Abstract
Importance
The use of autologous fat transfer (AFT) or lipofilling for correcting contour deformities is seen as one of the major breakthroughs in reconstructive plastic surgery. Its applications in facial reconstructive surgery have been of particular interest owing to the prospect of achieving autologous reconstruction by a minimally invasive approach. However, its unpredictability and variable degree of resorption have limited its utility and much skepticism still exists regarding its efficacy. Furthermore, more than 2 decades of clinical research have produced a highly fragmented body of evidence that has not been able to provide definite answers.
Objective
To investigate the safety and efficacy of AFT in facial reconstruction through a systematic review and meta-analysis.
Data Sources
A literature search was performed in PubMed, Embase, and the Cochrane Library from inception to October 11, 2017.
Study Selection
All published studies investigating the efficacy and safety of AFT in facial reconstructive surgery.
Data Extraction and Synthesis
Two independent reviewers performed data extraction systematically, adhering to the PRISMA guidelines. Summary measures were pooled in a random-effects model meta-analysis.
Main Outcomes and Measures
The patient and surgeon satisfaction, graft survival, number of AFT sessions, and the incidence of AFT-related complications were the main outcomes of interest in this meta-analysis.
Results
This systematic review resulted in the inclusion 52 relevant studies consisting of 1568 unique patients. These included 4 randomized clinical trials, 11 cohort studies, and 37 case series. The overall follow-up averaged 1.3 years after AFT. Meta-analysis revealed a very high overall patient satisfaction rate of 91.1% (95% CI, 85.1%-94.8%) and overall surgeon satisfaction rate of 88.6% (95% CI, 83.4%-92.4%). The number of AFT sessions required to achieve the desired result was 1.5 (95% CI, 1.3-1.7) and 50% to 60% of the injected volume was retained at 1 year. Only 4.8% (95% CI, 3.3%-6.9%) of procedures resulted in clinical complications.
Conclusions and Relevance
To our knowledge, this study provides the first overview of the current knowledge about AFT in facial reconstructive surgery. Our results confirm that AFT is an effective technique for treating soft-tissue deformities in the head and neck, with low rate of minor complications.
Level of Evidence
NA.
This systematic review and meta-analysis investigates the safety and efficacy of autologous fat transfer in facial reconstruction.
Key Points
Question
Is autologous fat transfer (lipofilling) a safe and effective treatment for soft-tissue deformities in the face?
Findings
This systematic review and meta-analysis of 52 studies and 1568 patients found autologous fat transfer to be associated with a high patient satisfaction rate (91.1%) and surgeon satisfaction rate (88.6%) after 1.5 sessions. Volume retention stabilized at 50% to 60% at 1 year, and only 4.8% of procedures resulted in clinical complications.
Meaning
This meta-analysis of the combined data from all published studies confirms that autologous fat transfer is a safe and effective treatment in facial reconstructive surgery.
Introduction
Autologous fat transfer (AFT), also known as lipofilling or fat grafting, is regarded as an elegant technique that excels in correcting soft-tissue contour deformities and volume deficits in almost any body part. Its application in the face has always been of particular interest because it allows for precise titration and injection of the patient’s own fat by means of a minimally invasive approach. Autologous fat transfer can play an important role in the treatment of disfiguring congenital syndromes such as progressive hemifacial atrophy (Parry-Romberg syndrome) or hemifacial (craniofacial) microsomia. For these disorders, the only viable alternative for correcting volume deficits remains the free-flap transfer, which often leads to disappointing results in the long term due to sagging and disruption of facial musculature. Another disorder that can greatly benefit from AFT is human immunodeficiency virus (HIV)-associated lipodystrophy, where antiretroviral therapy can lead to devastating facial wasting. The prospect of restoring facial volume without relying on allogenic fillers of implants makes AFT a promising solution in these patients. Owing to these properties, fat transfer has also been increasingly applied in the treatment of acquired soft-tissue defects in the face such as adhesive scars and even burns (Figure 1).
Figure 1. Examples of Indications That Could Potentially Benefit From Autologous Fat Transfer.
This is an original illustration by Todor K. Krastev, MD, depicting patients with acquired soft-tissue defects who could benefit from the autologous fat transfer technique.
However, the unpredictability of long-term graft retention has hampered the widespread acceptance and application of AFT in reconstructive plastic surgery. The loss of a significant portion of the grafted volume seems to be an inherent limitation with AFT, which has been the main criticism with this technique. This has engendered divergent and even polarized opinions among plastic surgeons worldwide with respect to whether AFT offers a reliable solution for correcting soft-tissue deformities in facial reconstructive surgery. Unfortunately, more than two decades of clinical research have failed to provide convincing evidence on this matter. The reason is that published literature comprises of a large number of small studies investigating a diversity of outcome measures in different patient populations, producing a body of evidence that is highly fragmented and difficult to analyze.
This has inspired us to perform the first systematic review and meta-analysis on the subject, aimed to facilitate interpretation of the evicence on the efficacy and safety of AFT in facial reconstructive surgery by clinicians, guideline committees, and policy makers.
Methods
This systematic review adhered to the standards of the Cochrane Handbook of Systematic Reviews of Interventions1 and the Meta-analysis Of Observational Studies in Epidemiology2 and was written in the format provided by the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) statement3 (eTable 1 in the Supplement). A comprehensive, reproducible electronic search was conducted in PubMed, Embase, and the Cochrane Library to identify all published studies with human subjects receiving AFT for facial reconstruction (eTables 2 and 3 in the Supplement). The search was performed on October 11, 2017. Eligibility assessment was performed in a standardized manner. The retrieved hits were screened and reviewed by 2 independent reviewers (T.K.K. and J.B.) based on the title and abstract using predefined inclusion and exclusion criteria. Disagreements were solved through discussion until consensus was reached. An additional assessment was performed based on the full-text versions of all selected records and those with insufficient information in the title and abstract. All references were stored in Endnote Reference Management Tool version X7.3.1 (Thomson Reuters).
