Key Points
Question
With increasing importance of genetic testing for uveal melanoma, how can we maximize the yield rate for fine-needle aspiration biopsy, especially for small lesions?
Findings
In this case series, a combined yield rate of 90.9% was achieved for transscleral and transvitreal biopsies of tumors with a median apical height of 2.7 mm.
Meaning
These findings suggest that an intraoperative adequacy check of biopsied samples can ensure enough cells were harvested for both cytologic and genomic analyses.
Abstract
Importance
Intraoperative adequacy check of biopsy samples can lead to a higher biopsy yield rate, and subsequent genomic analysis can provide patients with valuable prognostic information.
Objectives
To examine the yield rates for transscleral and transvitreal fine-needle aspiration biopsies of small uveal melanoma less than 3.6 mm in apical height and to discuss techniques that would maximize yield rates and minimize complications.
Design, Setting, and Participants
A retrospective, consecutive observational case series was conducted from January 29, 2013, to May 23, 2017, at Retina Consultants of Houston and Houston Methodist Hospital among 44 patients with uveal melanoma of the ciliary body or choroid.
Interventions or Exposures
Fine-needle aspiration biopsy and intraoperative histopathologic analysis prior to iodine 125 brachytherapy.
Main Outcomes and Measures
Tumor locations and dimensions were identified by histopathologic analysis and B-scan ultrasonography. Either transscleral or transvitreal biopsy was performed for tumors anterior to the equator and posterior to the equator, respectively. Biopsy specimens were checked for adequacy intraoperatively. Specimens were examined using hematoxylin-eosin, double immunostain with human melanoma black 45 and Ki67, and gene expression profile.
Results
A total of 44 patients were included in the study, with a mean (SD) age of 63.3 (12.7) years (21 men [47.7%]; 23 women [52.3%]). Median tumor height was 2.7 mm (interquartile range, 2.3- 2.9 mm). Forty of 44 biopsy samples (90.9%; 95% CI, 82.4%-99.4%) yielded adequate cells for gene expression profile analysis. Transscleral and transvitreal yield rates were 11 of 11 (100%) and 29 of 33 (87.9%), respectively. Most localized vitreous hemorrhages were resolved by 3 months. There was a moderate association between localized vitreous hemorrhage and transvitreal biopsy method, for which the phi value was −0.526 (95% CI, −0.712 to −0.157; P < .001).
Conclusions and Relevance
These findings suggest intraoperative adequacy evaluation of fine-needle aspiration biopsy specimens leads to high yield and is more informative for patients.
This case series examines the yield rates for transscleral and transvitreal fine-needle aspiration biopsies of small uveal melanoma.
Introduction
Uveal melanoma (UM) is the most common primary intraocular cancer in the adult population, with 1200 to 2000 new cases every year in the United States alone.1 Up to half of the patients with UM develop hematogenous metastases, approximately 90% of which first occur in the liver.2 Prior to 1990, there was no way to reliably predict based on clinical or histopathologic features which patients would develop metastases and which would not. However, starting in the 1990s chromosomal abnormalities including monosomy 3 were discovered to be associated with an increased risk of metastases.3 In recent years, various methods of genomic profiling, including gene expression profiling (GEP) analysis and multiplex ligation-dependent probe amplification (MLPA), have been adopted routinely in a clinical context to provide patients with individualized probabilities of survival.4,5 Mutations in key driver genes, including BAP1, SF3B1, and EIF1AX, have been identified to play a significant role in tumor prognosis.6 Therefore, most patients now expect cytologic confirmation of their diagnoses as well as genomic results after surgery. As genomic analysis of UM has become a critical portion of management of the disease, the importance of reliable techniques for fine-needle aspiration biopsy (FNAB) has also increased drastically.
