Key Points
Question
Is there a survival benefit to a shorter time from surgery to the start of radiation (TS-RT) for patients with head and neck squamous cell carcinoma?
Finding
In this observational cohort study of 25 216 patients from the National Cancer Database, a TS-RT of 42 days or less was associated with improved survival compared with 50 days or longer; a delay of 1 week resulted in inferior outcomes for patients with tonsil tumors. Accelerated fractionation was associated with improved survival compared with standard fractionation.
Meaning
Efforts should be made to prevent delays in TS-RT, and there may be a role for intensifying therapy when delays are unavoidable.
Using data from the National Cancer Database, this cohort study examines the association of delayed time to radiotherapy with survival outcomes in patients with head and neck squamous cell carcinoma.
Abstract
Importance
Shortening the time from surgery to the start of radiation (TS-RT) is a consideration for physicians and patients. Although the National Comprehensive Cancer Network recommends radiation to start within 6 weeks, a survival benefit with this metric remains controversial.
Objective
To determine the association of delayed TS-RT with overall survival (OS) using a large cancer registry.
Design, Setting, and Participants
In this observational cohort study, 25 216 patients with nonmetastatic stages III to IV head and neck cancer were identified from the National Cancer Database (NCDB).
Exposures
Patients received definitive surgery followed by adjuvant radiation therapy, with an interval duration defined as TS-RT.
Main Outcomes and Measures
Overall survival as a function of TS-RT and the effect of clinicopathologic risk factors and accelerated fractionation.
Results
We identified 25 216 patients with nonmetastatic squamous cell carcinoma of the head and neck. There were 18 968 (75%) men and 6248 (25%) women and the mean (SD) age of the cohort was 59 (10.9) years. Of the 25 216 patients, 9765 (39%) had a 42-days or less TS-RT and 4735 (19%) had a 43- to 49-day TS-RT. Median OS was 10.5 years (95% CI, 10.0-11.1 years) for patients with a 42-days or less TS-RT, 8.2 years (95% CI, 7.4-8.6 years; absolute difference, −2.4 years, 95% CI, −1.5 to −3.2 years) for patients with a 43- to 49-day TS-RT, and 6.5 years (95% CI, 6.1-6.8 years; absolute difference, −4.1 years, 95% CI, −3.4 to −4.7 years) for those with a 50-days or more TS-RT. Multivariable analysis found that compared with a 42-days or less TS-RT, there was not a significant increase in mortality with a 43- to 49-day TS-RT (HR, 0.98; 95% CI, 0.93-1.04), although there was for a TS-RT of 50 days or more (HR, 1.07; 95% CI, 1.02-1.12). A significant interaction was identified between TS-RT and disease site. Subgroup effect modeling found that a delayed TS-RT of 7 days resulted in significantly worse OS for patients with tonsil tumors (HR, 1.22; 95% CI, 1.05-1.43) though not other tumor subtypes. Accelerated fractionation of 5.2 fractions or more per week was associated with improved survival (HR, 0.93; 95% CI, 0.87-0.99) compared with standard fractionation.
Conclusions and Relevance
Delayed TS-RT of 50 days or more was associated with worse overall survival. The multidisciplinary care team should focus on shortening TS-RT to improve survival. Unavoidable delays may be an indication for accelerated fractionation or other dose intensification strategies.
Introduction
Adjuvant radiation and chemoradiation therapy are well known to improve outcomes for certain groups of patients with head and neck cancer. The ideal time to start postoperative radiation therapy (PORT) has been an issue of debate and study for decades. In the United States, the National Comprehensive Cancer Network (NCCN) recommends initiating radiotherapy within 6 weeks from surgery. The time from surgery to radiation therapy (TS-RT) has not been tested in a randomized fashion, and although many studies have shown improved oncologic outcomes with shorter TS-RT, the issue remains controversial. We sought to determine whether a delayed TS-RT impacted survival using the National Cancer Database (NCDB) registry, how clinicopathologic risk factors were associated with the TS-RT effect, and if accelerated radiation was associated with improved mortality rates.
Methods
Data Source
The NCDB is a hospital-based registry and is a combined program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society that captures over 70% of cancer cases in the United States. Hospitals included in the registry are Commission on Cancer-accredited. This study was granted an exemption from the institutional review board of Stanford University owing to the deidentified, publically available nature of the data source.
Study Population and Covariates
We identified patients from the NCDB from 2004 to 2013 who had nonmetastatic stages III to IV head and neck squamous cell carcinoma treated with surgical resection and adjuvant radiation. Definitive surgical resection was required as indicated by codes 30 to 80 for the site-specific Surgical Procedure of the Primary Site variable, which represents the cumulative effect of all surgeries to the primary site. Cancer subsites included: tonsil, nontonsil oropharynx, oral cavity, larynx, and hypopharynx. The TS-RT was defined as the days between primary surgical resection and the first day of radiation therapy. To reflect real world practice patterns, patients were included if they received 45 to 76 Gy and had a TS-RT of 21 to 90 days. Intensity modulated (IMRT) and 3-dimensional conformal (3DCRT) radiation techniques were included.
Patient clinical and demographic variables included age, sex, race, and Charlson/Deyo comorbidity index. Median household income and percent of adults without a high school education by zip code of residence were classified by quartile of the US population. Primary health insurance coverage was grouped as Medicaid, Medicare, none, other (private care/managed care/governmental insurance), or unknown. The NCCN collectively defines high-risk factors as an oral cavity tumor, T3 to T4 or N2 to N3 disease, positive surgical margins, extracapsular extension (ECE), lymphovascular invasion (LVI), and perineural invasion. Perineural invasion status is not collected by the NCDB, but all other high-risk factors were included for analysis. Lymphovascular invasion status was collected starting in 2010. Stage was according to the AJCC Cancer Staging Manual (6th and 7th Editions) depending on the year of diagnosis.
Surgical center was defined as academic, Commission on Cancer designated Comprehensive Community Cancer Program (CCCP), community, or other. Radiation was delivered either at the same center as surgery or one different from the reporting facility, and this distinction was included in the treating facility classification. The distance from the patient’s residence to the reporting hospital was categorized as distance traveled. The time from diagnosis to surgery (TD-S) was categorized with a 68-day cutoff, and although there is no threshold for tumor growth prior to treatment, this cutoff has previously correlated with outcomes with NCDB data. To better characterize the delay prior to radiation treatment we included the length of hospital stay from surgery and 30-day unplanned readmission status. Chemotherapy status was categorized as none, adjuvant, neoadjuvant, neoadjuvant plus adjuvant, or unknown.
Statistical Analyses
The recommended 42-days or less TS-RT was compared with a 43- to 49-day TS-RT and a 50-days or more TS-RT to determine the relative importance of an additional week or more of delay. Differences between covariates were tested with χ2 tests. The primary endpoint was overall survival (OS) from the date of diagnosis and was estimated with the Kaplan-Meier method. Differences in OS were tested with log-rank tests, and confidence intervals (CI) in differences between median OS were established using resampling methods. A multivariate Cox regression was performed with TS-RT modeled as a continuous variable using a restricted cubic spline with 3 knots with a 42-day TS-RT as the reference. Hazard ratios (HR) for mortality were determined using Cox multivariable regression models. Adjustments in multivariable models were made for socioeconomic variables, comorbidity, risk factors, type of facility, distance traveled, TD-S, length of hospital stay after surgery, unplanned hospital readmission, chemotherapy, radiation dose, and use of IMRT. The proportional hazards assumption was tested using Schoenfeld residuals. Covariates representing higher risk for recurrence were tested for interaction with TS-RT, including site of disease, stage, ECE, margin status, LVI, and receipt of chemotherapy. A complete case analysis was performed for patients with complete information on pathologic high-risk factors (disease site, stage, ECE, margin status, and LVI data).
