Key Points
Question
What are the incidence and risk factors of age-related macular degeneration (AMD) in a French population older than 72 years?
Findings
In this population-based cohort study of 659 French patients 73 years or older, the incidence of early and advanced AMD was 79.9 and 18.6, respectively, per 1000 person-years over a mean follow-up of 3.8 years. Progression from early to advanced AMD was significantly associated with early AMD stage in fellow eye, smoking, and genetic background, while incident early AMD was associated with early AMD stage in the fellow eye and a higher plasma high-density lipoprotein cholesterol level.
Meaning
This study confirms that risk factors appear different for progression from early to advanced AMD compared with incident early AMD and suggests a high risk for incident early AMD in individuals with high plasma high-density lipoprotein cholesterol levels.
This population-based cohort study describes the incidence and associated risk factors of age-related macular degeneration in elderly French individuals.
Abstract
Importance
While the prevalence of age-related macular degeneration (AMD) differs according to continents and races/ethnicities, its incidence in the European continent has been scarcely documented.
Objective
To describe the incidence and associated risk factors of AMD in elderly French individuals.
Design, Setting, and Participants
This population-based cohort study of 963 residents of Bordeaux, France, who were 73 years or older at baseline and participated in the Antioxydants, Lipides Essentiels, Nutrition et Maladies Oculaires (ALIENOR) Study between October 2, 2006, and December 21, 2012. Of 829 participants at risk for incident AMD, 659 (79.5%) were observed for a mean (SD) duration of 3.8 (1.1) years. Data were analyzed from August 2016 to March 2017.
Main Outcomes and Measures
Age-related macular degeneration was graded from retinal photographs and spectral-domain optical coherence tomography into 5 exclusive stages: no AMD, early AMD1, early AMD2, late atrophic AMD, and late neovascular AMD.
Results
Of the 659 eligible participants, 413 (62.7%) were women, and the mean (SD; range) age was 79.7 (4.4; 73-94) years. A total of 120 incident cases of early AMD and 45 incident cases of advanced AMD were recorded. Incidence rates of early and advanced AMD were 79.9 (95% CI, 66.8-95.5) per 1000 person-years and 18.6 (95% CI, 13.9-24.9) per 1000 person-years, respectively, corresponding to 5-year risks of 32.9% and 8.9%. Incidence of advanced AMD per 1000 eye-years was 1.5 in eyes without any AMD at baseline, 42.4 in those with early AMD1, and 85.1 in those with early AMD2. In multivariate analysis without correction for multiple testing, progression from early to advanced AMD was associated with AMD grade in the fellow eye (hazard ratio [HR] according to grade, 13.0 [95% CI, 2.8-61.2] to 22.5 [95% CI, 2.6-195.9]), having smoked at least 20 pack-years (calculated as number of smoking years × mean number of cigarettes per day / 20; HR, 3.0; 95% CI, 1.4-6.5), and complement factor H (CFH) Y402H genotype (CC genotype: HR, 2.3; 95% CI, 1.0-5.3; TC genotype: HR, 1.5; 95% CI, 0.6-3.7). Incidence of early AMD was associated with early AMD in the fellow eye (early AMD1: HR, 2.6; 95% CI, 1.6-4.2; early AMD2: HR, 5.6; 95% CI, 3.3-9.4) and high plasma high-density lipoprotein cholesterol levels (HR, 1.2; 95% CI, 1.0-1.4).
Conclusions and Relevance
In this cohort, AMD incidence rates were similar to those observed in other European populations. This study suggests a high risk for incident early AMD in individuals with high plasma high-density lipoprotein cholesterol levels while confirming the high risk for progression from early to advanced AMD in heavy smokers and carriers of CFH Y402H at-risk genotypes.
Introduction
Age-related macular degeneration (AMD) continues to be the leading cause of blindness in elderly populations in developed countries.1 The worldwide prevalence of AMD among individuals aged 45 to 85 years is 8.7%, with a projected number of affected people of 196 million in 2020, increasing to 288 million in 2040 if the incidence remains stable.2
While prevalence measures the proportion with disease in the population, incidence is also a key measure to plan the demand for health care systems. In addition, prospective studies allow the study of the natural history of disease as well as assessment of the associations with risk factors, which limits the risk of reverse causality (ie, the risk factor being modified by the disease itself). Relatively few population-based prospective studies of AMD have been conducted worldwide3,4,5,6,7,8,9,10,11 and even fewer in the European continent (ie, the Rotterdam Study in the Netherlands,12 the Copenhagen City Eye Study in Denmark,13 the Reykjavik Eye Study in Iceland,14 and the Pathologies Oculaire Liées à l’Age [POLA] Study in France15).
Advanced forms of the disease (ie, atrophic and neovascular AMD), which are associated with visual loss, are usually preceded and predicted by early retinal abnormalities,16 such as soft distinct and indistinct drusen, reticular pseudodrusen, or pigmentary abnormalities. Several classification systems have been proposed, with varying definitions and stages of early AMD.17,18,19,20
The major risk factors for AMD are age, smoking, and genetic background (in particular, single-nucleotide polymorphisms in the complement factor H [CFH] Y402H gene and the age-related maculopathy susceptibility 2 [ARMS2] A69S gene).16 Associations have also been reported with cardiovascular risk factors, including body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), hypertension, plasma lipid levels, and diabetes.21,22,23,24
The purpose of this report is to describe the incidence of early and advanced AMD and the related demographic, clinical, and genetic risk factors in a population-based study of elderly French individuals.
Methods
Study Sample
Participants of the ALIENOR (Antioxydants, Lipides Essentiels, Nutrition et Maladies Oculaires) Study were recruited from an ongoing population-based study (Three-City [3C] Study25) on the vascular risk factors for dementia. In 1999-2001, the 3C Study recruited 9294 participants 65 years or older from 3 French cities (Bordeaux, Dijon, and Montpellier) randomly selected from electoral rolls. In Bordeaux, 2104 participants were included, with a response rate of 39%. Since baseline, 3C Study participants have been followed-up with about every 2 years. The 630 individuals still participating in the 3C Bordeaux cohort in 2009 (10-year follow-up) were representative of the 19 232 inhabitants of Bordeaux 75 years or older.26 This research followed the tenets of the Declaration of Helsinki. Participants gave written consent prior to enrollment. The design of the ALIENOR Study was approved by the Ethical Committee of Bordeaux (Comité de Protection des Personnes Sud-Ouest et Outre-Mer III) in May 2006.
