Key Points
Question
What is the efficacy associated with using inhaled corticosteroids and long-acting β-agonists (LABAs) together as both the controller and the quick relief therapy termed single maintenance and reliever therapy (SMART) in patients with persistent asthma?
Findings
In this meta-analysis that included 22 524 patients aged 12 years or older and 341 children aged 4 to 11 years with persistent asthma, SMART was associated with a significantly lower risk of asthma exacerbations compared with a higher dose of inhaled corticosteroids and LABA as controller therapy, absolute risk difference, −2.8% for the older age group and −12.0% for the younger age group, although less robust data were available for this group.
Meaning
SMART was associated with better clinical outcomes than conventional approaches in patients with persistent asthma.
Abstract
Importance
Combined use of inhaled corticosteroids and long-acting β-agonists (LABAs) as the controller and the quick relief therapy termed single maintenance and reliever therapy (SMART) is a potential therapeutic regimen for the management of persistent asthma.
Objective
To conduct a systematic review and meta-analysis of the effects of SMART in patients with persistent asthma.
Data Sources and Study Selection
The databases of MEDLINE via OVID, EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews were searched from database inception through August 2016 and updated through November 28, 2017. Two reviewers selected randomized clinical trials or observational studies evaluating SMART vs inhaled corticosteroids with or without a LABA used as the controller therapy and short-acting β-agonists as the relief therapy for patients aged 5 years or older with persistent asthma and reporting on an outcome of interest.
Data Extraction and Synthesis
Meta-analyses were conducted using a random-effects model to calculate risk ratios (RRs), risk differences (RDs), and mean differences with corresponding 95% CIs. Citation screening, data abstraction, risk assessment, and strength of evidence grading were completed by 2 independent reviewers.
Main Outcomes and Measures
Asthma exacerbations.
Results
The analyses included 16 randomized clinical trials (N = 22 748 patients), 15 of which evaluated SMART as a combination therapy with budesonide and formoterol in a dry-powder inhaler. Among patients aged 12 years or older (n = 22 524; mean age, 42 years; 14 634 [65%] were female), SMART was associated with a reduced risk of asthma exacerbations compared with the same dose of inhaled corticosteroids and LABA as the controller therapy (RR, 0.68 [95% CI, 0.58 to 0.80]; RD, −6.4% [95% CI, −10.2% to −2.6%]) and a higher dose of inhaled corticosteroids and LABA as the controller therapy (RR, 0.77 [95% CI, 0.60 to 0.98]; RD, −2.8% [95% CI, −5.2% to −0.3%]). Similar results were seen when SMART was compared with inhaled corticosteroids alone as the controller therapy. Among patients aged 4 to 11 years (n = 341; median age, 8 [range, 4-11] years; 69 [31%] were female), SMART was associated with a reduced risk of asthma exacerbations compared with a higher dose of inhaled corticosteroids as the controller therapy (RR, 0.55 [95% CI, 0.32 to 0.94]; RD, −12.0% [95% CI, −22.5% to −1.5%]) or the same dose of inhaled corticosteroids and LABA as the controller therapy (RR, 0.38 [95% CI, 0.23 to 0.63]; RD, −23.2% [95% CI, −33.6% to −12.1%]).
Conclusions and Relevance
In this meta-analysis of patients with persistent asthma, the use of single maintenance and reliever therapy compared with inhaled corticosteroids as the controller therapy (with or without a long-acting β-agonist) and short-acting β-agonists as the relief therapy was associated with a lower risk of asthma exacerbations. Evidence for patients aged 4 to 11 years was limited.
This systematic review and meta-analysis of randomized trials compares the efficacy of combined use of inhaled corticosteroids and long-acting β-agonists (LABAs) as the controller and the quick relief therapy (SMART) vs the same or higher doses of inhaled corticosteroids with or without a LABA used as the controller therapy and short-acting β-agonists as the relief therapy in patients with persistent asthma.
Introduction
Among patients with persistent asthma that remains suboptimally controlled with the use of inhaled corticosteroids, US guidelines suggest a stepwise approach to modifying therapy that includes increasing the dose of inhaled corticosteroids or adding adjunctive therapies. Inhaled long-acting β-agonists (LABAs) are the preferred adjunctive therapy for patients aged 12 years or older and are considered 1 of several potential adjunctive therapies for patients aged 5 to 11 years.
When inhaled corticosteroids and LABA are used as controller therapies, the doses are scheduled on a daily basis and the patient continues to use short-acting β-agonists (SABAs) for as-needed quick relief of symptoms (Box). Given the fast onset of action with the LABA formoterol, it has been hypothesized that the combined use of inhaled corticosteroids and formoterol as needed instead of SABAs would provide quick symptom relief and also deliver steroid sooner during the course of symptom deterioration, thereby effectively managing asthma symptoms and reducing exacerbation risk.
Box. Key Definitions.
Inhaled Corticosteroids
The prescribed use of inhaled corticosteroids is not the same on a daily basis. As prescribed, intermittent dosing of inhaled corticosteroids may specify variations in the dose or frequency of administration. The determinant of intermittent dosing of inhaled corticosteroids may be the patient’s decision based on need, an index of worsening asthma, or some other predefined criteria.
Inhaled Corticosteroids as Controller
Medications recommended to be taken daily on a long-term basis to achieve and maintain control of persistent asthma.
Quick Relief
Medications to be used on an as-needed basis for acute symptom relief. Commonly referred to as rescue therapy.
Single Maintenance and Reliever Therapy (SMART)
Use of inhaled corticosteroids and long-acting β-agonists together as both the controller and the quick relief therapy.
The use of a combination of inhaled corticosteroids and LABA inhaler as both the controller and quick relief therapy is a strategy that has been termed single inhaler therapy or single maintenance and reliever therapy (SMART) and is recommended for asthma management by international guidelines. However, currently there are no combinations of inhaled corticosteroids and LABA approved for combined controller and quick relief therapy in the United States.
The objective of this review was to systematically identify and analyze data from trials that compared use of SMART among patients with persistent asthma vs inhaled corticosteroids with or without a LABA used as controller therapy and SABAs as reliever therapy among patients aged 5 years or older.
Methods
A standard protocol was developed and followed and can be found online in its entirety. This article represents 1 of 6 research questions posed in the protocol. Two questions examined the effect of intermittent inhaled corticosteroids among children with recurrent wheezing or among children and adults with persistent asthma and 3 questions evaluated the comparative effectiveness of long-acting muscarinic antagonists vs standard inhaled controllers among patients with uncontrolled and persistent asthma. The final full report addressing all 6 research questions is available on the Agency for Healthcare Research and Quality website.
