Key Points
Question
What is the childhood presentation and course of transient receptor potential cation channel subfamily M member 1 (TRPM1)–associated complete congenital stationary night blindness (cCSNB)?
Findings
In this longitudinal study, preschool-aged patients with TRPM1-associated cCSNB presented with myopia with strabismus, nystagmus, or both, initially without nyctalopia. Goldmann visual field results demonstrated constriction of finer stimuli (I2e), dark-adapted bright-flash full-field electroretinogram results were electronegative, and the full-field stimulus threshold was moderately elevated.
Conclusion
These findings suggest that attention to specific phenotypic features may lead to a prompt diagnosis and avoid unnecessary neurosystemic evaluation; TRPM1-associated cCSNB is a channelopathy that should be suspected in preschool-aged children with high levels of myopia even in the absence of night blindness.
Abstract
Importance
Congenital stationary night blindness (CSNB) implies a stable condition, with the major symptom being nyctalopia present at birth. Pediatric clinical presentation and the course of different genetic subtypes of CSNB have not, to our knowledge, been well described in the era of molecular genetic diagnosis.
Objective
To describe the presentation and longitudinal clinical characteristics of pediatric patients with molecularly confirmed TRPM1-associated complete CSNB (cCSNB).
Design, Setting, Participants
This study was conducted at the University of Iowa from January 1, 1990, to July 1, 2015, and was a retrospective, longitudinal case series of 7 children (5 [71.4%] female) with TRPM1-associated cCSNB followed up for a mean (SD) of 11.1 (2.8) years.
Main Outcomes and Measures
History, ophthalmologic examination findings, full-field electroretinogram (ffERG) results, full-field stimulus threshold testing results, Goldmann visual field results, optical coherence tomography results, and molecular genetic results were evaluated. Presenting symptoms and signs, the correlation of refractive error with electroretinography, and clinical evolution were analyzed.
Results
Seven patients (5 [71.4%] female) presented early in childhood with strabismus (n = 6 [86%]), myopia (n = 5 [71%]), and/or nystagmus (n = 3 [43%]). The mean (SD) age at presentation was 8 (4) months and for receiving a diagnosis by ffERG was 7.3 years, with molecular diagnosis at 9.7 years. The mean (SD) length of follow-up was 11 (2.8) years. The best-corrected visual acuity at the most recent visit averaged 20/30 in the better-seeing eye (range, 20/20-20/60). The mean (SD) initial refraction was −2.80 (4.42) diopters (D) and the mean refraction at the most recent visit was −8.75 (3.53) D (range, −4.00 to −13.75 D), with the greatest rate of myopic shift before age 5 years. Full-field electroretinogram results were electronegative, consistent with cCSNB, without a significant change in amplitude over time. No patient or parent noted night blindness at presentation; however, subjective nyctalopia was eventually reported in 5 of 7 patients (71%). The full-field stimulus threshold testing results were moderately subnormal (−29.7 [3.8] dB; normal −59.8 [4.0] dB). Goldmann visual field results were significant for full I-4e, but constricted I-2e isopter. Eight different mutations or rare variants in TRPM1 predicted to be pathogenic were detected, with 3 novel variants.
Conclusions and Relevance
Children with TRPM1-associated cCSNB presented before school age with progressive myopia as well as strabismus and nystagmus (but not nyctalopia), with stable, electronegative ffERG results, mildly subnormal full-field stimulus threshold testing results, and a constricted I2e isopter on perimetry. These findings suggest that ffERG and cCSNB genetic testing should be considered for children who present with early-onset myopia, especially in the presence of strabismus and/or nystagmus, and that TRPM1-associated cCSNB is a channelopathy that may present without complaints of night blindness in childhood.
This study describes the pediatric presentation and childhood course of TRPM1-associated complete congenital stationary night blindness.
Introduction
Congenital stationary night blindness (CSNB) is a group of genetically and clinically heterogeneous retinal disorders described as manifesting nonprogressive nyctalopia and an electronegative full-field electroretinogram [ffERG] result. Recently, genetic testing has been added to the diagnostic armamentarium, with at least 17 genes found to be associated with CSNB. This list includes genes encoding proteins involved in phototransduction, photoreceptor to bipolar cell signaling cascades, and retinoid recycling (https://www.omim.org/phenotypicSeries/PS310500).
Complete CSNB (cCSNB) can be X-linked or autosomal recessive and is caused by mutations in genes encoding proteins involved in the ON-bipolar signaling cascade, including NYX (Xp11.4; OMIM 300278), GRM6 (5q35.3; OMIM 257270), GPR179 (17q12; OMIM 614515), LRIT3 (4q25; OMIM 615058), and TRPM1 (15q13.3; OMIM 603576). Patients with cCSNB present with early high myopia, nystagmus, and strabismus. Visual acuity ranges from 20/20 to 20/125, with most patients requiring no academic accommodations due to vision. Not all patients report nyctalopia initially, especially when living in environments with artificial illumination; however, some patients report difficulty navigating in dim light. To our knowledge, accounts of subjective nyctalopia have rarely been stratified based on molecular genetic subtype. In patients who present with moderate to high myopia in early childhood, both primary ocular disorders and systemic disorders associated with myopia are considered. Ocular causes include keratoconus, infantile glaucoma, retinopathy of prematurity, or history of persistent macular hemorrhage. Systemic diagnoses, such as Stickler syndrome, Knobloch syndrome, Cohen syndrome, Ehlers Danlos syndrome type 6, and other connective tissue disorders, including those associated with ectopia lentis, may be investigated. For early-onset nystagmus without apparent ocular cause, neuroimaging is often completed. Unnecessary testing can be avoided by a careful history combined with salient clinical features, ffERG, and molecular confirmation.
We present a series of pediatric patients with TRPM1-associated cCSNB who had long-term clinical follow-up starting in infancy or early childhood. To our knowledge, this is one of the first studies to document longitudinal visual function and serial electroretinography and to assess dark-adapted (DA) retinal light sensitivity using full-field stimulus threshold testing (FST) in children who have received a molecular diagnosis.