The ALIENOR Study consists of eye examinations, which have been offered to all participants of the 3C Study cohort in Bordeaux since 2006. Among the 1450 participants of the 3C Study re-examined from October 2, 2006, to May 23, 2008, 963 (66.4%) participated in the ALIENOR Study. Detailed characteristics of participants and nonparticipants have been described elsewhere.27
Eye Examination
The eye examinations took place in the Department of Ophthalmology of the University Hospital of Bordeaux from October 2, 2006, to May 23, 2008 (baseline), May 21, 2008, to June 3, 2009 (wave 1), April 29, 2009, to January 17, 2011 (wave 2), and February 21, 2011, to December 21, 2012 (wave 3). They included a recording of ophthalmic history, measures of visual acuity, refraction, two 45° nonmydriatic color retinal photographs (one centered on the macula and the other centered on the optic disc), measures of intraocular pressure and central corneal thickness, and a tear breakup time test.
Retinal photographs were performed using a high-resolution digital nonmydriatic retinograph (TRC NW6S; Topcon). Photographs were interpreted in duplicate by 2 specially trained technicians according to international classification28 and to a modification of the grading scheme used in the Multi-Ethnic Study of Atherosclerosis29 for drusen size, location, and area (eMethods 1 in the Supplement). Inconsistencies between the 2 interpretations were adjudicated by a senior grader (C.D.) for classification of AMD and other retinal diseases. All cases of advanced AMD, other retinal diseases, and glaucoma were reviewed and confirmed by specialists (J.F.K., M.N.D., or M.B.R. for AMD and retinal diseases).
In addition to retinal photographs, at waves 2 and 3, a spectral-domain optical coherence tomography (SD-OCT) examination of the macula and the optic nerve was performed using Spectralis, version 5.4.7.0 (Heidelberg Engineering). All SD-OCT assessments were performed by the same experienced technician. For the macular thickness acquisition, the following conditions were used: resolution mode, high speed; scan angle, 20°; number of B-scans, 19; pattern size, 20 × 15 degrees; and distance between B-scans, 236 μm. A single horizontal and vertical B-scan image (1536 A-scans) centered on the fovea was also performed.
Assessment of Risk Factors
Clinical risk factors (ie, smoking, hypertension, diabetes, and BMI) were collected during the baseline home visit of the 3C Study by trained psychologists or nurses. For participants who were current or past smokers, the number of pack-years was calculated as the number of smoking years × mean number of cigarettes per day / 20. Diabetes was defined as a fasting blood glucose level of 126.1 mg/dL (to convert to millimoles per liter, multiply by 0.0555) or greater, a nonfasting blood glucose level of 198.2 mg/dL or greater, use of antidiabetic medication, or self-reported diabetes. Hypertension was defined as a self-reported medical follow-up for hypertension, systolic blood pressure of 140 mm Hg or greater, diastolic blood pressure of 90 mm Hg or greater, or antihypertensive treatment. Body mass index was calculated using weight and height measurements obtained during the baseline home visit. Plasma lipid levels were measured at the Biochemistry Laboratory of the University Hospital of Dijon from baseline fasting blood samples using routine enzymatic techniques. Genetic polymorphisms (CFH Y402H and ARMS2 A69S) were determined by the Lille Génopôle in Lille, France, from the DNA samples collected at baseline.30,31
Statistical Analysis
Characteristics of participants of the ALINEOR Study with and without follow-up were compared using logistic regression (eTable 1 in the Supplement). Incidence rates of early and advanced AMD per person were obtained by dividing the number of incident cases by the number of person-years (Table 1). The number of person-years was calculated by adding each person’s contribution of follow-up time (eMethods 2 in the Supplement).
Table 1. Incidence of Early and Advanced Age-Related Macular Degeneration (AMD) per Person According to Sex and Age Groups in the ALIENOR Study, 2006-2012.
| Characteristic | No. | 5-y Risk, % | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex Group | Age Group | Total | ||||||||||||||
| Men | Women | 73-79 y | ≥80 y | |||||||||||||
| Total PYs | Cases | Incidence per 1000 PYs (95% CI) | Total PYs | Cases | Incidence per 1000 PYs (95% CI) | Total PYs | Cases | Incidence per 1000 PYs (95% CI) | Total PYs | Cases | Incidence per 1000 PYs (95% CI) | Total PYs | Cases | Incidence per 1000 PYs (95% CI) | ||
| Advanced AMD | 911 | 13 | 14.3 (8.3-24.6) | 1506 | 32 | 21.2 (15.0-30.0) | 1072 | 13 | 12.1 (7.0-20.9) | 1345 | 32 | 23.8 (16.8-33.6) | 2417 | 45 | 18.6 (13.9-24.9) | 8.9 |
| Neovascular AMD | 934 | 2 | 2.1 (0.5-8.6) | 1531 | 20 | 13.1 (8.4-20.3) | 1078 | 9 | 8.3 (4.3-16.0) | 1387 | 13 | 9.4 (5.4-16.1) | 2465 | 22 | 8.9 (5.9-13.6) | 4.4 |
| Atrophic AMD | 911 | 11 | 12.1 (6.7-21.8) | 1506 | 13 | 8.6 (5.0-14.9) | 1072 | 4 | 3.7 (1.4-9.9) | 1345 | 20 | 14.9 (9.6-23.0) | 2417 | 24 | 9.9 (6.7-14.8) | 4.8 |
| Early AMD | 615 | 42 | 68.3 (50.5-92.4) | 888 | 78 | 87.8 (70.4-109.7) | 745 | 49 | 65.8 (49.7-87.0) | 758 | 71 | 93.7 (74.2-118.2) | 1503 | 120 | 79.9 (66.8-95.5) | 32.9 |
| Distinct soft drusen | 773 | 35 | 45.3 (32.5-63.0) | 1191 | 76 | 63.8 (51.0-79.9) | 925 | 44 | 47.6 (35.4-63.9) | 1039 | 67 | 64.5 (50.7-81.9) | 1964 | 111 | 56.5 (46.9-68.1) | 24.6 |
| Indistinct soft drusen | 807 | 28 | 34.7 (23.9-50.2) | 1257 | 44 | 35.0 (26.1-47.0) | 953 | 35 | 36.7 (26.4-51.2) | 1112 | 37 | 33.3 (24.1-45.9) | 2064 | 72 | 34.9 (27.7-43.9) | 16.0 |
| Reticular pseudodrusen | 882 | 9 | 10.2 (5.3-19.6) | 1414 | 25 | 17.7 (11.9-26.2) | 1043 | 8 | 7.7 (3.8-15.3) | 1253 | 26 | 20.8 (14.1-30.5) | 2296 | 34 | 14.8 (10.6-20.7) | 7.1 |
| Large area of soft drusen | 807 | 16 | 19.8 (12.2-32.4) | 1178 | 32 | 27.2 (19.2-38.4) | 928 | 18 | 19.4 (12.2-30.8) | 1057 | 30 | 28.4 (19.9-40.6) | 1985 | 48 | 24.2 (18.2-32.1) | 11.4 |
| Intermediate soft drusen | 316 | 66 | 209.2 (164.4-266.3) | 368 | 90 | 244.3 (198.7-300.3) | 386 | 84 | 217.5 (175.6-269.3) | 298 | 72 | 241.9 (192.0-304.7) | 684 | 156 | 228.1 (195.0-266.9) | 68.0 |
| Pigmentary abnormalities | 565 | 31 | 54.9 (38.6-78.1) | 899 | 69 | 76.7 (60.6-97.1) | 708 | 32 | 45.2 (32.0-63.9) | 756 | 68 | 89.9 (70.9-114.0) | 1464 | 100 | 68.3 (56.1-83.1) | 28.9 |
Abbreviation: PY, person-year.
