Key Points
Question
Do repeated ranibizumab injections worsen macular perfusion in a compromised capillary network, compared with laser monotherapy and combination ranibizumab and laser therapy?
Findings
This post hoc analysis of clinical trial data of biannual fluorescence angiography images of 240 patients and found that capillary dropout and foveal avascular zone regularity did not worsen significantly in eyes treated with ranibizumab injections compared to those treated with laser or combination therapy.
Meaning
Repeated ranibizumab injections were not associated with impaired macular perfusion.
This post hoc analysis of data from a randomized clinical trial evaluates the association of repeated ranibizumab injections on macular perfusion in patients with diabetic macular edema as compared with laser monotherapy or ranibizumab and laser combination therapy.
Abstract
Importance
Anti–vascular endothelial growth factor treatment is the first-line therapy in the treatment of center-involving diabetic macular edema. Data on capillary perfusion changes under repeated treatment in a possibly compromised vascular network are limited.
Objective
To evaluate the association of repeated ranibizumab injections on macular perfusion in patients with diabetic macular edema.
Design, Setting, and Participants
This study analyzed prospectively collected data from the 12-month RESTORE core study and the 24-month open label RESTORE extension study, which assessed the efficacy and safety of ranibizumab in patients with visual impairment due to diabetic macular edema. Of 345 patients with center-involving diabetic macular edema who had enrolled in the 12-month RESTORE core study, 240 entered the 24-month RESTORE extension study. Of these, 83 (34.6%) received ranibizumab, 83 (34.6%) received ranibizumab and laser combination therapy, and 74 (30.8%) received laser monotherapy in the first year of the study; 208 completed the 24-month extension study. Fluorescence angiography images were taken from each participant twice each year graded by Vienna Reading Center on severity of capillary loss in the parafoveal area, regularity of the foveal avascular zone outline, and measurement of the size of the foveal avascular zone, following a standardized protocol. Data analysis took place from July 2014 through December 2017.
Main Outcomes and Measures
Change in 3 fluorescence angiography perfusion parameters over the course of treatment.
Results
Mean (SD) patient age was 62.6 (8.8) years; 124 of 208 (59.2%) were male and 197 of 208 (94.6%) were white. The number of patients with definite altered foveal avascular zone regularity at baseline was 103 of 240 patients (42.9%); another 118 patients (49.2%) had questionably altered regularity at baseline. Definitive capillary loss was found in 65 of 240 patients (27.1%) at baseline. Mean (SD) foveal avascular zone size at baseline was 0.261 (0.232) mm2 in ranibizumab monotherapy, 0.231 (0.219) mm2 in ranibizumab and macular laser combination therapy, and 0.201 (0.13) mm2 in laser monotherapy. No treatment arm experienced significant increase in foveal avascular zone size at any time in the study period. At month 36, ranibizumab monotherapy resulted in a mean increase of 0.073 mm2 (95% CI, 0.005-0.142 mm2) and combination therapy resulted in a mean increase of 0.117 mm2 (95% CI, 0.045-0.188 mm2), but no changes were statistically significant. No changes occurred in foveal avascular zone regularity in any treatment group, and no differences were found in capillary loss around the fovea in the 3 treatment groups; neither element could be correlated with visual acuity or central retinal thickness.
Conclusions and Relevance
Repeated ranibizumab treatment was not associated with impaired macular perfusion in our study cohort. Because our data do not suggest a harmful effect of anti–vascular endothelial growth factor therapy on capillary integrity, patients with severe microangiopathy and advanced capillary dropout should not be denied these treatments.
Introduction
In the past decade, several clinical studies have demonstrated the effectiveness of intravitreally injected anti–vascular endothelial growth factor (VEGF) in the treatment of visual impairment related to center-involving diabetic macula edema (DME). Though elevated levels of VEGF are associated with ocular neovascular disease and DME, a physiological level of intraocular VEGF is essential for the integrity of microcirculation and contributes to neuroprotection in ischemic conditions. Therefore it is logical that blocking VEGF might be detrimental to vascular integrity and retinal function, especially in patients with preexisting microangiopathy. In fact, case reports published in the last decade attribute a negative effect on retinal perfusion to a single injection of bevacizumab. As a precaution, some clinical trials evaluating the effect of anti–VEGF agents for the treatment of DME have excluded patients with signs of pronounced macular ischemia.
