Key Points
Question
Does a treat-and-extend regimen of aflibercept or bevacizumab injections have outcomes similar to monthly injections in participants with macular edema associated with central retinal vein occlusion or hemiretinal vein occlusion who have exhibited a good response to 6 months of monthly injections?
Findings
In this secondary analysis of a randomized clinical trial, meaningful visual acuity differences at 12 months were not present in comparisons of participants who had received a treat-and-extend regimen vs patients who received monthly injections between months 6 and 12.
Meaning
Because of the wide confidence intervals on visual acuity differences between the monthly and the treat-and-extend groups, caution is warranted before concluding that the 2 regimens are associated with similar vision outcomes.
This secondary analysis of a randomized clinical trial compares visual acuity letter score and central subfield thickness outcomes in participants who received either monthly injections or a treat-and-extend regimen of aflibercept or bevacizumab.
Abstract
Importance
Comparisons of monthly vs treat-and-extend anti–vascular endothelial growth factor (anti-VEGF) regimens for macular edema from central retinal vein occlusion or hemiretinal vein occlusion is needed.
Objective
To compare visual acuity letter score and central subfield thickness outcomes of participants in the Study of Comparative Treatments for Retinal Vein Occlusion 2 (SCORE2) trial who then received either monthly injections or treat-and-extend (TAE) regimens of aflibercept or bevacizumab after a good response at month 6.
Design, Setting, and Participants
This randomized clinical trial enrolled participants from 66 private practice or academic centers in the United States. All participants had macular edema associated with central retinal vein occlusion or hemiretinal vein occlusion, had enrolled in the SCORE2 trial, and had a protocol-defined good response to monthly injections in the first 6 months of the trial. Participants initially assigned to receive monthly aflibercept were randomized to aflibercept on a monthly or TAE schedule, and participants initially assigned to receive monthly injections of bevacizumab were randomized to receive bevacizumab on a monthly or TAE schedule. The first participant was randomized in the SCORE2 trial on September 17, 2014, and the last month 12 visit occurred on October 24, 2016.
Main Outcomes and Measures
Change from month 6 to month 12 in best–corrected electronic visual acuity letter score (per the Early Treatment Diabetic Retinopathy Study).
Results
The 293 participants had a mean (SD) age of 68.9 (11.9) years; 127 (43.3%) were female. Of these, 79 were randomized to aflibercept on a monthly schedule, 80 to aflibercept on a TAE schedule, 67 to monthly bevacizumab, and 67 to bevacizumab on a TAE schedule. Mean treatment group difference (the change in visual acuity letter score in the monthly group minus the change in the TAE group) from month 6 to month 12 was 1.88 (97.5% CI, −1.07 to 4.83; P = .15) for aflibercept and 1.98 (97.5% CI, −1.08 to 5.03; P = .15) for bevacizumab. In the aflibercept arm, the mean number of injections between months 6 and 11 was 5.8 in the monthly injection group (95% CI, 5.6 to 5.9) and 3.8 in the TAE group (95% CI, 3.5 to 4.1; P < .001); in the bevacizumab arm, the mean number of injections was 5.8 (95% CI, 5.6 to 5.9) in the monthly group and 4.5 in the TAE group (95% CI, 4.2 to 4.8; P < .001).
Conclusions and Relevance
One to 2 fewer injections of aflibercept or bevacizumab were given to the TAE groups than the monthly groups in months 6 to 12 for macular edema associated with central retinal or hemiretinal vein occlusion. Because of wide confidence intervals on the differences between the groups, caution is warranted before concluding that the regimens are associated with similar vision outcomes.
Trial Registration
www.clinicaltrials.gov identifier: NCT01969708
Introduction
Several clinical trials have demonstrated efficacy of monthly intravitreal anti–vascular endothelial growth factor (anti-VEGF) therapy for the treatment of macular edema associated with central retinal vein occlusion (CRVO). The Study of Comparative Treatments for Retinal Vein Occlusion 2 (SCORE2) trial demonstrated that, among participants with macular edema associated with CRVO or hemiretinal vein occlusion (HRVO), monthly intravitreal bevacizumab was noninferior to monthly intravitreal aflibercept with respect to visual acuity after 6 months of treatment. We are not aware of prior studies comparing monthly vs treat-and-extend (TAE) injections of anti-VEGF therapy for macular edema associated with retinal vein occlusion after initial successful treatment with anti-VEGF therapy for 6 months. The purpose of the current study was to compare monthly dosing with TAE dosing of aflibercept or bevacizumab from months 6 to 12 with respect to visual acuity and central subfield thickness at month 12 in participants in the SCORE2 trial who had a protocol-defined good response at month 6.