Confidence intervals of incidence rates were calculated with Poisson regression models (PROC GENMOD). The cumulative 5-year incidence (CI) was derived from the incidence rate (IR) using the following exponential formula17: CI(t) = 1 − exp(−IR × t). Incidence rates and 95% CIs of early and advanced AMD per eye were obtained using Poisson regression models for correlated data (PROC GLIMIX with random effect for eye).
The associations of early AMD characteristics with incidence of advanced AMD were analyzed using Cox proportional hazards models with delayed entry and age as the time scale.32 The individual eye was used as the unit of analysis by using PROC PHREG with the covariance aggregate option. This survival model allows the use of correlated data in eye-specific analyses and uses a robust estimate of the variance of estimated parameters to account for correlation between right and left eyes (Table 2).33
Table 2. Associations of Early Abnormalities With Incidence of Advanced Age-Related Macular Degeneration (AMD) per Eye in the ALIENOR Study, 2006-2012.
| Characteristic | No. | Incidence of Late AMD per 1000 Eye-Years (95% CI) | 5-y Risk, % | Age-Adjusted and Sex-Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|---|
| Early AMD stagea | |||||
| No AMD | 902 | 1.5 (0.6-3.5) | 0.7 | 1 [Reference] | NA |
| Early AMD1 | 181 | 42.4 (27.4-65.5) | 19.1 | 27.42 (10.17-73.94) | <.001 |
| Early AMD2 | 113 | 85.1 (55.9-129.5) | 34.7 | 59.83 (22.64-158.11) | <.001 |
| Intermediate soft drusen | |||||
| No | 644 | 4.4 (2.4-8.1) | 2.2 | 1 [Reference] | NA |
| Pericentral | 340 | 13.4 (8.0-22.3) | 6.5 | 2.64 (1.15-6.07) | .02 |
| Central | 196 | 40.3 (26.7-61.2) | 18.2 | 10.31 (4.62-23.03) | <.001 |
| Distinct soft drusen | |||||
| No | 1096 | 10.1 (7.3-14.0) | 4.9 | 1 [Reference] | NA |
| Pericentral | 84 | 37.4 (19.6-71.4) | 17.1 | 4.46 (2.49-8.01) | <.001 |
| Central | 15 | 39.0 (11.1-136.4) | 17.7 | 7.36 (3.00-18.05) | <.001 |
| Indistinct soft drusen | |||||
| No | 1105 | 7.8 (5.5-11.2) | 3.8 | 1 [Reference] | NA |
| Pericentral | 65 | 83.0 (45.3-151.9) | 34.0 | 9.44 (5.05-17.65) | <.001 |
| Central | 25 | 88.5 (37.7-208.1) | 35.8 | 18.28 (8.14-41.01) | <.001 |
| Reticular pseudodrusen | |||||
| No | 1184 | 11.6 (8.6-15.6) | 5.6 | 1 [Reference] | NA |
| Pericentral | 11 | 65.2 (14.1-302.5) | 27.8 | 4.65 (1.73-12.51) | .002 |
| Large area of soft drusen | |||||
| No | 1039 | 6.4 (4.3-9.6) | 3.1 | 1 [Reference] | NA |
| Pericentral | 127 | 62.9 (39.3-100.8) | 27.0 | 9.23 (4.85-17.56) | <.001 |
| Central | 29 | 65.6 (27.3-157.3) | 28.0 | 16.77 (6.94-40.53) | <.001 |
| Hyperpigmentation | |||||
| No | 805 | 5.6 (3.4-9.1) | 2.8 | 1 [Reference] | NA |
| Pericentral | 59 | 126.4 (76.4-209.1) | 46.8 | 21.43 (11.13-41.28) | <.001 |
| Central | 82 | 74.5 (44.4-124.9) | 31.1 | 12.49 (5.93-26.33) | <.001 |
| Hypopigmentation | |||||
| No | 792 | 6.3 (4.0-10.1) | 3.1 | 1 [Reference] | NA |
| Pericentral | 64 | 84.3 (48.4-146.9) | 34.4 | 13.35 (6.85-26.03) | <.001 |
| Central | 93 | 63.9 (38.3-106.5) | 27.3 | 10.19 (5.01-20.71) | <.001 |
| Pigmentary abnormalities | |||||
| No | 770 | 5.2 (3.1-8.7) | 2.6 | 1 [Reference] | NA |
| Presence | 176 | 75.2 (52.8-107.2) | 31.3 | 14.20 (7.51-28.84) | <.001 |
Abbreviations: HR, hazard ratio; NA, not applicable.