On the other hand, repeated anti–VEGF treatment was previously shown to prevent diabetic retinopathy progression or even lead to regression of the Early Treatment Diabetic Retinopathy Study (ETDRS) severity score, as was reflected in the ETDRS trial (which used severity grading based on color fundus photography). Still, detailed evaluation of macular perfusion parameters in fluorescein angiography (FA) images have rarely been part of multicenter trials. Therefore, the purpose of this post hoc subevaluation was to investigate angiographic changes in macular perfusion in patients with DME during the course of treatment with repeated intravitreal ranibizumab injections.
Methods
Study Design
Details about core and extension studies of the 12-month RESTORE core trial have been reported previously. In brief, the RESTORE core study was a phase 3 multicenter trial that enrolled 345 diabetic patients who were experiencing visual impairment as a result of center-involving DME.
The RESTORE study adhered to the tenets of the Declaration of Helsinki and was registered at clinicaltrials.gov (NCT00687804). All analyses presented in this article were approved by the institutional review board of Medical University Vienna. All study patients gave written informed consent prior to study inclusion.
Patients were randomized to 3 treatment arms: intravitreal ranibizumab, 0.5 mg, monotherapy with sham laser treatment; intravitreal ranibizumab, 0.5 mg, combined with macular laser therapy; or laser therapy with sham ranibizumab monotherapy. (Sham treatments were designed to mask clinicians and participants to the treatment arms to which patients were assigned.) After a loading dose of 3 monthly ranibizumab injections or baseline laser therapy, patients were treated following prespecified retreatment criteria. Patients were seen monthly for best-corrected visual acuity (BCVA) testing and time-domain optical coherence tomography (Stratus OCT; Carl Zeiss Meditec) for central retinal thickness measurements.
After 12 months, 240 patients were enrolled in the 2-year open-label RESTORE extension study, during which all patients were eligible for intravitreal ranibizumab, 0.5 mg, treatment and laser therapy based on the same retreatment criteria used during the core study, regardless of their prior treatment assignment. All analyses of the FA subevaluation were performed on this subset, which comprised 83 persons who had previously been assigned to ranibizumab monotherapy, 83 persons who had been in a ranibizumab plus laser treatment arm, and 74 persons who had been treated with laser only. Of these 240, 208 patients (86.7%) completed the extension study. Throughout the study period (36 months), investigators were masked to the treatment group to which their patients had been primarily assigned.
Fluorescein Angiography
Fluorescein angiography was performed at baseline and every 6 months thereafter throughout the study period, and FA images were centrally evaluated by a reading center (Vienna Reading Center [VRC]) that was masked to patient treatment arms. Images with a minimum resolution of 1024 × 1024 pixels were captured by various FA camera systems after device and operator certification by the VRC and were graded using custom VRC software. The comparability of measurements was ensured by image calibration with VRC test eye procedures.
Image acquisition was performed in accordance with a predefined imaging protocol, which required at least 8 images of the study eye in the first minute after dye injection from early arterial filling was begun. Further images were taken after 1, 2, 5, and 10 minutes.
All FA images were anonymized, uploaded digitally to the VRC, and automatically checked for image integrity. Image grading was performed following a predefined reading protocol, which was based on the grading protocol previously used for the ETDRS diabetic retinopathy FA images. First, a grid was manually centered on the fovea of an early phase 30° F2 image. The same grid position was maintained in follow-up angiograms by a standardized VRC procedure. The following parameters of macular ischemia were graded in early-phase images: size and outline regularity of the foveal avascular zone (FAZ) and capillary dropout within the central and the 4 inner ETDRS subfields.
Fluorescein angiographic perfusion parameters were graded in strict adherence to the ETDRS FA grading protocol, which involved comparing clinical findings to ETDRS standard photographs. The viability of these grading variables were tested for intergrader and intragrader reproducibility by the ETDRS group, which resulted in an agreement (within 1 step of the grading protocol) of 89%, 74%, and 95% for FAZ size, FAZ outline, and capillary loss, respectively. The area of FAZ was calculated automatically after a reader manually outlined the innermost capillaries around the fovea (eFigure 1 in the Supplement). The FAZ outline was graded on a 5-step grading scale based on the ETDRS FA grading protocol (grade 0: outline of the FAZ is normal; grade 1: outline is questionable; grade 2: outline is clearly destroyed for up to half the original circumference; grade 3: outline is destroyed for half of the original circumference or more, but some remnants remain; grade 4: capillary outline is completely destroyed). Capillary dropout was graded in each of the central and 4 inner ETDRS subfields and classified on a 5-step grading scale that referred to specified subfields of ETDRS standard photographs (grade 0: dropout is absent; grade 1: dropout is questionable; grade 2: dropout is mild but clearly present; grade 3: dropout is moderate; grade 4: dropout is severe; grade 5: dropout is complete). The maximum grade in any of the 5 ETDRS subfields was used to represent the eye. The inner 4 ETDRS subfields were chosen because capillaries within approximately 1000 μm of the center of the macula are usually easily recognizable in good-quality angiograms, but those located further away are frequently unrecognizable.
