation of caspase, increased levels of p53 and p21, bcl-2 pressing, apoptosis induction, and the death of necrosis cell (Bolton et al., 2000; Inayat-Hussain et al., 2001: Morin et al., 2001; Salvi et al., 2002; Shen et al., 2004; Lee et al., 2011). Alkaloid compounds are toxic to humans, but most of them have physiological activities that are prominent and can be widely used in the treatment (Harborne, 1987).
A cytotoxicity study of the J. gendarussa leaves extract was conducted to ensure the safety. Cytotoxicity of 70% ethanol extract and fractionated-70% ethanol extracton MOLT-4 cells with 3-day incubation using colorimetric methods with reagent WST-1 showed relatively non-toxic results with CC50> 41 µg/mL (Widiyanti et al., 2016).
Studies on antiviral activities of J. gendarussa leaves extract and water extract of J. gendarussa leaves have shown their potential as an anti-HIV. 70% ethanol extract of J. gendarussa leaves demonstrated anti-HIV activities by decreasing the amount of p24 antigen and holding back both syncytium formation and reverse transcriptase enzyme (Prajogo et al., 2016; Widiyanti et al., 2016). Water extract of J. gendarussa leaves also showed the inhibition ratio of HIV-1 reverse transcriptase of more than 90%, where the inhibition rate was higher than the ethanol extract (Woradulayapinij et al., 2005).
Virucidal activity test needs to be done to verify the ability of the J. gendarussa leaves extract in terminating the HIV-1 virus. This test is to determine the ability of the J. gendarussa leaves extract in disabling HIV particles. Test results on Justicia reptans extracts showed virucidal activities on HIV, which were related to its major components, flavonoid glycosides (Bedoya et al., 2008). Some plant substances such as tannins and phenolic are known to have virucidal effects. These substances interact with a protein of viral particles to reduce and prevent adsorption of the virus to the host cell (Singh et al., 2005).
In this research cytotoxicity assay and virucidal activity assay were conducted with varying incubation periods to determine the effect of incubation period on the cytotoxicity’s potential and virucidal activities of the J. gendarussa leaves extract on MOLT-4 cells. As a result, information regarding the safety and efficacy of J. gendarussa leaves extract in its development as an anti-HIV was obtained.
Materials and Methods
Plant
J. gendarussa leaves were obtained from plants cultivated in the area of Pacet, Mojokerto, East Java, Indonesia. This plant was identified by the Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, Airlangga University (voucher no. 18/H3.1.5/DT/2012).
Extraction Procedure
J. gendarussa leaves powder was divided into two groups. The first group was leaves powder acidified to release the alkaloids, and the second one group was the leaves powder that was not acidified. Then both were extracted using 70% ethanol for 3x24 hours in a macerator device, and then the filtrate from the extraction was concentrated using a rotary evaporator. The extract was dried at a temperature of 50°C to obtain a 70% ethanol extract (17.4% w/w) and fractionated-70% ethanol extract (6.4% w/w) of J. gendarussa leaves.
Water extract (1.8% w/w) was obtained by blending fresh J. gendarussa leaves into cold water, and then the filtrate was collected and dried by using the freeze dry method.
Alkaloid and Flavonoid Screening of J. gendarussa Leaves Extract
Alkaloids on 70% ethanol extract, fractionated-70% ethanol extract and water extract of J. gendarussa leaves were screened by using a GF254 TLC plate with chloroform mobile phase: methanol (9:1), with staining using Dragendorff reagent. The alkaloid test results will be positive if there are orange stains.
Flavonoids on 70% ethanol extract, fractionated-70% ethanol extract and water extract of J. gendarussa leaves were screened by using GF254 TLC stationary phase with a mobile phase of butanol-glacial acetic acid-water (4: 1: 5), with staining using citric borate. Flavonoid test results will be positive if there are yellow-green fluorescent stains using UV 366 nm (Indonesia, 2008).