Early AMD1 defined as presence of soft distinct drusen without pigmentary abnormalities or of pigmentary abnormalities without large drusen (>125 μm). Early AMD2 defined as presence of soft indistinct drusen and/or reticular pseudodrusen and/or soft distinct drusen associated with pigmentary abnormalities (hyperpigmentation or hypopigmentation).
Associations of clinical and genetic factors with incidence of early AMD and progression from early to late AMD were estimated using Cox models. First, the association with each risk factor was assessed independently using models adjusted for age and sex. Second, stepwise models were applied using variables with P ≤ .20 in models adjusted for age and sex, and a P ≥ .05 was considered for exiting the model. Age and sex were forced into the models (Table 3 and Table 4).
Table 3. Associations of Progression From Early to Advanced Age-Related Macular Degeneration (AMD) per Eye With Potential Risk Factors in the ALIENOR Study, 2006-2012.
| Characteristic | No. (%) | Age-Adjusted and Sex-Adjusted HR (95% CI) | P Value | Final Multivariate-Adjusted HR (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| Progressing Eyes (n = 52 Eyes) | Nonprogressing Eyes (n = 212 Eyes) | |||||
| Study eye, AMD gradea | .02 | NA | ||||
| Early AMD1 | 22 (42.3) | 138 (65.1) | 1 [Reference] | NA | ||
| Early AMD2 | 30 (57.7) | 74 (34.9) | 2.14 (1.15-3.98) | NA | ||
| Fellow eye, AMD gradea | <.001 | <.001 | ||||
| None | 3 (6.0) | 92 (48.7) | 1 [Reference] | 1 [Reference] | ||
| Early AMD1 | 17 (34.0) | 52 (27.5) | 13.36 (2.91-61.39) | 13.05 (2.78-61.20) | ||
| Early AMD2 | 26 (52.0) | 39 (20.6) | 25.39 (5.77-111.85) | 20.18 (4.69-86.75) | ||
| Atrophic AMD | 2 (4.0) | 3 (1.6) | 11.93 (1.71-83.21) | 18.60 (2.45-141.14) | ||
| Neovascular AMD | 2 (4.0) | 3 (1.6) | 19.09 (2.44-149.38) | 22.54 (2.59-195.94) | ||
| Missing | 2 (4.0) | 23 (10.8) | NA | NA | ||
| Smoking, pack-yearsb | .002 | .002 | ||||
| Never | 30 (57.7) | 145 (68.4) | 1 [Reference] | 1 [Reference] | ||
| <20 | 5 (9.6) | 28 (13.2) | 1.24 (0.50-3.05) | 1.16 (0.38-3.52) | ||
| ≥20 | 17 (32.7) | 39 (18.4) | 3.23 (1.61-6.49) | 2.99 (1.38-6.50) | ||
| Hypertension | .25 | NA | ||||
| No | 8 (15.4) | 52 (24.5) | 1 [Reference] | NA | ||
| Yes | 44 (84.6) | 160 (75.5) | 1.63 (0.71-3.74) | NA | ||
| Diabetes | .34 | NA | ||||
| No | 45 (86.5) | 193 (91.0) | 1 [Reference] | NA | ||
| Yes | 7 (13.5) | 19 (9.0) | 1.71 (0.56-5.18) | NA | ||
| BMIc | .55 | NA | ||||
| ≤25 | 23 (44.2) | 98 (46.2) | 1 [Reference] | NA | ||
| 25-30 | 23 (44.2) | 90 (42.5) | 1.21 (0.61-2.41) | NA | ||
| >30 | 6 (11.6) | 24 (11.3) | 1.23 (0.50-3.03) | NA | ||
| Plasma lipid concentration, mean (SD), mg/dL | ||||||
| HDL cholesterol | 65.09 (16.17) | 66.16 (16.51) | 0.91 (0.67-1.25)d | .57 | NA | NA |
| LDL cholesterol | 144.48 (31.34) | 139.05 (35.26) | 1.12 (0.84-1.50)d | .43 | NA | NA |
| Total cholesterol | 232.24 (36.90) | 223.95 (39.72) | 1.13 (0.84-1.50)d | .43 | NA | NA |
| Triglyceride | 108.67 (47.36) | 99.81 (41.91) | 1.31 (0.96-1.80)d | .09 | NA | NA |
| CFH Y402H | .002 | .008 | ||||
| TT | 13 (25.0) | 93 (43.9) | 1 [Reference] | 1 [Reference] | ||
| TC | 23 (44.2) | 95 (44.8) | 1.66 (0.77-3.60) | 1.48 (0.60-3.68) | ||
| CC | 16 (30.8) | 24 (11.3) | 3.48 (1.61-7.55) | 2.26 (0.97-5.28) | ||
| ARMS2 A69S, No./total No. (%) | .48 | NA | ||||
| GG | 23/47 (48.9) | 110/186 (59.1) | 1 [Reference] | NA | ||
| GT | 19/47 (40.4) | 60/186 (32.3) | 1.30 (0.65-2.61) | NA | ||
| TT | 6/47 (12.8) | 15/186 (8.1) | 1.35 (0.41-4.38) | NA | ||
Abbreviations: ARMS2, age-related maculopathy susceptibility 2; BMI, body mass index; CFH, complement factor H; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein; NA, not applicable.
SI conversion factor: To convert HDL, LDL, and total cholesterol levels to millimoles per liter, multiply by 0.0259. To convert triglyceride level to millimoles per liter, multiply by 0.0113.
Early AMD1 defined as presence of soft distinct drusen without pigmentary abnormalities or of pigmentary abnormalities without large drusen (>125 μm). Early AMD2 defined as presence of soft indistinct drusen and/or reticular pseudodrusen and/or soft distinct drusen associated with pigmentary abnormalities (hyperpigmentation or hypopigmentation).
Pack-year calculated as number of smoking years × mean number of cigarettes per day / 20.
Calculated as weight in kilograms divided by height in meters squared.
HRs were estimated for 1-SD increases.
