Abstract
Triterpenoid saponins, which are present in leguminous plants and some marine animals, possess a broad range of biological actions. We have earlier reported the extraction of avicins, a family of triterpenoid saponins obtained from the Australian desert tree Acacia victoriae (Leguminosae: Mimosoideae) that inhibit tumor cell growth and induce apoptosis, in part, by perturbing mitochondrial function. These saponins have also been found to prevent chemical-induced carcinogenesis in mice. This study examines the effect of a triterpene mixture (F094) and a single molecular species (avicin G) isolated from the mixture on tumor necrosis factor (TNF)-induced activation of nuclear transcription factor-κB (NF-κB) in Jurkat cells (human T cell leukemia). Both F094 and avicin G were found to be potent inhibitors of TNF-induced NF-κB. Treatment of Jurkat cells with avicin G resulted in a much slower accumulation of the p65 subunit of NF-κB into the nucleus whereas the degradation of IκBα was unaffected. Avicin G also impaired the binding of NF-κB to DNA in in vitro binding assays. Treatment of cells with DTT totally reversed the avicin G-induced inhibition of NF-κB activity, suggesting that sulfhydryl groups critical for NF-κB activation were being affected. Avicin G treatment resulted in decreased expression of NF-κB-regulated proteins such as inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX-2). Thus, the avicins may prove important for reducing both oxidative and nitrosative cellular stress and thereby suppressing the development of malignancies and related diseases.
Nuclear transcription factor-κB (NF-κB), a ubiquitous transcription factor evolutionarily conserved from flies to mammals (1), is one of the central regulators of an organism's response to various stress signals (2). NF-κB regulates the transcription of a number of genes involved in immune and inflammatory pathways such as various proinflammatory cytokines, adhesion molecules, and apoptosis (3, 4). Dysregulation of NF-κB contributes to a variety of pathological conditions such as septic shock, acute inflammation, viral replication, and some malignancies (4, 5). The most abundant and active forms of NF-κB are dimeric complexes of p50/RelA (p50/p65). In unstimulated cells, these factors are held in the cytoplasm in a complex with inhibitory proteins (IκBs) that mask its nuclear localization signal. In response to an extracellular signal (e.g., inflammatory cytokines, mitogens, bacterial products, or oxidative stress), IκBα undergoes phosphorylation at specific serine residues, which then signals its ubiquitination and degradation by the proteosome pathway. Degradation of IκBα allows an inhibitor-free NF-κB complex to translocate into the nucleus, bind to DNA, and activate the transcription of specific genes. Because of NF-κB's role in inflammation, carcinogenesis, and other immunological disorders, it follows that down-modulators of NF-κB could have important therapeutic implications.
We have recently reported the extraction, and characterization of a mixture of triterpenoid saponins (designated fraction 35; F035) from above-ground plant parts of Acacia victoriae (6). We have also extracted a seedpod sample of A. victoriae to obtain a comparable triterpenoid saponin mixture (F094). Both F035 and F094 are comprised of multiple molecular species of triterpene glycosides. Pure triterpenoid glycoside species designated avicins D and G were then fractionated from F094 (7). All data comparing F035 and F094 with avicins D and G demonstrate consistently that they share properties of inhibiting the growth of cancer cells in vitro.
Extracted triterpene compositions and avicins purified from them inhibit cell proliferation, block phosphatidylinositol-3-kinase activity (6), and induce apoptosis by directly perturbing the mitochondria in a Jurkat cell line (human T cell leukemia; ref. 7). An in vivo study demonstrates that F035 effectively inhibited the chemical-induced initiation and promotion of skin tumors in mice by decreasing DNA damage caused by free radicals (8).
Based on the findings that avicins could be potentially antioxidant as well as antiinflammatory, the present study was designed to evaluate the possible inhibitory effects of triterpenoid saponin mixtures and a purified avicin on the activation of NF-κB as well as downstream mediators of inflammation such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). The inhibition of NF-κB activation and the decrease in the levels of iNOS and COX-2 after treatment with avicins provide insight into a probable mechanism underlying the antiinflammatory and therapeutic properties of avicins.
Methods
Cell Culture.
Jurkat cell line (human T cell leukemia) and RAW 264.7 cells (human macrophage cell line) were grown in RPMI-1640 medium supplemented with 10% FBS, 200 mM glutamine, and 0.05% gentamicin.
Treatment of Cells with Avicins.