Fractionation data were available for a subset of patients and were categorized as standard (<5.2 fractions per week) or accelerated (≥5.2 fractions per week). The relative importance of altered fractionation was tested using univariate Kaplan-Meier estimates and a log-rank test. A multivariable Cox regression model tested the survival impact of accelerated fractionation and was adjusted for TS-RT as well as the same confounders used in the other multivariable models. An interaction term between TS-RT and fractionation schedule was tested.
Significance threshold was set at P<.05, and all tests were 2 sided. Effect size estimates with 95% CIs were preferentially reported over P values to express precision. A clinically meaningful difference in median survival was set at 3 months from that of the TS-RT of 42-days or less group. This difference corresponded to an HR of 1.02 or more when survival times were fitted using a Weibull distribution. Analyses were performed with SAS statistical software (version 9.4; SAS Institute Inc) and R (version 3.3.3, R Foundation).
Results
From the 234 861 patients identified from the NCDB with nonmetastatic squamous cell carcinoma of the head and neck, 99 901 did not have stages III to IV disease, 81 116 did not receive definitive surgery, 23 995 did not receive adjuvant external beam radiation, and 4633 did not have a 21- to 90-day TS-RT, which resulted in a final cohort of 25 216 patients. There were 9318 patients (37%) who died by the end of this study. Of the 25 216 patients, 9765 (39%) had a TS-RT of 42 days or less, 4735 (19%) patients had a 43- to 49-day TS-RT, and 10 716 (42%) patients had a TS-RT of 50 days or more. Characteristics were compared between patients with a 42-days or less TS-RT vs a 43- to 49-day and a 50-days or longer TS-RT (Table 1). To highlight a few significant differences, patients in the 42-days or less TS-RT cohort were more likely to have been white and from higher education and income areas, and these patients were more likely to have private/managed care/government insurance, fewer comorbidities, tonsil or nontonsil oropharynx cancers, stage III disease, residences closer to the reporting facility, a shorter postsurgery hospital stay, higher radiation doses, and adjuvant chemotherapy. Compared with those with a TS-RT of 42 days or less, more patients with a 43- to 49-day TS-RT or a TS-RT of 50 days or more had all treatment at an academic facility (difference, 2%; 95% CI, 1% to 4%; and difference, 1%; 95% CI, −1% to 2%, respectively) or surgery at an academic site with radiation delivered elsewhere (difference, 5%; 95% CI, 3% to 6%; and difference, 6%; 95% CI, 5% to 7%, respectively). In addition, delays longer than 42 days increased over the study time period.
Table 1. Baseline Characteristics of Patients According to the Duration of Time From Surgery to Radiation (TS-RT).
| Characteristic | TS-RT | ||||
|---|---|---|---|---|---|
| No. (%) | % (95% CI) | ||||
| ≤42 d | 43-49 d | ≥50 d | Difference Between 43-49 d and ≤42 d | Difference Between ≥50 d and ≤42 d | |
| Sex | |||||
| Male | 7585 (78) | 3537 (75) | 7846 (73) | −3.0 (−4.5 to −1.5) | −4.5 (−5.6 to −3.3) |
| Female | 2180 (22) | 1198 (25) | 2870 (27) | 3.0 (1.5 to 4.5) | 4.5 (3.3 to 5.6) |
| Age, y | |||||
| ≤50 | 2389 (24) | 972 (21) | 2186 (20) | −3.9 (−5.4 to −2.5) | −4.1 (−5.2 to −2.9) |
| 51-60 | 3628 (37) | 1746 (37) | 3793 (35) | −0.3 (−2.0 to 1.4) | −1.8 (−3.1 to −0.4) |
| 61-70 | 2544 (26) | 1320 (28) | 2959 (28) | 1.8 (0.3 to 3.4) | 1.6 (0.3 to 2.8) |
| ≥71 | 1204 (12) | 697 (15) | 1778 (17) | 2.4 (1.2 to 3.6) | 4.3 (3.3 to 5.2) |
| Race | |||||
| White | 8788 (90) | 4147 (88) | 9239 (86) | −2.4 (−3.5 to −1.3) | −3.8 (−4.7 to −2.9) |
| Black | 657 (7) | 405 (9) | 1079 (10) | 1.8 (0.9 to 2.8) | 3.3 (2.6 to 4.1) |
| Other | 320 (3) | 183 (4) | 398 (4) | 0.6 (−0.1 to 1.2) | 0.4 (−0.1 to 0.9) |
| Educationa | |||||
| ≥21% | 1365 (14) | 744 (16) | 2005 (19) | 1.7 (0.5 to 3.0) | 4.7 (3.7 to 5.7) |
| 13%-20.9% | 2534 (26) | 1231 (26) | 2961 (28) | 0.1 (−1.5 to 1.6) | 1.7 (0.5 to 2.9) |
| 7%-12.9% | 3224 (33) | 1670 (35) | 3468 (32) | 2.3 (0.6 to 3.9) | −0.7 (−1.9 to 0.6) |
| <7% | 2540 (26) | 1032 (22) | 2158 (20) | −4.2 (−5.7 to −2.8) | −5.9 (−7.0 to −4.7) |
| Unknown | 102 (1) | 58 (1) | 124 (1) | 0.2 (−0.2 to 0.6) | 0.1 (−0.2 to 0.4) |
| Income, $b | |||||
| <38,000 | 1575 (16) | 847 (18) | 2109 (20) | 1.8 (0.4 to 3.1) | 3.6 (2.5 to 4.6) |
| 38 000-47 999 | 2292 (23) | 1183 (25) | 2758 (26) | 1.5 (0 to 3.0) | 2.3 (1.1 to 3.4) |
| 48 000-62 999 | 2678 (27) | 1288 (27) | 2834 (26) | −0.2 (−1.8 to 1.3) | −1.0 (−2.2 to 0.2) |
| ≥63 000 | 3114 (32) | 1359 (29) | 2886 (27) | −3.2 (−4.8 to −1.6) | −5.0 (−6.2 to −3.7) |