Several large centers in the United States and abroad have reported their experience with FNAB for UM.7,8,9,10,11,12,13,14,15,16 Most authors have reported a limited number of serious complications. In earlier years, before FNAB was widespread, a rare but serious complication was the extraocular extension of tumor through the biopsy needle tract. However, we reported a collaborative study examining the incidence of this complication, indicating that it was extremely rare with modern techniques.17 In addition, we published an in vivo prospective study that examined the FNAB needle tracts, and no iatrogenic extension of tumor cells was identified.18 In previously published FNAB studies on UM, the rate of cellular yield for cytology and genomic analysis has been variable among centers. More importantly, no publication to our knowledge focuses specifically on techniques that can maximize the positive cellular yield rate, especially for small lesions that are difficult to biopsy. In this article, we report our experience with both transscleral and transvitreal biopsies of UM with rapid intraoperative evaluation by our ophthalmic pathology team.
Methods
Patients
This was a single-center, retrospective, observational consecutive case series of 44 patients that was approved by the institutional review board at the Houston Methodist Hospital. Informed consent was waived because this was a retrospective study. The inclusion criteria for the study were patients older than 18 years, diagnosis of UM of the ciliary body or choroid, and tumor apical height of 3.6 mm or less. Patients included in the study had FNAB and iodine 125 episcleral plaque brachytherapy performed from January 29, 2013, to May 23, 2017.
General clinical variables extracted from the medical records included demographics—sex, age at diagnosis, laterality, the development of metastases, and survival. Details collected from the preoperative and postoperative ophthalmic evaluations included visual acuity, full ophthalmic examination results, fundus photographs, spectral-domain optical coherence tomography, A- and B-scan ultrasonography, and fluorescein angiography.
FNAB Technique
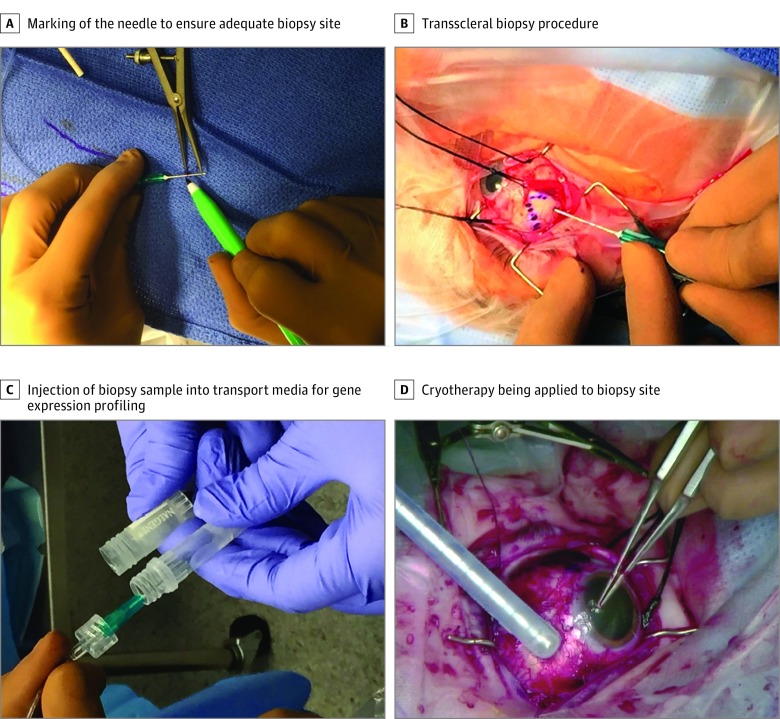
All surgery and biopsies were performed by the same experienced ocular oncologist (A.C.S.). Based on the tumor characteristics of each patient, including the tumor dimension, configuration, and location, either a transscleral or transvitreal approach was selected for the FNAB procedure. For tumors located anterior to the equator, providing direct access for the biopsy needle, a transscleral approach was chosen (Figure 1). Two to 3 rectus muscles were first isolated with nylon sutures. Transillumination was performed to confirm the location of the tumor and a sterile surgical marker was used to draw the outline of the tumor. For cases in which an amelanotic tumor did not cast a strong shadow on transillumination, indirect ophthalmoscopy with an O’Connor scleral depressor was used to localize the tumor, which was then marked with a sterile surgical marker. Half to two-thirds of the apical tumor height obtained from the B-scan was marked on a short 23- or 25-gauge needle with the surgical marking pen, which was connected to a 10-cm tubing (Accurate Surgical & Scientific Instruments Corp) and an aspiration biopsy syringe gun (Stryker Spine) to more easily visualize the maximum depth of penetration of the needle. After the biopsy, cryotherapy was performed as the biopsy needle was withdrawn to destroy any tumor cells present on the needle tip.
