Key Points
Questions
Do patients with vascular malformations have lower health-related quality of life than the US general population, and what factors contribute to this health-related quality of life?
Findings
This systematic review and meta-analysis of 11 studies that included 692 patients with vascular malformations found that patients with vascular malformations have more pain and mental distress than the US general population that contribute to a lower health-related quality of life.
Meaning
Psychosocial and mental health should be a vital component of care for patients with vascular malformations.
Abstract
Importance
Patients with vascular malformations (VAMs) and vascular overgrowth syndromes have lower health-related quality of life (HRQoL) attributable to social stigmatization, poor mental health, severity, and pain. However, the factors that contribute to this decreased HRQoL are not clear.
Objective
To perform a systematic review and meta-analysis of studies that used validated HRQoL instruments to compare the HRQoL of persons with VAMs with the US general population.
Data Sources
A comprehensive search was performed in MEDLINE, Embase, PsycINFO, CINAHL, and Scopus from 1946 to March 31, 2017, with the consultation of an experienced librarian.
Study Selection
All VAM studies with validated HRQoL instruments published in the English language were included. Case reports, review articles, non–English-language publications, and studies about the development of new HRQoL instruments were not included.
Data Extraction and Synthesis
Two reviewers assessed studies’ eligibility and the risk of bias and performed data extraction. The meta-analysis was performed using the random-effects model. Comparisons of means were performed using the unpaired, 2-sample t test.
Main Outcomes and Measures
The outcome was HRQoL.
Results
Eleven studies met the inclusion criteria for a total of 692 patients with VAMs. Six studies (320 patients) were included in the meta-analysis, whereas 5 studies were included in the qualitative analysis (372 patients). Those with VAMs had lower 36-Item Short-Form Health Survey scores in bodily pain (mean difference, −11.87; 95% CI, −21.45 to −2.29; I2 = 92%; P = .02) and mental health (mean difference, −6.04; 95% CI, −11.55 to −0.52; I2 = 83%; P = .03) compared with the US general population.
Conclusions and Relevance
Patients with VAMs had increased pain and psychosocial distress compared with the US general population. Pain and psychological morbidity are associated with poorer HRQoL and may serve as indicators for quality of life.
This systematic review and meta-analysis uses validated health-related quality-of-life instruments to compare the health-related quality of life of patients with vascular malformations with that of the US general population.
Introduction
Vascular malformations (VAMs) are abnormalities in blood vessel morphogenesis with an overall prevalence of approximately 1.2% to 1.5% among children1,2 but generally have a lifelong presentation. Malformations may include capillary malformation, venous malformation (VM), lymphatic malformation (LM), or arteriovenous malformation with or without overgrowth. Complications are common and may include thrombophlebitis, bleeding, ulcers, pain, and impairment of function attributable to VM involvement of joints, bones, or muscles.3
Patients with VAMs have a lower health-related quality of life (HRQoL) than the general population.4,5,6 The term HRQoL is used to describe an overall state of well-being and satisfaction with life, encompassing multiple dimensions, such as general health, society, work, and friendship. Factors such as social stigmatization, mental health, location, severity, and pain have been reported to affect the quality of life (QoL) in those with VAMs.5,7,8 However, the factors that contribute to this decreased QoL are largely unknown.
Several studies5,6,9 have examined HRQoL in children and adults with VAMs, but their HRQoL assessments have been largely inconclusive because of the use of nonvalidated QoL instruments. The goal of this systematic review was to obtain an accurate quantitative HRQoL measurement from patients with VAMs using only studies with validated HRQoL instruments and to compare this cohort with the US general population.
Methods
This systematic review and meta-analysis study was performed according to the MOOSE (Meta-analyses of Observational Studies in Epidemiology) guidelines.10 The PICOS (patient, problem, or population; intervention; comparison, control, or comparator; and outcome) framework was used to define the population (people of all ages), intervention or exposure (VAMs), comparison group (general US population vs those with VAMs), outcomes (HRQoL), and study (cohort and cross-sectional). The objective was to compare the HRQoL of patients with VAMs with that of the general US population using only studies with validated HRQoL instruments.