Data Sources and Searches
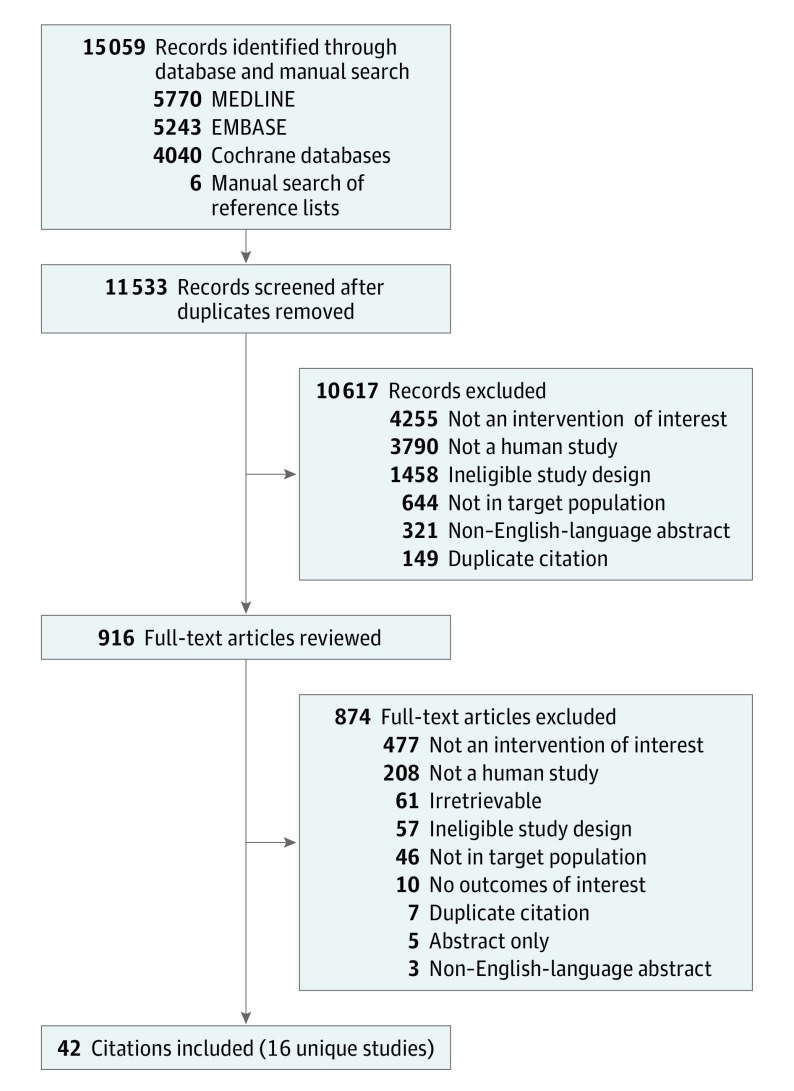
We searched MEDLINE via OVID (including in-process and other nonindexed citations), EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews via OVID from database inception through August 2016 and updated through November 28, 2017 (Figure 1 and eAppendix 1 in the Supplement). The bibliographic database searches were supplemented with backward-citation tracking of relevant publications.
Figure 1. Flow Diagram of Literature Search.
Additional searches included ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform for ongoing studies and those completed with reported results. The scientific resource center of the Agency for Healthcare Research and Quality also requested data from manufacturers.
Study Selection
Studies were included that evaluated patients aged 5 years or older with persistent asthma and compared SMART vs either inhaled corticosteroids alone or inhaled corticosteroids with LABA as the sole controller therapy and used SABA for reliever therapy. We required studies to assess 1 of the following outcomes: asthma exacerbations (systemic use of corticosteroids, hospitalization, emergency department [ED] visits, intensive care admission or intubation, or as defined by the study); all-cause or asthma-specific mortality; spirometry reported as peak, trough, or area under the curve; forced expiratory volume in the first second of expiration (FEV1); forced vital capacity (FVC); ratio of FEV1 to FVC; asthma control assessed by the Asthma Control Test or the 5-item Asthma Control Questionnaire (ACQ-5); asthma-related quality of life assessed by the Asthma Quality of Life Questionnaire; and health care use (additional medication use, additional health care use related to the intervention). A patient response was defined as a reduction in the ACQ-5 score by 0.5 points or greater. A minimally important difference in FEV1 was defined as a change of 0.2 L.
Studies considered for inclusion were randomized clinical trials (RCTs) (parallel or crossover design), prospective or retrospective observational cohort studies, and case-control studies. Crossover trials were included if the outcomes measured after the first treatment period were available and if the washout period was a minimum of 6 weeks for inhaled corticosteroids and 4 weeks for LABA. We did not apply restrictions in publication language or date.
Four independent investigators (D.M.S., E.R.W., E.N., W.L.B.) screened titles and abstracts to determine if the citation met eligibility criteria. Full-text publication review occurred when 2 of the reviewers agreed that a citation met the eligibility criteria. Disagreements were resolved through consensus in consultation with a third reviewer (D.M.S.). We contacted corresponding authors for clarification when needed to assess the inclusion criteria. Abstracts and meeting presentations were matched to their corresponding full-text publication and reviewed for supplemental data.
Data Extraction and Risk of Bias Assessment
One investigator (E.R.W. or E.N.) abstracted data into standardized collection forms and created tables for the evidence and outcomes. A second investigator (E.R.W. or E.N.) verified entries. For crossover trials, data from period 1 were abstracted when available, otherwise we contacted authors for period 1 outcomes.
Two independent reviewers (D.M.S., E.R.W., E.N., or W.L.B.) assessed risk of bias using the Cochrane Collaboration risk of bias tool for RCTs. The individual domains included (1) random-sequence generation, (2) allocation sequence concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) completeness of outcome data, (6) selective reporting, and (7) other sources of bias. Two investigators (D.M.S., E.R.W., E.N., or W.L.B.) independently classified each domain as either low, unclear, or high risk of bias.
Data Synthesis and Analysis
Data synthesis was based on pharmacological class rather than an individual drug. Studies were categorized as follows based on the comparator studied and in each case included a SABA reliever: inhaled corticosteroids alone as the controller therapy, both inhaled corticosteroids and LABA as the controller therapy, or either inhaled corticosteroids alone or inhaled corticosteroids and LABA together as the controller therapy. Studies were grouped and analyzed separately based on whether the estimated daily dose of inhaled corticosteroids in the comparator group was the same, higher, or lower than the intervention group. Some studies met criteria for more than 1 of these groups when multiple groups were reported. This article reports the results of SMART vs inhaled corticosteroids with or without a LABA at the same or higher dose of inhaled corticosteroids. The results of SMART vs a control group that allowed both inhaled corticosteroids alone or inhaled corticosteroids and LABA together as the controller therapy, or a lower comparative dose of inhaled corticosteroids appear in the full report.
Data synthesis also was based on age categories consistent with the previously used age cut points of 5 to 11 years and 12 years or older. Studies that fit into more than 1 predefined age category were included in the main analysis if they were the only source of data. These studies were added to the age category consistent with the reported mean or median age of the study as a sensitivity analysis. If a study fit into more than 1 age category and reported results separately for the different age subgroups, those subgroups were considered for the main analyses.
All analyses were performed using the meta package in R (version 3.4.3; R Project for Statistical Computing). We conducted a meta-analysis of the RCTs using the Hartung-Knapp random-effects model to estimate risk ratio (RR) and risk difference with corresponding 95% CIs for binary outcomes and mean difference with corresponding 95% CIs for continuous outcomes. For binary outcomes with rare events (<5%), odds ratios and 95% CIs were calculated using the method of Peto in place of an RR. Because of debate regarding the routine use of the Hartung-Knapp method, recommendations suggest conducting standard random-effects analyses as a sensitivity analysis. Therefore, we reanalyzed each end point using the DerSimonian and Laird random-effects model to determine if any of the conclusions were sensitive to model choice.