Figure 1. Clinical Photographs of Transscleral Biopsy Procedure.
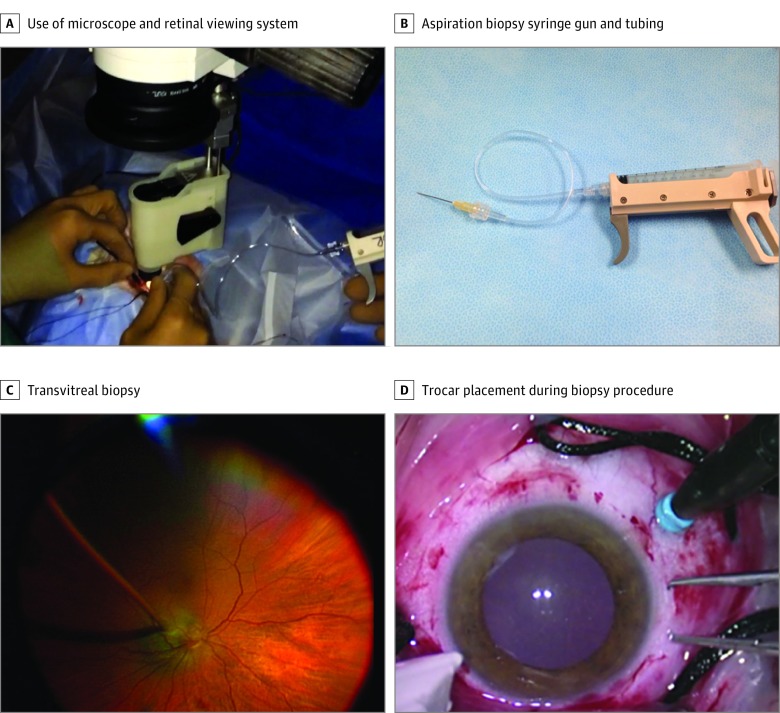
For tumors located posterior to the equator and therefore not easily accessible for a transscleral biopsy approach, the transvitreal method was used (Figure 2). A standard operating microscope was used with a noncontact retinal viewing system. A 23- or 25-gauge valved trocar was used to maintain the intraocular pressure and to prevent contact of the needle tip with the scleral bed. A second trocar was inserted 180° opposite to the tumor with chandelier lighting for visualization. Long 25- or 27-gauge needles were used most commonly to perform the biopsy. For the smallest posterior lesions (<2.5 mm in apical height) only, a 25- or 27-gauge needle was first used to create a small hole in the retina over the tumor, and then a 27-gauge cutter on a low cut rate (200-500 cuts/second) was used to harvest tumor cells. If a significant amount of hemorrhage was encountered and the need for a second pass was anticipated, intravitreal diathermy was applied to the biopsy site to minimize vitreous hemorrhage and an impaired view of the tumor.
Figure 2. Clinical Photographs of Transvitreal Biopsy Procedure.