Methods
The SCORE2 design and methods have been described in detail and are summarized here. The study adhered to the tenets of the Declaration of Helsinki and is registered at https://www.clinicaltrials.gov (identifier: NCT01969708). Institutional review board approval of the protocol was obtained at each of the 66 centers via either a site-specific or a centralized institutional review board, and written informed consent was obtained from all participants before eligibility screening and again before randomization into the study.
The study drug aflibercept (Eylea) was provided by Regeneron Pharmaceuticals and labeled for study use in single-use vials designed to deliver a 2.0-mg dose. Bevacizumab (Avastin) was purchased and repackaged by the University of Pennsylvania Investigational Drug Service into smaller, single-use vials labeled for the study and designed to deliver a 1.25-mg dose.
Major eligibility criteria for SCORE2 included a best-corrected electronic Early Treatment Diabetic Retinopathy Study (e-ETDRS) visual acuity letter score (VALS) between 19 and 73, a diagnosis on clinical examination of center-involved macular edema as a result of CRVO or HRVO, and central retinal thickness on spectral-domain optical coherence tomography (SD-OCT), defined as central subfield thickness (CST) of 300 μm or greater if measured with a Carl Zeiss Meditec Cirrus OCT machine or 320 μm or greater if measured with a Heidelberg Spectralis OCT machine.
The study enrolled 362 participants at 66 private practice or academic centers within the United States between September 17, 2014, and November 18, 2015. All were randomly assigned to receive injections of aflibercept (n = 180) or bevacizumab (n = 182). A total of 159 of 174 participants (91.4%) in the aflibercept arm and 134 of 173 participants (77.5%) in the bevacizumab arm had a protocol-defined good response at month 6 (χ21 = 12.8; P < .001) and were randomly assigned to continue treatment with the originally assigned drug on either a monthly schedule or a TAE schedule (eFigure 1 in the Supplement). The remaining participants, who had a protocol-defined poor response at month 6, were switched to an alternative treatment and are excluded from this analysis.
A good response was defined as either (1) a VALS of 58 or more (20/80 or better) and a VALS improvement of 5 or more letters from baseline; or (2) CST of less than 300 μm as measured by SD-OCT (or a CST of less than 320 μm on Heidelberg Spectralis) and neither intraretinal nor subretinal fluid on investigator assessment. Participants assigned to monthly injections were scheduled to receive 6 monthly injections of the originally assigned drug (aflibercept or bevacizumab) from month 6 to month 11. Participants assigned to either the aflibercept-TAE group or the bevacizumab-TAE group in months 6 through 11 were scheduled to receive an injection of the originally assigned drug at month 6. Then, those participants whose eyes did not have persistent retinal thickness at month 6 (defined as CST of greater than 300 μm as measured by SD-OCT or 320 μm as measured with Heidelberg Spectralis), intraretinal cystoid spaces on OCT, or subretinal fluid on OCT (on investigator assessment) were scheduled for a subsequent visit interval 2 weeks longer than the prior interval. For example, if a participant who was evaluated at a 6-week interval was without persistent retinal thickness, intraretinal cystoid spaces, or subretinal fluid, then treatment was provided and the next visit was scheduled to occur 8 weeks later. Visit intervals could be extended out to a maximum of 10 weeks through month 12. In contrast, participants whose eyes had any persistent retinal thickness, intraretinal cystoid spaces, or subretinal fluid on OCT were treated and then scheduled to return in 4 weeks. At each study visit, participants had visual acuity in both eyes assessed by the e-ETDRS method, intraocular pressure measurement, slit-lamp and dilated ophthalmoscopic examinations, and SD-OCT imaging.