Materials
70% Ethanol pharmaceutical grade, Methanol pro HPLC (Merck), sterile water for injection, distilled water (pure water) obtained from Lab CRC-EIRD, ITD Surabaya, RPMI-1640 medium (Gibco), sodium bicarbonate (Merck), Fetal Bovine Serum (FBS) (Gibco) inactivated at a temperature of 56ºC for 30 minutes, reagent 4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene Disulfonate (WST-1) (Roche), dimethyl sulfoxide (DMSO) (Sigma), 0.2 μm nitrocellulose membrane filter (Whatman), and Zidovudine+Lamivudine / ZDV+3TC (Duviral®).
Cells and Viruses
Cell line MOLT-4 clone 8 (Human T lymphocyte cancer cell line) and HIV isolates from a seropositive HIV donor that labeled IDU-18 were obtained from the Institute of Tropical Disease (ITD) laboratory, Airlangga University, Surabaya, Indonesia. MOLT-4 cells were cultured in RPMI-1640 media and equipped with 10% FBS. Cells were maintained in CCF T25 at a temperature of 37°C in 5% CO2 incubator.
Cytotoxicity Assay
Cytotoxicity from the extract on MOLT-4 cells was tested by a colorimetric method using a WST-1 reagent (Kangro and Mahy, 1996). In short, 50 µL of MOLT-4 cells at a concentration of 2 x 105 cells/well on a plate in each well in a 96-well microplate. Then, 50 µL of each test solution with varying concentrations (31.25; 62.5; 125; 250; 500; and 1000 µg/mL) was added to each well and incubated for 3 days and 5 days at 37°C in 5% CO2 incubator. ZDV+3TC was used as a positive control. Control cells without treatment and control of the media were also tested. The total volume per well was 100 µL. After incubation, 10 µL WST-1reagent was added into each of the wells and incubated for 2 hours at 37°C in 5% CO2 incubator. Absorbance was measured at a wavelength of 450 nm by using a microplate absorbance reader (Bio-Rad). The high absorbance read indicated the great amount of formazan formed, illustrating that the living cells are highly capable of metabolizing WST-1salt. The percentage of cell viability was determined by the following formula:
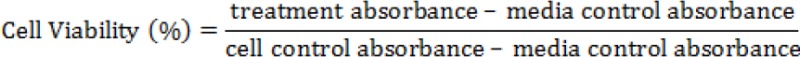
Virucidal Test
Virucidal activity testing on the J. Gendarussa leaves extract was determined by measuring the inhibitory activity against the formation of the syncytium (Kangro and Mahy, 1996; Tello et al., 2012). In short, 96-well microplates were filled with 100 µL growth culture medium into each well (except for row A). 200 µL of test solution with a concentration of 1000 µg/mL was added into row A (except the negative control column). Each test treatment group and negative control (without test solution) was done by applying two replications. Dilution was conducted to obtain various concentrations. Then, 50 µL of HIV with a concentration 2x104 cells/mL was added to each well containing the test solution. Then, the microplates were put in a 5% CO2 incubator at a temperature of 37ºC and incubated for 30 minutes. After the incubation, 96-well microplates were removed from the incubator, and 50 µL of cells MOLT-4 with a concentration 4x105 cells/mL was added to each well. Cytopathic effect was observed on days 3 and 5. The cytopathic effect, the formation of the syncytium, the virus with and without the J. gendarussa leaves extract were compared. The inhibition percentage of syncytium formation was calculated by using the following formula:
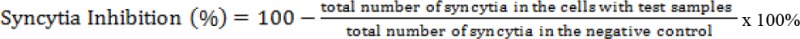
Statistical Analysis
Data analyzed by using probit regression analysis used Minitab 17 program to calculate the value of 50% cytotoxicity concentration (CC50), namely the concentration that could lower 50% of cell viability.