Additional sensitivity analyses were performed whereby studies considered to have at least 1 of 7 domains rated with high risk of bias (eg, those that did not blind participants or personnel) were removed and the analyses were rerun. The presence of statistical heterogeneity was assessed using the Cochrane P value (P < .10 was considered significant) and the degree of heterogeneity was assessed using the I2 statistic and a value greater than 50% was considered substantial. We planned to assess publication bias using funnel plot inspection and tests of plot asymmetry when 10 or more trials were pooled. However, none of the pooled analyses reached this threshold.
Strength of Evidence
Two senior investigators (D.M.S. and W.L.B.) assessed strength of evidence independently and via discussion arrived at the final grading based on 5 required domains: risk of bias, consistency, directness, precision, and publication bias. The strength of evidence for each outcome was designated as high, moderate, low, or insufficient (definitions appear in eAppendix 2 in the Supplement).
Results
Forty-two citations represent 16 RCTs (N = 22 748 patients), 15 of which evaluated SMART as a combination therapy with budesonide and formoterol in a dry-powder inhaler. Characteristics of the included studies appear in eTables 1 through 4 in the Supplement. For the comparison of SMART with other therapies, 4 RCTs evaluated inhaled corticosteroids alone as the controller therapy and 14 RCTs evaluated inhaled corticosteroids and LABA as the controller therapy. All studies included SABA as the relief therapy for patients aged 5 years or older with persistent asthma and reported an outcome of interest. All but 1 trial evaluated SMART as a combination of budesonide and formoterol in a dry-powder inhaler.
Risk of Bias
The risk of bias assessment for each included trial and the summative assessment by domain appear in eFigures 1 and 2 in the Supplement. Most RCTs had a low risk of bias for random-sequence generation (13 [81.3%]), allocation concealment (10 [62.5%]), incomplete reporting of data (14 [87.5%]), and other types of bias (16 [100%]). Of the 16 studies, 6 (37.5%) had a high risk of bias because of the blinding methods used for participants, personnel, and study outcomes. Most RCTs had an unclear risk of bias in the domain of selective outcome reporting (9 [56.2%]).
SMART vs Inhaled Corticosteroids Alone as Controller
Three RCTs compared SMART vs the same comparative daily dose of inhaled corticosteroids alone as the controller therapy among patients aged 12 years or older. Sovani et al enrolled patients with suboptimally controlled persistent asthma and required patients to have evidence of poor adherence to medications; therefore, the results are described separately from the other 2 trials. The remaining 2 trials enrolled symptomatic patients, one with mild to moderate persistent asthma and the other with moderate to severe persistent asthma.
One RCT provided a comparison of SMART vs a higher dose of inhaled corticosteroids alone as the controller therapy among patients aged 4 to 80 years with persistent symptomatic asthma. This trial also included a preplanned subgroup analysis among patients aged 4 to 11 years (median age, 8 [range, 4-11] years).
All trials compared SMART vs budesonide controller and SABA quick relief. Among patients aged 12 years or older, SMART was associated with a decreased risk of asthma exacerbations defined as a composite of those requiring use of systemic corticosteroids, hospitalization, or ED visit compared with the same dose of inhaled corticosteroids alone as the controller therapy (RR, 0.64 [95% CI, 0.53 to 0.78]; risk difference, −8.1% [95% CI, −11.5% to −4.5%]) and compared with a higher comparative dose of inhaled corticosteroids (RR, 0.59 [95% CI, 0.49 to 0.71]; risk difference, −11.0% [95% CI, −14.8% to −7.2%]; Table 1). Each of these results was based on a single RCT.
Table 1. Summary of Findings and Strength of Evidence in Studies Comparing SMART vs Inhaled Corticosteroids With or Without a LABA as Controller Therapy Among Patients Aged 12 Years or Older (n = 22 524)a.
| Outcome | Included Studiesb | SMART Group | Control Groupd | Absolute Risk Difference (95% CI), %e |
Effect Size (95% CI) | Strength of Evidencef | ||
|---|---|---|---|---|---|---|---|---|
| No. of Patients |
Outcomec | No. of Patients |
Outcomec | |||||
| SMART vs Same Dose of Inhaled Corticosteroids Alone as Controller Therapy | ||||||||
| Asthma exacerbations: required use of systemic corticosteroids, hospitalization, or ED visit | 947 | 137 | 943 | 212 | −8.1 (−11.5 to −4.5) | RR, 0.64 (0.53 to 0.78) | Moderate | |
| Death | ||||||||
| All cause | 947 | 1 | 943 | 2 | −0.1 (−0.7 to 0.4) | OR, 0.51 (0.05 to 4.92) | Insufficient | |
| Specific to asthma | 947 | 0 | 943 | 0 | No events occurred | Insufficient | ||
| Spirometry: FEV1, L | 947 | 2.54 (NR) | 943 | 2.44 (NR) | MD, 0.10 (0.07 to 0.13) | Moderate | ||
| Health care use: rescue medication, puffs/d | 355 | 1.04 (NR) | 342 | 1.48 (NR) | MD, −0.34 (−0.51 to −0.17) | Low | ||
| SMART vs Higher Dose of Inhaled Corticosteroids as Controller | ||||||||
| Asthma exacerbations: required use of systemic corticosteroids, hospitalization, or ED visit | 925 | 148 | 909 | 245 | −11.0 (−14.8 to −7.2) | RR, 0.59 (0.49 to 0.71) | Low | |
| SMART vs Same Dose of Inhaled Corticosteroids and LABA as Controller Therapy | ||||||||
| Asthma exacerbations | ||||||||
| Required use of systemic corticosteroids, hospitalization, or ED visit | 4226 | 263 | 4257 | 385 | −6.4 (−10.2 to −2.6) | RR, 0.68 (0.58 to 0.80) | High | |
| Mild | 3008 | 2166 | 3029 | 2328 | −4.6 (−15.9 to 6.6) | RR, 0.94 (0.81 to 1.09) | Moderate | |
| Death | ||||||||