To be able to conduct a meta-analysis, a thorough extraction of all relevant data was performed (eTable 4 in the Supplement). In some occasions, authors were contacted to send additional data. In cases when key data was reported only in graphs or figures, it was extracted using the WebPlotDigitizer software.4 Whenever necessary, units were transformed to a standard format to ensure comparability and allow pooling of data. Continuous variables reported in the form of median (range) were transformed into mean (SD) using the standard estimating equations used for meta-analyses.5 Custom outcome measurement scales and scores were also standardized accordingly. Finally, if studies from the same author or institution conducted in the same time period and reporting the same outcomes were suspected for overlap of more than 25% of the sample size, only the largest or most relevant study was included. Meta-analysis was performed using the metafor package6 of the R software (R Project).7 Summary measures were pooled in a standard random-effects model and presented as forest plots. Heterogeneity was assessed using the I2 statistic, which was tolerable if below 50%. Publication bias considered acceptable if on visual inspection the distribution of studies was approximately symmetrical.
Results
Study Selection
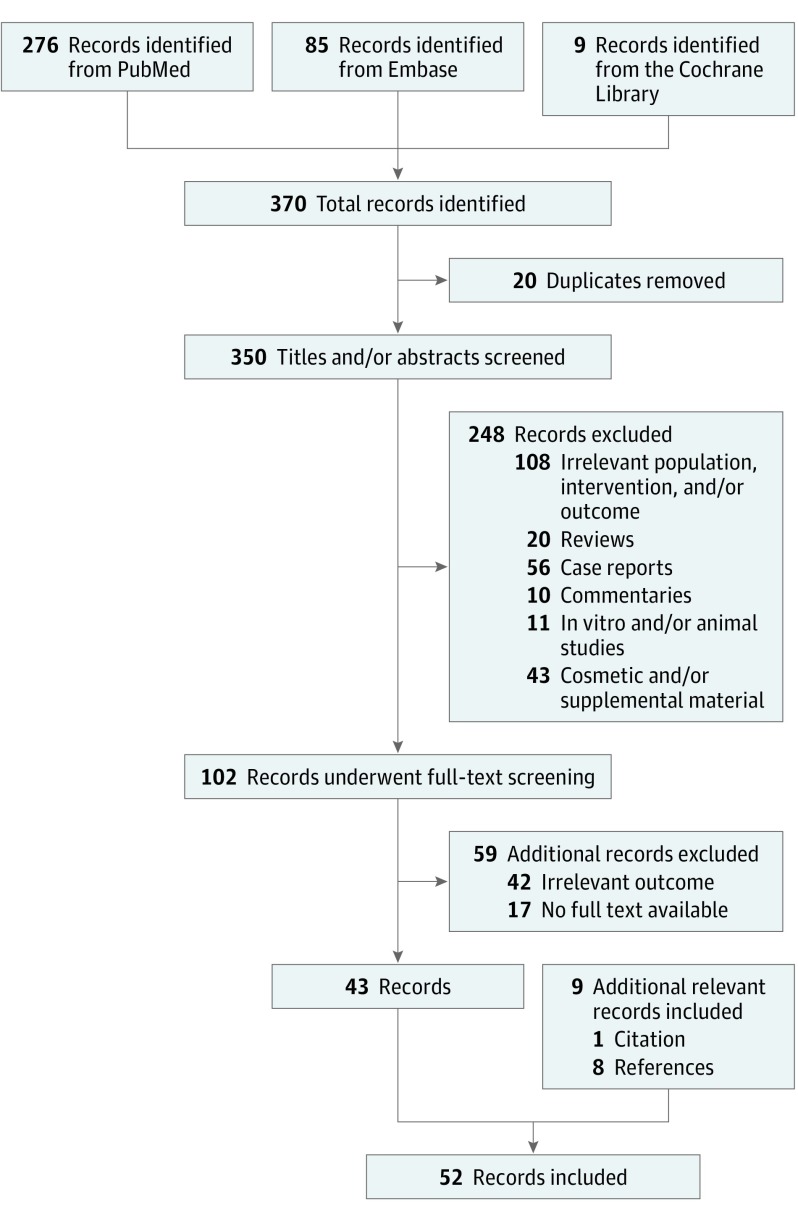
The electronic search yielded a total of 370 unique hits (Figure 2). Screening of the title and abstract lead to the inclusion of 102 studies for further evaluation. A total of 43 clinical trials were selected through further screening of the full-text. Reasons for exclusion consisted of the unavailability of the full text, secondary sources (reviews, commentaries), the use of AFT for cosmetic indication and enhancement with supplements (such as platelet-rich plasma or stromal-vascular fraction). Case reports (defined as reports of 5 cases or fewer) were also not considered for inclusion. Screening of the citations, references, and related articles of the 43 selected trials yielded 9 additional hits. It should be noted that some cohort studies consisting of 1 or more treatment arms, which did not meet the inclusion criteria of the predefined PICO (patient/problem/population, intervention, comparison/control/comparator, outcome) process, such as use of supplements or combination treatments, were not included in the analysis. This was also the case whenever outcomes of interest were only relevant or measured in one of the treatment groups.
Figure 2. Study Selection Process.
Flow diagram depicting the number of articles identified, retrieved, screened, and included in the final systematic review and meta-analysis.