Search System Methods
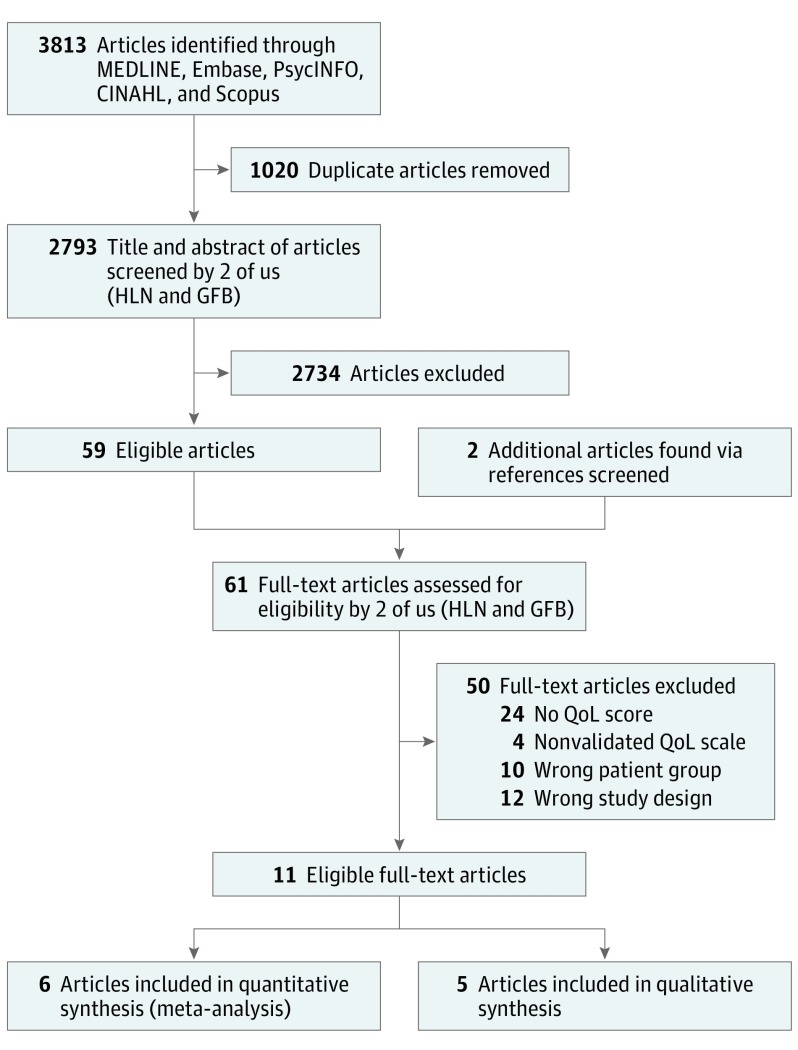
An experienced librarian (Patricia Erwin, MLS) was consulted to aid in designing a search strategy to include all studies on VAMs and HRQoL. A search was performed in the Ovid MEDLINE, Embase, PsycINFO, CINAHL, and Scopus electronic databases for English-language articles published between 1946 and March 31, 2017. Two of us (H.L.N. and G.F.B.) screened the title and abstract of all studies found through the search and afterward manually examined the references from the included articles for additional articles that the electronic search may have missed (Figure 1).
Figure 1. Flowchart of Search Methods.
QoL indicates quality of life.
Inclusion and Exclusion Criteria
All VAM studies that used validated HRQoL instruments published in the English language were included. Studies that examined the HRQoL in relation to a treatment modality were included if these studies reported the HRQoL score of patients with VAMs before starting treatment. Case reports, review articles, unpublished studies, publications not in the English language, abstracts, and studies about the development of new QoL instruments were excluded.
Study Quality Assessment
The Newcastle-Ottawa Scale (NOS)11 was used to assess the risk of bias by 2 of us (H.L.N. and G.F.B.). An adapted version of the scale was used in the cross-sectional studies. The NOS has 3 domains: selection, comparability, and outcome. The selection domain can have a maximum of 4 stars, the comparability domain can have 2 stars, and the outcome domain can have 3 stars for a combined total of 9 stars across all domains. A higher number of stars is associated with higher-quality articles and lower risks of bias (Table 1).
Table 1. Newcastle-Ottawa Quality Assessment Score for the 11 Studies Included in This Systematic Review.
| Source | Newcastle-Ottawa Quality Assessment Score, No. of Starsa | Risks of Bias | ||
|---|---|---|---|---|
| Selection | Comparability | Outcome | ||
| Durr et al,12 2017 | 3 | 1 | 2 | High or unclear |
| Hagen et al,8 2017 | 3 | 1 | 3 | High or unclear |
| Wohlgemuth et al,13 2017 | 3 | 1 | 3 | High or unclear |
| Ono et al,14 2016 | 3 | 1 | 3 | High or unclear |
| Sheikh et al,15 2015 | 3 | NA | 1 | High or unclear |
| Ramien et al,16 2014 | 2 | NA | 3 | High or unclear |
| Fahrni et al,4 2014 | 3 | NA | 2 | Moderate |
| Oduber et al,17 2010 | 3 | 1 | 3 | Moderate |
| Breugem et al,7 2004 | 3 | 1 | 3 | Moderate |
| Holme et al,18 2002 | 1 | 1 | 2 | High or unclear |
| Shakespeare et al,19 1998 | 2 | NA | 2 | High or unclear |
Abbreviation: NA, not available.
For selection, there are a maximum of 4 stars; comparability, maximum of 2 stars; and outcome, maximum of 3 stars. The higher the number of stars, the higher the quality of article.
Data Extraction
Data were extracted from the studies by 2 of us (H.L.N. and G.F.B.). Extracted data include authors, year of publication, country of study, study design, type of VAM, sample size, mean age, and HRQoL instruments and their scores. In the case that an eligible study lacked the necessary data, the first, second, and corresponding authors were contacted to obtain this information.
HRQoL Instruments
The following validated HRQoL instruments were used across the 11 studies in this systematic review.
Obstructive Sleep Apnea 18-Item Questionnaire
An obstructive sleep apnea 18-item questionnaire20 that assesses HRQoL in relation to the following 5 domains during the previous month was used: sleep disturbance, physical symptoms, emotional symptoms, daytime function, and caregiver concerns. The questionnaire also has a visual analog QoL global scale of 0 to 10, with 10 being the best possible QoL.