Images were sent to, collected at, and graded electronically at the VRC from July 2009 to March 2012. Dependent on the grader expertise, they were forwarded to and regraded by an experienced supervisor, in accordance with VRC quality control procedures used to affirm intergrader reproducibility. Additional quality control procedures included regular meetings for case discussions plus the forwarding of a randomly selected 10% of all FA images to the supervisor for review.
Statistical Analysis
Data analysis was done starting in July 2014 and continued through December 2017. All analyses were performed on data from patients for whom the treatment group assignment from the RESTORE core study was known at the time of analysis. Correlations were calculated using Pearson and Spearman correlation coefficients. Analysis between baseline treatment groups and subgroups were performed using mixed model repeated measures analysis and repeated measures logistic regression. Within-treatment analysis was performed using Wilcoxon signed rank tests and McNemar tests, depending on the type of variable (continuous or categorical) and on whether the data was normal or non-normal. Descriptive statistics and frequencies were also calculated. Investigation of baseline characteristics in analysis models were halted if differences did not satisfy the standard 2-tailed significance level of P < .05.
Results
For the RESTORE extension subgroup (n = 240), mean (SD) patient age was 62.6 (8.8) years; 142 members of the subset (59.2%) were male and 227 (94.6%) were white. Baseline demographics by treatment group are outlined in eTable 1 in the Supplement. Patient demographics and graded baseline perfusion variables (FAZ area, FAZ outline, and capillary dropout within the central and inner ETDRS subfields) were comparable between the 3 treatment groups of the RESTORE core study (eTable 1 in the Supplement).
In the course of 3 years, 1731 FA visits were uploaded and graded by the VRC for the following features: area of FAZ, outline of the FAZ, and capillary loss. A total of 3072 features were gradable. The rest of the features was not gradable because images from a given patient encounter were poor in quality, image positioning was incorrect, or insufficient images for grading were provided (ie, only early-phase or only late-phase images were available, or there was a lack of images necessary for the calibration of measurements). Table 1 and Table 2 provide details on the number of gradable features.
Table 1. Mean Area of Foveal Avascular Zone in Patients Assigned to Receive Ranibizumab Therapy, Laser Treatment, or Botha.
| Month | Patients With Data Available From Visit, No. (%)b | Patients With Measurable FAZ, No. (%)c,d | Gradable Images, No. (%)d,e | Gradable Features, No.f | Mean Area of Foveal Avascular Zone, mm2 (95% CI) | P Value (vs Laser Monotherapy)g | P Value (vs Ranibizumab Plus Laser Combination Therapy)g | P Value (vs Ranibizumab Monotherapy)g |
|---|---|---|---|---|---|---|---|---|
| Ranibizumab plus sham laser | 83 | |||||||
| 0 (Day 1) | 83 (100) | 53 (64) | 73 (88) | 193 | 0.261 (0.197-0.325) | |||
| 6 | 81 (98) | 52 (64) | 75 (93) | 194 | 0.234 (0.187-0.281) | |||
| 12 | 75 (90) | 50 (67) | 68 (91) | 181 | 0.239 (0.189-0.288) | .78 | .74 | NA |
| 18 | 64 (77) | 41 (64) | 53 (83) | 141 | 0.305 (0.233-0.377) | |||
| 24 | 68 (82) | 41 (60) | 55 (81) | 142 | 0.320 (0.231-0.410) | |||