Study participants were masked to the primary treatment assignment (ie, aflibercept or bevacizumab) through month 12. At month 6 and at month 12, visual acuity examiners, SD-OCT technicians, and reading center personnel were masked to primary treatment assignments. Masking the participant and clinical site staff to the dosing schedule (ie, monthly vs TAE) in the secondary randomization arms from month 6 to month 12 was not possible because of the varying visit schedules.
Statistical Analyses
The primary outcome was the difference in best-corrected VALS from the month 6 visit and the month 12 visit. Secondary outcomes included the mean change from month 6 to month 12 in CST, changes in VALS and CST from month 6 to all other post–month 6 visits, mean VALS and CST outcomes at the month 12 visit, and the percentage of participants whose eyes had resolution of macular edema at visits between month 7 and 12 (defined as CST of less than 300 μm, no subretinal or intraretinal fluid, and no cystoid spaces within the Early Treatment Diabetic Retinopathy Study grid on reading center evaluation). Statistical comparisons between the monthly and TAE arms were based on a contrast at month 12 from a normal repeated-measures regression (or, for resolution of macular edema, a logistic repeated-measures regression) that took into account secondary treatment assignment (ie, monthly or TAE groups), all visits the participant attended from month 7 to month 12, and the interaction between assignment and visit number. Furthermore, these models also provided statistical tests and P values for the unweighted mean across 6 months between months 7 and 12 of the estimated treatment effect at each month, comparing the monthly schedule with the TAE schedule.
To construct the visit number covariate, which was categorical in the models, a TAE visit was mapped to the integer month that was nearest to the month from randomization, but constrained to be between month 7 and month 11. Non-TAE visit covariates were taken to be the actual visit number. Temporal autocorrelations were modeled to decline exponentially with the temporal distance between the irregularly spaced actual visit dates. Any participant who attended a month 12 visit was considered to have completed the study.
The primary outcome involved 2 statistical tests of each monthly regimen and each TAE regimen, 1 for the aflibercept arm and 1 for the bevacizumab arm. We controlled for multiple testing for these 2 statistical tests by comparing the P value with .025 (ie, .05/2) and presenting 97.5% confidence intervals. For all secondary outcomes, we performed 25 statistical tests and therefore considered any P value less than .002 (.05/25) to be statistically significant. Confidence intervals for secondary outcomes were uncorrected for multiple testing and are intended only to describe the uncertainty in the estimates. Statistical analyses were conducted with SAS, versions 9.3 and 9.4 (SAS Institute). Analyses included data available as of July 10, 2017.
Results
At month 6, 79 participants were randomly assigned to the monthly group and 80 participants to the TAE group of the aflibercept arm, and 67 participants were randomly assigned to each of the monthly and TAE groups of the bevacizumab arm. The treatment groups were well balanced with respect to participant and ocular characteristics measured at the initial randomization (eTable 1 in the Supplement). The mean number of anti-VEGF treatments between randomization and month 6 was similar across the groups (eTable 1 in the Supplement). At month 6, before randomization to the monthly vs TAE groups, the mean (SD) VALS was 72.0 (16.3), with an approximate Snellen of 20/40, in the aflibercept arm, and the mean (SD) VALS was 74.1 (12.6), with an approximate Snellen of 20/32, in the bevacizumab arm (Table). Further, the mean (SD) CST prior to secondary randomization at month 6 was approximately 224 (45) μm in the aflibercept arm and 256 (75) μm in the bevacizumab arm (the Table presents treatment-specific means and standard deviations). Completion of the month 12 visits was between 95% and 99% across all participants in the monthly and TAE groups in the aflibercept and bevacizumab arms (eFigure 1 in the Supplement).
Table. Visual Acuity and Central Subfield Thickness Outcomes.