Study Characteristics
Fifty-two clinical trials were identified for inclusion8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59 (eTable 5 in the Supplement). They consisted of 37 case series, 11 cohort studies, and 4 randomized clinical trials. The majority of these had a retrospective design (n = 44), and all studies were conducted between January 1986 and December 2016.
Population
From the 52 included studies (1549 patients), only 1 study was suspected of significant overlap in patient populations,45 resulting in 51 studies with a total of 1533 unique patients. Most commonly, a study would include patients of a specific diagnosis. In fourteen studies,11,12,17,19,20,23,33,35,37,38,44,45,49,54 it involved patients with HIV-associated lipodystrophy, comprising 650 patients, thus representing the most common indication for which AFT is decribed in published literature. The majority of patients with HIV-associated lipodystrophy (n = 500 of 650 [76.9%]) were men, and the mean age at the time of the first AFT session was 42.6 years (95% CI, 34.4-46.7). Furthermore, 16 studies10,14,15,16,25,26,28,32,43,47,50,52,53,57,58,59 consisting of 409 patients evaluated AFT in patients with congenital disorders. From this group, 201 (49.1%) patients suffered from Parry-Romberg syndrome, 181 (44.3%) from craniofacial microsomia, and the remaining 27 patients (6.6%) represent a mixed group of craniofacial microsomia, Goldenhar syndrome, and Treacher Collins from a single study.32 The mean age at the time of treatment with AFT was 23.8 years (95% CI, 19.8-27.8) in this group. Another 17 studies8,9,13,18,21,22,24,27,29,30,39,40,41,46,48,55,56 formed a more heterogeneous group consisting 326 patients with a mean age of 36.8 years (95% CI, 31.5-42.1) treated with AFT for a variety of acquired facial deformities. In 7 studies, AFT was applied for treating small contour deformities such as facial scars (eg, traumatic, thermal injury), and in another 2 studies, AFT was performed for lip contouring after resection of epithelial cancer or cleft lip surgery. Five studies involved the restoration of contour deformities after maxillofacial reconstruction after trauma, and 2 studies examined use of AFT after autologous reconstruction of irradiated head and neck cancer. Finally, 1 study performed facial contouring with AFT in addition to conventional surgical treatment for facial burns involving large areas of the face. Each of the remaining 4 studies31,34,42,51 (148 patients) involved a mixed group of patients from any of the abovementioned indications.
Intervention
From the included trials, the vast majority adhered to the principles of AFT as described by Coleman,60 with the occasional minor variation in the surgical instruments used.
Control Group
Only 16 studies examining AFT included a control group, which most often involved patients receiving AFT with supplements (n = 9) such as platelet-rich plasma,19,21 stromal-vascular fraction,14,21,51,52,59 bone marrow–derived mesenchymal stem cells,26 or cultured adipose-derived stem cells.28 As the scope of this systematic review and meta-analysis is limited to the use of AFT without supplements, treatment arms that involved enhancement of AFT with the abovementioned supplements were excluded from the analysis. Another 4 studies involved additional treatment arms receiving (semi-) permanent fillers,36,37,38,44 2 studies compared AFT with autologous flaps,47,53 and the last study—with dermis-fat grafts.33
Outcome Measures
The satisfaction of the patient with respect to the result of the AFT reconstruction was measured in 30 trials. Twelve of these studies presented the result using categorical or Likert scores and reported the number of patients with a certain score (category) at the end of the treatment. The Likert scales consisted of a scale with 2 to 5 categories corresponding to degrees of satisfaction with the outcome (eg, very dissatisfied, dissatisfied, satisfied, very satisfied) or opinion about the rate of improvement (eg, very poor, poor, fair, good, excellent). To standardize the scales, scores were dichotomized into 2 distinct categories—satisfied vs dissatisfied. For all studies, such a cut-off point between the positive and negative categories was easily identifiable. Furthermore, 22 studies presented satisfaction scores in the form of custom-made visual analog scales (VAS), ranging from 4 to 50 levels, which were transformed into a standard 10-point VAS scale (0 = maximum dissatisfaction, 10 = maximum satisfaction).
Surgeon Satisfaction and/or Evaluation
Twenty-nine studies reported the surgeon’s satisfaction, which typically consisted of visual evaluation of preoperative and postoperative photographs to determine the rate of success of the AFT treatment. Sixteen studies reported surgeon’s evaluation using Likert scales with 2 to 5 categories describing the effect of AFT on the clinical result, which were subsequently dichotomized into good vs poor. Ten studies reported mean VAS scores (range 0-3 to 0-20), and these were also transformed to a standard scale from 0 (very poor result) to 10 (excellent result).
Volume Measurements
From 16 studies addressing fat resorption through volume measurements, 3 attempted to estimate volume changes by evaluating tissue thickness using ultrasonography or computed tomography (CT) scan and were excluded from the analysis.8,16,48 The remaining 13 studies used appropriate imaging techniques, such as 3-dimensional image capture devices25,28,36,50,53,58 or CT.14,19,20,43,52 Eight of these investigated volume retention in exclusively congenital patients (Parry-Romberg and craniofacial microsomia), while the remaining 5 involved patients with HIV-associated lipodystrophy. The percentage graft retention represents the fraction of the measured gain in volume, relative to the total volume of injected fat. The relationship between the graft retention and time (follow-up length) was investigated by means of a meta-regression.
AFT-Related Complications
Forty-five studies evaluated the safety of AFT in terms of immediate and late AFT-related complications. A total of 65 complications were documented in 1755 AFT procedures (3.7%), with the majority consisting of irregularities or asymmetry post-AFT (chiefly in patients with HIV). Only 2 infections (0.1%), 2 cases of fat necrosis, (0.1%) and 10 hematomas (0.6%) were reported, and these represent the only true complications encountered in this vast cohort. Additional meta-analysis was performed to derive the proportion of complications per AFT procedure.