| 30 | 70 (84) | 39 (56) | 56 (80) | 140 | 0.311 (0.219-0.40) | |||
| 36 | 72 (87) | 46 (64) | 58 (81) | 155 | 0.325 (0.252-0.397) | .25 | .45 | NA |
| Ranibizumab plus laser | 83 (100) | |||||||
| 0 (Day 1) | 83 | 48 (58) | 66 (80) | 178 | 0.234 (0.170-0.298) | |||
| 6 | 75 (90) | 47 (63) | 64 (85) | 167 | 0.212 (0.144-0.281) | |||
| 12 | 81 (98) | 50 (62) | 70 (86) | 180 | 0.236 (0.168-0.303) | .55 | NA | .74 |
| 18 | 70 (84) | 38 (54) | 50 (71) | 129 | 0.226 (0.176-0.277) | |||
| 24 | 65 (78) | 34 (52) | 52 (80) | 125 | 0.288 (0.160-0.416) | |||
| 30 | 65 (78) | 41 (63) | 43 (66) | 122 | 0.216 (0.172-0.260) | |||
| 36 | 71 (86) | 35 (49) | 49 (69) | 127 | 0.305 (0.242-0.368) | .07 | NA | .45 |
| Laser plus sham injections | 74 | |||||||
| 0 (Day 1) | 74 (100) | 43 (58) | 63 (85) | 162 | 0.202 (0.161-0.242) | |||
| 6 | 70 (95) | 43 (61) | 66 (94) | 167 | 0.197 (0.164-0.230) | |||
| 12 | 71 (96) | 38 (54) | 56 (79) | 139 | 0.214 (0.162-0.266) | NA | .55 | .78 |
| 18 | 59 (80) | 26 (44) | 40 (68) | 97 | 0.212 (0.158-0.266) | |||
| 24 | 56 (76) | 33 (59) | 42 (75) | 109 | 0.207 (0.162-0.252) | |||
| 30 | 61 (82) | 36 (59) | 44 (72) | 117 | 0.201) (0.158-0.243) | |||
| 36 | 63 (85) | 31 (49) | 43 (68) | 107 | 0.254 (0.207-0.301) | NA | .07 | .25 |
Abbreviations: FAZ, foveal avascular zone; NA, not applicable.
Ranibizumab was provided in a standard 0.5-mg dose by intraviteal injection.
Percentages are based on patients in the extension study and by core study treatment assignment.
Number signifies the number of patients with FAZ area data available.
Percentages are based on patients with data available at that visit.
Number signifies the number of fluorescein angiography images with at least 1 gradable feature.
Number signifies the number of total gradable features across all fluorescein angiography images.
P values refer to differences between treatment groups from mixed-model repeated-measures analysis with treatment, baseline, time, and the interactions between time and treatment and time and baseline as factors. P values were calculated at month 12 and month 36 of the 36-month trial. Values are presented redundantly per pairwise comparison.
Table 2. Foveal Avascular Zone Outline Regularity as Graded at Baseline.
| Characteristic | No. (%)a | ||
|---|---|---|---|
| Ranibizumab Plus Sham Laser | Ranibizumab Plus Laser | Laser Plus Sham Injection | |
| Patients | 80 | 80 | 74 |
| Foveal avascular zone outline | |||
| Normal | 8 (10) | 3 (4) | 8 (11) |
| Borderline | 18 (23) | 25 (31) | 14 (19) |
| <Half altered | 25 (31) | 22 (28) | 25 (34) |
| >Half destroyed | 8 (10) | 8 (10) | 6 (8) |
| Completely destroyed | 5 (6) | 3 (4) | 1 (1) |
| Cannot gradea | 16 (20) | 19 (24) | 20 (27) |
| Capillary loss | |||
| None | 18 (23) | 18 (23) | 27 (37) |
| Questionable | 19 (24) | 17 (21) | 11 (15) |
| Mild | 15 (19) | 20 (25) | 21 (28) |
| Moderate | 17 (21) | 19 (24) | 12 (16) |
| Severe | 7 (9) | 4 (5) | 2 (3) |
| Completely destroyed | 3 (4) | 1 (1) | 0 |
| Cannot grade | 1 (1) | 1 (1) | 1 (1) |
Abbreviation: ETDRS, Early Treatment Diabetic Retinopathy Study.
In around one-quarter of all images, the foveal avascular zone outline regularity could not be graded because of reduced image quality or because early-phase images were not available. Capillary loss was graded based on summary grading in which the central and the inner 4 ETDRS subfields were reviewed and the final grade was assigned based on the most severe capillary loss.