| Unknown | 106 (1) | 58 (1) | 129 (1) | 0.1 (−0.2 to 0.5) | 0.1 (−0.2 to 0.4) |
| Insurance | |||||
| Medicare | 2534 (26) | 1426 (30) | 3478 (32) | 4.2 (2.6 to 5.7) | 6.5 (5.3 to 7.7) |
| Medicaid | 754 (8) | 491 (10) | 1448 (14) | 2.6 (1.6 to 3.7) | 5.8 (5.0 to 6.6) |
| Private/managed care/other government | 5861 (60) | 2510 (53) | 4952 (46) | −7.0 (−8.7 to −5.3) | −13.8 (−15.2 to −12.5) |
| None | 456 (5) | 257 (5) | 680 (6) | 0.8 (−0 to 1.5) | 1.7 (1.1 to 2.3) |
| Unknown | 160 (2) | 51 (1) | 158 (1) | −0.6 (−0.9 to −0.2) | −0.2 (−0.5 to 0.2) |
| Charlson/Deyo score | |||||
| 0 | 7976 (82) | 3744 (79) | 8246 (77) | −2.6 (−4.0 to −1.2) | −4.7 (−5.8 to −3.6) |
| 1 | 1471 (15) | 783 (17) | 1946 (18) | 1.5 (0.2 to 2.7) | 3.1 (2.1 to 4.1) |
| ≥2 | 318 (3) | 208 (4) | 524 (5) | 1.1 (0.5 to 1.8) | 1.6 (1.1 to 2.2) |
| Disease site | |||||
| Tonsil | 4353 (45) | 1344 (28) | 2383 (22) | −16.2 (−17.8 to −14.6) | −22.3 (−23.6 to −21.1) |
| Nontonsil oropharynx | 1202 (12) | 527 (11) | 1094 (10) | −1.2 (−2.3 to −0.1) | −2.1 (−3.0 to −1.2) |
| Oral cavity | 2635 (27) | 1895 (40) | 4769 (45) | 13.0 (11.4 to 14.7) | 17.5 (16.2 to 18.8) |
| Larynx | 1360 (14) | 825 (17) | 2034 (19) | 3.5 (2.2 to 4.8) | 5.1 (4.0 to 6.1) |
| Hypopharynx | 215 (2) | 144 (3) | 436 (4) | 0.8 (0.3 to 1.4) | 1.9 (1.4 to 2.3) |
| Stage | |||||
| III | 2469 (25) | 1086 (23) | 2240 (21) | −2.3 (−3.8 to −0.9) | −4.4 (−5.5 to −3.2) |
| IV | 7296 (75) | 3649 (77) | 8476 (79) | 2.3 (0.9 to 3.8) | 4.4 (3.2 to 5.5) |
| Extracapsular extension | |||||
| No | 4563 (47) | 2056 (43) | 4393 (41) | −3.3 (−5.0 to −1.6) | −5.7 (−7.1 to −4.4) |
| Yes | 2513 (26) | 1370 (29) | 2955 (28) | 3.2 (1.6 to 4.8) | 1.8 (0.6 to 3.1) |
| Node negative | 1383 (14) | 893 (19) | 2396 (22) | 4.7 (3.4 to 6.0) | 8.2 (7.1 to 9.2) |
| Unknown | 1306 (13) | 416 (9) | 972 (9) | −4.6 (−5.6 to −3.5) | −4.3 (−5.2 to −3.4) |
| Margin status | |||||
| Negative | 6349 (65) | 3438 (73) | 7886 (74) | 7.6 (6.0 to 9.2) | 8.6 (7.3 to 9.8) |
| Positive | 1019 (10) | 423 (9) | 899 (8) | −1.5 (−2.5 to −0.5) | −2.0 (−2.8 to −1.2) |
| Unknown | 2397 (25) | 874 (18) | 1931 (18) | −6.1 (−7.5 to −4.7) | −6.5 (−7.6 to −5.4) |
| Lymphovascular invasion | |||||
| Absent | 2578 (26) | 1336 (28) | 3002 (28) | 1.8 (0.3 to 3.4) | 1.6 (0.4 to 2.8) |
| Present | 1119 (11) | 650 (14) | 1486 (14) | 2.3 (1.1 to 3.4) | 2.4 (1.5 to 2.7) |
| Unknown | 6068 (62) | 2749 (58) | 6228 (58) | −4.1 (−5.8 to −2.4) | −4.0 (−5.4 to −2.7) |
| Year of diagnosis | |||||
| 2004-2006 | 2377 (24) | 1089 (23) | 2378 (22) | −1.3 (−2.8 to 0.1) | −2.2 (−3.3 to −1.0) |
| 2007-2009 | 2505 (26) | 1254 (26) | 2870 (27) | 0.8 (−0.7 to 2.4) | 1.1 (−0.1 to 2.3) |
| 2010-2013 | 4883 (50) | 2392 (51) | 5468 (51) | 0.5 (−1.2 to 2.2) | 1.0 (−0.3 to 2.4) |
| TD-S, d | |||||
| <68 | 9221 (94) | 4368 (92) | 9661 (90) | −2.2 (−3.1 to −1.3) | −4.3 (−5.0 to −3.5) |
| ≥68 | 544 (6) | 367 (8) | 1055 (10) | 2.2 (1.3 to 3.1) | 4.3 (3.5 to 5.0) |
| Treating facilities | |||||
| Academic (surgery and radiation) | 3509 (36) | 1810 (38) | 3932 (37) | 2.3 (0.6 to 4.0) | 0.8 (−0.6 to 2.1) |
| Academic (surgery) | 877 (9) | 640 (14) | 1602 (15) | 4.5 (3.4 to 5.7) | 6.0 (5.1 to 6.9) |
| Comprehensive community (surgery and radiation) | 2730 (28) | 1159 (24) | 2594 (24) | −3.5 (−5.0 to −2.0) | −3.8 (−5.0 to −2.5) |
| Comprehensive community (surgery) | 655 (7) | 256 (5) | 581 (5) | −1.3 (−2.1 to −0.5) | −1.3 (−1.9 to −0.6) |
| Community (surgery and radiation) | 539 (6) | 244 (5) | 551 (5) | −0.4 (−1.1 to 0.4) | −0.4 (−1.0 to 0.2) |
| Community (surgery) | 198 (2) | 70 (1) | 151 (1) | −0.5 (−1.0 to −0.1) | −0.6 (−1.0 to −0.3) |
| Other (surgery and radiation) | 989 (10) | 423 (9) | 995 (9) | −1.2 (−2.2 to −0.2) | −0.8 (−1.7 to 0) |
| Other (surgery) | 268 (3) | 133 (3) | 310 (3) | 0.1 (−0.5 to 0.6) | 0.1 (−0.3 to 0.6) |
| Distance to facility, miles | |||||
| ≤10 | 4275 (44) | 1942 (41) | 4585 (43) | −2.8 (−4.5 to −1.1) | −1.0 (−2.4 to 0.4) |
| 11-20 | 2119 (22) | 1008 (21) | 2224 (21) | −0.4 (−1.8 to 1.0) | −0.9 (−2.1 to 0.2) |
| 21-50 | 1943 (20) | 1019 (22) | 2232 (21) | 1.6 (0.2 to 3.0) | 0.9 (−0.2 to 2.0) |
| 51-100 | 820 (8) | 466 (10) | 1036 (10) | 1.4 (0.4 to 2.5) | 1.3 (0.5 to 2.1) |
| >100 | 608 (6) | 300 (6) | 639 (6) | 0.1 (−0.7 to 1.0) | −0.3 (−0.9 to 0.4) |
| Hospital length of stay, d | |||||
| 0-7 | 6420 (66) | 2506 (53) | 4968 (46) | −12.8 (−14.5 to −11.1) | −19.4 (−20.7 to −18.1) |
| >7 | 1250 (13) | 1074 (23) | 3084 (29) | 9.9 (8.5 to 11.2) | 16.0 (14.9 to 17.1) |
| Unknown | 2095 (21) | 1155 (24) | 2664 (25) | 2.9 (1.5 to 4.4) | 3.4 (2.3 to 4.6) |
| 30-Day unplanned readmission status | |||||
| No | 9067 (93) | 4362 (92) | 9692 (90) | −0.7 (−1.7 to 0.2) | −2.4 (−3.2 to −1.7) |