| All cause | 3374 | 2 | 3408 | 5 | −0.1 (−0.2 to 0.1) | OR, 0.43 (0.04 to 4.49) | Moderate | |
| Specific to asthma | 3374 | 0 | 3408 | 0 | No events occurred | Insufficient | ||
| Asthma control | ||||||||
| Asthma Control Test scoreg | 32 | 6.9 (1.93) | 31 | 0.6 (2.61) | MD, 6.30 (5.15 to 7.45) | Insufficient | ||
| 5-Item Asthma Control Questionnaireh | ||||||||
| Score | 2177 | NA | 2198 | NA | MD, −0.16 (−0.39 to 0.06) | Low | ||
| Patient responsei | 1049 | 587 | 1042 | 511 | 6.9 (2.6 to 11.2) | RR, 1.14 (1.05 to 1.24) | Moderate | |
| Spirometry | ||||||||
| FEV1, L | 2177 | NA | 3215 | NA | MD, 0.04 (0 to 0.09) | Low | ||
| FEV1, % predicted | 133 | 5.5 (17.0) | 141 | 3.7 (21.3) | MD, 1.8 (−2.8 to 6.4) | Moderate | ||
| 15 | 7.6 (7.4) | 15 | 5.7 (9.1) | MD, 1.9 (−4.3 to 8.1) | ||||
| FVC, L | 852 | 0.11 (0.6) | 849 | 0.12 (0.6) | MD, −0.01 (−0.07 to 0.04) | Low | ||
| Health care use | ||||||||
| Rescue medication, puffs/d | 3014 | NA | 3032 | NA | MD, −0.16 (−0.45 to 0.14) | Low | ||
| SMART vs Higher Dose of Inhaled Corticosteroids and LABA as Controller Therapy | ||||||||
| Asthma exacerbations | ||||||||
| Required use of systemic corticosteroids, hospitalization, or ED visit | 2254 | 202 | 3371 | 394 | −2.8 (−5.2 to −0.3) | RR, 0.77 (0.60 to 0.98) | High | |
| Mild | Comparison 1,j | 1103 | 674 | 1099 | 687 | −1.4 (−5.5 to 2.7) | RR, 0.97 (0.91 to 1.04) | Moderate |
| Comparison 2,k | 1103 | 674 | 1119 | 660 | 0.2 (−2.0 to 6.2) | RR, 1.04 (0.97 to 1.11) | ||
| Death | ||||||||
| All cause | 2321 | 3 | 3436 | 1 | 0.1 (0 to 0.2) | OR, 5.19 (0.32 to 85.45) | Moderate | |
| Specific to asthma | 2321 | 0 | 3436 | 0 | No events occurred | Insufficient | ||
| Asthma control: 5-item Asthma Control Questionnaire scoreh | 1144 | 1.84 (NR) | 1145 | 1.89 (NR) | MD, −0.02 (−0.07 to 0.04) | High | ||
| Comparison 1,j | 1068 | NR | 1060 | NR | MD, −0.02 (−0.08 to 0.05) | |||
| Comparison 2,k | 1068 | NR | 1074 | NR | MD, 0.03 (−0.03 to 0.09) | |||
| Spirometry: FEV1, L | Comparison 1,j | 1103 | 2.69 (NR) | 1103 | 2.66 (NR) | MD, 0.01 (−0.03 to 0.04) | Moderate | |
| Comparison 2,k | 1103 | 2.69 (NR) | 1103 | 2.67 (NR) | MD, 0.01 (−0.03 to 0.04) | |||
| Quality of life: Asthma Quality of Life Questionnaire scorel | Comparison 1,j | 1103 | NR | 1103 | NR | MD, 0.01 (−0.07 to 0.08) | Moderate | |
| Comparison 2,k | 1103 | NR | 1103 | NR | MD, −0.02 (−0.09 to 0.06) | |||
| Health care use: rescue medication, puffs/d | 1144 | 0.95 (NR) | 1145 | 1.01 (NR) | MD, −0.04 (−0.12 to 0.04) | High | ||
| Comparison 1,j | 1068 | 1.02 (NR) | 1119 | 1.05 (NR) | MD, −0.03 (−0.12 to 0.06) | |||
| Comparison 2,k | 1068 | 1.02 (NR) | 1099 | 0.96 (NR) | MD, 0.07 (−0.02 to 0.16) | |||
Abbreviations: ED, emergency department; FEV1, forced expiratory volume in the first second of expiration; FVC, forced vital capacity; LABA, long-acting β-agonist; MD, mean difference; NA, not available; NR, not reported; OR, odds ratio; RR, risk ratio; SMART, single maintenance and reliever therapy.
The mean age of patients was 42 years and 14 634 (65%) were female.
Meta-analyses were performed when 3 or more studies reported on the same outcome. When data were available for 2 or fewer studies, the results from each study are individually shown. Thus, the groups are treated as a separate comparison with individual results shown.
Outcome data are expressed as mean (SD) or No. of patients with an event. The mean outcome values represent the mean change from baseline for each study group. The mean difference represents the between-group difference in change from baseline.
The control group used a short-acting β-agonist as the reliever therapy.
Indicates between-group risk (SMART group minus control group).
Based on domains of risk of bias, consistency, directness, precision, and publication bias. Additional information appears in eAppendix 2 in the Supplement. Some rows do not have an evidence rating because ratings were given for each outcome as a whole rather than for the individual studies.
Patient self-administered tool for assessing overall asthma control; range, 5 (worse) to 25 (better control). The minimally important difference is a 3-point change.
Patient self-administered tool for assessing overall asthma control; range, 0 (worse) to 6 (better control). The minimally important difference is a 0.5-point change.
Defined as an improved score by at least 0.5 points.
SMART vs combination budesonide and formoterol.
SMART vs combination fluticasone and salmeterol.
Patient self-administered tool for assessing asthma-specific quality of life; range, 1 (severe impairment) to 7 (no impairment). The minimally important difference is a 0.5-point change.
Compared with the same dose of inhaled corticosteroids alone, single trials demonstrated that SMART was associated with improved FEV1 (mean difference, 0.10 [95% CI, 0.07 to 0.13]) and decreased need for rescue medication inhalations per day (mean difference, −0.34 [95% CI, −0.51 to −0.17]). All-cause mortality was rare in the single trial reporting this outcome. One death (0.1%) occurred in the SMART group and 2 deaths (0.2%) occurred in the inhaled corticosteroids group (odds ratio, 0.51 [95% CI, 0.05 to 4.92]; risk difference, −0.1% [95% CI, −0.7% to 0.4%]), none of which were asthma-specific deaths. No other outcomes of interest were reported.
Among patients aged 4 to 11 years, an analysis of a similar composite for asthma exacerbations (including increases in asthma medications with or without a peak expiratory flow [PEF] component) suggests a reduced risk in the association with SMART based on a subgroup analysis in a single trial (Table 2). No significant association of SMART with the risk of mild asthma exacerbations was seen.