36-Item Short-Form Health Survey
The 36-Item Short-Form Health Survey21 (SF-36) that assesses HRQoL in the following 8 domains during the previous month was used: physical functioning (ability to perform physical activities, including bathing and dressing), role physical (problems with work or other daily activities as a result of physical health), bodily pain (pain scale and how it interferes with activities), general health (subjective overall health perception), vitality (energy level, how easily one gets tired), social functioning (interference of normal social activities attributable to physical and emotional problems), role emotional (problems with work or other daily activities as a result of emotional problems), and mental health (feelings of happiness, peacefulness, nervousness, or depression). Scores of 0 to 100 are reported, with higher scores representing a better HRQoL.
10-Item Short-Form Health Survey
A generic parent-completed 10-Item Short-Form Health Survey22 that assesses HRQoL in the past 4 weeks was used. The survey has been validated for patients between 5 and 17 years of age. Scores are reported as a physical and psychosocial health summary score of 0 to 100, with a higher score indicating better HRQoL.
Pediatric Quality of Life Inventory
A generic 21- to 23-item Pediatric Quality of Life Inventory23 that assesses HRQoL in the following 4 domains during the previous month was used: physical functioning (8 items), emotional functioning (5 items), social functioning (5 items), and school functioning (3 of 5 items). The mean sum of the last 3 domains makes up the psychosocial health summary score, and the physical functioning score is the physical health summary score. Scores of 0 to 100 are reported, with a higher score indicating better HRQoL.
Dermatology Life Quality Index
The Dermatology Life Quality Index24 was used to assess HRQoL on a scale of 30 points for patients older than 16 years during the previous week. Each item is scored on a 4-point Likert scale from 0 to 3. At the end, all items are summed to yield a total score of 0 through 30. Scores from 0 through 1 indicate no influence on the patient’s life; 2 through 5, small influence on the patient’s life; 6 through 10, moderate influence on the patient’s life; 11 through 20, very large influence on the patient’s life; and 21 through 30, extremely large influence on the patient’s life.
Children’s Dermatology Life Quality Index
The Children’s Dermatology Life Quality Index25 was used to assess HRQoL in patients 4 through 16 years of age during the previous week. The index is scored from 0 through 30 points. Scores from 0 through 1 indicate no influence on the child’s life; 2 through 6, small influence; 7 through 12, moderate influence; 13 through 18, very large influence; and 19 through 30, extremely large influence.
Skindex-29
Skindex-2926 was used to assess HRQoL during the previous month using the following 3 domains: symptoms (7 items), emotions (10 items), and functioning (12 items). A total score is calculated by averaging the scores from the 3 domains. Each item is scored on a 5-point Likert scale: never, rarely, sometimes, often, or all the time. All responses are transformed to a linear scale from 0 to 100, with higher scores indicating lower HRQoL.
Statistical Analysis
The mean, SD, sample size, and 95% CIs were extracted or calculated for the 6 studies in the meta-analysis. The Dersimonian and Laird random-effects pooling method was used for the pooled analysis of the SF-36 scores of the 8 domains.27 The random-effects model was chosen because these VAM studies measure the same outcome (SF-36 scores) but were conducted by separate groups from different countries; thus, there exists some degree of heterogeneity, which is more consistent with clinical situations.28 Heterogeneity was calculated using the I2 statistic. The I2 statistic threshold should always be interpreted with care, but a rough estimate of 25% denotes low heterogeneity, 50% denotes moderate heterogeneity, and 75% denotes high heterogeneity.29 Statistical analyses were performed using Review Manager 5.3 (Nordic Cochrane Center). For the qualitative analysis, an unpaired, 2-sample t test was used for the comparison of means.
Results
Literature Search
A search of MEDLINE found 550 publications; Embase, 1597; Scopus, 1646; PsycINFO, 0; and CINAHL, 20. The total number of publications found was 3813, of which 1020 were duplicates. As a result, there were 2793 unique publications, and after the review of abstracts and titles, 59 studies met the inclusion criteria. Screening the references of these 59 studies yielded 2 more publications that were not in the computer search. The full texts of the 61 eligible studies were reviewed by 2 of us (H.L.N. and G.F.B.). In total, 11 studies were included in the analyses (5 in the qualitative analysis and 6 in the quantitative analysis) (Figure 1).
Study Characteristics and Quality
The characteristics of each study are outlined in Table 2. Of the 11 studies included in this systematic review, 6 were cross-sectional studies and 5 were cohort studies. Four studies were from North America, 6 from Europe, and 1 from Japan. The 11 studies combined included 692 patients. The 6 studies that were included in the quantitative meta-analysis included 320 patients, whereas the 5 studies included in the qualitative synthesis included 372 patients.