| Yes | 262 (3) | 140 (3) | 414 (4) | 0.3 (−0.3 to 0.9) | 1.2 (0.7 to 1.7) |
| Unknown | 436 (4) | 233 (5) | 610 (6) | 0.5 (−0.3 to 1.2) | 1.2 (0.6 to 1.8) |
| Radiation dose, Gy | |||||
| 45.00-49.99 | 87 (1) | 56 (1) | 165 (2) | 0.3 (−0.1 to 0.7) | 0.6 (0.4 to 0.9) |
| 50.00-53.99 | 321 (3) | 171 (4) | 392 (4) | 0.3 (−0.3 to 1.0) | 0.4 (−0.1 to 0.9) |
| 54.00-59.99 | 687 (7) | 427 (9) | 1047 (10) | 2.0 (1.0 to 2.9) | 2.7 (2.0 to 3.5) |
| 60.00-65.99 | 3746 (38) | 2193 (46) | 4893 (46) | 8.0 (6.2 to 9.7) | 7.3 (6.0 to 8.6) |
| 66.00-69.99 | 2570 (26) | 1262 (27) | 2768 (26) | 0.3 (−1.2 to 1.9) | −0.5 (−1.7 to 0.7) |
| 70.00-76.00 | 2354 (24) | 626 (13) | 1451 (14) | −10.9 (−12.2 to −9.6) | −10.6 (−11.6 to −9.5) |
| Radiation modality | |||||
| 3-D Conformal | 242 (2) | 108 (2) | 295 (3) | −0.2 (−0.7 to 0.3) | 0.3 (−0.2 to 0.7) |
| IMRT | 5634 (58) | 2751 (58) | 6104 (57) | 0.4 (−1.3 to 2.1) | −0.7 (−2.1 to 0.6) |
| Unknown | 3889 (40) | 1876 (40) | 4317 (40) | −0.2 (−1.9 to 1.5) | 0.5 (−0.9 to 1.8) |
| Chemotherapy | |||||
| Not given | 3314 (34) | 1956 (41) | 4618 (43) | 7.4 (5.7 to 9.1) | 9.2 (7.8 to 10.5) |
| Adjuvant | 6148 (63) | 2630 (56) | 5679 (53) | −7.4 (−9.1 to −5.7) | −10.0 (−11.3 to −8.6) |
| Neoadjuvant | 102 (1) | 39 (1) | 124 (1) | −0.2 (−0.5 to 0.1) | 0.1 (−0.2 to 0.4) |
| Adjuvant and neoadjuvant | 51 (1) | 37 (1) | 79 (1) | 0.3 (0 to 0.5) | 0.2 (0 to 0.4) |
| Unknown | 150 (2) | 73 (2) | 216 (2) | 0 (−0.4 to 0.4) | 0.5 (0.1 to 0.8) |
Abbreviations: 3-D, 3-dimensional; IMRT, intensity modulated radiation therapy; TD-S, time from diagnosis to surgery.
Education indicates the percent of people with no high school degree in the patient’s zip code of residence.
Income indicates the median household income in the patient’s zip code of residence.
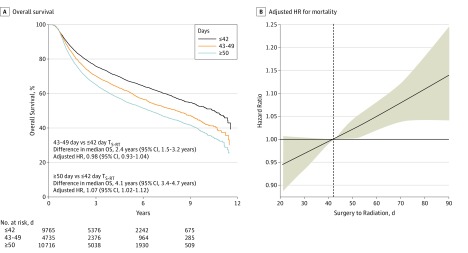
The median follow-up time for surviving patients was 49 months (range, 2-143 months). Of 9765 patients, 3026 (31%)with a TS-RT of 42 days or less, 1770 (37%) of 4735 patients with a 43- to 49-day TS-RT, and 4522 (42%) of 10 716 patients with a TS-RT of 50 days or more had died by the end of the study. There was a median OS of 10.5 years (95% CI, 10.0-11.1 years) for patients with a TS-RT of 42 days or less, 8.2 years (95% CI, 7.4-8.6 years; absolute difference,−2.4 years; 95% CI, −1.5 to −3.2 years) for patients with a 43-49-day TS-RT, and 6.5 years (95% CI, 6.1-6.8 years; absolute difference, −4.1 years; 95% CI, −3.4 to −4.7 years) for those with a TS-RT of 50 days or more (Figure 1). Multivariable Cox regression for mortality was performed with TS-RT modeled as a continuous variable (Figure 1). Compared with a 42-day TS-RT, there was a statistically significant but not clinically meaningful association between worse OS and a longer TS-RT; for example, at 49 days the HR was 1.02 (95% CI, 1.00-1.04). The HR became clinically meaningful when TS-RT reached 61 days (HR, 1.05; 95% CI, 1.02-1.09). Conversely, there was neither a statistically significant nor clinically meaningful association between OS and a shorter TS-RT; for example, at 35 days the HR was 0.98 (95% CI, 0.96-1.00).
Figure 1. Association of Delay From Surgery to Radiation (TS-RT) With Overall Survival.
A, Kaplan-Meier estimates of overall survival according to a TS-RT of 42 days or less, 43 to 49 days, or 50 days or more. The adjusted hazard ratio (HR) was determined from a Cox multivariable regression model with a TS-RT of 42 days or less as the reference. B, Adjusted HR for mortality by TS-RT modeled as a continuous variable with a restricted cubic spline with 3 knots. A 42-day TS-RT was used as the reference. Shading indicates the 95% CI for HR estimates.
The TS-RT was then tested with a multivariable Cox regression model (Table 2). Compared with a TS-RT of 42 days or less, a TS-RT of 50 days or more resulted in inferior OS (HR, 1.07; 95% CI, 1.02-1.12). No clinically meaningful association was seen with a 43- to 49-day TS-RT (HR, 0.98; 95% CI, 0.93-1.04). Other covariates with both a statistically significant and clinically meaningful association with worse OS included a hospital stay longer than 7 days (HR, 1.24; 95% CI, 1.18-1.30), radiation doses higher or lower than 60 to 66 Gy, and not receiving chemotherapy (HR, 1.10; 95% CI, 1.05-1.15). Having private/managed care/governmental insurance was associated with improved OS compared with Medicare insurance (HR, 0.73; 95% CI, 0.69-0.77). Compared with patients receiving all care at an academic center, patients who transferred care after surgery at an academic center had worse OS (HR, 1.11; 95% CI, 1.04-1.19), and patients receiving all care at a CCCP also had comparatively inferior outcomes (HR, 1.06; 95% CI, 1.01-1.12).