Methods
We obtained approval for a retrospective medical record review from the University of Iowa institutional review board. Written consent for research genetic testing and possible publication had also been obtained on a previous University of Iowa institutional review board approval. Ophthalmologic records of children who presented to the pediatric genetic eye disease service from January 1, 2008, to July 1, 2015, were reviewed. Inclusion criteria were a clinical diagnosis of cCSNB, 2 or more complete eye examinations at least 1 year apart with electroretinography, and TRPM1 variants predicted to be pathologic on genetic testing results.
Clinical information extracted included age at presentation, sex, race/ethnicity, initial diagnosis at presentation, best-corrected visual acuity (BCVA) at age of first optotype acuity and at most recent visit (MRV), initial and final cycloplegic refraction (cyclopentolate, 1%, with retinoscopy at ≥30 minutes postinstallation with manifest in older children), fundoscopic appearance, color vision (Ishihara Color Plates; Kanehara & Co), age at receiving a diagnosis of CSNB, electroretinography (ERG) (Espion E2 V5; Diagnosys), spectral-domain optical coherence tomography of the macula (Spectralis; Heidelberg Engineering), Goldmann visual field results (Haag Streit), and full-field stimulus threshold testing (FST) results (Epsion E; Diagnosys LLC). Parents of preverbal children were routinely asked if their children had difficulty visually locating them in a dark room or tended to cling to parents when walking at night. Older children were asked if they could see the stars at night and whether their eyes took longer to adjust in a dark movie theater or street than their friends’. Full-field ERG testing was conducted in a manner consistent with the International Society for Clinical Electrophysiology of Vision guidelines.
Longitudinal changes in amplitudes for DA combined response 3.0 ERG (3.0), light-adapted 3.0 ERG (light-adapted 3.0) and 30 Hz flicker response were analyzed. A linear mixed-effects model was used to compare initial and final amplitudes of the DA 3.0 b wave, light-adapted 3.0 b wave, and 30-Hz flicker amplitudes. Longitudinal changes in myopic refractive error were compared with ERG b-wave amplitude for DA 3.0- and 30-Hz flicker amplitudes for each patient, also using a linear mixed-effects model. Statistical significance was set at P ≤ .05.
Full-field stimulus threshold testing was performed as previously described. Full-field stimulus threshold testing measures the sensitivity of the entire retina by estimating the lowest luminance (duration, 200 milliseconds) flash that elicits a visual response after dark adaptation. The mean (SD) threshold in unaffected participants in our laboratory is −59.8 (4.0) dB, which is similar to published normative data, with intertest variability of ±3.1 dB.
Molecular Studies
Blood samples had been sent for commercial testing to the Carver Laboratory at the University of Iowa, GeneDx (Gaithersburg, Maryland), or the Casey Eye Institute at the Molecular Diagnostic Laboratory, and all were confirmed by the Carver Non-profit Genetic Testing Laboratory at the University of Iowa.
Results
Seven patients (5 [71.4%] female) from 5 families were included. Self-reported races/ethnicities included white/Northern European, Hispanic, and Middle Eastern/Ashkenazi Jewish. Patients presented for initial ophthalmologic examination at a mean (SD) age of 8 (4) months (range, 2-15 months) with a mean (SD) follow-up of 11 (2.8) years (range, 7.5-14 years) (Table 1). Presenting signs included strabismus (6 of 7 [85.7%]), myopia (5 of 7 [71.4%]), and/or nystagmus (3 of 7 [42.9%], with two-thirds resolving by age 2 years. Systemic evaluation was completed in 5 of 7 patients (71.4%) and included magnetic resonance imaging (3 of 7 [42.9%]; all normal results), and clinical and molecular evaluations for connective tissue disorders associated with high myopia (3 of 7 [42.9%]; all negative results). Initial diagnoses included pathologic myopia, congenital motor nystagmus, spasmus nutans, strabismus, retinitis pigmentosa, Stickler syndrome, Ehlers Danlos syndrome type 6, opsoclonus, and ametropic amblyopia (Table 2). At the MRV, all patients had strabismus and decreased stereopsis (Table 1). Congenital stationary night blindness was not initially suspected, and a clinical diagnosis was made at the mean (SD) age of 7.4 (2.4) years (Table 2) following a diagnostic ERG. Five previously reported mutations and 3 rare sequence variations, including 2 deletions predicted to be pathogenic, were identified (Table 2).
Table 1. Clinical Characteristics in Pediatric Patients With Complete CSNB With TRPM1 Mutations.