Table 2. Summary of Findings and Strength of Evidence in Studies Comparing SMART vs Inhaled Corticosteroids With or Without a LABA as Controller Therapy Among Patients Aged 4 to 11 Years (n = 341)a.
| Outcome | Included Studies | SMART Group | Control Groupb | Absolute Risk Difference (95% CI), %c |
Risk Ratio (95% CI) | Strength of Evidenced | ||
|---|---|---|---|---|---|---|---|---|
| No. of Patients |
No. With Event |
No. of Patients |
No. With Event |
|||||
| SMART vs Higher Dose of Inhaled Corticosteroids as Controller Therapy | ||||||||
| Asthma exacerbations | ||||||||
| Required use of systemic corticosteroids, hospitalization, ED visit, increase in inhaled corticosteroids or other asthma medication, or having PEF <70% | 118 | 17 | 106 | 28 | −12.0 (−22.5 to −1.5) | 0.55 (0.32 to 0.94) | Low | |
| Required use of systemic corticosteroids, hospitalization, ED visit, or increase in inhaled corticosteroid or other asthma medication | 118 | 10 | 106 | 21 | −11.3 (−20.7 to −2.2) | 0.43 (0.21 to 0.87) | Low | |
| Mild | 118 | 74 | 106 | 77 | −9.9 (−21.7 to 2.4) | 0.86 (0.72 to 1.04) | Low | |
| SMART vs Same Dose of Inhaled Corticosteroids and LABA as Controller Therapy | ||||||||
| Asthma exacerbations | ||||||||
| Required use of systemic corticosteroids, hospitalization, ED visit, increase in inhaled corticosteroids or other asthma medication, or having PEF <70% | 118 | 17 | 117 | 44 | −23.2 (−33.6 to −12.1) | 0.38 (0.23 to 0.63) | Low | |
| Required use of systemic corticosteroids, hospitalization, ED visit, or increase in inhaled corticosteroid or other asthma medication | 118 | 10 | 117 | 36 | −22.3 (−31.9 to −12.3) | 0.28 (0.14 to 0.53) | Low | |
| Mild | 118 | 74 | 117 | 98 | −21.1 (−31.6 to −9.8) | 0.75 (0.64 to 0.88) | Low | |
Abbreviations: ED, emergency department; LABA, long-acting β-agonist; PEF, peak expiratory flow; SMART, single maintenance and reliever therapy.
The median age of patients was 8 (range, 4-11) years and 69 (31%) were female.
The control group used a short-acting β-agonist as the reliever therapy.
Indicates between-group risk (SMART group minus control group).
Based on domains of risk of bias, consistency, directness, precision, and publication bias. Additional information appears in eAppendix 2 in the Supplement.
SMART vs Inhaled Corticosteroids and LABA Controller
Fourteen RCTs compared SMART vs inhaled corticosteroids and LABA as the controller therapy and SABA as the quick relief. Nine trials (n = 12 902) used the same comparative dose of inhaled corticosteroids in both groups and all but 1 RCT fit into the age group of 12 years or older. O’Byrne et al enrolled patients aged 4 to 80 years and provided subgroup data for patients aged 4 to 11 years.
Five trials (n = 7605) compared SMART vs a higher dose of inhaled corticosteroids and LABA controller therapy. Four of these 5 trials included patients aged 12 years or older (mean age range, 38-45 years). The other trial by Lundborg et al studied patients aged 6 years or older. However, this trial used formoterol instead of SABA as the quick relief therapy in the control group; therefore, the trial was not pooled with the others.
One trial further specified persistent asthma severity as moderate to severe. Nine trials enrolled patients with symptomatic asthma, 2 trials enrolled patients regardless of presence of asthma symptoms, and 4 trials specified that enrolled patients had uncontrolled asthma. Most trials used budesonide and formoterol in a dry-powder inhaler in both groups. Two trials compared combined budesonide and formoterol in a dry-powder inhaler and quick relief with combined fluticasone and salmeterol. One trial compared combined beclomethasone and formoterol in both groups.
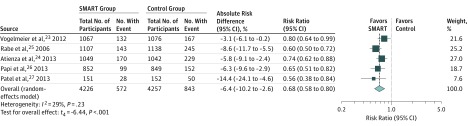
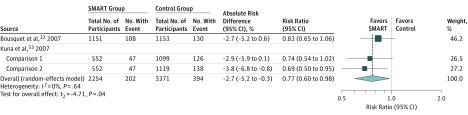
Among patients aged 12 years or older, asthma exacerbations were most commonly defined as a composite requiring use of systemic corticosteroids, hospitalization, or an ED visit. SMART was associated with a lower risk of asthma exacerbations compared with the same dose of inhaled corticosteroids and LABA controller therapy (RR, 0.68 [95% CI, 0.58 to 0.80]; risk difference, −6.4% [95% CI, −10.2% to −2.6%]; Figure 2) and a higher dose of inhaled corticosteroids and LABA controller therapy (RR, 0.77 [95% CI, 0.60 to 0.98], risk difference, −2.7% [95% CI, −5.2% to −0.3%]; Figure 3).
Figure 2. Association of SMART With Exacerbations Requiring Systemic Corticosteroids, Hospitalization, or ED Visits Among Patients Aged 12 Years or Older vs the Same Dose of Inhaled Corticosteroids and LABA Controller Therapy.
The box sizes are proportional to study weight and the horizontal lines indicate 95% CIs. The I2 value indicates the percentage of variability across the pooled estimates attributable to statistical heterogeneity (range, 0%-100%), and the P value for heterogeneity is a test of heterogeneity across all studies (P < .10 indicates likely variation across pooled estimates related to statistical heterogeneity). ED indicates emergency department; LABA, long-acting β-agonist; SMART, single maintenance and reliever therapy.
Figure 3. Association of SMART With Exacerbations Requiring Systemic Corticosteroids, Hospitalization, or ED Visits Among Patients Aged 12 Years or Older vs a Higher Dose of Inhaled Corticosteroids and LABA Controller Therapy.
The box sizes are proportional to study weight and the horizontal lines indicate 95% CIs. The I2 value indicates the percentage of variability across the pooled estimates attributable to statistical heterogeneity (range, 0%-100%), and the P value for heterogeneity is a test of heterogeneity across all studies (P < .10 indicates likely variation across pooled estimates related to statistical heterogeneity). ED indicates emergency department; LABA, long-acting β-agonist; SMART, single maintenance and reliever therapy.
No significant association of SMART with risk of mild exacerbation was seen (RR, 0.94 [95% CI, 0.81 to 1.09]; risk difference, −0.05 [95% CI, −0.16 to 0.07]; eFigure 3 in the Supplement). There was no association between SMART and all-cause mortality regardless of comparative dose of inhaled corticosteroids (2/3374 [0.06%] for SMART vs 5/3408 [0.15%] for the same dose of inhaled corticosteroids; 3/2321 [0.13%] for SMART vs 1/3436 [0.03%] for a higher dose of inhaled corticosteroids; eFigures 4-5 in the Supplement). There were no asthma-specific deaths.
No significant association of SMART with asthma symptom control measured by the ACQ-5 was seen compared with the same dose of inhaled corticosteroids and LABA controller therapy (eFigure 6 in the Supplement) or with a higher dose of inhaled corticosteroids (Table 1). However, in a single trial, SMART was associated with an improved ACQ-5 response rate vs the same dose of inhaled corticosteroids and LABA controller therapy (RR, 1.14 [95% CI, 1.05 to 1.24]; risk difference, 6.9% [95% CI, 2.6% to 11.2%]).
There was no significant association of SMART with changes in FEV1, FVC, or percentage of predicted FEV1 (Table 1 and eFigure 7 in the Supplement) vs inhaled corticosteroids and LABA controller therapy at either the same or a higher dose of inhaled corticosteroids. Two trials evaluated asthma-related quality of life using the Asthma Quality of Life Questionnaire and found no significant association with SMART vs a higher dose of inhaled corticosteroids and LABA controller therapy (Table 1). SMART was not associated with lower use of rescue medication as inhalations per day vs either the same or a higher dose of inhaled corticosteroids and LABA controller therapy (Table 1 and eFigure 8 in the Supplement).