Table 2. Multivariable Cox Regression for All-Cause Mortality.
| Variable | Hazard Ratio (95% CI)a |
|---|---|
| TS-RT, d | |
| ≤42 | 1 [Reference] |
| 43-49 | 0.98 (0.93-1.04) |
| ≥50 | 1.07 (1.02-1.12) |
| Sex | |
| Male | 1 [Reference] |
| Female | 0.90 (0.86-0.94) |
| Age, y | |
| ≤50 | 1 [Reference] |
| 51-60 | 1.20 (1.13-1.28) |
| 61-70 | 1.29 (1.20-1.38) |
| ≥71 | 1.76 (1.62-1.91) |
| Race | |
| White | 1 [Reference] |
| Black | 1.03 (0.96-1.10) |
| Other | 0.98 (0.88-1.10) |
| Educationb | |
| ≥21% | 1.06 (0.97-1.15) |
| 13%-20.9% | 1.07 (0.99-1.15) |
| 7%-12.9% | 1.05 (0.98-1.12) |
| <7% | 1 [Reference] |
| Unknown | 1.78 (0.44-7.19) |
| Income, $c | |
| <38,000 | 1.18 (1.09-1.28) |
| 38 000-47 999 | 1.09 (1.02-1.17) |
| 48 000-62 999 | 1.06 (1.00-1.13) |
| ≥63 000 | 1 [Reference] |
| Unknown | 1.07 (0.27-4.29) |
| Insurance | |
| Medicare | 1 [Reference] |
| Medicaid | 1.02 (0.95-1.10) |
| Private/managed care/other government | 0.73 (0.69-0.77) |
| None | 0.90 (0.82-0.99) |
| Unknown | 0.85 (0.71-1.01) |
| Charlson/Deyo score | |
| 0 | 1 [Reference] |
| 1 | 1.11 (1.05-1.17) |
| ≥2 | 1.52 (1.40-1.66) |
| Disease site | |
| Tonsil | 0.44 (0.41-0.49) |
| Nontonsil oropharynx | 1 [Reference] |
| Oral cavity | 1.91 (1.77-2.05) |
| Larynx | 1.53 (1.41-1.66) |
| Hypopharynx | 1.62 (1.45-1.82) |
| Stage | |
| III | 1 [Reference] |
| IV | 1.26 (1.20-1.33) |
| Extracapsular extension | |
| No | 1 [Reference] |
| Yes | 1.39 (1.32-1.46) |
| Node negative | 0.75 (0.71-0.79) |
| Unknown | 1.02 (0.94-1.10) |
| Margin status | |
| Negative | 1 [Reference] |
| Positive | 1.36 (1.27-1.46) |
| Unknown | 1.25 (1.18-1.32) |
| Lymphovascular invasion | |
| Absent | 1 [Reference] |
| Present | 1.36 (1.26-1.47) |
| Unknown | 1.02 (0.93-1.13) |
| Year of diagnosis | |
| 2004-2006 | 1 [Reference] |
| 2007-2009 | 0.92 (0.88-0.98) |
| 2010-2013 | 0.84 (0.76-0.92) |
| TD-S, d | |
| <68 | 1 [Reference] |
| ≥68 | 1.02 (0.95-1.10) |
| Treating facilities | |
| Academic (surgery and radiation) | 1 [Reference] |
| Academic (surgery) | 1.11 (1.04-1.19) |
| Comprehensive community (surgery and radiation) | 1.06 (1.01-1.12) |
| Comprehensive community (surgery) | 1.06 (0.97-1.17) |
| Community (surgery and radiation) | 1.06 (0.96-1.16) |
| Community (surgery) | 1.16 (0.98-1.37) |
| Other (surgery and radiation) | 0.99 (0.91-1.07) |
| Other (surgery) | 1.07 (0.94-1.22) |
| Distance to facility, mile | |
| ≤10 | 1 [Reference] |
| 11-20 | 0.96 (0.90-1.01) |
| 21-50 | 0.93 (0.88-0.98) |
| 51-100 | 0.87 (0.80-0.94) |
| >100 | 0.87 (0.79-0.95) |
| Hospital length of stay, d | |
| 0-7 | 1 [Reference] |
| >7 | 1.24 (1.18-1.30) |
| Unknown | 1.11 (1.05-1.17) |
| 30-Day unplanned readmission status | |
| No | 1 [Reference] |
| Yes | 1.06 (0.95-1.18) |
| Unknown | 1.06 (0.97-1.16) |
| Radiation dose, Gy | |
| 45.00-49.99 | 1.68 (1.43-1.97) |
| 50.00-53.99 | 1.16 (1.04-1.28) |
| 54.00-59.99 | 1.13 (1.05-1.21) |
| 60.00-65.99 | 1 [Reference] |
| 66.00-69.99 | 1.08 (1.02-1.13) |
| 70.00-76.00 | 1.26 (1.18-1.34) |
| Radiation modality | |
| 3-D Conformal | 1 [Reference] |
| IMRT | 0.92 (0.82-1.03) |
| Unknown | 0.94 (0.83-1.05) |
| Chemotherapy | |
| Not given | 1.10 (1.05-1.15) |
| Adjuvant | 1 [Reference] |
| Neoadjuvant | 1.16 (0.97-1.39) |
| Adjuvant and neoadjuvant | 1.02 (0.80-1.31) |
| Unknown | 0.86 (0.73-1.01) |
Abbreviations: 3-D, 3-dimensional; IMRT, intensity modulated radiation therapy; TD-S, time from diagnosis to surgery; TS-RT, time from surgery to radiation.
Multivariable model included TS-RT, sex, age, race, education, income, insurance type, Charlson/Deyo score, disease site, stage, extracapsular extension, margin status, lymphovascular invasion, year of diagnosis, TD-S, facility type, distance to facility, hospital length of stay, readmission status, radiation dose, radiation modality, and chemotherapy use.
Education indicates the percent of people with no high school degree in the patient’s zip code of residence.
Income indicates the median household income in the patient’s zip code of residence.
A complete case analysis for patients with complete LVI, margin, and ECE data was performed. This cohort consisted of 7859 patients, who were diagnosed after 2010 when LVI status became available. After adjusting for confounding covariates, estimates for the risk of mortality with a 43- to 49-day TS-RT (HR, 1.03; 95% CI, 0.91-1.16) and a TS-RT of 50 days or more (HR, 1.10; 95% CI, 0.99-1.21) were similar in direction and magnitude to those from the initial model (eTable 1 in the Supplement).
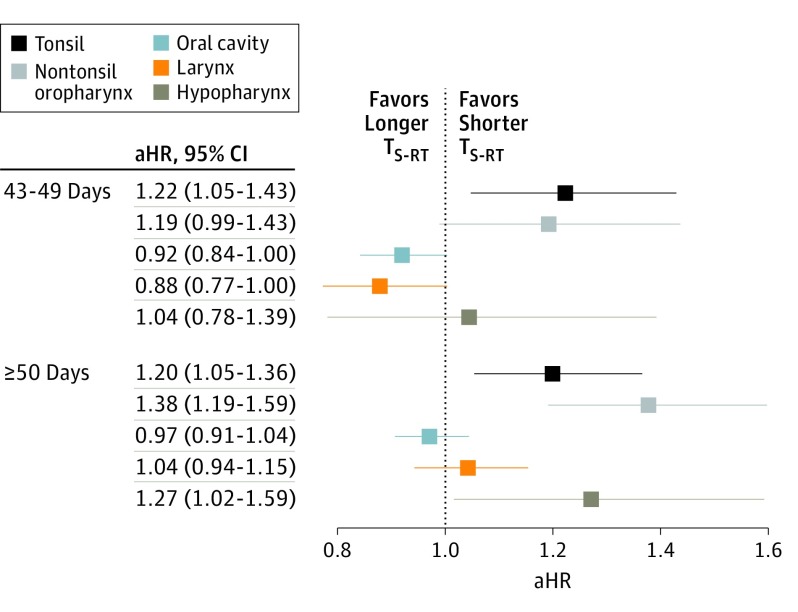
Subgroup effects of interactions between TS-RT and covariates coding for high-risk factors were tested with multivariable Cox regressions. There was a significant interaction between TS-RT group and site of disease, but not disease stage, LVI, margin status, ECE, or chemotherapy use. The multivariable regression model that included this interaction term demonstrated that, compared with a TS-RT of 42 days or less, having a TS-RT of 50 days or more resulted in inferior OS for patients with hypopharynx (HR, 1.27; 95% CI, 1.02-1.59), tonsil (HR, 1.20; 95% CI, 1.05-1.36), and nontonsil oropharynx cancers (HR, 1.38; 95% CI, 1.19-1.59) (Figure 2). A 43- to 49-day TS-RT was significantly associated with worse OS for patients with tonsil cancer (HR, 1.22; 95% CI, 1.05-1.43). The effect of delayed TS-RT was neither statistically significant nor clinically meaningful for patients with oral cavity or larynx cancers.