| Clinical Features | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Presenting age | 8 mo ± 4 mo | 8 mo ± 4 mo | 8 mo ± 4 mo | 8 mo ± 4 mo | 8 mo ± 4 mo | 8 mo ± 4 mo | 8 mo ± 4 mo |
| Strabismus | Yes; X(T)a (stereo 6/9) | Yes; E(T) and CN 4 palsy (stereo 0/9) | Yes; X(T) (stereo 5/9) | X (stereo 8/9)b | Yes; X(T)a | Yes; E(T) (stereo 3/9) | Yes; X(T) (stereo 0/9) |
| Nystagmus | Yesc | No | No | No | Yesc | No | Yes |
| Initial refractive error (SE) | OD: −2.00; OS: −3.50 |
OD: +1.00; OS: 0.25 |
OD: −8.50; OS: −8.50 |
OD: −8.25; OS: −7.75 |
OD: −1.00; OS: −1.50 |
OD: −3.50; OS: −3.50 |
OD: +3.75; OS: +3.75 |
| Systemic studies | Yes (MRI normal) | No | Yes (Stickler syndrome evaluation) | Yes (Stickler syndrome evaluation) | No | Yes (MRI normal) | Yes (MRI normal) |
| Initial clinical diagnosis | Spasmus mutans | CN 4 palsy | Pathologic myopia | Pathologic myopia | Myopia | Infantile nystagmus | Opsoclonus |
| Age nyctalopia reported | Progressive, preteen | Progressive, child | Progressive, child | None reported | None reported | Toddler | Child |
| First BCVA, y | 5 | 5 | 5 | 5 | 6 | 4 | 4 |
| First BCVA (Snellen/logMAR) | OD:20/50 (0.400); OS:20/30 (0.176) | OD: 20/50 (0.400); OS:20/70 (0.544) | OD:20/40 (0.301); OS:20/40 (0.301) | OD:20/20 (0.000); OS:20/25 (0.097) | OD: 20/70 (0.544); OS: 20/70 (0.544) | OD: 20/40 (0.301); OS: 20/30 (0.176) | OD: 20/100 (0.699); OS: 20/200 (1.00) |
| Age final, y | 15 | 14 | 11 | 9 | 9 | 15 | 10 |
| BCVA final, Snellen (logMAR) | OD: 20/20 (0.000); OS: 20/20 (0.000) | OD: 20/40 (0.301); OS: 20/70d (0.544) | OD: 20/25 (0.097); OS: 20/20 (0.000) | OD: 20/20 (0.000); OS: 20/20 (0.097) | OD: 20/80d (0.602); OS: 20/60 (0.477) | OD: 20/20 (0.000); OS: 20/20 (0.000) | OD: 20/40 (0.301); OS: 20/50 (0.398) |
| Final refractive error (SE) | OD: −4.00; OS: −4.25 |
OD: −5.50; OS: −6.50 |
OD: −13.75; OS: −14.00 |
OD: −9.00; OS: −8.00 |
OD: −6.50; OS: −6.00 |
OD: −13.50; OS: −14.00 |
OD: −9.00; OS: −10.25 |
| Color discrimination | Full | Full | Full | Full | Full | Full | Full |
| Fundus | ON: tilted; myopic; few hypo-pigmented areas | ON: tilted, temporal pallor; myopic fundus | ON: tilted; myopic, lattice | ON: tilted; myopic, cystic retinal tuft OD | ON: tilted; tessellated, myopic fundus | ON: tilted; myopic | ON: tilted; tessellated, myopic fundus |
| GVF | NML OU |
Mild I-2e depression (full to I-4e) OU; small central scotoma OU | Mild I-2e depression (full to I-4e) OU | Mild I-2e depression (full to I-4e) OU | Mild I-2e depression (full to I-4e) OU | Mild I-2e depression (full to I-4e) OU; small central scotoma OU | Mild I-2e depression (full to I-4e) OU |
| FST, dBe | OD: −34.5; OS: −31.5 |
OD: −28.2; OS: −26.6 |
OD: −31.4; OS: −29.6 |
OD: −26.6; OS: −28.9 |
OD: −22.5; OS: −27.8 |
OD: −29.2; OS: −27.4 |
OD: −36.0; OS: −35.5 |
| ffERG final | Electronegative DA 3.0 ERG | Electronegative DA 3.0 ERG | Electronegative DA 3.0 ERG | Electronegative DA 3.0 ERG | Electronegative DA 3.0 ERG | Electronegative DA 3.0 ERG | Electronegative DA 3.0 ERG |
| SD-OCT macula, CST, um; CV, mm2 | OD: 226 μm, 8.4 mm2; OS: 250 μm, 8.5 mm2 | OD: 246 μm, 6.01 mm2; OS: 234 μm, ND | OD: ND; OS: 387 μm, 11.68 mm2 | OD: 237 μm, 7.85 mm2; OS: 236 μm, 7.82 mm2 | OD: 259 μm, 8.29 mm2; OS: 275 μm, 8.01 mm2 | ND | OD: 233 μm, 8.56 mm2; OS: 235 μm, 7.91 mm2 |
Abbreviations: BCVA, best-corrected visual acuity; CN 4, congenital fourth nerve palsy; CSNB, congenital stationary night blindness; CST, central subfield thickness; CV, central volume; DA, dark-adapted; ERG, electroretinogram; ffERG, full field electroretinogram; ffFST, full-field sensitivity threshold test; FST, sensitivity threshold test; GVF, Goldmann visual field; E(T), intermittent esotropia; MRI, magnetic resonance imaging; ND, not done; NML, normal; SD-OCT, spectral-domain optical coherence tomography; SE, spherical equivalent; Stereo, stereopsis by Titmus testing given as number correct; Strab, strabismus; X(T), intermittent exotropia.
Had strabismus surgery.
Initially had orthophoria and then developed exophoria.
Nystagmus resolved by age 2 years in patients 1 and 5.
Amblyopia.
Normal FST is less than or equal to −55.00 dB.
Table 2. Longitudinal Clinical Diagnoses and Molecular Characteristicsa.
| Initial Clinical Diagnosis | Age of cCSNB Diagnosis | Intron/Exon | Nucleotide | Protein Effect | Reported Mutation | |
|---|---|---|---|---|---|---|
| Patient 1 | Spasmus mutans | Preteen | IVS8, exon 5 | c.1023 + 1 G>A, c.1406 t > C | Splice site, p.L469S | Yes, EPP3 |
| Patient 2 | 4th Nerve palsy | Child | IVS8, exon 5 | c.1023 + 1 G>A, c.1406 t > C | Splice site, p.L469S | Yes, EPP3 |
| Patient 3 | Pathologic myopia | Child | Exon 4, exon 4 | c.215A>G, c.215A>G | p.Y72C, p.Y72C | Yes, yes |
| Patient 4 | Pathologic myopia | Toddler | Exon 4; exon 4 | c.215A>G, c.215A>G | p.Y72C, p.Y72C | Yes, yes |
| Patient 5 | Nystagmus | Child | Exon 4; exon 20 | c.296T>C, c.2597-2599del | p.L99P, p.Ser866del | Yes, EPP3 |
| Patient 6 | High myopia | Child | Exon 20 | c.2894A>C, deletion of exon 2-7 | p.D965A, no functional protein | No-novel VUS, Yes |
| Patient 7 | Opsoclonus | Child | Exon 16, deletion of TRPM1 | c.1871G>A, undefined large deletion encompassing TRPM1 | p.R624H, lack of protein | Yes, no |
Abbreviations: CSNB, congenital stationary night blindness; EPP, estimate of pathologic probability; NA, not applicable; VUS, variation of unknown significance; XL, x-linked.