Table 2. Characteristics and Scores of the 11 Studies Included in This Systematic Reviewa.
| Source | Study Design | VAM Type | Patient Population | HRQoL Measure | Results, Mean (SD) |
|---|---|---|---|---|---|
| Durr et al,12 2017 | Cross-sectional | VM or LM | 19 Patients with head and neck VM, mean (SD) age, 7.1 (4.7) years; 64 patients with disease in other locations, mean (SD) age, 7.3 (4.9) years | OSA-18 | Head and neck VMs or LMs: QoL: 7.6 (1.6); OSA-18: 2.1 (1.0); sleep disturbance: 9.2 (4.8); physical symptoms: 8.3 (4.7); emotional distress: 6.6 (4.3); daytime function: 6.7 (4.4); caregiver concerns: 6.8 (4.4); VMs or LMs at other locations: QoL: 8.9 (1.5); OSA-18: 1.6 (0.82); sleep disturbance: 6.1 (3.5); physical symptoms: 6.6 (3.6); emotional distress: 4.9 (3.5); daytime function: 4.7 (2.6); caregiver concerns: 6.2 (4.9) |
| Wohlgemuth et al,13 2017 | Cohort | VMs of the lower extremities | 16 Adults and 15 children, mean age, 23.42 y | SF-36 version 2 and SF-10 | SF-36 version 2: PF: 75.99 (17.34); RP: 71.66 (21.50); BP: 40.33 (16.35); GH: 58.33 (19.07); VT: 58.75 (17.95); SF: 79.16 (26.16); RE: 82.77 (20.76); MH: 74.33 (17.20); SF-10: PHS: 25.25 (9.59); PSS: 51.08 (8.84) |
| Hagen et al,8 2017 | Cross-sectional | Facial CM | 244 Patients, mean (SD) age, 38.9 (13.2) years | Skindex-29 | Skindex-29 symptoms: 14.9 (18.4); emotions: 34.4 (25.8); functioning: 24.3 (22.3); total: 24.6 (19.1) |
| Ono et al,14 2016 | Cohort | VM | 28 Patients, mean age, 25.3 y | SF-36 version 2 | SF-36 version 2: PF: 90 (24.34); RP: 81 (39.16); BP: 63 (26.03); GH: 68 (22.16); VT: 63 (18.78); SF: 85 (27.58); RE: 88 (31.91); MH: 73 (19.98) |
| Sheikh et al,15 2015 | Cross-sectional | LM neck mass | 13 Patients, mean age NA | PedsQL | Physical: 83; psychosocial health summary: 78; emotional: 78; social: 79; school: 71 |
| Ramien et al,16 2014 | Cohort | VM | 6 Patients, mean age NA | CDLQI | CDLQI: 5.5 |
| Fahrni et al,4 2014 | Cross-sectional | AVM, VM, CM, LM | 71 Patients, mean (SD) age, 40 (16) years | SF-36 | SF-36: PF: 81 (23); RP: 72 (39); BP: 64 (28); GH: 63 (18); VT: 57 (20); SF: 80 (25); RE: 80 (35); MH: 70 (18) |
| Oduber et al,17 2010 | Cross-sectional | KTS | 78 Patients, mean age, 39.3 y | SF-36 and Skindex-29 | SF-36: PF: 67.5 (30.5); RP: 59.9 (43); BP: 60.9 (22.8); GH: 57.4 (22.6); VT: 63.6 (18.8); SF: 77.3 (21.2); RE: 84.7 (34.2); MH: 77.9 (16.2); Skindex-29: symptoms: 26 (19.7); emotions: 21.9 (18.9); functioning: 17.3 (17.5); total: 20.8 (16.2) |
| Breugem et al,7 2004 | Cross-sectional | VM, LM, AVM, CM, KTS | 81 Patients, mean (SD) age, 30.4 (10.5) years | SF-36 | SF-36: PF: 83.2 (18.7); RP: 73.1 (40.7); BP: 68.9 (25.6); GH: 67 (26.6); VT: 62.7 (18.0); SF: 79.7 (22.5); RE: 78.2 (37.0); MH: 73.6 (17.6) |
| Holme et al,18 2002 | Cohort | Thread veins, CM | 11 Patients, mean age, 50 y | DLQI | DLQI: 8 (5.7) |
| Shakespeare et al,19 1998 | Cohort | VM and CM | 46 Patients, mean age NA | SF-36 | SF-36: PF: 88.60 (23.12); RP: 86.50 (30.00); BP: 82.02 (25.69); GH:75.60 (21.02); VT: 58.10 (21.11); SF: 78.46 (27.26); RE: 75.92 (37.57); MH: 64.96 (22.66) |
| Vascular malformations pooled cohort4,7,13,14,17,19 | NA | CM, VM, LM, AVM, KTS | 320 Patients | SF-36 | SF-36: PF: 79.9 (24.5); RP: 72.2 (39.3); BP: 65.8 (26.0); GH: 69.5 (22.2); VT: 64.3 (18.8); SF: 81.2 (27.6); RE: 75.8 (32.1); MH: 67.6 (20.1) |
| US general population21 | NA | NA | 2474 Patients | SF-36 | SF-36: PF: 84.2 (23.3); RP: 81.0 (34.0); BP: 75.2 (23.7); GH: 72.0 (20.3); VT: 60.9 (21.0); SF: 83.3 (22.7); RE: 81.3 (33.0); MH: 74.7 (18.1) |
Abbreviations: AVM, arteriovenous malformation; BP, bodily pain; CDLQI, Children’s Dermatology Life Quality Index; CM, capillary malformation; DLQI, Dermatology Life Quality Index; GH, general health; KTS, Klippel-Trenaunay syndrome; LM, lymphatic malformation; MH, mental health; NA, not available; OSA-18, obstructive sleep apnea 18-item questionnaire; PedsQL, Pediatric Quality of Life Inventory; PF, physical functioning; QoL, quality of life; RE, role emotion; RP, role physical; SF, social functioning; SF-10, 10-Item Short-Form Health Survey; SF-36, 36-Item Short-Form Health Survey; VAM, vascular malformation; VM, venous malformation; VT, vitality.