Area of the FAZ
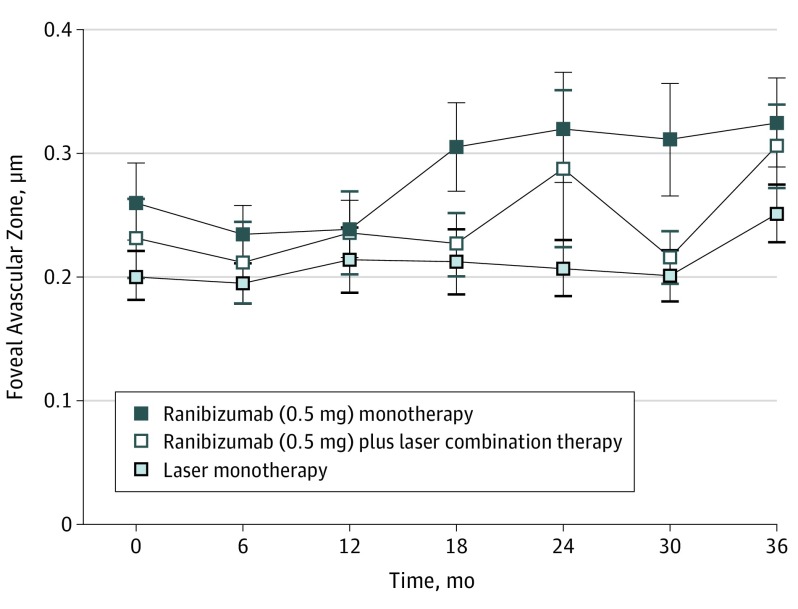
At baseline, the mean (SD) area of FAZ was similar in all 3 treatment arms, with measurements of 0.261 (0.232) mm2 in ranibizumab monotherapy, 0.231 (0.219) mm2 in ranibizumab and macular laser combination therapy, and 0.201 (0.13) mm2 in laser monotherapy (eTable 1 in the Supplement). There was no evidence of linear nor monotonic correlations between the area of FAZ or the regularity of FAZ outline and the central retinal thickness or BCVA in any group at baseline.
There was no evidence to suggest a difference in the area of FAZ between all 3 treatment arms at 12 months. However, after 3 years, the area of FAZ increased in patients primarily assigned to ranibizumab monotherapy (mean increase, 0.073 mm2; 95% CI, 0.005-0.142 mm2) and ranibizumab and macular laser combination therapy (mean increase, 0.117 mm2;95% CI, 0.045-0.188 mm2) but stayed stable in patients primarily assigned to laser monotherapy at baseline and switched to ranibizumab 0.5 mg monotherapy after month 12 (mean increase, 0.029 mm2; 95% CI, 0.031-0.089 mm2) (Table 1; Figure 1). Enlargement of the FAZ size within 36 months of both arms treated with ranibizumab from baseline (ranibizumab monotherapy and ranibizumab and macular laser combination therapy, pooled) was not correlated with changes in BCVA.
Figure 1. Changes in Foveal Avascular Zone Area Over 3 Years of Treatment.
Treatment arms refer to primary assigned treatment in the RESTORE core study.
None of the baseline characteristics (sex, age, hemoglobin A1c level, DME duration, diabetes duration, and history of hypertension) had any effect on the change of FAZ area or FAZ outline from baseline to month 36 in any treatment arm in a mixed model repeated measures analysis. As a result, they were removed from the final model.
Outline of the FAZ
At baseline, the FAZ outline was normal in 19 of 240 patients (7.9%). In 103 of 240 patients (42.9%), the FAZ outline was clearly altered or destroyed (grades 2-4) (eTable 1 in the Supplement and Table 2). The remaining 118 patients (49.2%) had grade 1, or questionable, FAZ outlines.
After 12 months and 24 months of open-label treatment, no treatment arm experienced changes in FAZ outline regularity compared with baseline measurements. The regularity of FAZ outlines did not differ between the treatment arms at any point (eTable 2 in the Supplement).