Figure 2. Subgroup Effects of Covariates With Significant Interactions With TS-RT.
Adjusted hazard ratio for mortality according to a TS-RT of 43 to 49 days or 50 days or more compared with 42 days or less varied by disease site. TS-RT indicates time from surgery to the start of radiation.
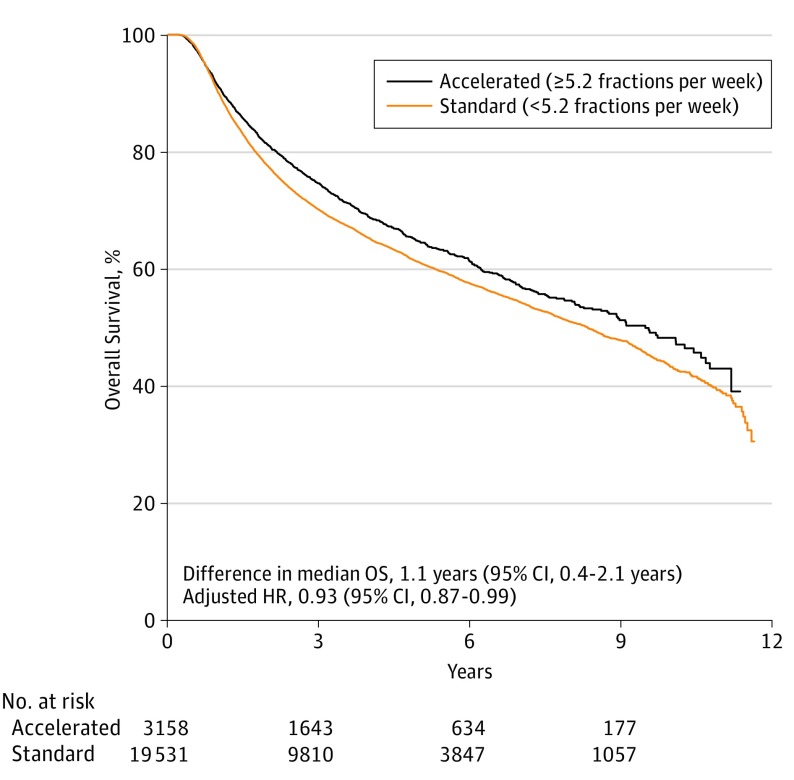
A total of 22 689 patients had radiation fractionation data available. Patients receiving accelerated fractionation (≥5.2 fractions per week) had a median OS of 9.5 years (95% CI, 8.7-10.4 years), and those receiving standard fractionation had a median OS of 8.3 years (95% CI, 8.0-8.6 years; absolute difference, −1.1 years; 95% CI, −0.4 to −2.1 years) (Figure 3). In a multivariable Cox regression an interaction term between fractionation schedule and TS-RT group was not significant. After adjusting for confounders, accelerated fractionation remained associated with improved survival (HR, 0.93; 95% CI, 0.87-0.99) (eTable 2 in the Supplement).
Figure 3. Association of Accelerated Fractionation With Overall Survival (OS).
Kaplan-Meier estimates of OS according to standard (<5.2 average fractions per week) or accelerated (≥5.2 average fractions per week) fractionation. The adjusted hazard ratio was determined from a Cox multivariable regression model with standard fractionation as the reference.
Discussion
We found that for patients with head and neck squamous cell carcinoma treated with definitive surgical resection followed by adjuvant radiation, improved survival was associated with a shorter interval from the date of surgery to the start of radiation (shorter TS-RT). A TS-RT within 42 days resulted in improved survival compared with greater than 50 days on multivariable analysis. For patients with tonsil tumors, even a 1-week delay resulted in comparatively inferior survival, although for other patients this additional week was not a significant factor.
Patients with head and neck cancer and high-risk pathologic features have well-established indications for adjuvant radiation or chemoradiation. However, many are not receiving the recommended adjuvant therapy, resulting in increased mortality. When adjuvant radiation is indicated, efforts should be made to start as quickly as reasonably possible. We found that radiation started within 42 days of surgery for 39% of patients, and compared with those with a longer delay, a greater proportion of these patients were treated at nonacademic centers. Other previously identified factors associated with delayed TS-RT include patient socioeconomic status, medical comorbidities, and a longer postsurgical hospital stay.
The 6-week cutoff originated from an analysis of patients treated at Memorial Sloan Kettering in the 1970s. Interestingly, a follow-up study with more patients found that the 6 week TS-RT cutoff predicted outcomes only for a subset of patients receiving doses under 60 Gy. However, the importance of the 6-week cutoff for locoregional control was demonstrated in other retrospective studies, as well as in a meta-analysis. Other groups have studied the benefit using shorter cutoffs, longer cutoffs, and no specific cutoff when TS-RT is modeled as a continuous variable. Studies of prospective trials have similarly demonstrated a benefit to starting radiation therapy within 4 or 6 weeks after surgery. However, the issue remains controversial because there was not always a demonstrable benefit to a shorter TS-RT.
Prior to treatment initiation, head and neck cancers grow rapidly. Models of oropharynx cancer have shown a decreased probability of local control with a longer delay for intact tumors. Additional time between surgery and the start of radiation (longer TS-RT) allows residual tumor clonogens to repopulate, increasing the risk of local failure. Some models of clonogenic doubling time would suggest that there may be a time period of accelerated repopulation immediately after surgery owing to the response from cellular depopulation. Accelerated repopulation during definitive radiation has been well established. In the postoperative setting, one could suggest that the decrease in the tumor mass results in a relative increased availability of nutrients for residual tumor cells, arguing for as short a TS-RT as can be achieved reasonably.
Patients in our study with a shorter TS-RT were less likely to receive treatment at an academic center than those with a more delayed TS-RT. However, in a multivariable Cox regression model adjusting for TS-RT, patients receiving surgery and radiation at an academic center had better outcomes compared with those who left the academic center for radiation elsewhere or had all treatment at a Comprehensive Community Cancer Program. Similar results were seen in a cancer registry study from the Netherlands, which found that initiation of treatment of head and neck cancer patients was longer for patients referred to 1 of 8 national head and neck oncology centers, but despite the delay these patients had better overall survival compared with those treated elsewhere. In addition, after surgery at the University of California, San Francisco, postoperative radiation received at nonacademic centers was associated with inferior radiation quality metrics: lower dose, lower fractional dose, longer TS-RT, increased radiation breaks, and higher rates of early termination of the radiation course. For a cohort of patients with oral cavity cancer, radiation treatment at the academic site translated into a survival benefit. Although the national pattern identified in our study and another registry study is that treatment at academic centers is associated with longer delays to starting radiation, it is reassuring that shorter TS-RT at academic sites can be achieved. In addition, when controlling for TS-RT, we found that there was improved survival with treatment at academic sites, suggesting a benefit to specialized care for head and neck cancer patients.