Jurkat cells (1 × 106/ml) in complete medium were treated with 2 μg/ml of F094 or avicin G for 8–16 h at 37°C. At the end of the treatment, cells were washed in complete medium and counted. An equal number of viable cells was used for different experiments.
Electrophoretic Mobility Shift Assays (EMSAs).
Jurkat cells (2 × 106/ml) treated with F094 or avicins were exposed to TNF (1 nM for 15 min) at 37°C. Four micrograms of nuclear extracts that were prepared as described previously (9) were incubated with 16 fmol of 32P-end-labeled NF-κB binding probe. The DNA–protein complex was separated from the free oligonucleotide on a 7.5% polyacrylamide gel. The specificity of NF-κB DNA binding was examined by competition with a double-stranded mutated oligonucleotide, unlabeled oligonucleotide, and by supershift of the band by anti-p65 antibody. The radioactive bands from dried gels were visualized on a PhosphorImager (Molecular Dynamics) and quantitated by using imagequant software.
Western Blot Analysis.
Degradation of IκB and nuclear translocation of the p65 subunit of NF-κB were studied by Western blot analysis of cytoplasmic and nuclear extracts of avicin G-treated Jurkat cells. Rabbit anti-IκB or rabbit anti-p65 antibody (Santa Cruz Biotechnology) followed with anti-rabbit antibody conjugated to horseradish peroxidase were used for immunoblotting. Protein bands were detected by chemiluminescence (ECL, Amersham Pharmacia).
Transfection and Assay of Luciferase Activity.
Jurkat cells were transfected with pGL3-NF-κB by electroporation. The cells were then treated with avicin G (1 μg/ml) for 16 h. NF-κB was activated by using 100 ng/ml of lipopolysaccharide (LPS), 5 ng/ml of phorbol 12-myristate 13-acetate (PMA), or 1 nM of tumor necrosis factor (TNF). LPS, PMA, and TNF were obtained from Sigma. Luciferase activity was measured by using the luciferase assay kit (Promega) according to the manufacturer's instructions.
Induction and Measurement of iNOS and COX-2.
RAW 264.7 cells (0.5 × 106/ml) were plated in 100-mm dishes and treated with F094 or avicins (2 μg/ml) for 16 h. Next, the cells were exposed to 100 ng/ml of LPS for 24 h. Cells were lysed in a buffer containing 50 mM Tris (pH 7.4), 100 mM NaCl, 0.5% Nonidet P-40, 10 μg/ml leupeptin, 5 μg/ml aprotinin, and 100 μM PMSF. Cellular proteins (70 μg) were resolved on a 7.5% SDS-polyacrylamide gel. Levels of iNOS and COX-2 were analyzed by Western blot analysis by using rabbit anti-iNOS (Santa Cruz Biotechnology) and goat anti-COX-2 antibodies, respectively.
Results
Mixture of Triterpenoid Saponins F035 and Avicin G Inhibit TNF-Induced NF-κB in a Time- and Dose-Dependent Manner.
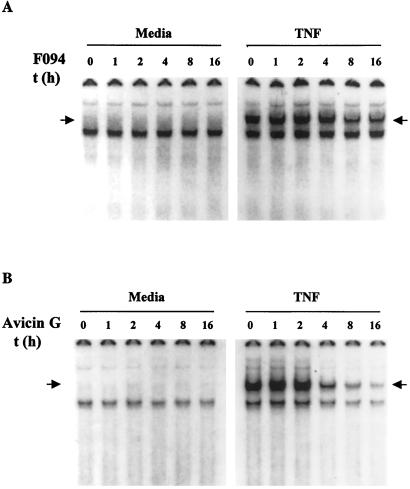
Addition of TNF to Jurkat cells activates NF-κB in electrophoretic mobility shift assays. Cells treated with F094 (Fig. 1A) or avicin G (Fig. 1B) for different time periods did not activate NF-κB in the absence of TNF (Left, Fig. 1 A and B). However, we demonstrated a time-dependent effect of F094 and avicin G on TNF-induced NF-κB (Right, Fig. 1 A and B). With avicin G, activation of NF-κB was inhibited within 4 h of treatment. Our earlier studies have shown that the various processes leading to apoptosis induced by the mixture of triterpenoid saponins or avicins were initiated as early as 30 min to 2 h posttreatment. To ensure that apoptosis of cells was not responsible for the decrease in the observed NF-κB activation, cells were counted at the end of treatment, and an equal number of viable cells was studied for TNF-induced NF-κB activation. Also, treatment of cells with zVAD-fmk (z-Val-Ala-Asp-CH2F), a broad cell-permeable irreversible inhibitor of caspases, did not affect the inhibition of activated NF-κB levels, suggesting that this inhibition was independent of caspase activity (data not shown).