Among patients aged 4 to 11 years, a subgroup analysis from a single RCT reported that SMART was associated with a lower risk of asthma exacerbations using 3 different composite definitions vs the same dose of inhaled corticosteroids alone as the controller therapy (Table 2).
Sensitivity Analysis
The results of the sensitivity analyses, whereby all outcomes were reanalyzed using different methods appear in eTable 5 in the Supplement. Three analyses were shown to be sensitive to model choice and the 95% CIs were more narrow than the base-case analysis, resulting in statistically significant findings.
When analyzed using the DerSimonian-Laird random-effects model and compared with the same dose of inhaled corticosteroids and LABA controller therapy, SMART was associated with a significant improvement in mild asthma exacerbations (RR, 0.94 [95% CI, 0.88 to 1.00]; eTable 5 in the Supplement; mean difference in asthma control assessed by the ACQ-5 score, −0.16 [95% CI, −0.24 to −0.09]; mean difference in rescue medication use, −0.16 [95% CI, −0.29 to −0.03 puffs/day]). All other outcomes remain unchanged.
When the studies rated as having a high risk of bias were removed during the sensitivity analyses, the overall findings remained consistent (eTable 6 in the Supplement).
Discussion
This systematic review supports the combined use of inhaled corticosteroids and LABA as both the controller and quick relief therapy (SMART) among patients aged 12 years or older compared with using either the same or a higher dose of inhaled corticosteroids alone as the controller therapy or the same or a higher dose of inhaled corticosteroids and LABA as the controller therapy with SABA as the quick relief therapy for patients aged 12 years or older with persistent asthma. Current evidence regarding children aged 4 to 11 years is limited but also suggests similar efficacy associated with SMART.
Persistent asthma severity was not usually defined further in the studies; however, all of the studies required patients to be taking inhaled corticosteroids prior to enrollment and many patients also were taking an LABA. Based on reported mean doses of inhaled corticosteroids prior to enrollment, most patients were taking medium to high doses of inhaled corticosteroids and thus represent patients with asthma severity that is worse than mild persistent asthma.
Efficacy associated with SMART among patients aged 12 years or older is primarily based on a composite outcome including asthma exacerbations requiring systemic corticosteroids, hospitalization, or ED visits. SMART was associated with reduced RRs regardless of the comparator. Although it is recommended that trials report the individual components of a composite outcome, this was rarely done. Some insight is provided from the comparison of SMART vs the same dose of inhaled corticosteroids and LABA controller therapy for which SMART was associated with a reduced risk in a similar magnitude for each of the 3 components of the composite outcome.
Other outcomes including all-cause mortality were rare and there were no asthma-specific deaths among the patients studied. Trials typically lasted 6 to 12 months and thus may be limited in the analysis of such long-term outcomes. Several postmarketing trials mandated by the US Food and Drug Administration that were designed to evaluate the safety of LABA used in combination with inhaled corticosteroids in patients with asthma, albeit traditional dosing not SMART, have not detected any concern regarding serious asthma-related events. This has recently prompted the Food and Drug Administration to remove the black box warning regarding asthma-related deaths from all medications that contain both inhaled corticosteroids and LABA.
Current guidelines recommend that asthma management goals not only include risk reduction of asthma exacerbations but also improvement in asthma control. Among patients aged 12 years or older, measures of asthma control were much less frequently available for analysis. Spirometry was represented mostly by FEV1. Even though SMART was associated with significantly increased FEV1 compared with the same comparative dose of inhaled corticosteroids, the difference did not reach the minimally important difference of 0.2 L. Continuous composite measures of asthma symptom control (eg, Asthma Control Test and the ACQ-5) did not support improvement in asthma symptom control with SMART more often than not despite a single trial that found SMART was associated with increased chances of having a response on the ACQ-5 (achievement of a minimally important difference of 0.5).
There are several hypotheses regarding why changes in symptom control were negligible in this review. Based on a systematic review of the literature, some have concluded that a within-patient minimally important difference of 0.5 for the ACQ-5 score is not achievable in studies when controller therapies are added to inhaled corticosteroids. Bateman et al noted that the development of the ACQ-5 was largely based on a population of patients that had not taken steroids or were taking inhaled corticosteroids alone, and initial derivation of the questionnaire was based on physician-perceived improvement in patient symptoms. SMART delivers a fast-acting LABA; therefore, it is reasonable to expect patients to obtain symptom relief in place of a SABA. However, unlike traditional dosing of inhaled corticosteroids and LABA, SMART delivers inhaled corticosteroids sooner during symptom deterioration, which may in part have a role in improvement of other outcomes such as asthma exacerbations.
Despite the lack of symptom control found in this review, a retrospective analysis of 5 trials included in this review found that ACQ-5 scores continued to improve over 6 to 12 months of therapy during the trials, regardless of the treatment group. The rates of controlled asthma according to various ACQ-5 cut points also were similar regardless of intervention group and continued to improve over the course of the trials. The data support improved asthma control as measured through validated composite scores with SMART.
SMART was not associated with changes in asthma-related quality of life; however, this outcome was rarely reported and should be a target for future studies evaluating this intervention. In addition, no significant association between SMART and changes in the need for rescue medication inhalations was seen across the various comparators in this review, and only favored SMART vs a higher dose of inhaled corticosteroids alone as the controller therapy, albeit not reaching the minimally important difference of 0.8 inhalations per day.
Composite asthma exacerbations were the only outcomes available for analysis among patients aged 4 to 11 years based on data from a subgroup analysis of a single trial. Even though a composite outcome for asthma exacerbations was used, it was broader than the literature base for patients aged 12 years or older and included asthma exacerbations requiring systemic steroids, hospitalization, ED visit, a change in PEF to less than 70%, or an increase in inhaled corticosteroids or other asthma medications.
Although lung function can document reduced airflow associated with asthma exacerbations, PEF is less sensitive than FEV1. Furthermore, not all reductions in PEF will result in medical intervention as was found in the 1 trial in which 87% of the asthma exacerbations meeting the PEF criterion were retrospectively found not to lead to medical intervention. When the PEF component of the composite outcome was removed, a significant association in favor of SMART was still found; however, the criterion of an increase in inhaled corticosteroids or other medication remained. Even in younger children, this characteristic is not 1 of the recommended definitions of asthma exacerbations.
Taken together, the strength of evidence for asthma exacerbation outcomes among patients aged 4 to 11 years was low and more limited than the evidence in support of SMART among patients aged 12 years or older. Future research is needed to confirm the similar efficacy of SMART seen in the prior trial on asthma exacerbations as well as to evaluate other important outcomes such as asthma control and quality of life.
Applicability
The target population for this review included patients aged 5 years or older with persistent asthma. Consistent with the National Asthma Education and Prevention Program’s Expert Panel Report 3 guidelines, this systematic review aimed to evaluate patients aged 5 years or older and separately analyzed patients aged 4 to 11 years and aged 12 years or older. The evidence base for patients aged 4 to 11 years was limited to a single subgroup analysis (n = 341) from a single trial and included patients as young as 4 years of age with uncontrolled asthma while receiving inhaled corticosteroids alone.