Together TS-RT and the duration of radiation therapy are defined as the overall package time. Retrospective studies have demonstrated the total package should be delivered within 87 to 100 days, and analysis from a prospective trial found better outcomes for those with package times under 77 to 91 days. Often, the duration of TS-RT is out of the hands of the radiation oncologist and medical team. From a radiation perspective, options to improve tumor control after a delayed TS-RT include escalated total radiation dose or accelerated fractionation schedules.
In the definitive setting, accelerated fractionation has been associated with improved progression-free survival in a meta-analysis of randomized trials, and overall survival in an NCDB study. In the postoperative setting, improved outcomes can also be attained with accelerated fractionation. With regard to the interaction of TS-RT with altered fractionation, studies have noted a decrease in the negative effect from delayed TS-RT with dose intensification or altered fractionation. Similarly, a randomized trial of fractionation schedules in PORT found a trend for improved local control with accelerated fractionation for those patients with a TS-RT of more than 48 days. Additional detrimental delays during radiation therapy are introduced with any breaks or a split-course fractionation schedule. We found that there was improved survival when accelerated fractionation was used. Thus, this study supports considering accelerated fractionation for patients at high risk for locoregional failure, and supportive measures during treatment should be employed to prevent breaks.
We found a significant interaction between TS-RT and tumor site, but not stage, ECE, margin status, or presence of LVI. Patients with oropharynx and hypopharynx tumors had a more pronounced response to delayed TS-RT. In contrast, some studies have shown that the association between shorter treatment time and locoregional control and survival can depend on the presence of high-risk features, although the strength of the relationship is not well established. Hypothetically those with larger or more aggressive tumors might have a greater chance for larger deposits of residual tumor cells after surgery, making local control more challenging after a longer TS-RT delay. However, this relationship warrants further exploration.
Limitations
The benefit of using the NCDB to address the effect of TS-RT was the large patient cohort. However, owing to the observational nature of the study there may be unmeasured confounders associated with delayed radiation and worse outcomes. One example would be patients with serious postoperative complications, and although we accounted for length of hospital stay and hospital readmissions in the multivariable models, there may be residual confounding. In addition, retrospective studies inherently may have unavoidable selection bias, and registry studies may have problems from errors in registrar coding or reporting. Another limitation was that although radiation dose was accounted for in multivariable models, data were unavailable for specific doses to the surgical cavity or nodal regions as well as normal tissue dosimetric parameters. These parameters may account for the benefit seen with treatment at academic centers. The database also lacks recurrence and toxic effects data, so future nonregistry studies are certainly warranted.
Conclusions
To our knowledge, there has never been a randomized trial to test the effect of TS-RT. However, given the preponderance of evidence from retrospective and prospective studies, as well as the plausible biological model that shorter TS-RT results in improved outcomes, we believe it would be unethical to carry out such a trial. This study supports the use of adjuvant radiation therapy and current national guidelines to prevent delays over 50 days. A multitude of factors contribute to delayed radiation, so multidisciplinary teams and patients must be educated about the importance of timing, with all efforts made to avoid unnecessary delays. Measures to intensify therapy including with accelerated fractionation should be considered when delays are unavoidable.
eTable 1. Multivariate Cox regression for mortality adjusted for covariates for patients with complete disease, stage, ECE, margin status, and LVI data.
eTable 2. Multivariate Cox regression for mortality for accelerated versus standard radiation fractionation
References
- 1.Kramer S, Gelber RD, Snow JB, et al. Combined radiation therapy and surgery in the management of advanced head and neck cancer: final report of study 73-03 of the Radiation Therapy Oncology Group. Head Neck Surg. 1987;10(1):19-30. [DOI] [PubMed] [Google Scholar]
- 2.Peters LJ, Goepfert H, Ang KK, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 1993;26(1):3-11. [DOI] [PubMed] [Google Scholar]
- 3.Frank JL, Garb JL, Kay S, et al. Postoperative radiotherapy improves survival in squamous cell carcinoma of the hypopharynx. Am J Surg. 1994;168(5):476-480. [DOI] [PubMed] [Google Scholar]
- 4.Trotti A, Klotch D, Endicott J, Ridley M, Cantor A. Postoperative accelerated radiotherapy in high-risk squamous cell carcinoma of the head and neck: long-term results of a prospective trial. Head Neck. 1998;20(2):119-123. [DOI] [PubMed] [Google Scholar]
- 5.Lundahl RE, Foote RL, Bonner JA, et al. Combined neck dissection and postoperative radiation therapy in the management of the high-risk neck: a matched-pair analysis. Int J Radiat Oncol Biol Phys. 1998;40(3):529-534. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):571-578. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JS, Pajak TF, Forastiere AA, et al. ; Radiation Therapy Oncology Group 9501/Intergroup . Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937-1944. [DOI] [PubMed] [Google Scholar]
- 8.Bernier J, Domenge C, Ozsahin M, et al. ; European Organization for Research and Treatment of Cancer Trial 22931 . Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945-1952. [DOI] [PubMed] [Google Scholar]
- 9.Vikram B. Importance of the time interval between surgery and postoperative radiation therapy in the combined management of head & neck cancer. Int J Radiat Oncol Biol Phys. 1979;5(10):1837-1840. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network Head and Neck Cancers (Version 2.2017). https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed July 31, 2017. [DOI] [PubMed]
- 11.Mantravadi RV, Haas RE, Liebner EJ, Skolnik EM, Applebaum EL. Postoperative radiotherapy for persistent tumor at the surgical margin in head and neck cancers. Laryngoscope. 1983;93(10):1337-1340. [DOI] [PubMed] [Google Scholar]
- 12.Vikram B, Strong EW, Shah JP, Spiro R. Failure in the neck following multimodality treatment for advanced head and neck cancer. Head Neck Surg. 1984;6(3):724-729. [DOI] [PubMed] [Google Scholar]
- 13.Kajanti M, Holsti LR, Holsti P. Radical surgery and postoperative split-course radiotherapy in squamous cell carcinoma of the mobile tongue: factors influencing local control and the time to recurrence. Radiother Oncol. 1991;22(3):174-179. [DOI] [PubMed] [Google Scholar]
- 14.Byers RM, Clayman GL, Guillamondequi OM, Peters LJ, Goepfert H. Resection of advanced cervical metastasis prior to definitive radiotherapy for primary squamous carcinomas of the upper aerodigestive tract. Head Neck. 1992;14(2):133-138. [DOI] [PubMed] [Google Scholar]
- 15.Dixit S, Vyas RK, Toparani RB, Baboo HA, Patel DD. Surgery versus surgery and postoperative radiotherapy in squamous cell carcinoma of the buccal mucosa: a comparative study. Ann Surg Oncol. 1998;5(6):502-510. [DOI] [PubMed] [Google Scholar]
- 16.Muriel VP, Tejada MR, de Dios Luna del Castillo J. Time-dose-response relationships in postoperatively irradiated patients with head and neck squamous cell carcinomas. Radiother Oncol. 2001;60(2):137-145. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21(3):555-563. [DOI] [PubMed] [Google Scholar]
- 18.Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, Maciejewski B. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys. 2003;56(2):399-412. [DOI] [PubMed] [Google Scholar]
- 19.Amdur RJ, Parsons JT, Mendenhall WM, Million RR, Stringer SP, Cassisi NJ. Postoperative irradiation for squamous cell carcinoma of the head and neck: an analysis of treatment results and complications. Int J Radiat Oncol Biol Phys. 1989;16(1):25-36. [DOI] [PubMed] [Google Scholar]
- 20.Schiff PB, Harrison LB, Strong EW, et al. Impact of the time interval between surgery and postoperative radiation therapy on locoregional control in advanced head and neck cancer. J Surg Oncol. 1990;43(4):203-208. [DOI] [PubMed] [Google Scholar]
- 21.Bastit L, Blot E, Debourdeau P, Menard J, Bastit P, Le Fur R. Influence of the delay of adjuvant postoperative radiation therapy on relapse and survival in oropharyngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2001;49(1):139-146. [DOI] [PubMed] [Google Scholar]
- 22.Marshak G, Rakowsky E, Schachter J, et al. Is the delay in starting postoperative radiotherapy a key factor in the outcome of advanced (T3 and T4) laryngeal cancer? Otolaryngol Head Neck Surg. 2004;131(4):489-493. [DOI] [PubMed] [Google Scholar]
- 23.Le Tourneau C, Jung G-M, Borel C, Bronner G, Flesch H, Velten M. Prognostic factors of survival in head and neck cancer patients treated with surgery and postoperative radiation therapy. Acta Otolaryngol. 2008;128(6):706-712. [DOI] [PubMed] [Google Scholar]
- 24.Suwiński R, Bańkowska-Woźniak M, Majewski W, et al. Randomized clinical trial on 7-days-a-week postoperative radiotherapy for high-risk squamous cell head and neck cancer. Radiother Oncol. 2008;87(2):155-163. [DOI] [PubMed] [Google Scholar]
- 25.American College of Surgeons National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb. Accessed October 1, 2017.