EPP of 3 and 2 mean chance probability of mutation causing disease is highly likely and possibly likely, respectively. EPP of 0 being a known benign polymorphism, and EPP 1 being a potentially low penetrance or modifying allele. Nucleotide numbering is based on reference sequence of TRPM1 (NM_002420.5), where A of the ATG initiation codon is 1.
Sibling patients 1 and 2 shared 2 mutations, one known to be pathologic and the other novel and predicted to be pathologic. Sibling patients 3 and 4 were homozygous for mutation c.215A>G (p.Y72C); each parent was heterozygous. Patient 5 had 1 known pathologic mutation and a novel inframe deletion predicted to be pathologic on separate alleles based on parental testing results. Patient 6 had 1 known mutation and 1 variant of uncertain significance. Patient 7 was heterozygous for a reported rare variation predicted by PolyPhen-2 to be deleterious and a large deletion predicted to be deleterious, which are on separate alleles based on parental testing results.
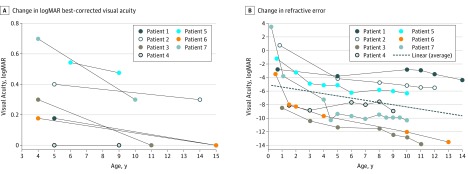
Best-corrected visual acuity improved with age (Figure 1A). At MRV, the average visual acuity of the better-seeing eye for all patients was 20/30 (range, 20/20-20/60; logMAR, 0.19 ± 0.25). The average initial spherical equivalent was −2.75 diopters (D) (range, +3.75 to −8.50) and the final spherical equivalent at MRV was −8.75 D (range, −4.00 to −14.00) at the mean age of 12 years (Figure 1B). Average change in myopic refractive error from the initial visit to 5 years of age was −1.07 D/y and between age 5 years and MRV age was −0.25 D/y.
Figure 1. Visual Acuity and Refractive Error.
A, Change in logMAR best-corrected visual acuity in the better-seeing eye of patients from time of initial recognition acuity to most recent visit. B, Change in refractive error from initial examination to most recent visit. The dotted line represents the average myopic change of the entire cohort.
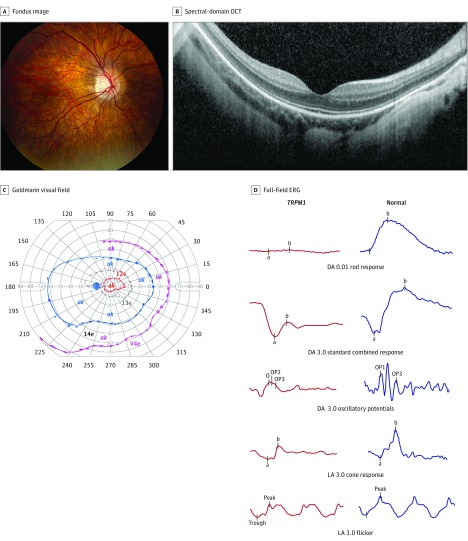
Fundus examination results were similar in all patients, with myopic tilting of the optic nerves, tessellation of the posterior pole, and absence of peripheral pigmentary changes (Figure 2A). The spectral-domain optical coherence tomography of the macula demonstrated a normal lamination pattern with an intact ellipsoid zone, with staphylomatous excavation in several of the patients (Figure 2B). In all patients, Goldmann visual field results were full to Stimulus I4e, with constriction of the I2e isopter in 6 of 7 patients (85.7%). One patient had small central scotomas. Mild constriction of the I2e isopter was found whether patients were tested with glasses or contact lenses (Figure 2C; eFigure 1 in the Supplement).
Figure 2. Representative Composite of Imaging and Visual Function Studies for Patients With TRPM1-Associated Complete Congenital Stationary Night Blindness.
A, Typical fundus appearance of a patient with TRPM1 mutations. The right eye demonstrates myopic fundus with tilting of the optic disc and a peripapillary crescent. These features were universal, and no patients had bone spicule–like pigmentation or arteriolar narrowing. B, Spectral-domain optical coherence tomography (OCT) of right eye of patient 5. Note the normal lamination pattern with preservation of the outer retinal layers and staphylomatous excavation of the overall contour. All OCTs were similar. C, Goldmann visual field results for patient 5. Patient 5 demonstrates a small central scotoma and constriction of the I2e isopter. The central scotoma was not uniformly present; however, constriction of the I2e isopter was present in 6 of 7 patients (85.7%) (eFigure 1 in the Supplement). Ok indicates that the area was tested and was within normal limits. D, Representative full-field electroretinography (ERG) waveforms of patient 5 at age 13 years compared with a normal ERG waveform. Note the electronegative standard combined response (dark-adapted [DA] 3.0), biphasic oscillatory potentials and flattened, broad a wave on light-adapted (LA) 3.0 bright flash (LA 3.0).