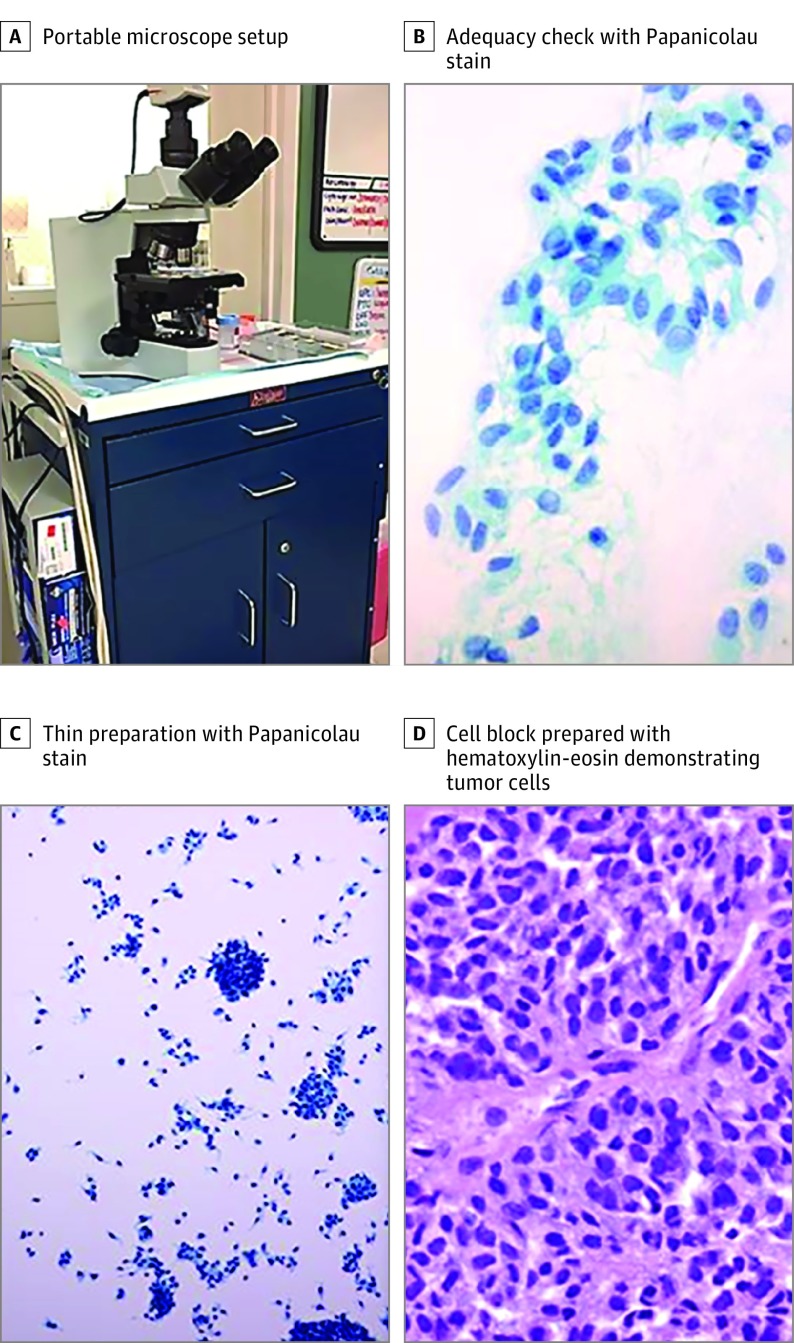
An onsite ophthalmic pathology team led by a senior fellowship-trained ophthalmic pathologist (P.C.-B.) performed an immediate intraoperative adequacy check for each acquired biopsy sample with a portable microscope and staining apparatus (Figure 3). For adequacy check, slides were stained with Diff-Quick. If inadequate samples were obtained, the ocular oncologist repeated the biopsy so that acquisition of adequate tumor cells was confirmed. Cytologic preparation and a cell block were then created. Hematoxylin-eosin stain and double immunostain with human melanoma black 45 and Ki67 were used to examine the cell block. A separate vial of tumor cells was collected for further genomic analysis.
Figure 3. Biopsy Adequacy Check in the Operating Room Using a Portable Microscope.