Figure 1.
Effect of F094 and avicin G on TNF-induced NF-κB activation. Jurkat cells (1 × 106/ml) were treated with 2 μg/ml of F094 (A) or 2 μg/ml of avicin G (B) for 1–16 h at 37°C. At the end of the treatment, cells were washed, resuspended at 2 × 106/ml in complete medium, and treated with 1 nM of TNF for 15 min at 37°C. Nuclear extracts were prepared and assayed for activation of NF-κB as described in Methods. The arrow indicates the gel location of NF-κB bound to DNA.
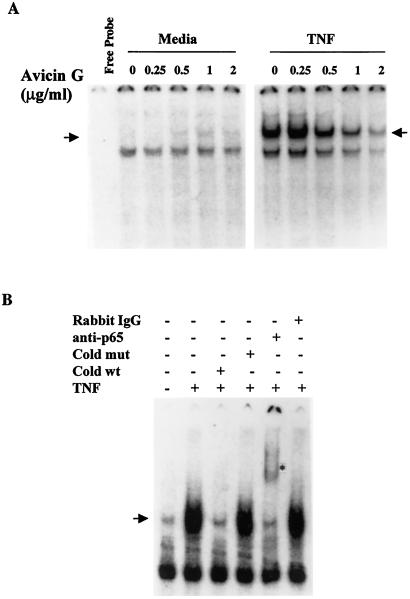
We next investigated the dose-response of avicin G. In the absence of TNF, avicin G alone was unable to activate any NF-κB (Fig. 2A Left). Jurkat cells treated with 0.5 μg/ml of avicin G for 16 h showed almost a 50% decrease in TNF-induced NF-κB, and treatment with 2 μg/ml of avicin G resulted in almost complete inhibition (Fig. 2A Right). The NF-κB DNA complex in the treated cells could be competed out with an unlabeled oligonucleotide and was supershifted by anti-p65 antibody, both of which indicate the specificity of the NF-κB band (Fig. 2B).
Figure 2.
(A) Dose-response of inhibition of TNF-induced NF-κB activation by avicin G. Jurkat cells (1 × 106/ml) were treated with different concentrations of avicin G for 16 h at 37°C. At the end of the treatment, cells were washed, resuspended at 2 × 106/ml in complete medium, and treated with 1 nM of TNF for 15 min at 37°C. Nuclear extracts were prepared and assayed for activation of NF-κB as described in Methods. (B) Supershift and specificity analysis of NF-κB. Nuclear extracts from TNF-treated cells were incubated for 15 min with mutant NF-κB oligo, unlabeled NF-κB oligo, anti-p65 antibody, and preimmune rabbit serum. Activation of NF-κB was then assayed as described earlier. The arrow indicates the gel location of NF-κB bound to DNA, and the asterisk indicates the supershifted band.
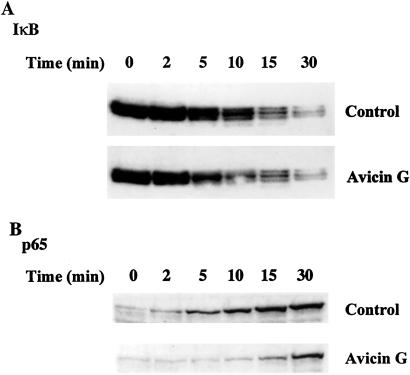
Avicin G Does Not Inhibit Degradation of IκB but Inhibits the Nuclear Accumulation of the p65 Subunit of NF-κB.
Activation of NF-κB involves two important steps: (i) the release of the inhibitory IκB subunit, and (ii) the nuclear accumulation of the activated NF-κB. To elucidate the effect of avicin G on either or both of these steps, control and avicin G-treated Jurkat cells were exposed to TNF for different time periods. The kinetics of IκB degradation were studied by Western blot analysis of the cytoplasmic extracts. As shown in Fig. 3A, we did not observe any difference in the pattern of IκB degradation after treatment with avicin G. Next, to study the accumulation of the activated NF-κB into the nucleus, we evaluated the appearance of the p65 subunit of NF-κB in the nuclear extracts of control and avicin G-treated cells. The results shown in Fig. 3B indicate that avicin G treatment results in delayed and reduced nuclear localization of the p65 subunit of NF-κB. Treatments up to 90 min with TNF did not increase the levels of p65 in the nucleus (data not shown).