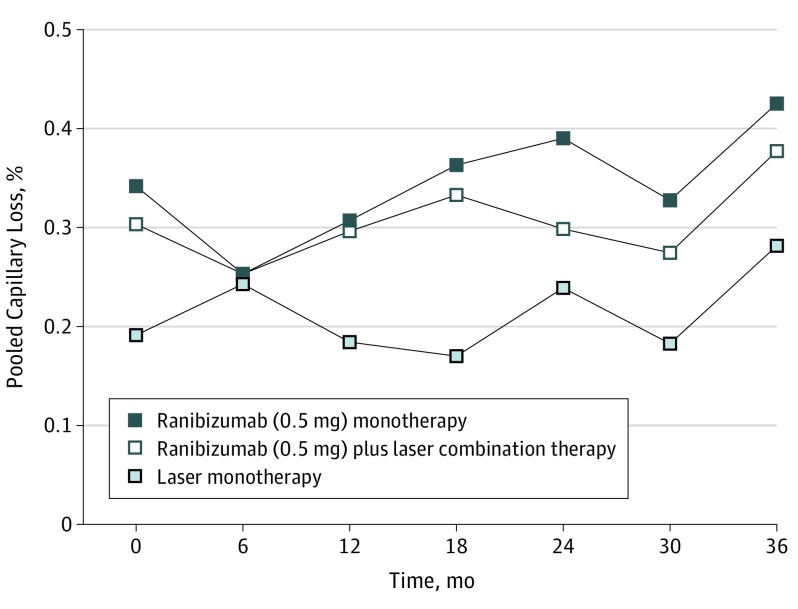
Capillary Loss
Definitive capillary loss (grades 3-5) was present in 65 of 240 patients (27.1%) at baseline and was equally distributed between the 3 treatment arms (eTable 1 in the Supplement; Table 2). Within the first year of the RESTORE study, there was no difference evident between the ranibizumab monotherapy and laser treatment monotherapy groups. Despite some worsening across treatment groups, there was no strong evidence of worsening to moderate or severe capillary loss at any point during the study (eTable 3 in the Supplement). At the end of study, severe capillary loss (grades 3-5) was present in 26 of the 83 patients in the ranibizumab monotherapy group (36%), 20 of the 83 patients in the combination therapy group (28%), and 13 of the 74 patients in the laser monotherapy group (21%). However, there was no evidence to suggest a difference between the 3 baseline treatment arms at any point (Figure 2). In all treatment arms, capillary dropout in the central subfield was most frequently graded moderate or severe; in total, this was observed in 351 visits for FA imaging overall, with 148 such observations in the ranibizumab monotherapy group, 124 in the combination therapy group, and 79 in the laser monotherapy group. Capillary dropout was least frequently seen in the inner inferior ETDRS subfield; in total, this was observed 38 times across all study patients and all FA imaging visits, including 17 observations in the ranibizumab monotherapy group, 14 in the combination therapy group, and 7 in the laser monotherapy group. There was no correlation found between change in BCVA score and capillary loss in the central subfield (eFigure 2 in the Supplement).
Figure 2. Changes in Capillary Dropout on Fluoroscein Angiography Over 3 Years of Treatment.
Treatment arms refer to primary assigned treatment in the RESTORE core study.
Discussion
The effectiveness of ranibizumab treatment in DME has been repeatedly demonstrated through reductions in central retinal thickness and improvements in BCVA. However, with the exception of optical coherence tomography analysis, only a few multicenter clinical trials have performed FA at regular intervals, even though diabetic retinopathy is known for its microangiopathic pathogenesis. The multicenter RESTORE core and extension study is therefore distinct; the VRC graded qualitative as well as quantitative macular perfusion parameters in FA images of enrolled diabetic patients.
Studies examining short-term macular perfusion after anti–VEGF injections provide contradicting results, ranging from severe ischemia after a single injection to a good response to repeated anti–VEGF injections in diabetic eyes with severe central capillary loss. Campochiaro et al assessed the area of retinal nonperfusion within a radius of 6 mm from the fovea in a large cohort from A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus (or RISE and RIDE) trials and followed up with these participants for more than 3 years. This study group assessed the portion of fundus devoid of retinal arterioles or capillaries in the measure of disc areas. More than 90% of study patients in the RISE and RIDE trials had no signs or minimal signs of retinal nonperfusion at baseline. In that analysis, there was evidence that retinal nonperfusion worsened in the laser therapy group compared to patients treated with ranibizumab, 0.5 mg, as early as 3 months after initial treatment. Twelve months after the study implemented a treatment crossover to offer ranibizumab, 0.5 mg, to all patients who had been assigned to other arms, the difference diminished.