Results
Results of screening by TLC (Table 1) alkaloid showed orange stains in 70% ethanol extract and water extract of J. gendarussa leaves, indicating that they contained alkaloids. At the flavonoid screening results, greenish yellow fluorescence stains were found in 70% ethanol extract, fractionated-70% ethanol extract and water extract of J. gendarussa leaves, indicating that they contained flavonoids.
Table 1.
Alkaloid and flavonoid screening of J. gendarussa leaves extract
| Extract | Alkaloid | Flavonoid |
|---|---|---|
| 70% ethanol extract | + | + |
| Fractionated-70% ethanol extract | - | + |
| Water extract | + | + |
(+) Detected; (-) Not Detected
Cytotoxicity test results showed that the J. gendarussa leaves extract had low cytotoxicity in the MOLT-4 cells both after 3-day and 5-day incubations. The increase of concentrations of each ethanol extract of J. gendarussa leaves could lower the percentage of MOLT-4 cell viability (Table 2).
Table 2.
Results of cytotoxicity assay in MOLT-4 cell culture after 3 and 5day incubations
| Concentration (μg/mL) | Cell Viability (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| EE | FEE | WE | ZDV+3TC | |||||
| 3 days | 5 days | 3 days | 5 days | 3 days | 5 days | 3 days | 5 days | |
| 1000 | 59.680 | 51.597 | 79.135 | 64.742 | 67.293 | 67.293 | 91.259 | 91.259 |
| 500 | 59.962 | 55.528 | 81.579 | 67.813 | 70.771 | 70.771 | 91.447 | 91.447 |
| 250 | 73.120 | 63.514 | 84.586 | 68.305 | 78.477 | 78.477 | 91.823 | 91.823 |
| 125 | 74.342 | 66.339 | 86.936 | 69.656 | 79.793 | 79.793 | 94.549 | 94.549 |
| 62.5 | 76.410 | 70.147 | 89.944 | 74.447 | 84.586 | 84.586 | 95.019 | 95.019 |
| 31.25 | 78.853 | 76.781 | 90.320 | 76.781 | 88.346 | 88.346 | 97.086 | 97.086 |
EE: 70% ethanol extract of J. gendarussa leaves; FEE: fractionated-70% ethanol extract of J. gendarussa leaves; WE: Water extract of J. gendarussa leaves; ZDV/3TC: Zidovudine-Lamivudine
The percentage of cell viability was then used to determine the values of CC50 of each test material. CC50 values were calculated by probit regression analysis using Minitab 17 software. The CC50 value was presented in Table 3.
Table 3.
CC50 in MOLT-4 cell culture after 3 and 5day incubations
| Extract | CC50(μg/mL) | |
|---|---|---|
| 3day incubation | 5day incubation | |
| 70% ethanol extract | 3928.620 | 3176.581 |
| Fractionated-70% ethanol extract | 81782.428 | 12175.870 |
| Water extract | 16372.689 | 2946.117 |
| ZDV+3TC | 1154489.530 | 462711.187 |
The test results of virucidal activities (Table 4) were calculatedby the inhibition percentage of syncytium formation performed at various concentrations. Table 4 shows that the increase of the concentration of J. gendarussa leaves extract was able to increasec the inhibition percentage of syncytium formation in MOLT-4 cells infected with HIV.
Table 4.