Time Frame and Meta-analysis Results
The average clinical follow-up after AFT was 1.3 years (95% CI, 0.9-1.6). Results of individual studies are presented in eTable 4 in the Supplement. A random-effects model meta-analysis was performed with pooled data of each outcome. Categorical data was dichotomized and presented as a meta-analysis of proportions, which can be treated as percentages.
Patient Satisfaction
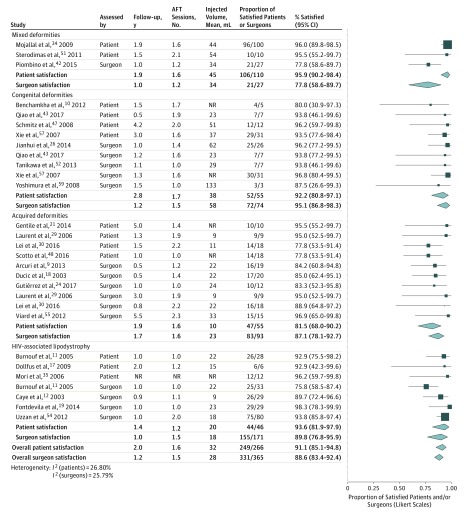
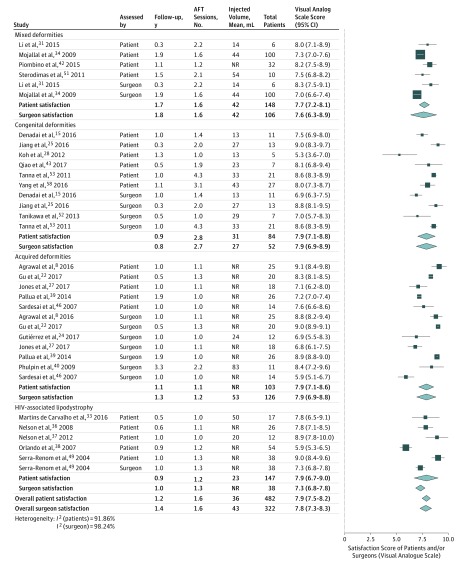
Meta-analysis of categorical data revealed an overall proportion of satisfied patients of 91.1% (95% CI, 85.4%-94.8%), after a mean follow-up of 2.0 years (Figure 3). It was highest in studies with mixed patients (95.9%), followed by patients with HIV-associated lipodystrophy (93.6%) and congenital deformities (92.2%), and it was lowest in studies investigating patients with acquired deformities (81.5%). Meta-analysis of reported VAS scores resulted in an average score of 7.9 (95% CI, 7.5-8.2), with little variation between the mean scores of individual studies or subgroups (Figure 4).
Figure 3. Patient and Surgeon Satisfaction.
This model is based on categorical data. AFT indicates autologous fat transfer; NR, data not reported.
Figure 4. Meta-analysis of Patient and Surgeon Satisfaction.
This model is based on continuous data. AFT indicates autologous fat transfer; NR, data not reported.
Surgeon Satisfaction and/or Evaluation
As with patient satisfaction, a very high proportion of the plastic surgeons were satisfied with the result—88.6% (95% CI, 83.4%-92.4%) after a mean follow-up of 1.2 years (Figure 3). The highest overall satisfaction rate was noted in congenital deformities (95.1%), followed by patients with HIV-associated lipodystrophy (89.8%) and acquired deformities (87.1%). The mixed patients displayed the lowest surgeon satisfaction rate (77.8%), though this subgroup consisted of a single study with only 27 patients. Subsequent meta-analysis of VAS scores led to an overall score of 7.8 (95% CI, 7.3-8.3) and similarly to the scores reported by patients, surgeon scores varied little between relevant subgroups (range, 7.3-7.9; Figure 4).
Volume Measurements
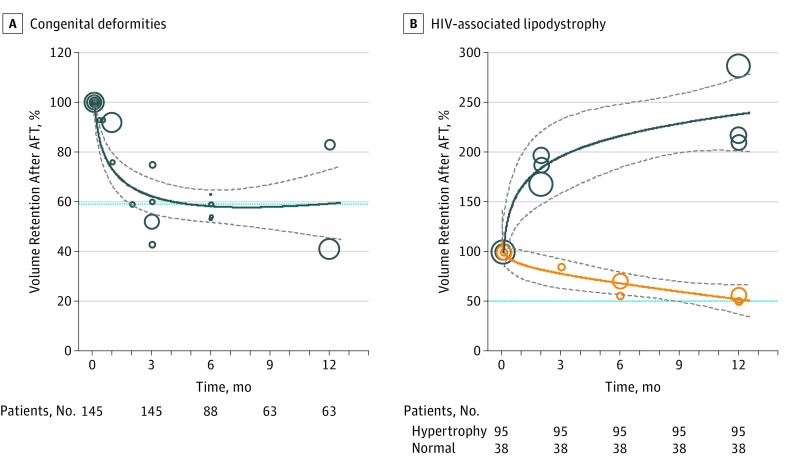
Twelve studies provided data on volume retention, which was plotted against time in a meta-regression model. Patients with congenital deformities displayed a trend toward gradual decrease, stabilizing at 59.0% (95% CI, 51.1%-64.6%) after 1 year of follow-up (Figure 5A). On the contrary, conflicting patterns of volume changes were seen in patients with HIV-associated lipodystrophy, where volume retention decreased to 50.9% (95% CI, 37.2%-66.2%) in some studies and or increased drastically to 238.1% (95% CI, 201.5%-274.3%) in others (Figure 5B).