Table 4. Associations of Incidence of Early Age-Related Macular Degeneration (AMD) per Eye With Potential Risk Factors in the ALIENOR Study, 2006-2012.
| Characteristic | No. (%) | Age-Adjusted and Sex-Adjusted HR (95% CI) | P Value | Final Multivariate-Adjusted HR (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| Incident Eyes (n = 176 Eyes) | Nonincident Eyes (n = 622 Eyes) | |||||
| AMD grade in fellow eyea | <.001 | <.001 | ||||
| None | 116 (65.9) | 509 (81.8) | 1 [Reference] | 1 [Reference] | ||
| Early AMD1 | 28 (15.9) | 42 (6.8) | 2.78 (1.78-4.34) | 2.62 (1.65-4.16) | ||
| Early AMD2 | 17 (9.7) | 8 (1.3) | 5.73 (3.39-9.66) | 5.59 (3.31-9.44) | ||
| Neovascular AMD | 0 | 1 (0.2) | NA | NA | ||
| Missing | 15 (8.5) | 62 (10.0) | NA | NA | ||
| Smoking, pack-yearsb | .31 | NA | ||||
| Never | 115 (65.3) | 405 (65.1) | 1 [Reference] | NA | ||
| <20 | 31 (17.6) | 122 (19.6) | 1.05 (0.66-1.65) | NA | ||
| ≥20 | 30 (17.1) | 95 (15.3) | 1.30 (0.81-2.10) | NA | ||
| Hypertension | .13 | NA | ||||
| No | 56 (31.8) | 156 (25.1) | 1 [Reference] | NA | ||
| Yes | 120 (68.2) | 466 (74.9) | 0.76 (0.53-1.08) | NA | ||
| Diabetes | .14 | NA | ||||
| No | 168 (95.5) | 582 (93.6) | 1 [Reference] | NA | ||
| Yes | 8 (4.5) | 40 (6.4) | 0.71 (0.45-1.12) | NA | ||
| BMIc | .09 | NA | ||||
| ≤25 | 81 (46.0) | 222 (35.7) | 1 [Reference] | NA | ||
| 25-30 | 72 (40.9) | 291 (46.8) | 0.76 (0.52-1.12) | NA | ||
| >30 | 23 (13.1) | 109 (17.5) | 0.70 (0.43-1.13) | NA | ||
| Plasma lipid concentration, mean (SD), mg/dL | ||||||
| HDL cholesterol | 63.43 (14.32) | 59.55 (14.92) | 1.28 (1.09-1.50)d | .003 | 1.21 (1.02-1.44)d | .003 |
| LDL cholesterol | 141.41 (32.62) | 141.40 (32.10) | 1.00 (0.85-1.17)d | .96 | NA | NA |
| Total cholesterol | 225.16 (35.92) | 222.71 (36.86) | 1.04 (0.89-1.22)d | .59 | NA | NA |
| Triglyceride | 102.41 (34.02) | 109.68 (54.40) | 0.84 (0.71-0.99)d | .045 | NA | NA |
| CFH Y402H | .12 | NA | ||||
| TT | 78 (44.3) | 315 (50.6) | 1 [Reference] | NA | ||
| TC | 75 (42.6) | 255 (41.0) | 1.08 (0.76-1.55) | NA | ||
| CC | 23 (13.1) | 52 (8.4) | 1.67 (0.99-2.82) | NA | ||
| ARMS2 A69S, No./total No. (%) | .78 | NA | ||||
| GG | 109/149 (73.2) | 386/558 (69.2) | 1 [Reference] | NA | ||
| GT | 36/149 (24.2) | 165/558 (29.6) | 0.83 (0.55-1.26) | NA | ||
| TT | 4/149 (2.7) | 7/558 (1.3) | 1.86 (0.59-5.92) | NA | ||
Abbreviations: ARMS2, age-related maculopathy susceptibility 2; BMI, body mass index; CFH, complement factor H; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein; NA, not applicable.
SI conversion factor: To convert HDL, LDL, and total cholesterol levels to millimoles per liter, multiply by 0.0259. To convert triglyceride level to millimoles per liter, multiply by 0.0113.
Early AMD1 defined as presence of soft distinct drusen without pigmentary abnormalities or of pigmentary abnormalities without large drusen (>125 μm). Early AMD2 defined as presence of soft indistinct drusen and/or reticular pseudodrusen and/or soft distinct drusen associated with pigmentary abnormalities (hyperpigmentation or hypopigmentation).
Pack-year calculated as number of smoking years × mean number of cigarettes per day / 20.
Calculated as weight in kilograms divided by height in meters squared.
HRs were estimated for 1-SD increases.
Statistical significance was set at P < .05, and all P values were 2-tailed. Statistics were calculated using SAS, version 9.4 (SAS Institute).
Results
Of the 963 participants included in the ALIENOR Study, 134 participants (13.9%) were not eligible for AMD incidence; 49 participants had prevalent advanced AMD in one or both eyes, and 85 participants had no available AMD status at baseline. Among the 829 participants at risk for incident AMD in at least 1 eye, 138 (16.6%) did not participate in any of the follow-up examinations (waves 1, 2, and 3). Of the remaining 691 participants (83.4%), 32 (4.6%) had ungradable AMD status in both eyes at all available follow-ups. This left 659 eligible participants (1189 eyes) who had available AMD status during follow-up. The mean (SD) and median (range) follow-up period for these participants was 3.8 (1.1) years and 4.5 (0.7-5.0) years, respectively.
The mean (SD; range) baseline age for these 659 participants was 79.7 (4.4; 73-94) years, and 413 (62.7%) were women. Nonparticipants were older than participants (eTable 1 in the Supplement). However, other characteristics were not statistically different between participants and nonparticipants except for ARMS2 A69S genotypes (eTable 1 in the Supplement).
Incidence of AMD
Table 1 presents the incidence rates of early and advanced AMD (per-person analysis). A total of 45 participants (6.8%) developed incident advanced AMD during the 4-year follow-up period, corresponding to an incidence rate of 18.6 (95% CI, 13.9-24.9) per 1000 person-years (PYs) and to a 5-year risk of 8.9%. The incidence of atrophic AMD (9.9 per 1000 PYs; 95% CI, 6.7-14.8) was slightly higher than the incidence of neovascular AMD (8.9 per 1000 PYs; 95% CI, 5.9-13.6). The incidence of advanced AMD was about 2-fold higher in individuals 80 years or older than in individuals aged 73 to 79 years (23.8 [95% CI, 16.8-33.6] vs 12.1 [95% CI, 7.0-20.9] per 1000 PYs).