Figure 3.
Effect of avicin G on TNF-induced degradation of IκB (A) and nuclear translocation of p65 (B). Control or avicin G- (2 mg/ml for 16 h) treated cells were washed and incubated with 1 nM of TNF for the listed time periods. The cytoplasmic and nuclear extracts of these cells were used to study the levels of IκB and p65, respectively, by Western blot analysis as described in Methods. A was probed with anti-IκB antibody and B was probed with an anti-p65 antibody.
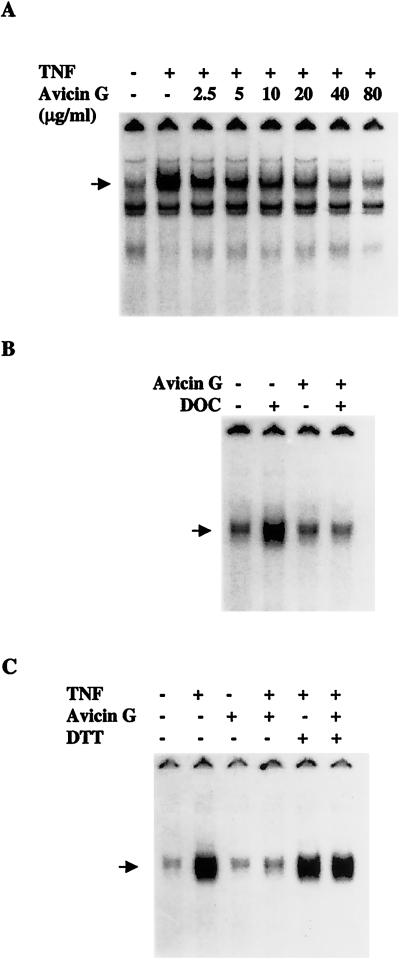
Avicin G Inhibits the Binding of NF-κB to DNA.
We next used a cell-free system to examine the effect of avicin G on the ability of active NF-κB to bind to a radioactively labeled oligonucleotide containing κB DNA elements. Nuclear extracts from TNF-stimulated cells were incubated with increasing concentrations of avicin G, inhibiting the binding of NF-κB to DNA in a dose-dependent manner (Fig. 4A). However, higher concentrations of avicin G were required in vitro as compared with whole cells for comparable inhibition of NF-κB binding to DNA. This result could suggest that either avicin G needs to be metabolized by cells to an active form, or its action requires a cellular cofactor. Deoxycholate (DOC) is known to dissociate NF-κB from IκB, thereby making it available in an active nuclear form. On treatment with DOC (0.8%), the cytoplasmic extracts of avicin G-treated cells showed decreased DNA binding as compared with the extracts of control cells (Fig. 4B). Taken together with the fact that IκBα degradation is not affected by avicin G (Fig. 3A), these results suggest that avicin G modifies the NF-κB in a way such that it no longer can bind to DNA.
Figure 4.
(A) Effect of in vitro addition of avicin G on NF-κB DNA binding. Extracts from TNF-stimulated Jurkat cells were treated with different concentrations of avicin G for 30 min at 37°C and analyzed for NF-κB binding by electrophoretic mobility shift assay (EMSA). (B) Effect of avicin G on deoxycholate (DOC)-induced activation of NF-κB. Cytoplasmic extracts from untreated cells were treated with DOC in the presence or absence of avicin G and then analyzed for NF-κB activation. (C) Effect of DTT on avicin G-induced inhibition of NF-κB activation. The arrow indicates the gel location of NF-κB bound to radioactively labeled DNA.
The x-ray structure of p65 has defined its DNA-binding domain and reveals the presence of cysteine residues. We therefore evaluated the potential role of sulfhydryl groups in the action of avicin G by treating Jurkat cells with 100 μM of DTT for 2 h before treating them with avicin G (2 μg/ml for 8 h). Cells were then exposed to TNF as described earlier. As shown in Fig. 4C, DTT did not modify the TNF-induced activation of NF-κB; instead, it reversed the action of avicin G. These results suggest that avicin G could be modifying NF-κB by alkylation of free sulfhydryls and this reaction is prevented by DTT.