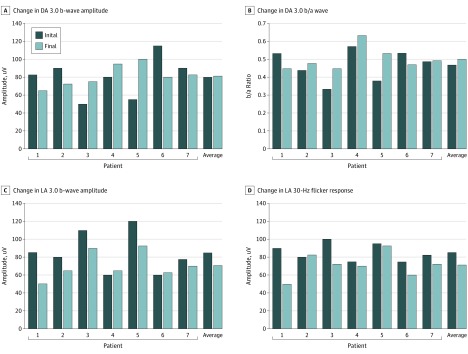
All patients had more than 1 ffERG performed over an average of 44 months (range, 12-60 months). The ERG results were consistent with cCSNB, and ffERGs were almost superimposable among patients (Figure 2D; eFigure 2 in the Supplement). All patients demonstrated very low-amplitude DA dim flash (DA 0.01) responses, and electronegative DA bright flash response (DA 3.0) as well as biphasic oscillatory potentials and flattened photopic a-waves (eFigure 2 in the Supplement). When viewed individually, patients 1, 2, and 6 appear to have clinically meaningful decline (≥20%-25% change in amplitudes) in DA 3.0 b wave amplitudes (Figure 3). However, when analyzed collectively, there was no statistically significant decline in the DA 3.0 b-wave amplitude (95% CI, −20.136 to 23.050; P = .90) or b/a ratio (95% CI, −0.078 to 0.056; P = .76) (Figure 3). The photopic light-adapted 3.0 b wave amplitude (light-adapted 3.0) and 30-Hz flicker response declined more than the DA amplitudes; however, they were not statistically significant (95% CI, −28.183 to −0.817; P = .06; and 95% CI, −24.552 to −0.264; P = .07, respectively) (Figure 3). The decline in DA 3.0 b-wave and 30-Hz flicker amplitudes did not correlate with increase in myopic refraction (95% CI, −0.030 to 0.027; P = .92; and 95% CI, −0.039 to 0.004; P = .16, respectively) after controlling for the association of age. There is a significant association of age with the change in myopic refraction (95% CI, −1.17 to −0.30; P = .01) after controlling for b wave difference (95% CI, −1.02 to −0.14; P = .03 after controlling for 30-Hz difference).
Figure 3. Change in Electroretinogram for Each Individual Patient and Average for Cohort.
A, Change in dark-adapted (DA) 3.0 b-wave amplitude (P = .90). B, Change in DA 3.0 b/a wave (P = .78). C, Change in light-adapted (LA) 3.0 b-wave amplitude (P =.07). D, Change in LA 30-Hz flicker response (P = .07). While the average change for the cohort was greatest for LA responses, it was not statistically significant.
No patient or parent noted nyctalopia at presentation; however, 5 of 7 patients (71%) eventually endorsed some nyctalopia with a mean (SD) age at onset of 6 (3.1) years (range, 2-10 years). Two patients identified it as disrupting their activities, and 3 noted progressive nyctalopia. Dark-adapted FST results were subnormal in all patients who were tested. The mean (SD) FST scores were −29.7 (3.6) dB OU, −29.8 (4.1) dB OD (range, −36 to −22.5), and −28.6 dB OS (range, −35.5 to −26.6) compared with the normal mean (SD) of −59.8 (3.6) dB OD and −59.9 (4.5) dB OS for our laboratory (eFigure 3 in the Supplement). Patients without complaints of nyctalopia did not have different FST results from those who did.
Discussion
Our study describes the pediatric presentation and childhood course of TRPM1-associated cCSNB. This is one of the first reports, to our knowledge, of serial ffERGs and FST testing in children with this disorder. Our data suggest that young children presenting with high myopia, strabismus, and nystagmus should be offered ERG evaluation even in the absence of complaints of night blindness. It is possible that the night vision deficit is only noticed at older ages when more activities are done independently vs a true worsening of night vision. In other disorders with congenital or early-onset night blindness, such as rhodopsin-associated retinitis pigmentosa or RPE65-associated Leber congenital amaurosis (LCA), parents notice from the earliest months of life that the child cannot see them in a dark room; that was not the case for these infants with TRPM1 mutations, although most parents and children noted decreased vision in the dark.
Strengths and Limitations
The limitations of this study that limit the confidence in the definitiveness of the conclusions include the small number of patients evaluated and the retrospective nature of the data collection. TRPM1 encodes the transient receptor potential melastatin 1 (TRPM1) cation channel located in the dendritic tips of ON-bipolar cells. Failed expression or localization of TRPM1 leads to loss of the ON-bipolar response and cCSNB. Transient receptor potential melastatin 1 localization and function depends on its protein-to-protein interactions with a large multiprotein complex, including cCSNB-implicated proteins glutamate receptor metabotropic 6 (GRM6), probable G-protein coupled receptor 179 (GPR179), leucine-rich repeat immunoglobin-like and transmembrane domains 3 (LRIT3), and nyctalopin (NYX). In the dark, increased calcium influx into the rod axon leads to glutamate release in the synapse and is detected by GRM6, which subsequently binds the heterotrimeric G-protein, leading to the closure of the nonselective TRPM1 channel and hyperpolarization of the ON-bipolar cell. In response to light, glutamate concentration is decreased in the synapse and leads to TRPM1 channel opening, depolarizing the ON-bipolar cells that contribute to the b wave. The DA 0.01 pathway originates in the rod photoreceptors and is transmitted to rod ON-bipolar cells then to A11 amacrine cells (and cone bipolar cells) and finally to the ganglion cells. The absence or dysfunction of any proteins involved with the expression, localization, or function of TRPM1, or from mutations affecting the TRPM1 channel itself, leads to diminished signal transduction of rod ON-bipolar cells, leading to diminished DA 0.01 dim flash amplitudes and an electronegative DA 3.0 bright flash response. To date, 73 nonsense, missense, frameshift, splice site, and large or microdeletions have been identified in TRPM1, with 67 of those causing a CSNB phenotype. Some mutations, such as splice site, result in loss of TRPM1 function, while some missense mutations lead to a mislocalization of TRPM1. Genotype-phenotype studies will help delineate the mechanisms for specific mutations in TRPM1.
Visual acuity in our cohort at MRV averaged 20/30 in the better-seeing eye (range, 20/20-20/70). Vision improved, likely reflecting improved testing performance with advancing age (Figure 1A). The BCVA in this cohort concurs with other studies of patients with cCSNB; however, a range of BCVA from 20/20 to 20/125 has been reported. Our study also concurs with others that report nystagmus diminishing or resolving over time; however, 1 of the patients had large-amplitude, persistent nystagmus that was initially suspected of being opsoclonus. This patient had a gross deletion of 1 copy of TRPM1, which may have led to a more severe phenotype, or the patient’s genetic background may have influenced the severity (Table 2).
No decline between initial and final ERG amplitudes for the group of patients occurred, consistent with a stationary disorder (Figure 3). Some individual patients, such as patient 1, had a decline in amplitudes over time, which might have led the clinician to suspect photoreceptor degeneration. High intertest variability inherent to ERG (up to 20%-25%) and our small sample size make interpretation difficult.