The incidence was higher in women (21.2 per 1000 PYs; 95% CI, 15.0-30.0) than in men (14.3 per 1000 PYs; 95% CI, 8.3-24.6). The difference between women and men was particularly marked for neovascular AMD (13.1 [95% CI, 8.4-20.3] vs 2.1 [95% CI, 0.5-8.6] per 1000 PYs). Conversely, the incidence of atrophic AMD was somewhat higher in men (12.1 per 1000 PYs; 95% CI, 6.7-21.8) than in women (8.6 per 1000 PYs; 95% CI, 0.5-14.9). Taking into consideration only the color retinal photographs, 34 participants (incidence, 14.0 per 1000 PYs; 95% CI, 10.0-19.5) developed advanced AMD vs 45 participants (incidence, 18.6 per 1000 PYs; 95% CI, 13.9-24.9) with a diagnosis based on retinal photographs and SD-OCT examination.
A total of 120 cases of incident early AMD were recorded during follow-up, corresponding to an incidence rate of 79.9 (95% CI, 66.8-95.5) per 1000 PYs and a 5-year risk of 32.9% (Table 1). As for advanced AMD, early AMD incidence increased with age and was slightly higher in women than in men. Incidence of individual retinal lesions (types and areas of drusen and pigmentary abnormalities) similarly increased with age and was higher in women, except for soft indistinct drusen, the incidence of which was similar in both sex and both age groups.
Risk of Incident Late AMD According to Early Abnormalities
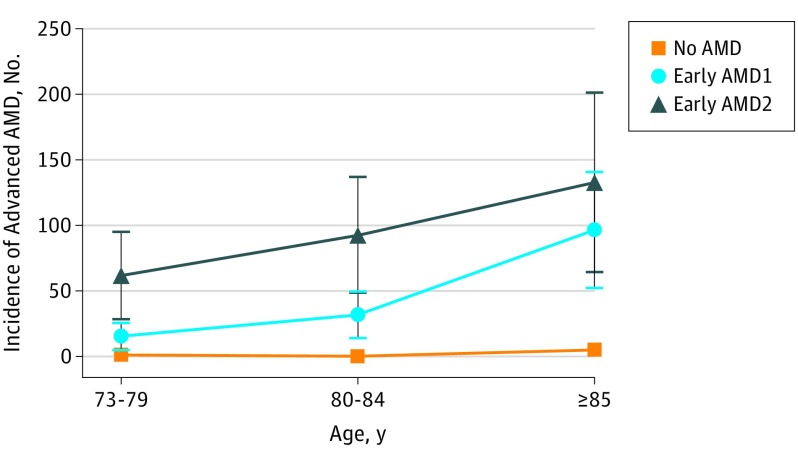
Table 2 presents the eye-specific associations between the baseline early AMD abnormalities and the incidence of advanced AMD. The incidence of advanced AMD in eyes with early AMD1 at baseline was 42.4 (95% CI, 27.4-65.5) per 1000 PYs and was 85.1 (95% CI, 55.9-129.5) per 1000 PYs in eyes with early AMD2 compared with 1.5 (95% CI, 0.6-3.5) per 1000 PYs in those without any AMD. The corresponding 5-year risks were 0.5% in eyes without AMD, 19.1% in those with early AMD1, and 34.7% in those with early AMD2. As shown in the Figure, the incidence rates of advanced AMD were very low in eyes without AMD at baseline, whatever the age group (less than 5 per 1000 PYs). For eyes with early AMD1, incidence rates increased with age from 15 per 1000 PYs for individuals aged 73 to 79 years to 96 per 1000 PYs for individuals 85 years or older. For eyes with early AMD2, incidence rates increased from 62 per 1000 PYs for those aged 73 to 79 years to 133 per 1000 PYs for those 85 years or older. Associations of incident advanced AMD were statistically significant for all types of early abnormalities (ie, intermediate, soft distinct and soft indistinct drusen, reticular drusen, and pigmentary abnormalities), with generally higher hazard ratios for central location of drusen.
Figure. Eye-Specific Incidence of Age-Related Macular Degeneration (AMD) According to Age Group and AMD Grade at Baseline.
Incidence of advanced AMD calculated as number of cases per 1000 eye-years. Early AMD1 defined as presence of soft distinct drusen without pigmentary abnormalities or of pigmentary abnormalities without large drusen (>125 μm). Early AMD2 defined as presence of soft indistinct drusen and/or reticular pseudodrusen and/or soft distinct drusen associated with pigmentary abnormalities (hyperpigmentation or hypopigmentation). The error bars indicate 95% CIs.
Association With Risk Factors
Because almost all cases of advanced AMD developed in eyes with early AMD at baseline, we assessed the associations of potential risk factors with the progression from early to advanced AMD. As shown in Table 3, after adjustment for age and sex, progression to advanced AMD was associated with AMD grade of the studied eye (early AMD2 vs early AMD1: HR, 2.1; 95% CI, 1.1-4.0) but also of the fellow eye, with HRs ranging from 11.9 to 25.4 according to the grade. Associations were also statistically significant with smoking more than 20 pack-years and with CFH Y402H genotype. These association maintained statistical significance in the multivariate analysis except for AMD grade in the same eye. No statistically significant associations were observed with hypertension, diabetes, BMI, plasma lipid levels, or ARMS2 A69S genotype.
In age-adjusted and sex-adjusted analysis, the association of incident early AMD was statistically significant with early AMD in the fellow eye and with high plasma high-density lipoprotein (HDL) cholesterol concentration (HR, 1.3; 95% CI, 1.1-1.5). These associations were maintained in the final multivariate model. Associations of incident early AMD with other risk factors were not statistically significant.
Discussion
In the present study, incidence rates of advanced and early AMD were 18.6 and 79.9 per 1000 PYs, respectively, corresponding to 5-year risks of 8.9% and 32.9%. Progression from early to advanced AMD was significantly associated with AMD grade in the fellow eye, smoking, and CFH Y402H genotype, while incidence of early AMD was associated with AMD grade in the fellow eye and plasma HDL cholesterol concentration.