Figure 5. Meta-analysis of Graft Retention Over Time for Patients Who Underwent Facial Reconstruction.
A, Volume retention in patients with congenital deformities (circles). B, Volume retention in patients with HIV-associated lipodystrophy. Studies were categorized based on whether patients experienced a normal (expected) rate of resorption (yellow circles) or extreme hypertrophy (blue circles). The size of the circles corresponds to sample size, thick lines represent meta-regression effect estimates and their respective 95% CI (gray dotted lines). The total number of patients with volume measurements at each time point in the follow-up is shown below. HIV indicates human immunodeficiency virus.
Number of AFT Sessions
Meta-analysis of pooled data from 50 studies (1525 patients) showed that a mean of 1.5 sessions (95% CI, 1.3-1.7) were needed to achieve the desired result and on average 27 mL of fat was injected per procedure (eFigure 1 in the Supplement). Subgroup analysis revealed some variation between indications with the highest number of procedures being performed for the correction of congenital deformities (1.8 sessions) and the lowest in HIV-associated facial lipodystrophy (1.1 sessions).
Complications
The incidence of AFT-related clinical complications based on our meta-analysis was low—4.8% (95% CI, 3.3%-6.9%) of the AFT procedures (eFigure 2 in the Supplement). Only in patients with HIV-associated lipodystrophy, a higher rate of complications was reported (6.9%); however, these were not commonly encountered AFT-related complications as in other indications but consisted mainly of asymmetry or overcorrection due to lipohypertrophy.
Discussion
This meta-analysis aims to provide a clinically relevant, quantifiable measure on the overall efficacy of AFT in facial reconstructive surgery. Our results demonstrate a clear trend toward a very-high rate of patient (91.1%) and surgeon (88.6%) satisfaction, confirmed by high satisfaction scores of 7.9 and 7.8, respectively. A successful result was achieved after a mean of 1.5 procedures with a low rate (4.8%) of minor complications. The volume retention in patients with congenital facial deformities showed a wide variation between studies, ranging between 40% to over 80% with pooled effect estimate of 59.0% at 1 year. In patients with HIV-associated lipodystrophy, deviations were even more extreme, and 2 distinct patterns of volume changes were observed. The former resembled the resorption pattern of congenital patients, gradually decreasing to 50.9% at 1 year. The latter, on the other hand, led to a spontaneous increase in volume to more than 238% at 1 year. This could, in theory, be attributed to extreme lipohypertrophy of grafted fat, which has been commonly reported as an adverse event in studies of patients with HIV. Whether this effect is the result of the choice of a donor site affected by lipohypertrophy (cervical hump, abdomen, or breast area), metabolic stimuli or medication must be evaluated in future studies.
By a thorough examination of the totality of the evidence, it can be deduced that AFT seems to be an effective procedure that leaves the vast majority of patients and surgeons satisfied with the result after about a year of clinical follow-up. It is clear that future studies will play a crucial role in providing higher quality evidence that would allow for more nuanced recommendations regarding specific subgroups or indications, evaluating factors influencing the (long-term) volume retention, as well as the added value of supplements. As setting up randomized clinical trials in some of these areas can be difficult, they should be evaluated whenever possible by prospective study design with the use of control groups and validated outcome measurement instruments such as 3-dimensional volume assessments and quality-of-life scales (eTable 6 in the Supplement).
Limitations
The main limitation of this meta-analysis, as well as the accumulated body of knowledge on AFT in general, remains the low level of evidence of published studies. Unlike the widely accepted approach, where the adoption of a new therapy is based on the achievement of statistical significance for the primary outcome from well-executed randomized clinical trials or large cohorts, this has not been possible in the case of AFT. Its unique properties that give it competitive edge above conventional techniques, have also robbed it of an alternative treatment to perform in a control group. Setting up randomized clinical trials with AFT has therefore been difficult owing to practical and ethical challenges. Nonrandomized cohorts, on the other hand could potentially introduce bias by allocating the treatment based on the indication, severity or the patient and/or surgeon’s preference. Inevitably, this would produce groups that are not comparable, thereby reducing the methodological quality of the study. Nevertheless, studies involving control groups not treated with AFT are scarce, and the few such trials that have been conducted only compare different AFT techniques (with or without supplements) with each other. More than 2 decades after the popularization of AFT by Coleman,60 clinical research on the topic has not been successful in providing convincing evidence on its efficacy and safety.
Another important obstacle that hinders progress in the field of AFT is the lack of practical and reliable instruments to quantify the absolute effect of treatment. For many years, plastic surgeons have relied on nonvalidated, custom-made questionnaires to measure patient satisfaction and routinely used preoperative and postoperative photographs for a slightly more objective confirmation of the findings. These outcomes have been reported as either continuous (means [SD]) or categorical (frequencies) variables, based on the type of questionnaire or scale. Each of these presents with its own advantages and limitations. As most retrospective studies rarely measured outcomes preoperatively, determination of the absolute effect of AFT becomes difficult in the absence of baseline values. In addition, the use of a continuous scale is of limited value when attempting to quantify the effect of AFT with different baseline scores. It is arguable that the same change in different satisfaction scores can have different clinical significance. For example, an increase in a satisfaction from 1 to 4 out of 10 can be classified as an unsatisfactory result, while the same increase in another patient from a score of 4 to a score of 7 out of 10 would be seen as successful. When dealing with studies reporting of nominal (categorical) data, outcomes such as the rate of satisfaction are often reported as frequencies of patients falling in each category (eg, dissatisfied, satisfied, very satisfied). As such an approach does not rely on baseline values, it can more accurately reflect the absolute effectiveness of AFT, though it lacks the precision of continuous data.