Some variability was present between siblings sharing identical mutations. Patient 1 exhibited higher and earlier myopia and nystagmus than his sibling, patient 2, but ended with better BCVA and stereopsis and less myopia (Table 1). Both siblings had strabismus; patient 1 had exotropia and patient 2 had esotropia and fourth nerve palsy. Both complained of progressive nyctalopia. Patient 3 had better retinal sensitivity on FST than sibling patient 4; however, only patient 3 reported nyctalopia.
From the earliest reports of TRPM1-associated cCSNB in humans by Audo et al, high myopia has been consistently reported. Myopia has been reported to occur in all genetic types of cCSNB. Transient receptor potential melastatin 1 may play a role in emmetropization, supported by the finding that the patients did not have the usual course of juvenile myopia. In contrast to “school-age” or juvenile-onset myopia, in which myopic progression is most pronounced from ages 6 to 15 years (0.35-0.60 D per year), TRPM1-associated myopia progressed most rapidly in the first 5 years of life, with mean (SD) myopic correction at age 5 years of −7.3 (2.9) D and a mean (SD) change from initial visit to 5 years of age of −1.07 (.897) D/year. Refractive error change was minimal during the school-age years (−0.25 D/year). At MRV, average age 12 years, refraction was −8.8 (3.8) D. This suggests a different mechanism from typical juvenile myopia. Hendriks et al reported that disorders disrupting bipolar cells have the highest risk of high myopia and that the involved cell type in retinal dystrophies correlate with different refractive errors.
Full-field stimulus threshold testing is a psychophysical test that measures the DA retinal intensity threshold. Young children are able to perform FST, which has been used in clinical treatment trials for LCA. Full-field stimulus threshold testing in patients with TRPM1 revealed mild to moderate loss of sensitivity of −29.7 (3.8) dB compared with −59.8 (4.0) dB in controls. Other conditions causing nyctalopia, such as RPE65 LCA (FST, approximately −9 dB), CEP290 LCA (lack of rod sensitivity, variable cone sensitivity), and GUCY2D retinopathy (−19 dB [data not shown]; rod sensitivity reduced by 0.5 to 5 log units in LCA), show greater loss. Van Genderen et al reported that Goldmann Weekers dark adaptometry was abnormal in a patient with TRPM1-associated cCSNB. This test measures the length of time to recover function after a bleaching light; the rods were not recovered after 20 minutes. In contradistinction, FST measures retinal sensitivity after 45 minutes of dark adaptation. Dark-adapted retinal sensitivity is more mildly affected in young patients with TRPM1 than would be predicted based on previously reported dark adaptometry. Thus, while the dark adaptometry curve appears to show little rod response, the sensitivity of the retina after 45 minutes of dark adaptation is only 40% to 50% reduced in the pediatric patients. The variable nyctalopia in our cohort differs from previous studies by Bijveld et al in which 100% of Dutch patients with cCSNB reported nyctalopia. However, in other studies, the authors concluded that symptoms might be subtle and not disabling in modern artificial light conditions. In controlled lighting conditions, patients with cCSNB experienced blindness at low intensities (equal to starlight) and quickly regained function at light intensities equal to moonlight. Interestingly, in our cohort of 5 patients who eventually reported nyctalopia (at a mean [SD] age of 6 [3.1] years), 3 (60%) reported progression. Nyctalopia may not be progressive; older children may recognize night vision deficits with more independent activities at night. It is possible that the older age of participants (median, 18 years) in the Bijveld et al study resulted in their higher number of patients with nyctalopia.
Visual fields are reportedly normal in both incomplete and complete forms of CSNB. Six of the 7 patients (85.7%) had depression of the I2e isopter regardless of BCVA, refractive error, FST, or contact lens vs glasses correction, and 1 patient (14.3%) had small central scotomas (eFigure 1 in the Supplement). This may be related to myopic fundus changes with the stretching of the posterior pole changing the spacing between cone photoreceptors and the decreasing resolution of small targets, or to primary cone dysfunction. In the Appaloosa horse, a naturally occurring animal model of TRPM1-associated CSNB, acuity in bright light sometimes decreases over time.
Most of the patients presented with horizontal strabismus (esotropia or exotropia) and all had diminished stereopsis. The TRPM1 Appaloosa horse has also been reported to exhibit strabismus. To our knowledge, the effect of TRPM1 mutations on the oculomotor system is unknown. Retinal degenerations that decrease peripheral vision often exacerbate phorias or latent strabismus due to loss of peripheral fusion necessary to stabilize ocular alignment; however, the patients had full peripheral visual fields. The synaptic function of TRPM1 may contribute to retinogeniculate projections or other neuroretinal functions that play a role in oculomotor alignment.
Conclusions
The prominence of “night blindness” in the term CSNB should not lead physicians away from considering this diagnosis for children who present with preschool myopia without complaints of nyctalopia, especially if strabismus and/or nystagmus are also present. Full-field electroretinography should be offered, and if a characteristic electronegative pattern is identified, molecular genetic testing of cCSNB genes should be considered. If TRPM1 mutations are present, parents can be counseled to make subtle modifications, such as having children carry a flashlight or cellular phone to provide illumination if the conditions require it. Myopia and subjective nyctalopia may progress over time, while nystagmus may decrease.
eFigure 1. Goldmann Visual Fields of all patients.