Test results of inhibition of syncytium formation in MOLT-4 cell culture after 3 and 5 day incubations
| Concentration (μg/mL) | Syncytia Inhibition (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| EE | FEE | WE | ZDV+3TC | |||||
| 3 days | 5 days | 3 days | 5 days | 3 days | 5 days | 3 days | 5 days | |
| 1000 | 100 | cd | 96.534 | 91.497 | 68.803 | cd | 97.479 | 94.485 |
| 500 | 100 | cd | 96.218 | 88.510 | 48.004 | 56.798 | 96.218 | 91.497 |
| 250 | 95.903 | 91.727 | 94.958 | 85.063 | 15.231 | 22.788 | 95.903 | 81.386 |
| 125 | 13.341 | 31.750 | 49.895 | 52.202 | 9.559 | 22.328 | 92.437 | 78.169 |
| 62.5 | 7.983 | 30.142 | 44.223 | 47.376 | 10.820 | 11.528 | 91.807 | 80.927 |
| 31.25 | 10.820 | 2.566 | 39.181 | 38.874 | 4.517 | 3.485 | 94.643 | 60.245 |
EE: 70% ethanol extract of J. gendarussa leaves; FEE: fractionated-70% ethanol extract of J. gendarussa leaves; WE: Water extract of J. gendarussa leaves; ZDV/3TC: Zidovudine-Lamivudine; cd: cell death
Discussion
Cytotoxicity assay with the variations of incubation periods was performed to determine the effect of incubation periods on the cytotoxicity potentials of J. gendarussa leaves extract on MOLT-4 cells. Cytotoxicity assay is the colorimetric method using a tetrazolium salt reagent 4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene Disulfonate or better known as the WST-1 assay. The test is based on the reduction of the WST-1 by living cells that produce soluble formazan salt (Rode, 2008).The great amount of formazan formed reflects the high cell viability, namely the number of active living cells metabolizing WST-1 (Rampersad, 2012).
The observation of cytotoxic activities was done by calculating the percentage of MOLT-4 cell viability, and then a regression equation between the percentage of cell viability with log concentration was made. The number of cytotoxicity activities of the extract was determined by the CC50 values in which the concentration of the extract was able to lower 50% of cell viability.
The extract cytotoxicity level was evaluated based on the CC50 values. CC50 values below 20 µg/mL would mean cytotoxic; CC50 values ranging between 21-40 µg/mL would mean low cytotoxic; CC50 values of more than 41 µg/mL would mean non-cytotoxic (Mohamed et al., 2000; Rohaya et al., 2005; Ayob et al., 2013). In this study, observation cytotoxic activity was observed on different days, namely on day 3 and 5, because previous studies have reported that there was a significant difference from the values of CC50when calculated on different days (Kimura et al., 1999).
A calculation used the probit regression analysis showed that the values of CC50 in MOLT-4 cell culture by incubation period of 3 and 5 days with the test materials 70% ethanol extract, fractionated-70% ethanol extract, and water extract of J. gendarussa leaves demonstrated that all J. gendarussa leaves extract showed non-cytotoxic activities in MOLT-4 cells because of the values of CC50> 41 µg/mL (Table 3). Previous research on the cytotoxic activities of 70% ethanol extract and fractionated-70% ethanol extract of J. gendarussa leaves on MOLT-4 cells by an incubation period of 3 days using the WST-1 colorimetric method stated that CC50 values obtained from the two extracts also indicated non-toxicity (Widiyanti et al., 2016).
The differences CC50 values between incubation 3 and 5 days were probably due to the compounds of both extracts. The content of alkaloids probably gave a major influence on the cell death when compared to the content of flavonoids in the extracts (Astuti et al., 2006). CC50 values of 70% ethanol extract and water extract were lower than fractionated-70% ethanol extract. This is because the 70% ethanol extract and water extract did not release the alkaloids, which means that the extracts contained a high level of alkaloids (Table 1). Therefore, it gave an effect on MOLT-4 cell death.
The viability of cells in MOLT-4 cell culture showed a decline along with the increase in the concentration of 70% ethanol extract, fractionated-70% ethanol extract, and water extract of J. gendarussa leaves. A decrease in cell viability means that there was an inhibition of cell growth associated with suppression of cell proliferation activities so that the number of dividing cells or the living ones declined. Various activities of cell signaling that involved protein expression on programmed cell death such as bid, bax and bcl-2 were likely to be activated when the cell line was exposed to the active compound contained in the extracts (Singh, 2014). An assay using tetrazolium could only detect the formation of formazan (related to the activity of mitochondria of living cells) but not determine the cause of cell death (Paul and Manjula, 2014).