In the RESTORE core study, around 70% of patients were graded with mild or no capillary dropout at baseline based on the ETDRS grading scale, which is comparable with the results of Campochiaro et al. In general, capillary loss is known to be a risk factor for diabetic retinopathy progression. Although overall capillary dropout worsened in all treatment arms of the RESTORE study, patients with moderate or severe capillary loss did not worsen significantly within the 3-year study period. There was no evidence of a difference between the groups after the first end point (month 12). This evidence seems to suggest that ranibizumab treatment does not increase the risk of further malperfusion.
Though moderate or severe capillary loss was graded within a radius of 1.5 mm from the center of the fovea in only some of the patients in this study, more subtle changes (such as definite irregularities in the FAZ outline) were present in 44% of the patients at baseline. Obviously, the regularity of FAZ outline seems to be a sensitive marker of macular malperfusion. This is because nonperfused capillaries create sharp-angled bays that create the irregular appearance of the FAZ. Consequently, the maximum FAZ diameter is also a commonly used parameter for macular ischemia.
Nonetheless, there were no changes evident in FAZ regularity that depend on DME treatment. Neither parafoveal capillary dropout nor FAZ irregularities seemed to effect the BCVA score or central retinal thickness. Hence, visual acuity testing retains an independent diagnostic importance that cannot be replaced by retinal imaging alone.
A strength of our evaluation is that 3 different macula perfusion parameters were analyzed by a reading center masked to patient treatment. Perfusion parameters of the FAZ as well as ischemic dropout of the capillaries in the inner ETDRS subfields were analyzed according to a strict and standardized protocol.
Limitations
A limitation of our analysis is that grading of the perfusion status was not the primary statistical outcome of the RESTORE study. Nonetheless, all perfusion parameters evaluated were defined by the reading center beforehand. Additionally, though capillary changes within the central millimeter might not be representative for the perfusion status of the entire retina, capillary dropout close to the fovea might be responsible for loss in BCVA score (at least in theory). In addition, analysis and P values are to be interpreted in a descriptive fashion as there has been no adjustment for multiplicity.
Finally, contradictory results exist on the clinical significance of FAZ size. In our cohort, the mean size of FAZ area at baseline was smaller than described previously in diabetic patients, which could be associated with the different evaluation procedures used in these different studies. Moreover, we did not find a correlation between FAZ size and BCVA score or central retinal thickness. However, it is known that FAZ size can vary considerably even among healthy patients and that the regularity of FAZ outline might be a better indicator for microangiopathy.
Conclusions
In the future, optical coherence tomography angiography might provide a more detailed insight to capillary perfusion within a larger field of view than early phase FA images can provide. Thus a more detailed evaluation of the retinal vascular plexus will become feasible. Moreover, optical coherence tomography angiography allows a clear differentiation between the superficial and deep retinal capillary plexus, further expanding clinical and research opportunities.
Pending those future studies, we submit these conclusions. The RESTORE study found that repeated intravitreal ranibizumab injections did not aggravate the central capillary perfusion status in patients with clinically significant macular edema. Furthermore, macular malperfusion parameters based on the complex ETDRS FA grading progressed equally in all treatment arms within the first year. Two years after open-label treatment, capillary perfusion parameters in patients with initially moderate or severe capillary loss did not deteriorate under ranibizumab treatment. Based on these findings, ranibizumab treatment in patients with signs of macular malperfusion does not increase the risk of further macular ischemia associated with vision loss.