For the abovementioned reasons, performing a meta-analysis on this topic is highly challenging as the paucity of relevant control groups and preoperative vs postoperative data prevents applying widely accepted statistical measures of effect such as the relative risk or odds ratios. Therefore, this meta-analysis focused on providing pooled effect estimates that indicate the size, direction and confidence intervals for each outcome.
Dealing with heterogeneity is another major challenge with meta-analyses. Pooling a large number of studies with potentially different patient demographics, indications and outcome measurements can result in important heterogeneity that could reduce the validity of the effect estimates. To correct for this, a random-effects model was applied to all cases and whenever possible, subgroup meta-analyses were performed by pooling studies with similar indications together. It is important to note that despite the potential differences between included studies, the pooled effect estimates demonstrate a clear convergence toward a very-high rate of satisfaction with the procedure and a low rate of clinical complications. As the indications of AFT in facial reconstructive surgery consists of a heterogeneous group of indications and patients, it can be argued that such a general approach delivers evidence on precisely the population this meta-analysis aims to draw conclusions about.
Conclusions
Autologous fat transfer seems to be an effective treatment for contour deformities in facial reconstruction, as reflected by the high rate of patient (91.1%) and surgeon (88.6%) satisfaction, confirmed by high satisfaction scores of 7.9 and 7.8, respectively. The desired result was achieved after only 1.5 procedures. Volume retention was observed to decrease to 50% to 60% after 1 year but needs further investigation, especially in patients with HIV-associated facial lipodystrophy. Autologous fat transfer can be considered a safe treatment, with only 4.8% of procedures resulting in minor complications.
eFigure 1. Meta-analysis of the number of AFT sessions needed to achieve the desired result
eFigure 2. Meta-analysis of the rate of AFT-related complications per procedure
eTable 1. Prisma checklist
eTable 2. PICO design
eTable 3. Search syntax
eTable 4. Data extraction sheet
eTable 5. Baseline table of all studies
eTable 6. Recommendations for focus of future research on AFT in craniofacial reconstruction
References
- 1.Higgins JP. G.S.e.C.H.f.S.R.o.I.V.u.M.T.C.C. 2011. http://www.handbook.cochrane.org. Accessed January 31, 2018.
- 2.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 3.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39(2):91-92. [DOI] [PubMed] [Google Scholar]
- 4.WebPlotDigitizer http://arohatgi.info/WebPlotDigitizer. Accessed November 15, 2017.
- 5.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. [Google Scholar]
- 7.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing V, Austria. 2016. https://www.R-project.org/. Accessed January 31, 2018.
- 8.Agrawal KS, Bachhav M, Naik CS, Tanwar H, Sankhe SS. Autologous fat transfer for esthetic contouring of face in posttraumatic nonfunctional maxillofacial deformities. Craniomaxillofac Trauma Reconstr. 2016;9(2):113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arcuri F, Brucoli M, Baragiotta N, Stellin L, Giarda M, Benech A. The role of fat grafting in the treatment of posttraumatic maxillofacial deformities. Craniomaxillofac Trauma Reconstr. 2013;6(2):121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benchamkha Y, Ettalbi S, Droussi H, Bahechar N, Boukind EH. [Lipostructure for morphologic restauration in Parry-Romberg syndrome: about 12 cases]. Ann Chir Plast Esthet. 2012;57(3):273-280. [DOI] [PubMed] [Google Scholar]
- 11.Burnouf M, Buffet M, Schwarzinger M, et al. Evaluation of Coleman lipostructure for treatment of facial lipoatrophy in patients with human immunodeficiency virus and parameters associated with the efficiency of this technique. Arch Dermatol. 2005;141(10):1220-1224. [DOI] [PubMed] [Google Scholar]
- 12.Caye N, Le Fourn B, Pannier M. [Surgical treatment of facial lipoatrophy]. Ann Chir Plast Esthet. 2003;48(1):2-12. [DOI] [PubMed] [Google Scholar]
- 13.Cervelli D, Gasparini G, Moro A, et al. Lipofilling as refinement procedure in maxillo-mandibular malformations. Acta Otorhinolaryngol Ital. 2016;36(5):368-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Q, Li J, Dong Z, Liu L, Lu F. Quantitative volumetric analysis of progressive hemifacial atrophy corrected using stromal vascular fraction-supplemented autologous fat grafts. Dermatol Surg. 2013;39(10):1465-1473. [DOI] [PubMed] [Google Scholar]
- 15.Denadai R, Raposo-Amaral CA, Buzzo CL, Raposo-Amaral CE. Isolated autologous free fat grafting for management of facial contour asymmetry in a subset of growing patients with craniofacial microsomia. Ann Plast Surg. 2016;76(3):288-294. [DOI] [PubMed] [Google Scholar]