Avicin G Inhibits NF-κB-Dependent Expression of Luciferase, iNOS, and COX-2.
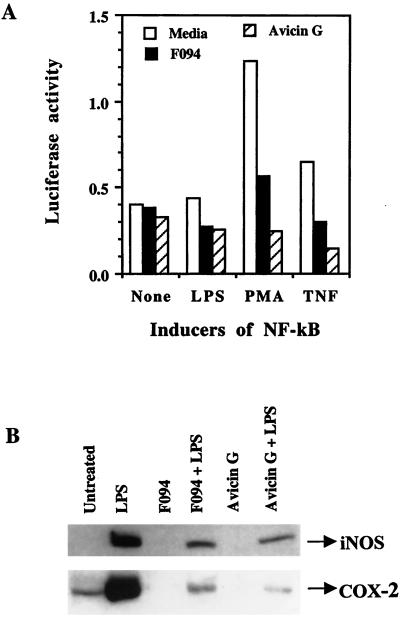
To determine the effect of avicin G on NF-κB-dependent gene expression, we used pGL3, a luciferase reporter gene with an NF-κB element from the IL-2 promoter linked to it. Jurkat cells transiently transfected with the pGL3-NF-κB were treated with LPS, PMA, or TNF to induce luciferase activity. As shown in Fig. 5A, pretreatment of the transfected cells with F094 or avicin G significantly inhibited the luciferase activity induced by the different agents.
Figure 5.
(A) Effect of avicin G on the activity of NF-κB-dependent luciferase gene expression. Jurkat cells were transfected with pGL3-NF-κB by electroporation. NF-κB was activated by using LPS (100 ng/ml), PMA (5 ng/ml), or TNF (1 nM). Luciferase activity was measured by using the luciferase assay kit (Promega) according to the manufacturer's instructions. (B) Effect of F094 and avicin G on LPS-induced expression of iNOS and COX-2. RAW 264.7 cells pretreated with F094 or avicin G were treated with LPS as described in Methods. Expression of iNOS and COX-2 was assayed in the cytoplasmic extracts of these cells by using Western blot analysis.
Next, we looked at the effect of F094 and avicin G on the induction of iNOS and COX-2, expressions that are regulated by NF-κB. Significant levels of iNOS and COX-2 were induced in RAW264.7 cells in response to LPS treatment. Pretreatment of cells with F094 or avicin G resulted in a dramatic decrease in the levels of LPS-induced iNOS and COX-2 (Fig. 5B).
Discussion
One of the major challenges of cancer prevention is the development of effective new drugs that have little or no effect on normal cells and tissues. This objective has led to a growing interest in the development of natural products for chemoprevention. One such family of natural compounds known for their medicinal value is the terpenoids (10, 11). They form the largest and most diverse class of organic compounds found in plants (12). They exhibit enormous chemical variety and complexity but have a common biosynthetic origin, the fusion of five-carbon units, each having an isoprenoid structure (13). Triterpenoids contain 30 carbon atoms and are formed in nature by the cyclization of squalene. We recently isolated mixtures of triterpenoid saponins (F035 and F094) from A. victoriae. Further purification of F094 resulted in two pure compounds called avicin D and avicin G, the structures and biological activities of which have been reported earlier (7). In another study, F035 was found to be very effective against the initiation and promotion of murine skin tumors in mice (8). Topical applications of F035 significantly inhibited epidermal hyperplasia in DMBA (7,12-dimethylbez[a]anthracene) and DMBA/TPA (TPA = phorbol 12-tetradecanoate 13-acetate) skin carcinogenesis models at 4 and 8 weeks of treatment (8). Application of F035 also reduced H-ras mutations at codon 61 and decreased skin 8-hydroxy-2′-deoxyguanosine (8-OH-dG) levels in the treated animals, suggesting a decrease in DNA damage caused by free radicals.