| Outcome | Aflibercept Study Arm | Bevacizumab Study Arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monthly Group | Treat-and-Extend Group | Estimate of Difference Between Groups (CI) | P Value | Monthly Group | Treat-and-Extend Group | Estimate of Difference Between Groups (CI) | P Value | |||||
| No. (%) | Mean (SD) | No. (%) | Mean (SD) | No. (%) | Mean (SD) | No. (%) | Mean (SD) | |||||
| Visual acuity letter score | ||||||||||||
| 6 mo | 79 (100) |
72.1 (15.7) |
80 (100) |
72.0 (17.0) |
67 (100) |
73.8 (12.3) |
67 (100) |
74.4 (13.0) |
||||
| 12 mo | 78 (99) |
72.7 (17.3) |
76 (95) |
71.6 (16.4) |
1.85 (−3.42 to 7.12)a |
.49 | 66 (99) |
75.2 (13.1) |
65 (97) |
74.0 (14.0) |
1.27 (−3.31 to 5.85)a |
.59 |
| Change from month 6 to 12b | 78 (99) |
0.8 (10.5) |
76 (95) |
−1.2 (9.2) |
1.88 (−1.07 to 4.86)c |
.15 | 66 (99) |
1.6 (8.9) |
65 (97) |
-0.4 (9.8) |
1.98 (−1.08 to 5.03)c |
.15 |
| Central subfield thickness (μm)d | ||||||||||||
| 6 mo | 77 (97) |
223 (43) |
74 (93) |
225 (48) |
66 (99) |
261 (79) |
67 (100) |
251 (71) |
||||
| 12 mo | 72 (91) |
237 (108) |
72 (90) |
238 (84) |
−3.52 (−28.25 to 21.21)a |
.78 | 64 (96) |
245 (64) |
64 (96) |
268 (130) |
−25.77 (−60.61 to 9.07)a |
.15 |
| Change from month 6 to 12 | 71 (90) |
12.5 (105.8) |
68 (85) |
15.1 (85.1) |
−5.2 (−26.87 to 16.47)a |
.64 | 64 (96) |
−16.2 (74.5) |
64 (96) |
17.1 (109.8) |
−35.01 (−63.95 to −6.07)a |
.02e |
| Resolution of macular edema | No./Total No. (%) | No./ Total No. (%) |
Odds Ratio (95% CI) |
No./ Total No. (%) | No./ Total No. (%) |
Odds Ratio (95% CI) |
||||||
| 6 mo | 50/78 (64) |
42/78 (54) |
23/67 (34) |
26/67 (39) |
||||||||
| 12 mo | 38/73 (52) |
28/74 (38) |
1.18 (0.61 to 2.26) |
.62 | 23/66 (35) |
26/64 (41) |
0.71 (0.35 to 1.42) |
.33 | ||||
95% CI.
The primary outcome was the mean difference in treatment group outcomes (monthly minus TAE) from month 6 to 12.
97.5% CI.
Central subfield thickness scores were obtained via spectral-domain optical coherence tomography.
Not significant after correction for multiple testing.
During the period between month 6 through month 12, more anti-VEGF injections were administered to study participants in the monthly group compared with the TAE group for both the aflibercept arm (monthly mean, 5.8; 95% CI, 5.6 to 5.9; vs TAE mean, 3.8; 95% CI, 3.5 to 4.1; P < .001) and the bevacizumab arm (monthly mean, 5.8; 95% CI, 5.6 to 5.9; vs TAE mean, 4.5; 95% CI, 4.2 to 4.8; P < .001) (eTable 2 in the Supplement). The mean number of days between successive injections for participants with 2 or more injections was approximately 17 days longer in the aflibercept–TAE group (mean, 46.8; 95% CI, 42.6 to 51.0) than in the aflibercept monthly group (mean, 29.6; 95% CI, 29.0 to 30.5; P < .001) and 10 days longer in the bevacizumab–TAE group (mean, 39.6; 95% CI, 37.5 to 41.7) than in the bevacizumab monthly group (mean, 29.4; 95% CI, 28.1 to 30.1; P < .001; eTable 2 in the Supplement). Extending the TAE schedules throughout the month 6 to month 12 period occurred in 45 participants receiving aflibercept (56%) and 21 participants receiving bevacizumab (31%), although the difference was not significant after correction for multiple testing. Compliance with the TAE and monthly injection schedules was high, with more than 90% of all injections occurring within 7 days of the target injection date for participants receiving aflibercept or bevacizumab (eTable 2 in the Supplement).