- 16.Denadai R, Raposo-Amaral CA, Pinho AS, Lameiro TM, Buzzo CL, Raposo-Amaral CE. Predictors of autologous free fat graft retention in the management of craniofacial contour deformities. Plast Reconstr Surg. 2017;140(1):50e-61e. [DOI] [PubMed] [Google Scholar]
- 17.Dollfus C, Blanche S, Trocme N, Funck-Brentano I, Bonnet F, Levan P. Correction of facial lipoatrophy using autologous fat transplants in HIV-infected adolescents. HIV Med. 2009;10(5):263-268. [DOI] [PubMed] [Google Scholar]
- 18.Ducic Y, Pontius AT, Smith JE. Lipotransfer as an adjunct in head and neck reconstruction. Laryngoscope. 2003;113(9):1600-1604. [DOI] [PubMed] [Google Scholar]
- 19.Fontdevila J, Guisantes E, Martínez E, Prades E, Berenguer J. Double-blind clinical trial to compare autologous fat grafts versus autologous fat grafts with PDGF: no effect of PDGF. Plast Reconstr Surg. 2014;134(2):219e-230e. [DOI] [PubMed] [Google Scholar]
- 20.Fontdevila J, Serra-Renom JM, Raigosa M, et al. Assessing the long-term viability of facial fat grafts: an objective measure using computed tomography. Aesthet Surg J. 2008;28(4):380-386. [DOI] [PubMed] [Google Scholar]
- 21.Gentile P, De Angelis B, Pasin M, et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical evaluation for cell-based therapies in patients with scars on the face. J Craniofac Surg. 2014;25(1):267-272. [DOI] [PubMed] [Google Scholar]
- 22.Gu Z, Li Y, Li H. Use of condensed nanofat combined with fat grafts to treat atrophic scars. JAMA Facial Plast Surg. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guaraldi G, De Fazio D, Orlando G, et al. Facial lipohypertrophy in HIV-infected subjects who underwent autologous fat tissue transplantation. Clin Infect Dis. 2005;40(2):e13-e15. [DOI] [PubMed] [Google Scholar]
- 24.Gutiérrez Santamaría J, Masiá Gridilla J, Pamias Romero J, Giralt López-de-Sagredo J, Bescós Atín MS. Fat grafting is a feasible technique for the sequelae of head and neck cancer treatment. J Craniomaxillofac Surg. 2017;45(1):93-98. [DOI] [PubMed] [Google Scholar]
- 25.Jiang T, Xie Y, Zhu M, et al. The second fat graft has significantly better outcome than the first fat graft for Romberg syndrome: A study of three-dimensional volumetric analysis. J Plast Reconstr Aesthet Surg. 2016;69(12):1621-1626. [DOI] [PubMed] [Google Scholar]
- 26.Jianhui Z, Chenggang Y, Binglun L, et al. Autologous fat graft and bone marrow-derived mesenchymal stem cells assisted fat graft for treatment of Parry-Romberg syndrome. Ann Plast Surg. 2014;73(suppl 1):S99-S103. [DOI] [PubMed] [Google Scholar]
- 27.Jones CM, Morrow BT, Albright WB, Long RE, Samson TD, Mackay DR. Structural fat grafting to improve reconstructive outcomes in secondary cleft lip deformity. Cleft Palate Craniofac J. 2017;54(1):70-74. [DOI] [PubMed] [Google Scholar]
- 28.Koh KS, Oh TS, Kim H, et al. Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann Plast Surg. 2012;69(3):331-337. [DOI] [PubMed] [Google Scholar]
- 29.Laurent F, Capon-Dégardin N, Martinot-Duquennoy V, Dhellèmmes P, Pellerin P. [Role of lipo-filling in the treatment of sequelae in craniosynostosis surgery]. Ann Chir Plast Esthet. 2006;51(6):512-516. [DOI] [PubMed] [Google Scholar]
- 30.Lei H, Ma GE, Liu Z. Evaluation of repairing facial depression deformities secondary to lupus erythematosus panniculitis with autologous fat grafting. J Craniofac Surg. 2016;27(7):1765-1769. [DOI] [PubMed] [Google Scholar]
- 31.Li GKH, Chung JHP, Liu LHL, Chow VLY, Lau GISK, Chan RCL. Fat grafting: a safe and effective treatment of craniofacial depression. Surg Pract. 2015;19(2):75-81. [Google Scholar]
- 32.Lim AA, Fan K, Allam KA, et al. Autologous fat transplantation in the craniofacial patient: the UCLA experience. J Craniofac Surg. 2012;23(4):1061-1066. [DOI] [PubMed] [Google Scholar]
- 33.Martins de Carvalho F, Casal D, Bexiga J, et al. HIV-Associated facial lipodystrophy: experience of a tertiary referral center with fat and dermis-fat compound graft transfer. Eplasty. 2016;16:e31. [PMC free article] [PubMed] [Google Scholar]
- 34.Mojallal A, Shipkov C, Braye F, Breton P, Foyatier JL. Influence of the recipient site on the outcomes of fat grafting in facial reconstructive surgery. Plast Reconstr Surg. 2009;124(2):471-483. [DOI] [PubMed] [Google Scholar]
- 35.Mori A, Lo Russo G, Agostini T, Pattarino J, Vichi F, Dini M. Treatment of human immunodeficiency virus-associated facial lipoatrophy with lipofilling and submalar silicone implants. J Plast Reconstr Aesthet Surg. 2006;59(11):1209-1216. [DOI] [PubMed] [Google Scholar]
- 36.Nelson L, Stewart KJ. Experience in the treatment of HIV-associated lipodystrophy. J Plast Reconstr Aesthet Surg. 2008;61(4):366-371. [DOI] [PubMed] [Google Scholar]
- 37.Nelson L, Stewart KJ. Psychological morbidity and facial volume in HIV lipodystrophy: quantification of treatment outcome. J Plast Reconstr Aesthet Surg. 2012;65(4):439-447. [DOI] [PubMed] [Google Scholar]
- 38.Orlando G, Guaraldi G, De Fazio D, et al. Long-term psychometric outcomes of facial lipoatrophy therapy: forty-eight-week observational, nonrandomized study. AIDS Patient Care STDS. 2007;21(11):833-842. [DOI] [PubMed] [Google Scholar]