The incidence of early and advanced AMD were in the range of previous studies performed in populations of European ancestry in the United States, Australia, and Europe (eTable 2 in the Supplement). For instance, in individuals aged 70 to 79 years, the 5-year risk of early and advanced AMD based on retinal photographs only (which was the case for all previous studies) were 28.0% and 4.1%, respectively, whereas previous studies ranged from 14.7% to 43.9% for early and from 0.6% to 4.4% for advanced AMD.3,4,6,14,15,17 As observed in previous studies,3,4,34 women had somewhat higher rates of early and advanced AMD (in particular, neovascular AMD) than men.
As expected, advanced AMD occurred almost exclusively in eyes with early AMD at baseline. Moreover, in multivariate models, AMD grade in the fellow eye showed particularly strong associations with progression from early to advanced AMD and with incident early AMD, highlighting the bilateral nature of AMD.
With regard to lifestyle and genetic risk factors, as expected, statistically significant associations of smoking and CFH Y402H genotype with progression from early to advanced AMD were detected.16 Surprisingly, associations of ARMS2 A69S with progression from early to advanced AMD and incident early AMD were not statistically significant, although it is well known to be associated with AMD and was associated with prevalent early and advanced AMD at the baseline examination of the ALIENOR Study.30,31 According to Shuler et al,35 individuals carrying 2 at-risk alleles for ARMS2 A69S may develop AMD at an earlier age. This may explain why ARMS2 A69S was not significantly associated with AMD in our older population (mean age at baseline, 80 years). Additionally, statistical power was relatively low, as shown by the wide confidence intervals.
High plasma HDL cholesterol concentration was significantly associated with incident early AMD. This is consistent with previous observations from the ALIENOR study21 showing that higher plasma HDL cholesterol concentration was significantly associated with an increased risk of early AMD at baseline. However, results are inconsistent in this field, and a meta-analysis23 did not evidence any significant association of plasma HDL cholesterol level with AMD in prospective, cross-sectional, or case-control studies, although a 2017 meta-analysis36 using mendelian randomization suggested a potential causal association of elevated HDL cholesterol concentration with risk of AMD.
Strengths of this study include its population-based older cohort sample with a follow-up at 3 time points, no major difference between participants and nonparticipants except their age, a good follow-up rate (78.9%), and the use of SD-OCT in addition to color retinal photography to document macular conditions. In particular, most previous studies1,7,8,10 used 5-year intervals between follow-up examinations. This may have led to survival bias and decreased the incidence rates, as some participants may have developed AMD and died within this 5-year interval and therefore would not be counted as incident cases. Our much shorter intervals (1 to 2 years between examinations) probably has decreased much of this survival bias and may at least partly explain the higher incidence rates observed in our study. Moreover, survival bias may lead to underestimation of associations between AMD and risk factors, in particular smoking, which is positively associated with AMD and with mortality.37 The effect of the competing risk with death will be evaluated in future analyses from our study, in particular using illness-death models for interval-censored time to event.38
Limitations
Our study had limitations. One limitation of our study is the small number of cases of incident advanced AMD (n = 45), which may have induced insufficient statistical power for detecting some associations with progression from early to advanced AMD. This is particularly true for infrequent risk factors, such as ARMS2 A69S polymorphism, and is reflected by the wide confidence intervals of some of the HRs. We also have not corrected for multiple testing. However, applying the Bonferroni correction to Table 3 and Table 4 would not affect many of the conclusions, leading to thresholds for statistical significance of .0042 for Table 3 and .0045 for Table 4.
Another limitation of our study could come from the representativeness of the sample. As previously discussed,27 the ALIENOR subsample tends to overrepresent younger individuals and higher socioeconomic status compared with the parent cohort (the 3C study). Accordingly, the individuals included in this study may be healthier and present different lifestyles, in particular concerning their diet and physical activity, compared with the general population. However, for most parameters of interest in our study, in particular smoking, BMI, diabetes, hypertension, and plasma lipid concentration, individuals included in the ALIENOR study were not different from those who did not participate.27
Conclusions
In conclusion, in this population-based study of elderly French individuals, the incidence rates of early and advanced AMD were 79.9 and 18.6 per 1000 PYs, which was similar to incidence rates observed in other populations of European descent. This study suggests a high risk for incident early AMD in those with high plasma HDL cholesterol concentration while confirming the high risk for progression from early to advanced AMD in heavy smokers and CFH Y402H at-risk genotype carriers.
eMethods 1. Classification of age-related macular degeneration.
eMethods 2. Statistical analysis.
eTable 1. Comparison of characteristics between participants with and without follow-up in the ALIENOR Study, 2006-2012.
eTable 2. Incidence of early and late age-related macular degeneration (AMD) in population-based studies performed in individuals of European ancestry.