Results
Patient Demographics and UM Characteristics
A total of 44 patients were analyzed for this study. Of these patients, 23 (52.3%) were female and 21 (47.7%) were male. Of the affected eyes, 24 (54.4%) were right eyes and 20 (45.5%) were left eyes. The median (range) age at the time of FNAB procedure was 63.6 (33.6) and the mean (SD) age at the time of FNAB procedure was 63.3 (12.7) years. The median (range) follow-up period was 25.3 (1.0) and the mean (SD) follow-up period was 24.7 (53.7) months. Only 1 patient is currently alive with metastatic UM. No patients have died of metastatic UM.
Tumor apical height at diagnosis on B-scan ultrasonography ranged from 1.9 mm to 3.6 mm (mean [SD], 2.7 [0.47] mm; median [interquartile range], 2.7 [2.3-2.9] mm). The largest basal diameter by B-scan had a median of 11.0 mm (range, 7.5-20.5 mm). In terms of the location of the tumor, 1 tumor was located in the iris and ciliary body region, 4 were located between the ciliary body and peripheral choroid, and 39 were in the equator to the posterior pole region (Table 1).
Table 1. Patient Demographics and Tumor Characteristics in This Series.
| Characteristics | Value |
|---|---|
| Sex, No. (%) | |
| Male | 21 (47.7) |
| Female | 23 (52.3) |
| Laterality of affected eye, No. (%) | |
| Right | 24 (54.5) |
| Left | 20 (45.5) |
| Age at FNAB, y | |
| Mean (SD) | 63.3 (12.7) |
| Median (range) | 63.6 (33.6-86.1) |
| Tumor apical height, mm | |
| Mean (SD) | 2.7 (0.47) |
| Median (range) | 2.7 (1.9-3.6) |
| Largest basal diameter, mm | |
| Mean (SD) | 11.1 (2.87) |
| Median (range) | 11.0 (7.5-20.5) |
| Tumor location, No. | |
| Iris and ciliary body | 1 |
| Ciliary body to peripheral choroid | 4 |
| Equator to the posterior pole | 39 |
| GEP classification, No. | |
| Class 1A | 26 |
| Class 1B | 7 |
| Class 2 | 7 |
| NA (no amplification of genes) | 4 |
| Follow-up period, mo | |
| Mean (SD) | 24.7 (14.9) |
| Median (range) | 25.3 (1.0-53.7) |
Abbreviations: FNAB, fine-needle aspiration biopsy; GEP, gene expression profiling; NA, not applicable.
Biopsy Yields for Transscleral and Transvitreal Approaches
A total of 44 FNAB procedures for 44 patients were performed for genomic and cytopathologic analyses. Regarding the biopsy instrument used, a 27-gauge needle was used for 16 patients, a 25-gauge needle was used for 18 patients, a 23-gauge needle was used for 6 patients, and a 27-gauge cutter was used for 4 patients.
A total of 40 of 44 biopsy samples (90.9%) contained adequate cells for genomic analyses (90.9%; 95% CI, 82.4%-99.4%). Forty-one of 44 biopsy samples (93.2%) had positive cytopathologic yields. For tumors biopsied via a transvitreal approach, the percentage of adequate cellular yield for GEP analysis was 29 of 33 (87.9%). For tumors biopsied via a transscleral approach, the cellular yield rate was 11 of 11 (100%). For GEP classification among 40 tumors, 26 tumors (65.0%) were classified as class 1A, 7 tumors (17.5%) as class 1B, 7 tumors (17.5%) as class 2. Four samples did not have successful amplification. Among successful biopsies, the mean discriminant value (measure of the reliability of the classification, based on quality of RNA for analysis; >0.1 corresponds with >95% confidence interval19) for GEP classification was 0.79 (range, 0.08-1.36). An average of 2.4 needle passes were performed per patient to acquire positive cytology yields.