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. [DOI] [PubMed] [Google Scholar]
- 27.Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol. 2007;84(1):5-10. [DOI] [PubMed] [Google Scholar]
- 28.Murphy CT, Galloway TJ, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the united states. J Clin Oncol. 2016;34(2):169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montori VM, Kleinbart J, Newman TB, et al. ; Evidence-Based Medicine Teaching Tips Working Group . Tips for learners of evidence-based medicine: 2. Measures of precision (confidence intervals). CMAJ. 2004;171(6):611-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen MM, Roman SA, Yarbrough WG, Burtness BA, Sosa JA, Judson BL. Trends and variations in the use of adjuvant therapy for patients with head and neck cancer. Cancer. 2014;120(21):3353-3360. [DOI] [PubMed] [Google Scholar]
- 31.Graboyes EM, Garrett-Mayer E, Sharma AK, Lentsch EJ, Day TA. Adherence to National Comprehensive Cancer Network guidelines for time to initiation of postoperative radiation therapy for patients with head and neck cancer. Cancer. 2017;123(14):2651-2660. [DOI] [PubMed] [Google Scholar]
- 32.Mackillop WJ, Bates JH, O’Sullivan B, Withers HR. The effect of delay in treatment on local control by radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34(1):243-250. [DOI] [PubMed] [Google Scholar]
- 33.Al-Dweri FMO, Guirado D, Lallena AM, Pedraza V. Effect on tumour control of time interval between surgery and postoperative radiotherapy: an empirical approach using Monte Carlo simulation. Phys Med Biol. 2004;49(13):2827-2839. [DOI] [PubMed] [Google Scholar]
- 34.Peters LJ, Withers HR. Applying radiobiological principles to combined modality treatment of head and neck cancer—the time factor. Int J Radiat Oncol Biol Phys. 1997;39(4):831-836. [DOI] [PubMed] [Google Scholar]
- 35.Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27(2):131-146. [DOI] [PubMed] [Google Scholar]
- 36.Hendry JH. Treatment acceleration in radiotherapy: the relative time factors and dose-response slopes for tumours and normal tissues. Radiother Oncol. 1992;25(4):308-312. [DOI] [PubMed] [Google Scholar]
- 37.van Harten MC, Hoebers FJP, Kross KW, et al. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol. 2015;51(3):272-278. [DOI] [PubMed] [Google Scholar]
- 38.George JR, Yom SS, Wang SJ. Combined modality treatment outcomes for head and neck cancer: comparison of postoperative radiation therapy at academic vs nonacademic medical centers. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1118-1126. [DOI] [PubMed] [Google Scholar]
- 39.George JR, Yom SS, Wang SJ. Improved outcomes in adjuvant radiotherapy for oral cavity carcinoma at an academic center: a matched-pair analysis. Laryngoscope. 2014;124(7):1603-1608. [DOI] [PubMed] [Google Scholar]
- 40.Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ, Million RR. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys. 1997;39(1):137-148. [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal DI, Liu L, Lee JH, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24(2):115-126. [DOI] [PubMed] [Google Scholar]
- 42.Tribius S, Donner J, Pazdyka H, et al. Survival and overall treatment time after postoperative radio(chemo)therapy in patients with head and neck cancer. Head Neck. 2016;38(7):1058-1065. [DOI] [PubMed] [Google Scholar]
- 43.Marshak G, Popovtzer A. Is there any significant reduction of patients’ outcome following delay in commencing postoperative radiotherapy? Curr Opin Otolaryngol Head Neck Surg. 2006;14(2):82-84. [DOI] [PubMed] [Google Scholar]
- 44.Lacas B, Bourhis J, Overgaard J, et al. ; MARCH Collaborative Group . Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 2017;18(9):1221-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaikh T, Handorf EA, Murphy CT, Mehra R, Ridge JA, Galloway TJ. The impact of radiation treatment time on survival in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2016;96(5):967-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanguineti G, Richetti A, Bignardi M, et al. Accelerated versus conventional fractionated postoperative radiotherapy for advanced head and neck cancer: results of a multicenter Phase III study. Int J Radiat Oncol Biol Phys. 2005;61(3):762-771. [DOI] [PubMed] [Google Scholar]
- 47.Barton MB, Keane TJ, Gadalla T, Maki E. The effect of treatment time and treatment interruption on tumour control following radical radiotherapy of laryngeal cancer. Radiother Oncol. 1992;23(3):137-143. [DOI] [PubMed] [Google Scholar]
- 48.Robertson C, Robertson AG, Hendry JH, et al. Similar decreases in local tumor control are calculated for treatment protraction and for interruptions in the radiotherapy of carcinoma of the larynx in four centers. Int J Radiat Oncol Biol Phys. 1998;40(2):319-329. [DOI] [PubMed] [Google Scholar]
- 49.Parsons JT, Bova FJ, Million RR. A re-evaluation of split-course technique for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1980;6(12):1645-1652. [DOI] [PubMed] [Google Scholar]
- 50.Amdur RJ, Parsons JT, Mendenhall WM, Million RR, Cassisi NJ. Split-course versus continuous-course irradiation in the postoperative setting for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1989;17(2):279-285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Multivariate Cox regression for mortality adjusted for covariates for patients with complete disease, stage, ECE, margin status, and LVI data.
eTable 2. Multivariate Cox regression for mortality for accelerated versus standard radiation fractionation