Also included are the pooled VAM cohort of the 6 quantitative studies and the general US population SF-36 scores.
With use of the NOS, 5 studies received a 7-star rating, which denotes satisfactory-quality articles with low risks of bias, whereas the rest of the studies had substantial risks of bias. However, we believed that the 3 domains of the NOS did not capture risks of bias accurately in these studies.30 Therefore, in addition to reporting the NOS score, we provided our own assessment of these studies’ risks of bias based on sample size, inclusion and exclusion criteria, control for other comorbidities, survey response rate, patients who were lost to or unavailable for follow-up, statistical methods, and whether the research questions and the outcome were clearly defined. On the basis of our judgment, 8 studies were rated as having high or unclear risks of bias and 3 studies as having moderate risks of bias (Table 1).
Quantitative Meta-analysis
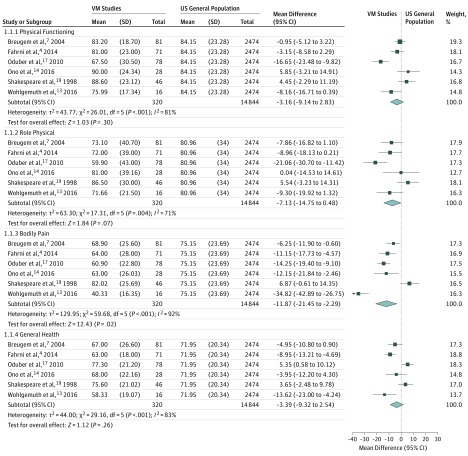
The results of the meta-analysis included 6 studies4,7,13,14,17,19 and are given in Figure 2 and Figure 3. One study14 did not provide explicit SF-36 scores and SD values. Two attempts were made to contact the authors, and the second author replied that he was not able to provide the data. Thus, the scores were extracted from the graph, and the combined SDs from the 5 studies4,7,13,17,19 were used because all 6 studies had similar patients’ characteristics.
Figure 2. Meta-analysis of 6 Studies Comparing the 36-Item Short-Form Health Survey Domains of Physical Functioning, Role Physical, Bodily Pain, and General Health Against the US General Population.
Bodily pain results are statistically significant. VM indicates venous malformation.
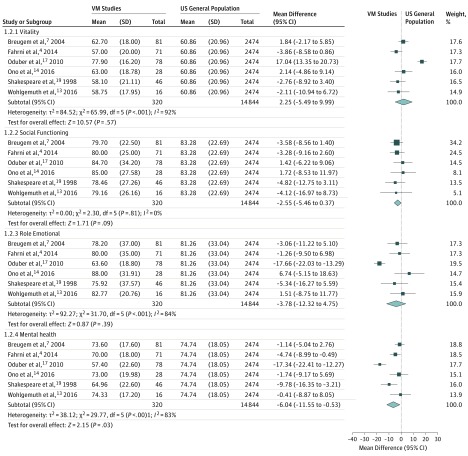
Figure 3. Meta-analysis of 6 Studies Comparing the 36-Item Short-Form Health Survey Domains of Vitality, Social Functioning, Role Emotional, and Mental Health Against the US General Population.
Mental health results are statistically significant. VM indicates venous malformation.
Significant associations were found in 2 domains of the SF-36 between patients with VAMs and the general US population. Patients with VAMs had lower scores in bodily pain (mean difference [MD], −11.87; 95% CI, −21.45 to −2.29; I2 = 92%; P = .02) and mental health (MD, −6.04; 95% CI, −11.55 to −0.52; I2 = 83%; P = .03). No significant association was found in the other 6 domains: physical functioning, role physical, general health, vitality, social functioning, and role emotional.
Qualitative Analysis
Durr et al12 performed a cross-sectional study to assess the HRQoL in those with VMs and LMs of the head and neck vs at other locations using the obstructive sleep apnea 18-item questionnaire. The study found that patients with VMs and LMs of the head and neck had lower mean (SD) QoL scores (7.6 [1.6] vs 8.9 [1.5]; P = .003), greater sleep disturbance (9.2 [4.8] vs 6.1 [3.5]; P = .004), and a more negative influence on daytime function (6.7 [4.4] vs 4.7 [2.6]; P = .01) than patients with VMs and LMs at other locations.
Holme et al18 examined a cohort of 11 individuals with thread veins and capillary malformations and found that they had a mean (SD) Dermatology Life Quality Index score of 8 (5.7), which according to the score stratification, indicates a moderate influence on HRQoL. Hagen et al8 performed a cross-sectional study on a cohort of 244 patients with facial port wine stains (PWSs) using Skindex-29 and found the following mean (SD) Skindex-29 scores: symptoms, 14.9 (18.4); emotions, 34.4 (25.8); functioning, 24.3 (22.3); and total, 24.6 (19.1). These findings signify that facial PWSs have a moderate influence on HRQoL.31
HRQoL in the Pediatric Population
Three studies15,16,32 investigated HRQoL of VAMs in the pediatric population. Sheikh et al15 surveyed 13 children with LM of the neck using the Pediatric Quality of Life Inventory and found that this cohort had a physical functioning score of 83, an emotional functioning score of 78, a social functioning score of 79, a school functioning score of 71, and a psychosocial health summary score of 78. The combination of small sample size and the lack of SD values in this study makes statistical analysis not possible; thus, no conclusion could be made. However, the scores do not differ much from those for the general population.32 Ramien et al16 derived a 5.5 Children’s Dermatology Life Quality Index score in a group of 6 children with VAMs. These children had a similar HRQoL level as those with acne vulgaris and urticaria.33 Wohlgemuth et al13 surveyed the parents of 15 children with VAMs from the age of 6 to 18 years using the 10-Item Short-Form Health Survey; the mean (SD) health summary score was 25.25 (9.59), and the mean (SD) psychosocial summary score was 51.08 (8.84).