eTable 1 Baseline characteristics of the participants of the RESTORE Extension Study
eTable 2 Change in FAZ outline over time
eTable 3 Patients with capillary loss graded moderate or greater pooled for the central and 4 inner ETDRS subfields
eFigure 1 Example of early phase fluorescence angiography image with the inner capillaries of the foveal avascular zone outlined manually and overlay of ETDRS grid centered on the foveae
eFigure 2 Change of visual acuity from baseline to month 36 by capillary loss in the central subfield at month 36
References
- 1.Schmidt-Erfurth U, Lang GE, Holz FG, et al. ; RESTORE Extension Study Group . Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121(5):1045-1053. [DOI] [PubMed] [Google Scholar]
- 2.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elman MJ, Aiello LP, Beck RW, et al. ; Diabetic Retinopathy Clinical Research Network . Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DM, Nguyen QD, Marcus DM, et al. ; RIDE and RISE Research Group . Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013-2022. [DOI] [PubMed] [Google Scholar]
- 5.Bandello F, De Benedetto U, Knutsson KA, Parodi MB, Cascavilla ML, Iacono P. Ranibizumab in the treatment of patients with visual impairment due to diabetic macular edema. Clin Ophthalmol. 2011;5:1303-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. ; RESTORE study group . The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615-625. [DOI] [PubMed] [Google Scholar]
- 7.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97(18):10242-10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med. 2005;9(4):777-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel N, Kumar V, Ghosh B. Ischemic maculopathy following intravitreal bevacizumab for refractory diabetic macular edema. Int Ophthalmol. 2011;31(1):39-42. [DOI] [PubMed] [Google Scholar]
- 10.Sabet-Peyman EJ, Heussen FMA, Thorne JE, Casparis H, Patel SJ, Do DV. Progression of macular ischemia following intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2009;40(3):316-318. [DOI] [PubMed] [Google Scholar]
- 11.Shimura M, Yasuda K. Macular ischaemia after intravitreal bevacizumab injection in patients with central retinal vein occlusion and a history of diabetes and vascular disease. Br J Ophthalmol. 2010;94(3):381-383. [DOI] [PubMed] [Google Scholar]
- 12.Mansour AM, Bynoe LA, Welch JC, et al. Retinal vascular events after intravitreal bevacizumab. Acta Ophthalmol. 2010;88(7):730-735. [DOI] [PubMed] [Google Scholar]
- 13.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078-1086.e2. [DOI] [PubMed] [Google Scholar]
- 14.Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130(9):1145-1152. [DOI] [PubMed] [Google Scholar]
- 15.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247-2254. [DOI] [PubMed] [Google Scholar]
- 16.Gerendas B, Simader C, Sponer U, et al. Comparability of pixel size in images of different cameras of the same type used for multi-center trials at different study sites evaluated with a new test eye (SISPOT). Invest Ophthalmol Vis Sci. 2011;52(14):4049. [Google Scholar]
- 17.Early Treatment Diabetic Retinopathy Study Research Group Classification of diabetic retinopathy from fluorescein angiograms: ETDRS report number 11. Ophthalmology. 1991;98(5)(suppl):807-822. [PubMed] [Google Scholar]
- 18.Michaelides M, Fraser-Bell S, Hamilton R, et al. Macular perfusion determined by fundus fluorescein angiography at the 4-month time point in a prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (Bolt study): report 1. Retina. 2010;30(5):781-786. [DOI] [PubMed] [Google Scholar]
- 19.Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121(9):1783-1789. [DOI] [PubMed] [Google Scholar]
- 20.Chung EJ, Roh MI, Kwon OW, Koh HJ. Effects of macular ischemia on the outcome of intravitreal bevacizumab therapy for diabetic macular edema. Retina. 2008;28(7):957-963. [DOI] [PubMed] [Google Scholar]
- 21.Bonini-Filho M, Costa RA, Calucci D, Jorge R, Melo LA Jr, Scott IU. Intravitreal bevacizumab for diabetic macular edema associated with severe capillary loss: one-year results of a pilot study. Am J Ophthalmol. 2009;147(6):1022-1030, 1030.e1-1030.e5. [DOI] [PubMed] [Google Scholar]
- 22.Retinopathy D; Early Treatment Diabetic Retinopathy Study Research Group . Fluorescein angiographic risk factors for progression of diabetic retinopathy: ETDRS report number 13. Ophthalmology. 1991;98(5)(suppl):834-840. [PubMed] [Google Scholar]
- 23.Di G, Weihong Y, Xiao Z, et al. A morphological study of the foveal avascular zone in patients with diabetes mellitus using optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2016;254(5):873-879. [DOI] [PubMed] [Google Scholar]
- 24.Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(6):1051-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansour AM, Schachat A, Bodiford G, Haymond R. Foveal avascular zone in diabetes mellitus. Retina. 1993;13(2):125-128. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Lee CS, Chao J, et al. Wide-field optical coherence tomography based microangiography for retinal imaging. Scientific Reports. 2016;6:22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160(1):35-44.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1 Baseline characteristics of the participants of the RESTORE Extension Study
eTable 2 Change in FAZ outline over time
eTable 3 Patients with capillary loss graded moderate or greater pooled for the central and 4 inner ETDRS subfields
eFigure 1 Example of early phase fluorescence angiography image with the inner capillaries of the foveal avascular zone outlined manually and overlay of ETDRS grid centered on the foveae
eFigure 2 Change of visual acuity from baseline to month 36 by capillary loss in the central subfield at month 36