- 39.Pallua N, Baroncini A, Alharbi Z, Stromps JP. Improvement of facial scar appearance and microcirculation by autologous lipofilling. J Plast Reconstr Aesthet Surg. 2014;67(8):1033-1037. [DOI] [PubMed] [Google Scholar]
- 40.Phulpin B, Gangloff P, Tran N, Bravetti P, Merlin JL, Dolivet G. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plast Reconstr Surg. 2009;123(4):1187-1197. [DOI] [PubMed] [Google Scholar]
- 41.Pinski KS, Roenigk HH Jr. Autologous fat transplantation: long-term follow-up. J Dermatol Surg Oncol. 1992;18(3):179-184. [DOI] [PubMed] [Google Scholar]
- 42.Piombino P, Marenzi G, Dell’Aversana Orabona G, Califano L, Sammartino G. Autologous fat grafting in facial volumetric restoration. J Craniofac Surg. 2015;26(3):756-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao J, Gui L, Fu X, et al. A novel method of mild to moderate parry-romberg syndrome reconstruction: computer-assisted surgery with mandibular outer cortex and fat grafting. J Craniofac Surg. 2017;28(2):359-365. [DOI] [PubMed] [Google Scholar]
- 44.Rauso R, Curinga G, Santillo V, Corvo G, Tartaro G. Comparison between lipofilling and a nonabsorbable filler for facial wasting rehabilitation in HIV-positive patients. J Craniofac Surg. 2011;22(5):1684-1688. [DOI] [PubMed] [Google Scholar]
- 45.Rauso R, Sangiovanni V, Cobellis G, Tartaro G. Lipofilling’s role in facial wasting rehabilitation of HIV+ patients. Eur Surg Res. 2010;45(3-4):285-286. [Google Scholar]
- 46.Sardesai MG, Moore CC. Quantitative and qualitative dermal change with microfat grafting of facial scars. Otolaryngol Head Neck Surg. 2007;137(6):868-872. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz S, Weis C, Morley S, Demey A, Dabernig J. Treatment of facial lipodystrophy syndromes. Lipofilling versus free flap surgery. Eur J Plast Surg. 2008;31(6):305-310. [Google Scholar]
- 48.Scotto di Santolo M, Sagnelli M, Tortora G, et al. The utility of the high-resolution ultrasound technique in the evaluation of autologous adipose tissue lipofilling, used for the correction of post-surgical, post-traumatic and post-burn scars. Radiol Med. 2016;121(6):521-527. [DOI] [PubMed] [Google Scholar]
- 49.Serra-Renom JM, Fontdevila J. Treatment of facial fat atrophy related to treatment with protease inhibitors by autologous fat injection in patients with human immunodeficiency virus infection. Plast Reconstr Surg. 2004;114(2):551-555. [DOI] [PubMed] [Google Scholar]
- 50.Slack GC, Tabit CJ, Allam KA, Kawamoto HK, Bradley JP. Parry-Romberg reconstruction: beneficial results despite poorer fat take. Ann Plast Surg. 2014;73(3):307-310. [DOI] [PubMed] [Google Scholar]
- 51.Sterodimas A, de Faria J, Nicaretta B, Boriani F. Autologous fat transplantation versus adipose-derived stem cell-enriched lipografts: a study. Aesthet Surg J. 2011;31(6):682-693. [DOI] [PubMed] [Google Scholar]
- 52.Tanikawa DY, Aguena M, Bueno DF, Passos-Bueno MR, Alonso N. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg. 2013;132(1):141-152. [DOI] [PubMed] [Google Scholar]
- 53.Tanna N, Wan DC, Kawamoto HK, Bradley JP. Craniofacial microsomia soft-tissue reconstruction comparison: inframammary extended circumflex scapular flap versus serial fat grafting. Plast Reconstr Surg. 2011;127(2):802-811. [DOI] [PubMed] [Google Scholar]
- 54.Uzzan C, Boccara D, Lacheré A, Mimoun M, Chaouat M. [Treatment of facial lipoatrophy by lipofilling in HIV infected patients: retrospective study on 317 patients on 9 years]. Ann Chir Plast Esthet. 2012;57(3):210-216. [DOI] [PubMed] [Google Scholar]
- 55.Viard R, Bouguila J, Voulliaume D, Comparin JP, Dionyssopoulos A, Foyatier JL. [Fat grafting in facial burns sequelae]. Ann Chir Plast Esthet. 2012;57(3):217-229. [DOI] [PubMed] [Google Scholar]
- 56.Vitagliano T, Curto LS, Greto Ciriaco A, Gareri P, Ribuffo D, Greco M. Two-thirds lip defects: a new combined reconstructive technique for patients with epithelial cancer. J Craniofac Surg. 2016;27(8):1995-2000. [DOI] [PubMed] [Google Scholar]
- 57.Xie Y, Li Q, Zheng D, Lei H, Pu LL. Correction of hemifacial atrophy with autologous fat transplantation. Ann Plast Surg. 2007;59(6):645-653. [DOI] [PubMed] [Google Scholar]
- 58.Yang X, Wu R, Bi H, et al. Autologous fat grafting with combined three-dimensional and mirror-image analyses for progressive hemifacial atrophy. Ann Plast Surg. 2016;77(3):308-313. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34(9):1178-1185. [DOI] [PubMed] [Google Scholar]
- 60.Coleman SR. Structural fat grafting. Aesthetic Surg J. 1998;18(5):386-388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Meta-analysis of the number of AFT sessions needed to achieve the desired result
eFigure 2. Meta-analysis of the rate of AFT-related complications per procedure
eTable 1. Prisma checklist
eTable 2. PICO design
eTable 3. Search syntax
eTable 4. Data extraction sheet
eTable 5. Baseline table of all studies
eTable 6. Recommendations for focus of future research on AFT in craniofacial reconstruction