Complications
No local recurrence or extraocular extension of UM was observed in any patient. No rhegmatogenous retinal detachment or hypotony occurred as a result of the FNAB procedure. One patient had a tumor with a large base diameter and later developed radiation retinopathy with neovascular glaucoma and chose to have an enucleation; however, this was unrelated to the biopsy procedure. Localized vitreous hemorrhage was a common transient ocular complication that occurred in 24 cases postoperatively, especially after transvitreal biopsies. Twelve cases were resolved by 3 months of follow-up, and 9 were resolved by 6 months. Three patients had vitreous hemorrhage that persisted beyond 6 months. One patient had diffuse vitreous hemorrhage after the biopsy and had vitrectomy 8 months after the biopsy and plaque brachytherapy. One patient developed UM metastases.
The effective individual variable of interest on outcomes was analyzed by Fisher exact test, 2-sample t test with equal variances, and Pearson χ2 test. Based on an independent t test, there was a difference between the mean age of the patients who had a positive biopsy yield and the mean age of the patients with a negative yield, with younger patients more likely to have a positive yield (mean age difference, 17.6 years; 95% CI, 5.2-30.0; P = .007). Sex, location of tumor, biopsy approach (ie, transvitreal vs transscleral), biopsy needle gauge type, tumor basal diameter, and tumor height were not shown to be related to biopsy yield. In terms of the incidence of localized vitreous hemorrhage at 1 day postoperatively, there was a statistically significant association between the mean patient age (independent t test, P = .04) with positive postoperative hemorrhage. Also, transvitreal biopsy was associated with localized hemorrhage at 1 day postoperatively, for which the phi value for correlation was −0.526 (95% CI, −0.712 to −0.157; P < .001). Sex, tumor location, needle gauge type, tumor height, and tumor basal width were not shown to have a relationship to postoperative hemorrhage.
Discussion
The purpose of this study was to examine the cellular yield rate for our unique approach to FNAB for UM using an intraoperative frozen section–type analysis. In this series, we achieved a combined GEP yield rate of 90.9% for transvitreal and transscleral methods, a value among the highest in the previously published articles on FNAB for UM (Table 2). Our yield was consistently high across all surgical approaches, transscleral (100%) and transvitreal (87.8%). Moreover, our median tumor apical height was 2.7 mm, which was significantly smaller than the average tumor heights reported in other studies (Table 2), demonstrating our ability to successfully obtain adequate tumor cells from even very thin lesions. In addition, our cytology yield rate was 93.2% (Table 2), which conveys a prognostic significance. Because the current GEP testing does not confirm the biopsied tissue type, low cytology yield raises the question of whether an incorrect tissue type, such as retinal cells, could have been sent for analysis. Therefore, a high rate of cytologic confirmation of tumor cells reinforces the validity of the GEP analysis.
Table 2. Comparison of Studies on Fine Needle Aspiration Biopsy Yield Rates.
| Source | Patients, No. | Mean Tumor Thickness, mm | No./Total No. (%) | |||
|---|---|---|---|---|---|---|
| Transvitreal Yield Rates | Transscleral Yield Rates | Genetic Yield | Cytopathologic Yield | |||
| Shields et al,9 2007 | 140 | 3.9 | 65/67 (97.0) | 55/73 (75.3) | 120/140 (85.7) | NA |
| Chang and McCannel,13 2014 | 38 | 4.8 | 27/38 (71.1) | 25/38 (65.8) | NA | 52/76 (68.4) |
| Singh et al,12 2016 | 143, Including transcorneal biopsy | 6.0a | Cytology: 55/64 (85.9); genomics: 44/64 (68.7) | Cytology: 68/71 (95.7); genomics: 70/71 (98.6) | 114/135 (84.4) | 123/135 (91.1) |
| Sellam et al,14 2016 | 217 | 8.4 | 30/32 (93.8) | 139/185 (75.1) | 169/217 (77.9) | NA |
| Correa and Augsburger,15 2014 | 159 | 5.8 | NA | NA | 158/159 (99.4) | 125/159 (78.6) |
| Current study | 44 | 2.7 | Cytology: 30/33 (90.9); genomics: 29/33 (87.8) | Cytology: 11/11 (100); genomics: 11/11 (100) | 40/44 (90.9) | 41/44 (93.2) |
Abbreviation: NA, not applicable.