However, even though this study was the only source of evidence for this age group, the data were included because the mean age was within the target range. Several of the trials fitting the age category of 12 years or older involved mainly older patients (mean ages in the 40s) and thus reflect adult populations rather than adolescents. The majority of trials required patients to either have uncontrolled asthma or required use of rescue SABA during the run-in period. Although SABA use is 1 criterion to measure impairment associated with asthma control, others such as nighttime awakenings, spirometry, and interference with activities of daily living are also suggested.
All but 1 trial evaluated the combination of inhaled corticosteroids and LABA as budesonide and formoterol delivered as a dry-powder inhaler; therefore, the results of this systematic review are most reflective of this particular inhaler. Combination budesonide and formoterol is only available in the United States as a metered-dose inhaler. Data suggest that budesonide and formoterol delivered via a metered-dose inhaler is therapeutically equivalent to the dry-powder inhaler based on morning PEF measured in patients aged 12 years or older with suboptimal asthma control while using inhaled corticosteroids alone. However, specific trials evaluating the metered-dose inhaler in SMART were not available.
Limitations
This study has several limitations. First, this review did not evaluate adverse events associated with administering SMART because it was not within the scope of the review. However, when selecting appropriate therapy for patients, decision makers should consider known harms and costs of these drug therapies.
Second, although there were many subgroups of interest, individual analyses could not be conducted due to the lack of reported data, which limits the ability of decision makers to further individualize recommendations. Third, some trials included in this review were determined to have medium risk of bias specifically due to the open-label nature of the study design. Fourth, due to the number of outcomes that were evaluated, some statistically significant associations may represent type I error.
Fifth, there is still debate surrounding the most appropriate statistical model to use within meta-analyses. Due to its more favorable handling of between-study heterogeneity, an a priori decision was made to use a random-effects model with a Hartung-Knapp estimator. However, the Hartung-Knapp estimator has received criticism and some recommend conducting a sensitivity analysis using standard random-effects analyses, such as the DerSimonian-Laird estimator. When each end point was reanalyzed using this method, the 95% CIs for a few outcomes were narrowed whereby they did not cross unity. However, because the changes seen did not reach the level of clinical relevance, the conclusions remain unchanged.
Conclusions
In this meta-analysis of patients with persistent asthma, the use of single maintenance and reliever therapy compared with inhaled corticosteroids as the controller therapy (with or without a long-acting β-agonist) and short-acting β-agonists as the relief therapy was associated with a lower risk of asthma exacerbations. Evidence for patients aged 4 to 11 years was limited.
eAppendix 1. Search strategies
eAppendix 2. Strength of Evidence
eTable 1. Study and population baseline characteristics for SMART vs. ICS controller (same ICS dose) RCTs
eTable 2. Study and population baseline characteristics for SMART vs. ICS controller (higher ICS dose) RCTs
eTable 3. Study and population baseline characteristics for SMART vs. ICS and LABA controller (same ICS dose) RCTS
eTable 4. Study and population baseline characteristics for SMART vs. ICS and LABA controller (higher ICS dose) RCTs
eTable 5. Sensitivity Analyses Evaluating Random-Effects Model Selection
eTable 6. Sensitivity Analyses Removing Studies with High Risk of Bias
eFigure 1. Assessment of Risk of Bias Using Cochrane Collaboration Tool
eFigure 2. Summative Assessment of Risk of Bias (No. Studies = 15)
eFigure 3. The association of SMART versus ICS and LABA controller (same dose) on mild exacerbations
eFigure 4. The association of SMART versus ICS and LABA controller (same ICS dose) on all-cause death
eFigure 5. The association of SMART versus ICS and LABA controller (higher ICS dose) on all-cause death
eFigure 6. The association of SMART versus ICS and LABA controller (same ICS dose) on ACQ-5 score
eFigure 7. The association of SMART versus ICS and LABA controller (same ICS dose) on FEV1
eFigure 8. The association of SMART versus ICS and LABA controller (same ICS dose) on rescue medication inhalations per day
References
- 1.National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. [Google Scholar]
- 2.Palmqvist M, Persson G, Lazer L, Rosenborg J, Larsson P, Lötvall J. Inhaled dry-powder formoterol and salmeterol in asthmatic patients: onset of action, duration of effect and potency. Eur Respir J. 1997;10(11):2484-2489. [DOI] [PubMed] [Google Scholar]
- 3.European Medicines Agency DuoResp Spiromax. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002348/human_med_001749.jsp&mid=WC0b01ac058001d124. Accessed January 9, 2018.
- 4.Global Initiative for Asthma Global strategy for asthma management and prevention, 2017. http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed January 9, 2018.
- 5.National Heart, Lung, and Blood Institute Advisory Council Asthma Expert Working Group Needs Assessment Report for Potential Update of the Expert Panel Report-3 (2007): Guidelines for the Diagnosis and Management of Asthma; Bethesda, MD: National Heart, Lung, and Blood Institute; 2015. [Google Scholar]
- 6.Agency for Healthcare Research and Quality Systematic review of intermittent inhaled corticosteroids and of long-acting muscarinic antagonists for asthma: research protocol. https://www.effectivehealthcare.ahrq.gov/topics/asthma-pharmacologic-treatment/research-protocol. Accessed January 9, 2018. [PubMed]
- 7.Sobieraj DM, Baker WL, Weeda ER, et al. Intermittent inhaled corticosteroids and long-acting muscarinic antagonists for asthma: comparative effectiveness review No. 194. https://effectivehealthcare.ahrq.gov/topics/asthma-pharmacologic-treatment/research-2017/. Accessed March 19, 2018. [PubMed]
- 8.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115(5):1265-1270. [DOI] [PubMed] [Google Scholar]
- 9.Santanello NC, Zhang J, Seidenberg B, Reiss TF, Barber BL. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J. 1999;14(1):23-27. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartung J, Knapp G. On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat Med. 2001;20(12):1771-1782. [DOI] [PubMed] [Google Scholar]
- 12.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20(24):3875-3889. [DOI] [PubMed] [Google Scholar]
- 13.Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26(1):53-77. [DOI] [PubMed] [Google Scholar]
- 14.Wiksten A, Rücker G, Schwarzer G. Hartung-Knapp method is not always conservative compared with fixed-effect meta-analysis. Stat Med. 2016;35(15):2503-2515. [DOI] [PubMed] [Google Scholar]