eFigure 2. Full-field ERG waveforms of all patients with TRPM1 variations compared to normal age-matched control
eFigure 3. Dark-adapted full-field stimulus threshold testing
References
- 1.Zeitz C, Robson AG, Audo I. Congenital stationary night blindness: an analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog Retin Eye Res. 2015;45:58-110. [DOI] [PubMed] [Google Scholar]
- 2.Bech-Hansen NT, Naylor MJ, Maybaum TA, et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000;26(3):319-323. [DOI] [PubMed] [Google Scholar]
- 3.Pusch CM, Zeitz C, Brandau O, et al. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet. 2000;26(3):324-327. [DOI] [PubMed] [Google Scholar]
- 4.Dryja TP, McGee TL, Berson EL, et al. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci U S A. 2005;102(13):4884-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Audo I, Bujakowska K, Orhan E, et al. Whole-exome sequencing identifies mutations in GPR179 leading to autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2012;90(2):321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peachey NS, Ray TA, Florijn R, et al. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2012;90(2):331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeitz C, Jacobson SG, Hamel CP, et al. ; Congenital Stationary Night Blindness Consortium . Whole-exome sequencing identifies LRIT3 mutations as a cause of autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2013;92(1):67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Audo I, Kohl S, Leroy BP, et al. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2009;85(5):720-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Sergouniotis PI, Michaelides M, et al. Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am J Hum Genet. 2009;85(5):711-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Genderen MM, Bijveld MM, Claassen YB, et al. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009;85(5):730-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dryja TP. Molecular genetics of Oguchi disease, fundus albipunctatus, and other forms of stationary night blindness: LVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2000;130(5):547-563. [DOI] [PubMed] [Google Scholar]
- 12.Traboulsi EI, Leroy BP, Zeitz C. Congenital stationary night blindness In: Traboulsi EI, ed. Genetic Diseases of the Eye. 2nd ed Oxford, England: Oxford University Press; 2012:476-483. [Google Scholar]
- 13.Bijveld MM, van Genderen MM, Hoeben FP, et al. Assessment of night vision problems in patients with congenital stationary night blindness. PLoS One. 2013;8(5):e62927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logan NS, Gilmartin B, Marr JE, Stevenson MR, Ainsworth JR. Community-based study of the association of high myopia in children with ocular and systemic disease. Optom Vis Sci. 2004;81(1):11-13. [DOI] [PubMed] [Google Scholar]
- 15.Marr JE, Halliwell-Ewen J, Fisher B, Soler L, Ainsworth JR. Associations of high myopia in childhood. Eye (Lond). 2001;15(pt 1):70-74. [DOI] [PubMed] [Google Scholar]
- 16.Bertsch M, Floyd M, Kehoe T, Pfeifer W, Drack AV. The clinical evaluation of infantile nystagmus: what to do first and why. Ophthalmic Genet. 2017;38(1):22-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrt O. Infantile and acquired nystagmus in childhood. Eur J Paediatr Neurol. 2012;16(6):567-572. [DOI] [PubMed] [Google Scholar]
- 18.Lavery MA, O’Neill JF, Chu FC, Martyn LJ. Acquired nystagmus in early childhood: a presenting sign of intracranial tumor. Ophthalmology. 1984;91(5):425-453. [DOI] [PubMed] [Google Scholar]
- 19.Papageorgiou E, McLean RJ, Gottlob I. Nystagmus in childhood. Pediatr Neonatol. 2014;55(5):341-351. [DOI] [PubMed] [Google Scholar]
- 20.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130(1):1-12. [DOI] [PubMed] [Google Scholar]
- 21.Klein M, Birch DG. Psychophysical assessment of low visual function in patients with retinal degenerative diseases (RDDs) with the diagnosys full-field stimulus threshold (D-FST). Doc Ophthalmol. 2009;119(3):217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roman AJ, Schwartz SB, Aleman TS, et al. Quantifying rod photoreceptor-mediated vision in retinal degenerations: dark-adapted thresholds as outcome measures. Exp Eye Res. 2005;80(2):259-272. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Sanuki R, Yasuma TR, et al. TRPM1 mutations are associated with the complete form of congenital stationary night blindness. Mol Vis. 2010;16:425-437. [PMC free article] [PubMed] [Google Scholar]
- 24.Philp AR, Jin M, Li S, et al. Predicting the pathogenicity of RPE65 mutations. Hum Mutat. 2009;30(8):1183-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone EM. Leber congenital amaurosis—a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144(6):791-811. [DOI] [PubMed] [Google Scholar]
- 26.Morgans CW, Zhang J, Jeffrey BG, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009;106(45):19174-19178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider FM, Mohr F, Behrendt M, Oberwinkler J. Properties and functions of TRPM1 channels in the dendritic tips of retinal ON-bipolar cells. Eur J Cell Biol. 2015;94(7-9):420-427. [DOI] [PubMed] [Google Scholar]
- 28.Koike C, Obara T, Uriu Y, et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010;107(1):332-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Y, Rampino MA, Carroll RC, Nawy S. G-protein-mediated inhibition of the Trp channel TRPM1 requires the Gβγ dimer. Proc Natl Acad Sci U S A. 2012;109(22):8752-8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5(10):747-757. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, Posokhova E, Martemyanov KA. TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. J Neurosci. 2011;31(32):11521-11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuillé M, Morgans CW, Cao Y, et al. LRIT3 is essential to localize TRPM1 to the dendritic tips of depolarizing bipolar cells and may play a role in cone synapse formation. Eur J Neurosci. 2015;42(3):1966-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearring JN, Bojang P Jr, Shen Y, et al. A role for nyctalopin, a small leucine-rich repeat protein, in localizing the TRP melastatin 1 channel to retinal depolarizing bipolar cell dendrites. J Neurosci. 2011;31(27):10060-10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray TA, Heath KM, Hasan N, et al. GPR179 is required for high sensitivity of the mGluR6 signaling cascade in depolarizing bipolar cells. J Neurosci. 2014;34(18):6334-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abouelhoda M, Faquih T, El-Kalioby M, Alkuraya FS. Revisiting the morbid genome of mendelian disorders. Genome Biol. 2016;17(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malaichamy S, Sen P, Sachidanandam R, et al. Molecular profiling of complete congenital stationary night blindness: a pilot study on an Indian cohort. Mol Vis. 2014;20:341-351. [PMC free article] [PubMed] [Google Scholar]