This is a calculated value from data provided in the article.
One unique aspect of our study that may have accounted for the high cellular yield rates and minimal serious procedural complications was performing rapid intraoperative frozen section–type analysis immediately after the acquisition of tumor samples. To our knowledge, no other studies have reported intraoperative assessments for UM FNAB. Intraoperative adequacy analysis not only allowed for instant feedback on whether FNAB was successful but also minimized the number of needle passes, which in turn decreased the possibility of complications and the extraocular extension of tumor cells.
In addition to the adequacy analysis, we used several key strategies for achieving maximal cellular yield rates and minimal complications while performing both transscleral and transvitreal approaches. For the transscleral approach, we marked the margin of each tumor using transillumination. We also marked the height of the tumor from B-scans on the needle before insertion for the exact target needle depth, a technique previously discussed in the scientific literature by Pelayes et al.20 After each biopsy, we simultaneously pulled out the needle and applied cryotherapy at the insertion site to prevent tumor cell tracking along the scleral tract. No cases of scleral necrosis due to post biopsy cryotherapy were reported in this series. For the transvitreal method, we used a long needle with short bevel to not only embed the bevel completely in the tumor but also avoid penetrating the sclera under the tumor. When performing FNAB on small lesions, we alternately used a 27-gauge cutter to increase the yield, as the small opening of the cutter allowed the cutter to be buried within the tumor (yield rate for each needle type and size is listed in the eTable in the Supplement).
Limitations
The study has limitations, including its retrospective nature, a relatively small sample size, short follow-up, and no comparative group. However, orbital recurrences after intraocular biopsy, albeit very rare, are generally thought to occur within 1 year of the biopsy,21 so we would have expected to see such a complication had it happened in our patient population, among whom all but 2 of the patients had 6 months of follow-up or more.
Conclusions
Some aspects of our biopsy technique are unique to our academic center (access to onsite ophthalmic pathology during surgery; access to specialized retinal and oncology instrumentation; the presence of a radiation oncology team experienced in brachytherapy) and may be difficult to recreate in other centers. Larger collaborative prospective multicenter trials comparing different approaches to biopsy are needed in the future to define how to achieve the best yield rates. We are currently part of a multicenter effort in this regard.
Results from this study further reaffirm the safety and efficacy of FNAB as a diagnostic modality for UM. High cellular yield rates were demonstrated for all FNAB approaches. In a world where the role for genomic analysis is expanding, patients with UM should be encouraged to undergo FNAB, given its high rate of successful cellular acquisition with a very low risk of serious ocular complications and no incidence of extraocular extension of tumor cells.