In this study, virucidal activities were also tested with the inhibition parameters of syncytium formation (Table 4). The test materials are considered to have virucidal activities if they inhibit the formation of syncytium ≥90% (Tello et al., 2012). Based on the calculation of the inhibition percentage of syncytium formation in MOLT-4 cultured cells on day 3, test materials that inhibited the formation of syncytium were ≥90% in 70% ethanol extract of J. gendarussa leaves at a concentration of 250 µg/mL, 500 µg/mL, and 1000 µg/mL; fractionated-70% ethanol extract of J. gendarussa leaves at a concentration of 250 µg/mL, 500 µg/mL and 1000 µg/mL; and ZDV+3TC at a concentration of 31.25 µg / mL, 62.5 µg /mL, 125 µg/mL, 250 µg/mL, 500 µg/mL, and 1000 µg/mL. Meanwhile, from the inhibition percentage of syncytium in MOLT-4 cell culture onday 5, it was found that test materials inhibiting the formation of syncytium were ≥90% in 70% ethanol extract of J. gendarussa leaves at a concentration of 250 µg/mL; fractionated-70% ethanol extract of J. gendarussa leaves at a concentration of 1000 µg /mL; and ZDV+3TC at a concentration of 500 µg /mL and 1000 µg /mL.
Virucidal activity test results showed that the fractionated-70% ethanol extract had better potentials in virucidal activities than other extracts. In fact, 70% ethanol extract of J. gendarussa leaves also have the potentials, but it caused death in MOLT-4 cells at the concentration of 500 µg/mL and 1000 µg /mL on day 5 of incubation. Cell death also occurred in the water extract of J. gendarussa leaves at a concentration of 1000 µg/mL. This is likely due to the alkaloid content in 70% ethanol extract and water extract of J. gendarussa leaves (Table 1).
Differences in virucidal activities from each test material were because each of the test materials has different chemical contents that could inhibit the development phase of the virus differently, such as the stage of viral entry until the stage of replication (Leteane et al., 2012). In J. gendarussa leaves extract, without alkaloids, the extracts were capable of providing anti-HIV activities better and lowered the cellular toxicity, compared to the extracts containing alkaloids.
Conclusion
The results obtained showed that 70% ethanol extract, fractionated-70% ethanol extract and water extract of J. gendarussa leaves were relatively nontoxic toward MOLT-4 cells after 3 and 5 days incubation. The results of the virucidal activity test showed that fractionated-70% ethanol extract had better potentials in virucidal activities than other extracts.
Acknowledgement:
The authors would like to thank the Collaborative Research Center for Emerging and Reemerging Infectious Disease (CRC-ERID), Institute of Tropical Disease (ITD), Airlangga University for supporting Biosafety Level-3 facility.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Astuti E, Pranowo D, Puspitasari S. D. Cytotoxicity of Phaleria macrocarpa (Scheff.) Boerl. Fruit Meat And Seed Ethanol Extract to Mononuclear Perifer Normal Cell Of Human Body. Indonesian Journal of Chemistry. 2006;6(2):212–218. [Google Scholar]
- 2.Ayob Z, Samad AA, Bohari SPM. Cytotoxicity Activities in Local Justicia gendarussa Crude Extracts against Human Cell Lines. Jurnal Teknologi (Sciences and Engineering) 2013;64(2):45–52. [Google Scholar]