- 37.Ellingford JM, Barton S, Bhaskar S, et al. Whole genome sequencing increases molecular diagnostic yield compared with current diagnostic testing for inherited retinal disease. Ophthalmology. 2016;123(5):1143-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor RL, Parry NRA, Barton SJ, et al. Panel-based clinical genetic testing in 85 children with inherited retinal disease. Ophthalmology. 2017;124(7):985-991. [DOI] [PubMed] [Google Scholar]
- 39.Retterer K, Juusola J, Cho MT, et al. Clinical application of whole-exome sequencing across clinical indications. Genet Med. 2016;18(7):696-704. [DOI] [PubMed] [Google Scholar]
- 40.Audo I, Bujakowska KM, Léveillard T, et al. Development and application of a next-generation-sequencing (NGS) approach to detect known and novel gene defects underlying retinal diseases. Orphanet J Rare Dis. 2012;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou L, Li T, Xing YQ, Li Y, Wu QS, Zhang MJ. Novel TRPM1 mutations in two Chinese families with early-onset high myopia, with or without complete congenital stationary night blindness. Int J Ophthalmol. 2016;9(10):1396-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bijveld MM, Florijn RJ, Bergen AA, et al. Genotype and phenotype of 101 dutch patients with congenital stationary night blindness. Ophthalmology. 2013;120(10):2072-2081. [DOI] [PubMed] [Google Scholar]
- 43.Al Oreany AA, Al Hadlaq A, Schatz P. Congenital stationary night blindness with hypoplastic discs, negative electroretinogram and thinning of the inner nuclear layer. Graefes Arch Clin Exp Ophthalmol. 2016;254(10):1951-1956. [DOI] [PubMed] [Google Scholar]
- 44.Patel N, Aldahmesh MA, Alkuraya H, et al. Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Genet Med. 2016;18(6):554-562. [DOI] [PubMed] [Google Scholar]
- 45.Fromer M, Pocklington AJ, Kavanagh DH, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu X, Zhang B, Liu W, et al. A survey of rare coding variants in candidate genes in schizophrenia by deep sequencing. Mol Psychiatry. 2014;19(8):857-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsunami N, Hensel CH, Baird L, et al. Identification of rare DNA sequence variants in high-risk autism families and their prevalence in a large case/control population. Mol Autism. 2014;5(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pieh C, Simonsz-Toth B, Gottlob I. Nystagmus characteristics in congenital stationary night blindness (CSNB). Br J Ophthalmol. 2008;92(2):236-240. [DOI] [PubMed] [Google Scholar]
- 49.Fishman GA, Chappelow AV, Anderson RJ, Rotenstreich Y, Derlacki DJ. Short-term inter-visit variability of erg amplitudes in normal subjects and patients with retinitis pigmentosa. Retina. 2005;25(8):1014-1021. [DOI] [PubMed] [Google Scholar]
- 50.Grover S, Fishman GA, Birch DG, Locke KG, Rosner B. Variability of full-field electroretinogram responses in subjects without diffuse photoreceptor cell disease. Ophthalmology. 2003;110(6):1159-1163. [DOI] [PubMed] [Google Scholar]
- 51.Kennedy RH. Progression of myopia. Trans Am Ophthalmol Soc. 1995;93:755-800. [PMC free article] [PubMed] [Google Scholar]
- 52.Brodstein RS, Brodstein DE, Olson RJ, Hunt SC, Williams RR. The treatment of myopia with atropine and bifocals. a long-term prospective study. Ophthalmology. 1984;91(11):1373-1379. [DOI] [PubMed] [Google Scholar]
- 53.Jensen H. Myopia progression in young school children. a prospective study of myopia progression and the effect of a trial with bifocal lenses and beta blocker eye drops. Acta Ophthalmol Suppl. 1991;(200):1-79. [PubMed] [Google Scholar]
- 54.Hendriks M, Verhoeven VJM, Buitendijk GHS, et al. ; RD5000 Consortium . Development of refractive errors—what can we learn from inherited retinal dystrophies? Am J Ophthalmol. 2017;182:81-89. [DOI] [PubMed] [Google Scholar]
- 55.Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231-2239. [DOI] [PubMed] [Google Scholar]
- 57.Jacobson SG, Cideciyan AV, Ratnakaram R, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130(1):9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Messias K, Jägle H, Saran R, et al. Psychophysically determined full-field stimulus thresholds (FST) in retinitis pigmentosa: relationships with electroretinography and visual field outcomes. Doc Ophthalmol. 2013;127(2):123-129. [DOI] [PubMed] [Google Scholar]
- 59.Jacobson SG, Cideciyan AV, Sumaroka A, et al. Outcome measures for clinical trials of leber congenital amaurosis caused by the intronic mutation in the CEP290 gene. Invest Ophthalmol Vis Sci. 2017;58(5):2609-2622. [DOI] [PubMed] [Google Scholar]
- 60.Jacobson SG, Cideciyan AV, Peshenko IV, et al. Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human Leber congenital amaurosis en route to therapy: residual cone-photoreceptor vision correlates with biochemical properties of the mutants. Hum Mol Genet. 2013;22(1):168-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992;12(2):127-133. [DOI] [PubMed] [Google Scholar]
- 62.Sandmeyer LS, Breaux CB, Archer S, Grahn BH. Clinical and electroretinographic characteristics of congenital stationary night blindness in the Appaloosa and the association with the leopard complex. Vet Ophthalmol. 2007;10(6):368-375. [DOI] [PubMed] [Google Scholar]
- 63.Witzel DA, Smith EL, Wilson RD, Aguirre GD. Congenital stationary night blindness: an animal model. Invest Ophthalmol Vis Sci. 1978;17(8):788-795. [PubMed] [Google Scholar]
- 64.Sandmeyer LS, Grahn BH, Breaux CB. Diagnostic ophthalmology. congenital stationary night blindness (CSNB). Can Vet J. 2006;47(11):1131-1133. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Goldmann Visual Fields of all patients.
eFigure 2. Full-field ERG waveforms of all patients with TRPM1 variations compared to normal age-matched control
eFigure 3. Dark-adapted full-field stimulus threshold testing