Carcinogenesis is a multistep process, with inflammation and oxidative stress forming integral components (14–17). A strong correlation has been shown between chronic inflammatory conditions and the onset of some cancers (17–19). Some examples of these related processes include chronic infections (schistosomiasis and bladder cancer, Helicobacter pylori and gastric cancer) or chronic tissue damage by chemical or physical agents (reflux esophagitis and esophageal cancer). Chronic inflammation and oxidative stress are related, perhaps inseparable, phenomena. Reactive oxygen species (ROS) have been thought to be genotoxic byproducts of inflammation (20) that may contribute to genetic instability and phenotypic changes leading to malignant transformation (21, 22). Also, increased levels of ROS maintain the inflammation, thereby enhancing the cellular assault leading to the progression of cancer. The NF-κB family of transcription factors, which play a fundamental role in the mediation of inflammation, have been found to be important regulators of cell proliferation and oncogenesis (23). Members of this family, such as v-rel, c-rel, p100, p105, Rel A, Bcl-3, and IκB, have all been associated with cell transformation as a result of overexpression, gene amplification, gene rearrangement, or translocation (24). NF-κB is known to induce gene products that suppress apoptotic cascades such as those induced by TNF (25) and expression of oncoproteins (26). NF-κB is also known to regulate expression of antiapoptotic members of the Bcl-2 family (27).
Based on the antioxidant and antiinflammatory properties of the avicins observed in a mouse skin model (8), we decided to evaluate their effects on the transcriptional regulatory activity of NF-κB in Jurkat cells. The wide-ranging effects of NF-κB are under the regulation of a complex network of inhibitors and coactivators. Activation of NF-κB by inflammatory cytokines, mitogens, bacterial products, or oxidative stress requires the degradation of Ικβ, which holds NF-κB in the cytoplasm in a dormant complexed state. After the degradation of IκB, NF-κB is released from the complex to translocate into the nucleus where it binds to κB DNA elements and activates different genes. The results shown in this manuscript indicate that avicins do not affect the degradation of IκB but do decrease the nuclear localization of the p65 subunit of NF-κB. Avicins also inhibit the binding of NF-κB to κB DNA elements in the nuclear extracts. This effect was observed when NF-κB was activated by either TNF or deoxycholate, and suggests that on avicin G treatment, NF-κB is subtly modified such that it is not caused to dissociate from the inhibitor complex, but once released by other factors it has modified DNA-binding affinity. Avicin-dependent inhibition of NF-κB binding to DNA is reversed by DTT, suggesting that avicins may modify a sulfhydryl group (28) critical for activation of NF-κB. The x-ray structure of p65 has shown the presence of cysteine residues, which are critical for optimal DNA–protein interactions (29, 30). Avicin G has α,β ester groups (two in the outer monoterpene chains and one at C28) that could react with nucleophiles, especially cysteine sulfhydryl groups by a Michael-type addition (31). Like the sesquiterpene lactone helanalin (32), avicins could be targeting the exposed thiol groups, such as the cysteine residues in the NF-κB molecule. This chemical property of avicins could also account in part for their chemopreventive effects (8), possibly by activating the transcription factor Nrf2 and thereby inducing phase 2 enzymes (33). An alternate but not mutually exclusive mechanism whereby avicins inhibit NF-κB activation is a reduction in reactive oxygen species such as H2O2. We have shown that avicins reduce H2O2 generation during apoptosis (7) as well as after UVA exposure (unpublished observations). Thus, it is likely that avicins enhance detoxification of H2O2 by activating peroxiredoxins (34) and catalase, and/or glutathione peroxidase (35) as well as suppress production of H2O2 by inhibiting phosphatidylinositol-3-kinase signaling.
There is an intensive search for molecules that inhibit activation of NF-κB. Inhibitors with various modes of action have been reported (36). Classic antiinflammatory compounds such as salicylates and glucocorticoids either prevent the degradation of IκB (37) or activate the transcription of IκB (38). Alternatively, inhibition of the nuclear translocation of p65 and/or binding of NF-κB to DNA as reported in this paper offers a new strategy for therapeutic intervention. Several agents, including three natural products (32, 39, 40) and a prostaglandin (41), have been shown to inhibit either nuclear translocation of p65 or binding of NF-κB to DNA.
NF-κB acts as a transcriptional regulator of other proteins, such as iNOS and COX-2, that form an essential component of an inflammatory cascade critical for the repair of oxidative damage and progression of carcinogenesis (42–45). Thus, specific inhibitors of COX-2 have been shown to have potent antiinflammatory effects in clinical studies (46), and there is a search for specific inhibitors of iNOS. Because NF-κB is an activator of iNOS and COX-2 expression, the ability of avicin G to inhibit induction of these proteins could have wide-ranging clinical importance. Supporting the notion that terpenoids could be significant platforms for drug discovery is the work of Sporn and colleagues, which reports synthetic terpenoids inhibiting iNOS and COX-2 (47). The potential of antiinflammatory agents as chemoprevention drugs has been recently bolstered with the identification of a phosphatidyl-serine-specific receptor on activated human macrophages that inhibits the inflammatory response after binding to apoptotic cells (48). Thus, avicins' dual effects of inhibiting upstream events of carcinogenesis, such as free radical generation and inflammation, and thereby oxidative and nitrosative cellular stress (49), as well as downstream events, such as apoptosis (7), mutagenesis, and chromosome instability (8), make them potentially exciting compounds for chemoprevention.