- 3.Bedoya L.M, Alvarez A, Bermejo M, Gonza´lez N, Beltra´n M, Sa´nchez-Palomino S, Cruz S.M, Gaita´n I, Olmo E, Escarcena R, Garcı´a P.A, Ca´ceres A, Feliciano A.S, Alcamı J. Guatemalan plants extracts as virucides against HIV-1 infection.Phytomedicine. Elsevier. 2008;15(6):520–524. doi: 10.1016/j.phymed.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of Quinones in Toxicology. Chemical research in toxicology. American Chemical Society (ACS) Publications. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarty AK, Dastidar P. P. G, Pakrashi S. C. Simple Aromatic Amines from Justicia gendarussa. 13C NMR Spectra of the Bases and Their Analogues. 12. Vol. 38. Tetrahedron: Elsevier; 1982. pp. 1797–1802. [Google Scholar]
- 6.Harborne J. B. Metode Fitokimia Penuntun Cara Modern Menganalisis Tumbuhan, Terbitan Kedua (in Indonesian) Institut Teknologi Bandung. 1987 [Google Scholar]
- 7.Inayat-Hussain SH, Winski SL, Ross D. Different Involvement of Caspase in Hydroquinone-induced Apoptosis in Human Leucemic HL-60 and Jurcat Cells. Toxicology and applied pharmacology. Elsevier. 2001;175(2):95–103. doi: 10.1006/taap.2001.9221. [DOI] [PubMed] [Google Scholar]
- 8.Indonesia D. K. R. Farmakope Herbal Indonesia, Edisi I (in Indonesian) Jakarta: Departemen Kesehatan RI; 2008. pp. 78–80. [Google Scholar]
- 9.Kangro H. O, Mahy B. W. J. Virology Methods Manual. Academic Press; 1996. pp. 293–308. [Google Scholar]
- 10.Kimura E, Koike T, Inouye Y. Macrocyclic Polyamines and Their Metal Complexes: A Novel Type of Anti-HIV Agent. Perspective on Bioinorganic Chemistry. 1999;4:145–164. [Google Scholar]
- 11.Kiren Y, Deguchi J, Hirasawa Y, Morita H, Prajogo B. Justidrusamides A-D, New 2-aminobenzyl Alcohol Derivatives from Justicia gendarussa. Journal of Natural Medicines. Springer. 2014;68(4):754–758. doi: 10.1007/s11418-014-0862-8. [DOI] [PubMed] [Google Scholar]
- 12.Ko Y-J, Oh H-J, Ahn H-M, Kang H-J, Kim J-H, Ko YH. Flavonoids as Potential Inhibitors of Retroviral Enzymes. Journal of the Korean Society for Applied Biological Chemistry. Springer. 2009;52(4):321–326. [Google Scholar]
- 13.Lee MH, Dan DW, Hyon SH, Park JC. Apoptosis of Human Fibrosarcoma HT-1080 Cell by Epigallocathecin-3-O-gallate via induction of p53 and Caspase as well as Suppression of Bcl-2 and Phosphorylated Nuclear Factor-κB. Apoptosis. Springer. 2011;16(1):75–85. doi: 10.1007/s10495-010-0548-y. [DOI] [PubMed] [Google Scholar]
- 14.Leteane MM, Ngwenya BN, Muzila M, Namushe A, Mwinga J, Musonda R, Moyo S, Mengestu YB, Abegaz BM, Andrae-Marobela K. Old Plants Newly Discovered Cassia sieberiana D.C. and Cassia abbreviate Oliv. Root Extract Inhibit in Vitro HIV-1c Replication in Peripheral Blood Mononuclear Cells (PBMCs) by Different Modes of Actions. Journal of Ethnopharmacology. Elsevier. 2012;141(1):48–56. doi: 10.1016/j.jep.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed SM, Ali AM, Rahmani M, Dhaliwal JS, Yusoff K. Journal Biochemistry Molecular Biology and Biopsychology. 4. Vol. 4. Harwood Academic Publishers; 2000. Apoptotic and Neurotic Cell death Manifestations in Leukemic Cell treated with Methylgerambulin a Sulphone from Glycosmis calcicola; pp. 253–26. [Google Scholar]