Patients With Klippel-Trenaunay Syndrome
Klippel-Trenaunay syndrome (KTS) is a vascular overgrowth syndrome that is characterized by PWSs, VMs, and bone or soft tissue overgrowth.34 Only 1 study17 assessed the HRQoL solely in patients with KTS using the SF-36 and the Skindex-29. Compared with the pooled VAM studies, patients with KTS had lower physical functioning (MD, −12.4; 95% CI, −18.80 to −6.00; P < .001), role physical (MD, −12.3; 95% CI, −22.24 to −2.36; P = .02), general health (MD, −12.1; 95% CI, −17.63 to −6.57; P < .001), role emotional (MD, 8.9; 95% CI, 0.83-16.97; P = .03), and mental health (MD, 10.3; 95% CI, 5.48-15.12; P < .001). Compared with the general US population, patients with KTS had lower physical functioning (MD, −16.7; 95% CI, −22.02 to −11.38; P < .001), role physical (MD, −21.1; 95% CI, −28.85 to −13.35; P < .001), bodily pain (MD, −14.3; 95% CI, −19.65 to −8.95; P < .001), general health (MD, −14.6; 95% CI, −19.20 to −10.00; P < .001), and social functioning (MD, −6.0; 95% CI, −11.12 to −0.88; P = .02). Overall, patients with KTS had lower HRQoL than both the general US population and patients with isolated VAMs.
According to the Skindex-29 (higher scores denote worse HRQoL), patients with KTS had lower HRQoL in the symptoms domain than those without skin disease, those with vitiligo, and those with facial PWS but better HRQoL than those with psoriasis, pemphigus, eczema, epidermolysis bullosa, tinea, lichen sclerosus, cutaneous lupus erythematosus, dermatomyositis, and vulvodynia. For the emotions domain, patients with KTS had poorer HRQoL than patients without skin disease but better HRQoL than those with vitiligo, facial PWS, rosacea, acne vulgaris, morphea, psoriasis, pemphigus, eczema, epidermolysis bullosa, tinea, lichen sclerosus, cutaneous lupus erythematosus, dermatomyositis, and vulvodynia. Finally, patients with KTS had poorer HRQoL in the functioning domain than patients without skin disease, warts, and nonmelanoma skin cancer or actinic keratosis but better HRQoL than those with facial PWS, chronic hand dermatitis, pemphigus, eczema, epidermolysis bullosa, tinea, lichen sclerosus, cutaneous lupus erythematosus, dermatomyositis, and vulvodynia (eTable in the Supplement).
Discussion
The results of this systematic review and meta-analysis suggest that patients with VAMs have higher bodily pain than the general US population, which greatly influences HRQoL. This influence is likely in large part attributable to complications that result in significant morbidity in this population.35 Of importance, patients with VAMs have lower SF-36 mental health scores, which represent overall mood and mental state, compared with the US general population.21 Although specialists who care for patients with VAMs often focus on the pressing medical morbidities that are often present, evaluation of psychosocial and mental health should be an important component of care, with appropriate referrals for support and counseling made as indicated.
A specific subset of patients with VAMs have KTS. In addition to the VMs and LMs, patients with KTS also have PWSs and overgrowth of bone or soft tissue, the latter of which can also pose challenging difficulties with physical health. This triad of findings has a significant influence on HRQoL. Those with KTS have lower scores in 5 of 8 SF-36 domains (physical functioning, role physical, general health, role emotion, and mental health) compared with the VAM cohort. This finding suggests that patients with KTS are more physically and mentally impaired compared with those with isolated VAMs. The KTS mental health score revealed no statistically significant difference vs the general US population but a statistically significant higher mental health score vs the VAM cohort. A possible explanation for this observation is that those who are born with KTS have developed resilience. In addition, robust patient advocacy groups currently exist for KTS, which may be helpful with the mental burden and resiliency. However, because this observation is based only on 1 European study,17 it may not be generalizable to all patients with KTS.
Nijsten et al31 statistically categorized Skindex-29 scores to distinct stratification based on the influence on HRQoL. This study revealed that KTS had a moderate impairment on HRQoL and a similar HRQoL score to vitiligo, onychomycosis, alopecia areata, and facial PWSs (eTable in the Supplement). Although this appears to be reassuring, the Skindex-29 has only been validated for QoL assessment of patients with skin diseases and likely underestimates the influence of the extracutaneous involvement in patients with KTS. However, it may be inferred that the cosmetic appearance of KTS has a minimal influence on patients’ HRQoL and that treatment can likely focus on alleviating functional impairment.