eTable. Comparison of Needle Type/Size and GEP Yield Rate
References
- 1.Schefler AC, Abramson DH. Update on ophthalmic oncology 2012: advances in retinoblastoma and uveal melanoma. Asia Pac J Ophthalmol (Phila). 2013;2(2):119-131. [DOI] [PubMed] [Google Scholar]
- 2.Eschelman DJ, Gonsalves CF, Sato T. Transhepatic therapies for metastatic uveal melanoma. Semin Intervent Radiol. 2013;30(1):39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horsthemke B, Prescher G, Bornfeld N, Becher R. Loss of chromosome 3 alleles and multiplication of chromosome 8 alleles in uveal melanoma. Genes Chromosomes Cancer. 1992;4(3):217-221. [DOI] [PubMed] [Google Scholar]
- 4.Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64(20):7205-7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damato B, Dopierala J, Klaasen A, van Dijk M, Sibbring J, Coupland SE. Multiplex ligation-dependent probe amplification of uveal melanoma: correlation with metastatic death. Invest Ophthalmol Vis Sci. 2009;50(7):3048-3055. [DOI] [PubMed] [Google Scholar]
- 6.Field MG, Harbour JW. Recent developments in prognostic and predictive testing in uveal melanoma. Curr Opin Ophthalmol. 2014;25(3):234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augsburger JJ. Diagnostic biopsy of selected intraocular tumors. Am J Ophthalmol. 2005;140(6):1094-1095. [DOI] [PubMed] [Google Scholar]
- 8.Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010;12(4):461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields CL, Ganguly A, Materin MA, et al. Chromosome 3 analysis of uveal melanoma using fine-needle aspiration biopsy at the time of plaque radiotherapy in 140 consecutive cases: the Deborah Iverson, MD, Lectureship. Arch Ophthalmol. 2007;125(8):1017-1024. [DOI] [PubMed] [Google Scholar]
- 10.Young TA, Rao NP, Glasgow BJ, Moral JN, Straatsma BR. Fluorescent in situ hybridization for monosomy 3 via 30-gauge fine-needle aspiration biopsy of choroidal melanoma in vivo. Ophthalmology. 2007;114(1):142-146. [DOI] [PubMed] [Google Scholar]
- 11.Midena E, Bonaldi L, Parrozzani R, Radin PP, Boccassini B, Vujosevic S. In vivo monosomy 3 detection of posterior uveal melanoma: 3-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2008;246(4):609-614. [DOI] [PubMed] [Google Scholar]
- 12.Singh AD, Medina CA, Singh N, Aronow ME, Biscotti CV, Triozzi PL. Fine-needle aspiration biopsy of uveal melanoma: outcomes and complications. Br J Ophthalmol. 2016;100(4):456-462. [DOI] [PubMed] [Google Scholar]
- 13.Chang MY, McCannel TA. Comparison of uveal melanoma cytopathologic sample retrieval in trans-scleral versus vitrectomy-assisted transvitreal fine needle aspiration biopsy. Br J Ophthalmol. 2014;98(12):1654-1658. [DOI] [PubMed] [Google Scholar]
- 14.Sellam A, Desjardins L, Barnhill R, et al. Fine needle aspiration biopsy in uveal melanoma: technique, complications, and outcomes. Am J Ophthalmol. 2016;162:28-34 e21. [DOI] [PubMed] [Google Scholar]
- 15.Correa ZM, Augsburger JJ. Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients, and relative prognostic significance of these classifications. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):131-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen VM, Dinakaran S, Parsons MA, Rennie IG. Transvitreal fine needle aspiration biopsy: the influence of intraocular lesion size on diagnostic biopsy result. Eye (Lond). 2001;15(Pt 2):143-147. [DOI] [PubMed] [Google Scholar]
- 17.Schefler AC, Gologorsky D, Marr BP, Shields CL, Zeolite I, Abramson DH. Extraocular extension of uveal melanoma after fine-needle aspiration, vitrectomy, and open biopsy. JAMA Ophthalmol. 2013;131(9):1220-1224. [DOI] [PubMed] [Google Scholar]
- 18.Kim RS, Chevez-Barrios P, Bretana ME, Wong TP, Teh BS, Schefler AC. Histopathologic analysis of transvitreal fine needle aspiration biopsy needle tracts for uveal melanoma. Am J Ophthalmol. 2017;174:9-16. [DOI] [PubMed] [Google Scholar]
- 19.Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelayes DE, Zárate JO, Biscotti CV, Singh AD. Calibrated needle for ophthalmic fine needle aspiration biopsy. Br J Ophthalmol. 2012;96(8):1147-1148. [DOI] [PubMed] [Google Scholar]
- 21.Caminal JM, Sanz S, Carreras M, Català I, Arruga J, Roca G. Epibulbar seeding at the site of a transvitreal fine-needle aspiration biopsy. Arch Ophthalmol. 2006;124(4):587-589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Comparison of Needle Type/Size and GEP Yield Rate