- 16.Morin D, Barthelemy S, Zini R, Labidalle S, Tillement JP. Curcumin Induces the Mitochondrial Permeability Transition Pore by Membrane Protein Thiol Oxidation. Federation of European Biochemical Societies (FEBS) letters.Wiley Online Library. 2001;495(1-2):131–136. doi: 10.1016/s0014-5793(01)02376-6. [DOI] [PubMed] [Google Scholar]
- 17.Paul A, Manjula Cytotoxic and Antiproliferative Activity of Indian Medicinal Plant in Cancer Cell. International Journal of Science and Research. 2014;3(6):88–93. [Google Scholar]
- 18.Prajogo B. Autentik Tanaman Justicia gendarussa Burm.f. Sebagai Bahan Baku Obat Kontrasepsi Pria (in Indonesian) Surabaya: Airlangga University Press; 2014. [Google Scholar]
- 19.Prajogo B, Guliet D, Queiroz F, Wolfernder J-L, Cholies N, Aucky H, Hostettmann K. Isolation of Male Antifertility Compound in n-Butanol Fraction of Justicia gendarussa Burm.f. Leaves. Folia Medica Indonesiana. 2009;45(1):28–31. [Google Scholar]
- 20.Prajogo B, Widiyanti P, Riza H. Effect Of Free Alkaloid And Non-Free Alkaloid Ethanol 70% Extract of Justicia gendarussa Burm.f. Leaves Against Reverse Transcriptase HIV Enzyme In Vitro And Chemical Compound Analysis. Indonesian Journal of Tropical and Infectious Disease. 2016;6(1):1–4. [Google Scholar]
- 21.Rampersad S. N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. Molecular Diversity Preservation International. 2012;12(9):12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rode H. J. Apoptosis, Cytotoxicity and Cell Proliferation. Roche Diagnostics GmbH. 2008;4:116–135. [Google Scholar]
- 23.Rohaya Abdul, Manaf Daud, Nor Hadiani, Khozirah, Nordin Antioxidant, Radical-Scavenging, Anti-inflammatory, Cytotoxic and Antibacterial Activities of Methanolic Extracts of Some Hedyotis Species. Life Sciences. Elsevier. 2005;76(17):1953–1964. doi: 10.1016/j.lfs.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 24.Salvi M, Brunati AM, Clari G, Toninello A. Interaction of Genistein with the Mitochondrial Electron Transport Chain Results in the Opening of the Membrane Transition Pore. Biochimica et Biophysica Acta (BBA)-Bioenergetics. Elsevier. 2002;1556:187–196. doi: 10.1016/s0005-2728(02)00361-4. [DOI] [PubMed] [Google Scholar]
- 25.Shen SC, Ko CH, Tseng SW, Tsai SH, Chen YC. Structurally Related Antitumor Effects of Flavanones in vitro and in vivo Involvement of Caspase 3 Activation, p21 Gene Expression, and Reactive Oxygen Species Production. Toxicology and Applied Pharmacology. Elsevier. 2004;197(2):84–95. doi: 10.1016/j.taap.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Singh IP, Bharate SB, Bhutani KK. Anti-HIV Natural Products. Current Science. Journal Storage (JSTOR) 2005;89(2):269–290. [Google Scholar]
- 27.Singh R. Interaction and Cytotoxicity of Compounds with Human Cell Lines. Romanian Journal of Biochemistry. 2014;51(1):57–74. [Google Scholar]
- 28.Veljkovic V, Mouscadet J-F, Veljkovic N, Glisic S, Debyser Z. Simple Criterion for Selection of Flavonoid Compounds With Anti-HIV Activity. Bioorganic and Medicinal Chemistry Letters. Elsevier. 2007;17(5):1226–1232. doi: 10.1016/j.bmcl.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 29.Widiyanti P, Prajogo B, Hikmawati N. P. E. Cytotoxicity of Justicia gendarussa Burm.f. Leaf Extracts on MOLT-4 Cell. Indonesian Journal of Tropical and Infectious Disease. 2016;6(1):24–28. [Google Scholar]