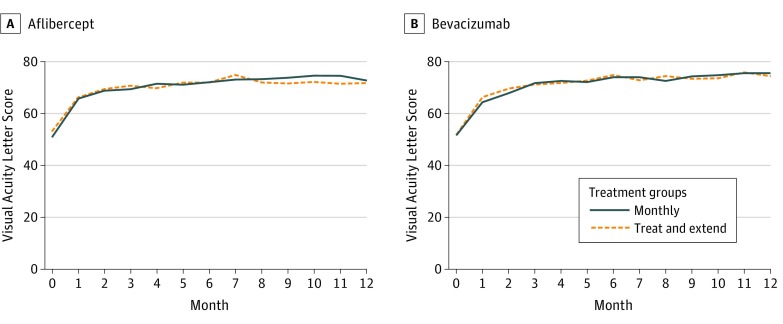
At month 12 for the aflibercept arm, the mean VALS was 72.7 in the monthly group and 71.6 for the TAE group (Figure 1), with a small improvement in VALS of 0.8 from month 6 to month 12 in the monthly group and a small decrease of 1.2 in the TAE group (eTable 3 in the Supplement). The estimated difference between treatment groups (monthly minus TAE) in the mean change in VALS from month 6 to month 12 was 1.88 (97.5% CI, −1.07 to 4.83; P = .15). For the bevacizumab arm, the mean VALS at month 12 was 75.2 in the monthly group and 74.0 for the TAE group, which represented a mean VALS decrease of 1.6 from month 6 to month 12 in the monthly group and a mean VALS decrease of 0.4 in the TAE group (eTable 3 in the Supplement). The estimated difference between treatment groups (monthly minus TAE) in the mean change in VALS from month 6 to month 12 was 1.98 (97.5% CI, −1.08 to 5.03; P = .15) (Table). The trends in VALS observed at month 12 in the monthly and TAE groups for both the aflibercept and bevacizumab arms was consistent with other visits before month 12 (eTable 3 in the Supplement, Figure 1 and Figure 2).
Figure 1. Mean Visual Acuity Letter Score Over Time.
A, Within the aflibercept group, the mean difference in the monthly minus treat-and-extend treatment group over months 7 through 12 was 2.67 (95% CI, −2.17 to 7.52; P = .28). B, Within the bevacizumab group, the mean difference in the monthly minus treat-and-extend treatment groups over months 7 to 12 was 0.65 (95% CI, −3.46 to 4.77; P = .75). Both images show means before and after the randomization at 6 months.
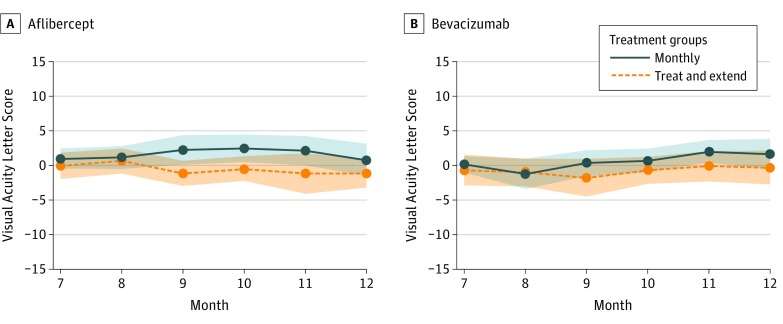
Figure 2. Visual Acuity Letter Score Mean Change in Months 7 Through 12.
A and B both depict means from months 7 through 12. Each mean line is surrounded by a shaded area depicting pointwise 95% CIs about the means. The overlap between the shaded areas roughly depicts times when the means do not statistically differ.
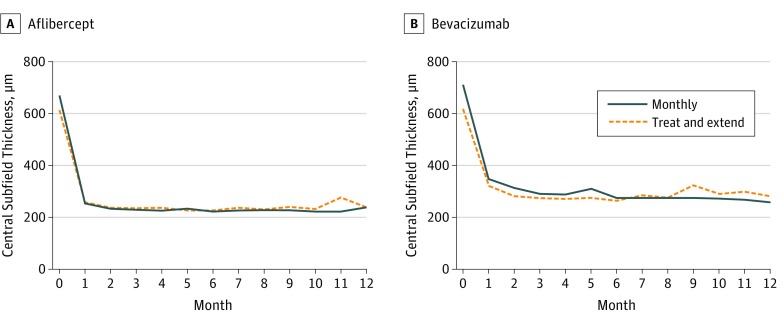
In the aflibercept arm, the mean CST measured by SD-OCT at month 12 was 237 μm in the monthly group and 238 μm in the TAE group (Figure 3). Both the monthly and TAE groups for the aflibercept arm had small increases (worsening) in CST as measured by SD–OCT from month 6 to month 12 (12.5 μm and 15.1 μm, respectively) (Figure 4; eTable 4 in the Supplement). The estimated difference between treatment groups (monthly minus TAE) in mean CST change from month 6 to month 12 was −5.2 μm (95% CI, −26.7 to 16.5; P = .64). Further, 38 participants in the monthly group (52%) and 28 in the TAE group (38%) had resolution of macular edema at month 12, although the odds of resolution of macular edema, averaged over months 7 to 12, was 2.32 times higher in the monthly group compared with the TAE group, which is statistically significant after correction for multiple testing (95% CI, 1.38 to 3.90; P = .002; eFigure 2 in the Supplement).