In addition to severity of the disease, the location of VAMs can have an influence on HRQoL. Those with VMs and LMs of the head and neck have more difficulty with sleeping and daytime functioning than those with VMs and LMs at other locations, and as a result they have lower HRQoL.12 This finding suggests that evaluating sleep hygiene and quality should be a focus when treating patients with head and neck VMs and LMs, which is often not a primary focus of those caring for these patients.
Patients with facial PWSs had lower HRQoL scores across all domains of the Skindex-29 compared with the population without skin disease8 (eTable in the Supplement). The emotions domain is influenced the most, followed by the functioning and the symptoms domain. The emotional influence of facial PWSs is similar to that of vitiligo, rosacea, epidermolysis bullosa, and psoriasis.8 This finding confirms that visible skin aberration, such as PWSs, on a highly visible area of the body has an influence on a person’s mental health, in accordance with prior studies.9,36
Strengths and Limitations
This systematic review has many strengths, particularly regarding methods. First, the search strategy was performed thoroughly with the consultation of an experienced librarian and is reproducible. Second, 2 of us screened for eligible studies, reviewed the full-text articles, performed quality assessments, and extracted the relevant data. Therefore, potential sources of biases and errors were minimized. Third, only studies that used validated HRQoL instruments were included, which resulted in a comprehensive analysis of HRQoL in patients with vascular anomalies with multiple findings that are meaningful and applicable to clinical practice.
There are several limitations regarding making inferences from this systematic review and meta-analysis. For nonrandomized, observational studies, the NOS is one of the few available tools to assess studies’ risk of bias37 and therefore is often used by many systematic reviews. Despite this, we and others have found that the NOS does not capture the nuances of the 11 studies adequately and therefore is an inaccurate indicator of risks of bias.30,38,39 Therefore, any conclusions regarding articles’ quality using NOS scores should be interpreted with caution. Second, there is significant heterogeneity among the 6 studies in this meta-analysis because VAM is an umbrella term that includes capillary malformation, VM, LM, arteriovenous malformation, and vascular overgrowth syndromes, all of which are heterogeneous in the presentation. We were unable to consider the severity, type, and location of the malformation on the body, time frame of diagnosis, treatments, other comorbidities and their severities, age distribution of those affected, and society differences in medical and mental health support in the analysis. Thus, it is possible that those with more severe VAMs or those who live in areas with less medical and mental health support may have even lower HRQoL. Furthermore, sampling bias may exist because those with more severe VAMs are more likely to get treatment and therefore are more likely to be enrolled in HRQoL studies. Finally, some of these studies have a small sample size of 30 or fewer patients13,14,15,16,18 and a disproportionate female to male ratio;13,16 thus, they may not represent the wider population.
Conclusions
Patients with VAMs have more pain and greater mental stress than the US general population, findings that are influenced by the location of involvement. Those with VMs or LMs of the head and neck have worse HRQoL than individuals with VMs or LMs at other locations because of visibility and influence on sleep function, and sleep evaluation must be considered in these patients. Vascular overgrowth syndromes, such as KTS, have a greater negative influence on HRQoL than do isolated VAMs because of decreased functionality, suggesting that decreased functionality should be a focus in evaluating and treating those with KTS. Although generally benign, PWSs can cause mental distress when they are present in a visible area of the body. Psychological morbidity and pain are associated with poorer HRQoL in patients with VAMs and may serve as predictive factors for HRQoL. Future research should focus on how pain, location, and type of VAM influence HRQoL. Quality of life is a crucial factor that should not be overlooked in patients with VAMs and should be an important part of clinical care. Understanding of the type, quality, and location of VAMs can be helpful to guide support and care of these patients.
eTable. Skindex-29 scores and comparisons of Patients With Klippel-Trenaunay Syndrome Against Those With Other Diseases
References
- 1.Tasnádi G. Epidemiology of vascular malformations In: Mattassi R, Loose DA, Vaghi M, eds. Hemangiomas and Vascular Malformations: An Atlas of Diagnosis and Treatment. Milan, Italy: Springer Milan; 2009:109-110. [Google Scholar]
- 2.Eifert S, Villavicencio JL, Kao TC, Taute BM, Rich NM. Prevalence of deep venous anomalies in congenital vascular malformations of venous predominance. J Vasc Surg. 2000;31(3):462-471. [PubMed] [Google Scholar]
- 3.Cox JA, Bartlett E, Lee EI. Vascular malformations: a review. Semin Plast Surg. 2014;28(2):58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahrni JO, Cho EY, Engelberger RP, Baumgartner I, von Känel R. Quality of life in patients with congenital vascular malformations. J Vasc Surg Venous Lymphat Disord. 2014;2(1):46-51. [DOI] [PubMed] [Google Scholar]
- 5.van der Ploeg HM, van der Ploeg MN, van der Ploeg-Stapert JD. Psychological aspects of the Klippel-Trenaunay syndrome. J Psychosom Res. 1995;39(2):183-191. [DOI] [PubMed] [Google Scholar]
- 6.Steiner F, FitzJohn T, Tan ST. Ethanol sclerotherapy for venous malformation. ANZ J Surg. 2016;86(10):790-795. [DOI] [PubMed] [Google Scholar]
- 7.Breugem CC, Merkus MP, Smitt JH, Legemate DA, van der Horst CM. Quality of life in patients with vascular malformations of the lower extremity. Br J Plast Surg. 2004;57(8):754-763. [DOI] [PubMed] [Google Scholar]
- 8.Hagen SL, Grey KR, Korta DZ, Kelly KM. Quality of life in adults with facial port-wine stains. J Am Acad Dermatol. 2017;76(4):695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanigan SW, Cotterill JA. Psychological disabilities amongst patients with port wine stains. Br J Dermatol. 1989;121(2):209-215. [DOI] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 11.Wells GSB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 10, 2017.