Finally, the inhibition of NF-κB activation by avicns may have important evolutionary significance as well. NF-κB is a ubiquitous family of transcription factors, evolutionarily conserved from flies to mammals (1), that is pivotal to various cellular and physiological processes, such as axis formation (50) and immune defense (4). Secondary plant metabolites that evolved as an important defense adaptation against predators (10), including infectious agents such as fungi (51), also target highly conserved and critical pathways in animals. An understanding of the mechanism of action of avicins against many of these pathways, some of which are critical events in oncogenesis, may yield new leads for drug discovery.
Acknowledgments
We thank Sarah Lock-Lim for her excellent technical assistance. Research support was provided by the Clayton Foundation for Research and the Biomedical Research Foundation.
Abbreviations
- NF-κB
nuclear factor-κB
- iNOS
inducible nitric oxide synthase
- COX
cyclooxygenase
- LPS
lipopolysaccharide
- PMA
phorbol 12-myristate 13-acetate
- TNF
tumor necrosis factor
- DOC
deoxycholate
Footnotes
See commentary on page 10986.
References
- 1.Tickle C. Nature (London) 1998;392:547–549. doi: 10.1038/33276. [DOI] [PubMed] [Google Scholar]
- 2.Mercurio F, Manning A M. Curr Opin Cell Biol. 1999;11:226–232. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baichwal V R. Adv Immunol. 1997;65:111–137. [PubMed] [Google Scholar]
- 4.Baeuerle P A, Henkel T. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 5.Siebenlist U, Franzoso G, Brown K. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 6.Mujoo K, Haridas V, Hoffman J J, Wachter G A, Hutter L K, Lu Y, Blake M E, Jayatilake G S, Bailey D, Mills G B, Gutterman J U. Cancer Res. 2001;61:5486–5490. [PubMed] [Google Scholar]
- 7.Haridas V, Higuchi M, Jayatilake G S, Bailey D, Mujoo K, Blake M E, Arntzen C J, Gutterman J U. Proc Natl Acad Sci USA. 2001;98:5821–5826. doi: 10.1073/pnas.101619098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanausek M, Ganesh P, Walaszek Z, Arntzen C J, Slaga T J, Gutterman J U. Proc Natl Acad Sci USA. 2001;98:11551–11556. doi: 10.1073/pnas.191363198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haridas V, Darnay B G, Natarajan K, Heller R, Aggarwal B B. J Immunol. 1998;160:3152–3162. [PubMed] [Google Scholar]
- 10.Hostettmann K, Marston A. Saponins. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 11.Hikino H, Ohsawa T, Kiso Y, Oshima Y. Planta Med. 1984;50:353–355. doi: 10.1055/s-2007-969730. [DOI] [PubMed] [Google Scholar]
- 12.Mahato S B, Sen S. Phytochemistry. 1997;44:1185–1236. doi: 10.1016/s0031-9422(96)00639-5. [DOI] [PubMed] [Google Scholar]
- 13.Wendt K U, Schulz G E, Corey E J, Liu D R. Agnew Chem Int Ed Engl. 2000;39:2812–2833. [PubMed] [Google Scholar]
- 14.Sporn M B, Roberts A B. J Clin Invest. 1986;78:329–332. doi: 10.1172/JCI112580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore R J, Owens D M, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, et al. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 16.Hensley K, Robinson K A, Gabbita S P, Salsman S, Floyd R A. Free Radical Biol Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 17.Ohshima H, Bartsch H. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 18.Spechler S J. Gastroenterology. 1999;117:218–228. doi: 10.1016/s0016-5085(99)70571-8. [DOI] [PubMed] [Google Scholar]
- 19.Talamini G, Falconi M, Bassi C, Sartori N, Salvia R, Caldiron E, Frulloni L, Di Francesco V, Vaona B, Bovo P, et al. Am J Gastroenterol. 1999;94:1253–1260. doi: 10.1111/j.1572-0241.1999.01075.x. [DOI] [PubMed] [Google Scholar]
- 20.Cordon-Cardo C, Prives C. J Exp Med. 1999;190:1367–1370. doi: 10.1084/jem.190.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weitzman S A, Gordon L I. Blood. 1990;76:655–663. [PubMed] [Google Scholar]