- 15.Jackson D, Law M, Rücker G, Schwarzer G. The Hartung-Knapp modification for random-effects meta-analysis: a useful refinement but are there any residual concerns? Stat Med. 2017;36(25):3923-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berkman ND, Lohr KN, Ansari M, et al. Grading the strength of a body of evidence when assessing health care interventions for the effective health care program of the Agency for Healthcare Research and Quality: an update, 2013. https://www.effectivehealthcare.ahrq.gov/. Accessed January 9, 2018. [PubMed]
- 19.Sovani MP, Whale CI, Oborne J, et al. Poor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help? Br J Gen Pract. 2008;58(546):37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Byrne PM, Bisgaard H, Godard PP, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171(2):129-136. [DOI] [PubMed] [Google Scholar]
- 21.Scicchitano R, Aalbers R, Ukena D, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin. 2004;20(9):1403-1418. [DOI] [PubMed] [Google Scholar]
- 22.Rabe KF, Pizzichini E, Ställberg B, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest. 2006;129(2):246-256. [DOI] [PubMed] [Google Scholar]
- 23.Vogelmeier C, Naya I, Ekelund J. Budesonide/formoterol maintenance and reliever therapy in Asian patients (aged ≥16 years) with asthma: a sub-analysis of the COSMOS study. Clin Drug Investig. 2012;32(7):439-449. [DOI] [PubMed] [Google Scholar]
- 24.Atienza T, Aquino T, Fernández M, et al. Budesonide/formoterol maintenance and reliever therapy via Turbuhaler versus fixed-dose budesonide/formoterol plus terbutaline in patients with asthma: phase III study results. Respirology. 2013;18(2):354-363. [DOI] [PubMed] [Google Scholar]
- 25.Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368(9537):744-753. [DOI] [PubMed] [Google Scholar]
- 26.Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med. 2013;1(1):23-31. [DOI] [PubMed] [Google Scholar]
- 27.Patel M, Pilcher J, Pritchard A, et al. ; SMART Study Group . Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med. 2013;1(1):32-42. [DOI] [PubMed] [Google Scholar]
- 28.Hozawa S, Terada M, Hozawa M. Comparison of the effects of budesonide/formoterol maintenance and reliever therapy with fluticasone/salmeterol fixed-dose treatment on airway inflammation and small airway impairment in patients who need to step-up from inhaled corticosteroid monotherapy. Pulm Pharmacol Ther. 2014;27(2):190-196. [DOI] [PubMed] [Google Scholar]
- 29.Ställberg B, Ekström T, Neij F, et al. ; SHARE trial group . A real-life cost-effectiveness evaluation of budesonide/formoterol maintenance and reliever therapy in asthma. Respir Med. 2008;102(10):1360-1370. [DOI] [PubMed] [Google Scholar]
- 30.Takeyama K, Kondo M, Tagaya E, et al. Budesonide/formoterol maintenance and reliever therapy in moderate-to-severe asthma: effects on eosinophilic airway inflammation. Allergy Asthma Proc. 2014;35(2):141-147. [DOI] [PubMed] [Google Scholar]
- 31.Bisgaard H, Le Roux P, Bjåmer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest. 2006;130(6):1733-1743. [DOI] [PubMed] [Google Scholar]
- 32.Bousquet J, Boulet LP, Peters MJ, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs high-dose salmeterol/fluticasone. Respir Med. 2007;101(12):2437-2446. [DOI] [PubMed] [Google Scholar]
- 33.Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavord ID, Jeffery PK, Qiu Y, et al. Airway inflammation in patients with asthma with high-fixed or low-fixed plus as-needed budesonide/formoterol. J Allergy Clin Immunol. 2009;123(5):1083-1089, 1089.e1-1089.e7. [DOI] [PubMed] [Google Scholar]
- 35.Lundborg M, Wille S, Bjermer L, et al. Maintenance plus reliever budesonide/formoterol compared with a higher maintenance dose of budesonide/formoterol plus formoterol as reliever in asthma: an efficacy and cost-effectiveness study. Curr Med Res Opin. 2006;22(5):809-821. [DOI] [PubMed] [Google Scholar]
- 36.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719-23.e1. [DOI] [PubMed] [Google Scholar]
- 37.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47(1):81-87. [DOI] [PubMed] [Google Scholar]
- 38.Bateman ED, Esser D, Chirila C, et al. Magnitude of effect of asthma treatments on Asthma Quality of Life Questionnaire and Asthma Control Questionnaire scores: systematic review and network meta-analysis. J Allergy Clin Immunol. 2015;136(4):914-922. [DOI] [PubMed] [Google Scholar]
- 39.Fuhlbrigge A, Peden D, Apter AJ, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012;129(3)(suppl):S34-S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stempel DA, Raphiou IH, Kral KM, et al. ; AUSTRI Investigators . Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822-1830. [DOI] [PubMed] [Google Scholar]
- 41.Stempel DA, Szefler SJ, Pedersen S, et al. ; VESTRI Investigators . Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375(9):840-849. [DOI] [PubMed] [Google Scholar]
- 42.Peters SP, Bleecker ER, Canonica GW, et al. Serious asthma events with budesonide plus formoterol vs budesonide alone. N Engl J Med. 2016;375(9):850-860. [DOI] [PubMed] [Google Scholar]
- 43.FDA Drug Safety Communication FDA review finds no significant increase in risk of serious asthma outcomes with long-acting beta-agonists (LABAs) used in combination with inhaled corticosteroids (ICS). https://www.fda.gov/Drugs/DrugSafety/ucm589587.htm. Accessed January 9, 2018.
- 44.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553-558. [DOI] [PubMed] [Google Scholar]
- 45.Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J. 2010;36(6):1410-1416. [DOI] [PubMed] [Google Scholar]
- 46.Bateman ED, Reddel HK, Eriksson G, et al. Overall asthma control: the relationship between current control and future risk. J Allergy Clin Immunol. 2010;125(3):600-608, 608.e1-608.e6. [DOI] [PubMed] [Google Scholar]
- 47.Morice AH, Peterson S, Beckman O, Osmanliev D. Therapeutic comparison of a new budesonide/formoterol pMDI with budesonide pMDI and budesonide/formoterol DPI in asthma. Int J Clin Pract. 2007;61(11):1874-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cornell JE, Mulrow CD, Localio R, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160(4):267-270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search strategies
eAppendix 2. Strength of Evidence
eTable 1. Study and population baseline characteristics for SMART vs. ICS controller (same ICS dose) RCTs
eTable 2. Study and population baseline characteristics for SMART vs. ICS controller (higher ICS dose) RCTs
eTable 3. Study and population baseline characteristics for SMART vs. ICS and LABA controller (same ICS dose) RCTS
eTable 4. Study and population baseline characteristics for SMART vs. ICS and LABA controller (higher ICS dose) RCTs
eTable 5. Sensitivity Analyses Evaluating Random-Effects Model Selection
eTable 6. Sensitivity Analyses Removing Studies with High Risk of Bias
eFigure 1. Assessment of Risk of Bias Using Cochrane Collaboration Tool
eFigure 2. Summative Assessment of Risk of Bias (No. Studies = 15)
eFigure 3. The association of SMART versus ICS and LABA controller (same dose) on mild exacerbations
eFigure 4. The association of SMART versus ICS and LABA controller (same ICS dose) on all-cause death
eFigure 5. The association of SMART versus ICS and LABA controller (higher ICS dose) on all-cause death
eFigure 6. The association of SMART versus ICS and LABA controller (same ICS dose) on ACQ-5 score
eFigure 7. The association of SMART versus ICS and LABA controller (same ICS dose) on FEV1
eFigure 8. The association of SMART versus ICS and LABA controller (same ICS dose) on rescue medication inhalations per day