- 12.Durr ML, Meyer AK, Kezirian EJ, Mamlouk MD, Frieden IJ, Rosbe KW. Sleep-disordered breathing in pediatric head and neck vascular malformations. Laryngoscope. 2017;127(9):2159-2164. [DOI] [PubMed] [Google Scholar]
- 13.Wohlgemuth WA, Müller-Wille R, Teusch V, Hammer S, Wildgruber M, Uller W. Ethanolgel sclerotherapy of venous malformations improves health-related quality-of-life in adults and children: results of a prospective study. Eur Radiol. 2017;27(6):2482-2488. [DOI] [PubMed] [Google Scholar]
- 14.Ono Y, Osuga K, Takura T, et al. Cost-effectiveness analysis of percutaneous sclerotherapy for venous malformations. J Vasc Interv Radiol. 2016;27(6):831-837. [DOI] [PubMed] [Google Scholar]
- 15.Sheikh F, Akinkuotu A, Olutoye OO, et al. Prenatally diagnosed neck masses: long-term outcomes and quality of life. J Pediatr Surg. 2015;50(7):1210-1213. [DOI] [PubMed] [Google Scholar]
- 16.Ramien ML, Ondrejchak S, Gendron R, et al. Quality of life in pediatric patients before and after cosmetic camouflage of visible skin conditions. J Am Acad Dermatol. 2014;71(5):935-940. [DOI] [PubMed] [Google Scholar]
- 17.Oduber CE, Khemlani K, Sillevis Smitt JH, Hennekam RC, van der Horst CM. Baseline quality of life in patients with Klippel-Trenaunay syndrome. J Plast Reconstr Aesthet Surg. 2010;63(4):603-609. [DOI] [PubMed] [Google Scholar]
- 18.Holme SA, Beattie PE, Fleming CJ. Cosmetic camouflage advice improves quality of life. Br J Dermatol. 2002;147(5):946-949. [DOI] [PubMed] [Google Scholar]
- 19.Shakespeare V, Shakespeare P, Cole RP. Measuring patient satisfaction with pulsed dye laser treatment of vascular lesions. Lasers Med Sci. 1998;13(4):253-259. [DOI] [PubMed] [Google Scholar]
- 20.Franco RA Jr, Rosenfeld RM, Rao M. First place–resident clinical science award 1999: quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;123(1, pt 1):9-16. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Kosinski M, Dewey JE, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality Metric Inc; 2000. [Google Scholar]
- 22.Optum. SF-10 Health Survey for Children. http://campaign.optum.com/optum-outcomes/what-we-do/pediatric-health-surveys/children.html. Accessed May 14, 2017.
- 23.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126-139. [DOI] [PubMed] [Google Scholar]
- 24.Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159(5):997-1035. [DOI] [PubMed] [Google Scholar]
- 25.Salek MS, Jung S, Brincat-Ruffini LA, et al. Clinical experience and psychometric properties of the Children’s Dermatology Life Quality Index (CDLQI), 1995-2012. Br J Dermatol. 2013;169(4):734-759. [DOI] [PubMed] [Google Scholar]
- 26.Chren MM. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatol Clin. 2012;30(2):231-236, xiii. xiii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 28.Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44(2):127-139. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605. [DOI] [PubMed] [Google Scholar]
- 31.Nijsten T, Sampogna F, Abeni D. Categorization of Skindex-29 scores using mixture analysis. Dermatology. 2009;218(2):151-154. [DOI] [PubMed] [Google Scholar]
- 32.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155(1):145-151. [DOI] [PubMed] [Google Scholar]
- 34.Volz KR, Kanner CD, Evans J, Evans KD. Klippel-Trénaunay syndrome: need for careful clinical classification. J Ultrasound Med. 2016;35(9):2057-2065. [DOI] [PubMed] [Google Scholar]
- 35.Lee A, Driscoll D, Gloviczki P, Clay R, Shaughnessy W, Stans A. Evaluation and management of pain in patients with Klippel-Trenaunay syndrome: a review. Pediatrics. 2005;115(3):744-749. [DOI] [PubMed] [Google Scholar]
- 36.Gupta MA, Gupta AK. Psychiatric and psychological co-morbidity in patients with dermatologic disorders: epidemiology and management. Am J Clin Dermatol. 2003;4(12):833-842. [DOI] [PubMed] [Google Scholar]
- 37.Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2-10. [DOI] [PubMed] [Google Scholar]
- 38.Hartling L, Milne A, Hamm MP, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol. 2013;66(9):982-993. [DOI] [PubMed] [Google Scholar]
- 39.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Skindex-29 scores and comparisons of Patients With Klippel-Trenaunay Syndrome Against Those With Other Diseases