- 22.Marnett L J. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 23.Gilmore T D, Koedwood M, Piffat K A, White D W. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- 24.Luque I, Gelinas C. Semin Cancer Biol. 1997;8:103–111. doi: 10.1006/scbi.1997.0061. [DOI] [PubMed] [Google Scholar]
- 25.Beg A A, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 26.Mayo M W, Baldwin A S. Biochim Biophys Acta. 2000;1470:M55–M62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 27.Lee H H, Dadgostar H, Cheng Q, Shu J, Cheng G. Proc Natl Acad Sci USA. 1999;96:9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voet D, Voet J. Biochemistry. New York: Wiley; 1990. [Google Scholar]
- 29.Chen Y-Q, Ghosh S, Ghosh G. Nat Struct Biol. 1998;5:67–73. doi: 10.1038/nsb0198-67. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Rabson A B, Gelinas C. Mol Cell Biol. 1992;12:3094–3106. doi: 10.1128/mcb.12.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt T J. Bioorg Med Chem. 1997;5:645–653. doi: 10.1016/s0968-0896(97)00003-5. [DOI] [PubMed] [Google Scholar]
- 32.Lyss G, Knorre A, Schmidt T J, Pahl H L, Merfort I. J Biol Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- 33.Dinkova-Kostova A T, Massiah M A, Bozak R E, Hicks R J, Talalay P. Proc Natl Acad Sci USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang S W, Chae H Z, Seo M S, Kim K, Baines I C, Rhee S G. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- 35.Cerutti P A. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 36.Epinat J-C, Gilmore T D. Oncogene. 1998;18:6896–6909. doi: 10.1038/sj.onc.1203218. [DOI] [PubMed] [Google Scholar]
- 37.Kopp E, Ghosh S. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 38.Scheinman R I, Cogswell P C, Lofquist A K, Baldwin A S. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 39.Natarajan K, Singh S, Burke T R, Jr, Grunberger D, Aggarwal B B. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'acquisto F, Lanzotti V, Carnuccio R. Biochem J. 2000;3:793–798. [PMC free article] [PubMed] [Google Scholar]
- 41.Straus D, Pascaul G, Li M, Welch J S, Ricote M, Hsiang C-H, Sengchanthalangsy L L, Ghosh G, Glass C K. Proc Natl Acad Sci USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Q W, Kashiwabara Y, Nathan C. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 43.Kojima M, Morisaki T, Izuhara K, Uchiyama A, Matsunari Y, Katano M, Tanaka M. Oncogene. 2000;19:1225–1231. doi: 10.1038/sj.onc.1203427. [DOI] [PubMed] [Google Scholar]
- 44.Vane J R, Mitchell J A, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby D A. Proc Natl Acad Sci USA. 1994;91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marnett L J. Curr Opin Chem Biol. 2000;4:545–552. doi: 10.1016/s1367-5931(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 46.Kalgutkar A S, Crews B C, Rowlinson S W, Marnett A B, Kozak K R, Remmel R P, Marnett L J. Proc Natl Acad Sci USA. 2000;97:925–930. doi: 10.1073/pnas.97.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suh N, Honda T, Finlay H J, Barchowsky A, Williams C, Benoit N E, Xie Q W, Nathan C, Gribble G W, Sporn M B. Cancer Res. 1998;58:717–723. [PubMed] [Google Scholar]
- 48.Fadok V A, Bratton D L, Rose D M, Pearson A, Ezekewitz R A, Henson P M. Nature (London) 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 49.Marshall H E, Merchant K, Stamler J S. FASEB J. 2000;14:1889–1900. doi: 10.1096/fj.00.011rev. [DOI] [PubMed] [Google Scholar]
- 50.Stein D, Goltz J S, Jurcsak J, Stevens L. Development. 1998;125:2159–2169. doi: 10.1242/dev.125.11.2159. [DOI] [PubMed] [Google Scholar]
- 51.Papadopoulou K, Melton R E, Leggett M, Daniels M J, Osbourn A E. Proc Natl Acad Sci USA. 1999;96:12923–12928. doi: 10.1073/pnas.96.22.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]