References
- 1.Bourne RR, Stevens GA, White RA, et al. ; Vision Loss Expert Group . Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339-e349. [DOI] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109(6):1092-1097. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104(1):7-21. [DOI] [PubMed] [Google Scholar]
- 5.Varma R, Foong AW, Lai MY, Choudhury F, Klein R, Azen SP; Los Angeles Latino Eye Study Group . Four-year incidence and progression of age-related macular degeneration: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):741-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukesh BN, Dimitrov PN, Leikin S, et al. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology. 2004;111(6):1176-1182. [DOI] [PubMed] [Google Scholar]
- 7.Leske MC, Wu SY, Hyman L, Hennis A, Nemesure B, Schachat AP; Barbados Eye Studies Group . Four-year incidence of macular changes in the Barbados Eye Studies. Ophthalmology. 2004;111(4):706-711. [DOI] [PubMed] [Google Scholar]
- 8.You QS, Xu L, Yang H, et al. Five-year incidence of age-related macular degeneration: the Beijing Eye Study. Ophthalmology. 2012;119(12):2519-2525. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda M, Kiyohara Y, Hata Y, et al. Nine-year incidence and risk factors for age-related macular degeneration in a defined Japanese population the Hisayama study. Ophthalmology. 2009;116(11):2135-2140. [DOI] [PubMed] [Google Scholar]
- 10.Cruickshanks KJ, Nondahl DM, Johnson LJ, et al. Generational differences in the 5-year incidence of age-related macular degeneration. JAMA Ophthalmol. 2017;135(12):1417-1423. doi: 10.1001/jamaophthalmol.2017.5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastawrous A, Mathenge W, Peto T, et al. Six-year incidence and progression of age-related macular degeneration in Kenya: Nakuru Eye Disease Cohort Study. JAMA Ophthalmol. 2017;135(6):631-638. doi: 10.1001/jamaophthalmol.2017.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klaver CC, Assink JJ, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2001;42(10):2237-2241. [PubMed] [Google Scholar]
- 13.Buch H, Vinding T, la Cour M, Jensen GB, Prause JU, Nielsen NV. Risk factors for age-related maculopathy in a 14-year follow-up study: the Copenhagen City Eye Study. Acta Ophthalmol Scand. 2005;83(4):409-418. [DOI] [PubMed] [Google Scholar]
- 14.Jonasson F, Arnarsson A, Peto T, Sasaki H, Sasaki K, Bird AC. 5-year incidence of age-related maculopathy in the Reykjavik Eye Study. Ophthalmology. 2005;112(1):132-138. [DOI] [PubMed] [Google Scholar]
- 15.Delcourt C, Lacroux A, Carrière I; POLA Study Group . The three-year incidence of age-related macular degeneration: the “Pathologies Oculaires Liées à l’Age” (POLA) prospective study. Am J Ophthalmol. 2005;140(5):924-926. [DOI] [PubMed] [Google Scholar]
- 16.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728-1738. [DOI] [PubMed] [Google Scholar]
- 17.van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam Study. Arch Ophthalmol. 2003;121(4):519-526. [DOI] [PubMed] [Google Scholar]
- 18.Ferris FL, Davis MD, Clemons TE, et al. ; Age-Related Eye Disease Study (AREDS) Research Group . A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123(11):1570-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006;113(2):260-266. [DOI] [PubMed] [Google Scholar]
- 20.Ferris FL III, Wilkinson CP, Bird A, et al. ; Beckman Initiative for Macular Research Classification Committee . Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cougnard-Grégoire A, Delyfer MN, Korobelnik JF, et al. Elevated high-density lipoprotein cholesterol and age-related macular degeneration: the ALIENOR Study. PLoS One. 2014;9(3):e90973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cougnard-Grégoire A, Delyfer MN, Korobelnik JF, et al. Long-term blood pressure and age-related macular degeneration: the ALIENOR Study. Invest Ophthalmol Vis Sci. 2013;54(3):1905-1912. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delcourt C, Michel F, Colvez A, Lacroux A, Delage M, Vernet MH; POLA Study Group . Associations of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA study. Ophthalmic Epidemiol. 2001;8(4):237-249. [DOI] [PubMed] [Google Scholar]
- 25.3C Study Group Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22(6):316-325. [DOI] [PubMed] [Google Scholar]
- 26.Tabue-Teguo M, Grasset L, Avila-Funes JA, et al. Prevalence and co-occurrence of geriatric syndromes in people aged 75 years and older in France: results from the Bordeaux Three-City Study. J Gerontol A Biol Sci Med Sci. 2017;73(1):109-116. [DOI] [PubMed] [Google Scholar]
- 27.Delcourt C, Korobelnik JF, Barberger-Gateau P, et al. Nutrition and age-related eye diseases: the ALIENOR (Antioxydants, Lipides Essentiels, Nutrition et Maladies Oculaires) Study. J Nutr Health Aging. 2010;14(10):854-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bird AC, Bressler NM, Bressler SB, et al. ; The International ARM Epidemiological Study Group . An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39(5):367-374. [DOI] [PubMed] [Google Scholar]
- 29.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis. Ophthalmology. 2006;113(3):373-380. [DOI] [PubMed] [Google Scholar]
- 30.Delcourt C, Delyfer MN, Rougier MB, et al. Associations of complement factor H and smoking with early age-related macular degeneration: the ALIENOR Study. Invest Ophthalmol Vis Sci. 2011;52(8):5955-5962. [DOI] [PubMed] [Google Scholar]
- 31.Delcourt C, Delyfer MN, Rougier MB, et al. ARMS2 A69S polymorphism and the risk for age-related maculopathy: the ALIENOR Study. Arch Ophthalmol. 2012;130(8):1077-1078. [DOI] [PubMed] [Google Scholar]
- 32.Lamarca R, Alonso J, Gómez G, Muñoz A. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53(5):M337-M343. [DOI] [PubMed] [Google Scholar]
- 33.Glynn RJ, Rosner B. Regression methods when the eye is the unit of analysis. Ophthalmic Epidemiol. 2012;19(3):159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119(3):571-580. [DOI] [PubMed] [Google Scholar]
- 35.Shuler RK Jr, Schmidt S, Gallins P, et al. Phenotype analysis of patients with the risk variant LOC387715 (A69S) in age-related macular degeneration. Am J Ophthalmol. 2008;145(2):303-307. [DOI] [PubMed] [Google Scholar]
- 36.Fan Q, Maranville JC, Fritsche L, et al. HDL-cholesterol levels and risk of age-related macular degeneration: a multiethnic genetic study using mendelian randomization. Int J Epidemiol. 2017;46(6):1891-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGuinness MB, Karahalios A, Kasza J, Guymer RH, Finger RP, Simpson JA. Survival bias when assessing risk factors for age-related macular degeneration: a tutorial with application to the exposure of smoking. Ophthalmic Epidemiol. 2017;24(4):229-238. [DOI] [PubMed] [Google Scholar]
- 38.Leffondré K, Touraine C, Helmer C, Joly P. Interval-censored time-to-event and competing risk with death: is the illness-death model more accurate than the Cox model? Int J Epidemiol. 2013;42(4):1177-1186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Classification of age-related macular degeneration.
eMethods 2. Statistical analysis.
eTable 1. Comparison of characteristics between participants with and without follow-up in the ALIENOR Study, 2006-2012.
eTable 2. Incidence of early and late age-related macular degeneration (AMD) in population-based studies performed in individuals of European ancestry.