Figure 3. Mean Central Subfield Thickness.
A, Within the aflibercept group, the mean difference between monthly minus treat-and-extend treatment groups over months 7 through 12 was −12.29 (95% CI, −28.28 to 3.69; P = .13). B, Within the bevacizumab group, the mean difference between monthly minus treat-and-extend treatment effect over months 7 through 12 was −14.77 (95% CI, −40.33 to 10.79; P = .26). Both images show means before and after secondary randomization at 6 months.
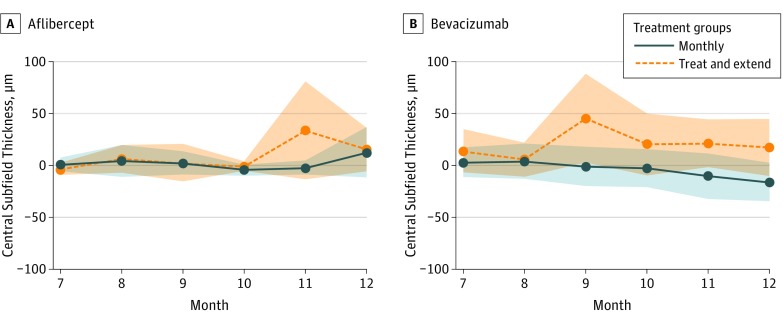
Figure 4. Central Subfield Thickness Mean Change in Months 7 Through 12.
A and B both depict means from months 7 through 12 only. Each mean line is surrounded by a shaded area depicting pointwise 95% CIs about the means. The overlap between the shaded areas roughly depicts times when the means do not statistically differ.
For the participants receiving bevacizumab, the mean CST measured by SD-OCT at month 12 was 245 μm in the monthly group and 268 μm in the TAE group (Figure 3). The monthly group in the bevacizumab arm had a small decrease (improvement) in CST as measured by SD-OCT from month 6 to month 12, of 16.2 μm; the TAE group of the bevacizumab arm experienced a small increase (worsening) in CST as measured by SD-OCT at month 12, of 17.1 μm (Figure 4; eTable 4 in the Supplement). The estimated difference in mean CST change by treatment groups (monthly minus TAE) from month 6 to month 12 was −35.0 μm, which was not significant after correction for multiple testing. The odds of resolution of macular edema did not significantly differ between the monthly and TAE arms (Table, eFigure 2 in the Supplement).
Between month 6 and month 12, ocular adverse events of interest in the study eye were rare (eTable 5 in the Supplement). One case of infectious endophthalmitis occurred in a participant in the monthly group taking aflibercept, and 1 case of culture-negative endophthalmitis occurred in a participant in the monthly group taking bevacizumab. Five or fewer participants across the 4 groups had intraocular pressure higher than 10 mm Hg over baseline. A nonfatal cerebrovascular accident occurred in 1 aflibercept-TAE participant (1.3%), and a non-fatal cerebrovascular accident and 2 deaths (caused by cardiorespiratory arrest and sepsis) occurred between months 6 and 12 in the aflibercept-TAE arm.
Discussion
In participants with macular edema secondary to CRVO or HRVO and a protocol-defined good response to 6 monthly injections of either aflibercept or bevacizumab, subsequent TAE dosing resulted in an average of 1 to 2 fewer injections over 6 months than monthly dosing. Meaningful visual acuity differences at 12 months were not present between TAE and monthly schedules. This is important as the TAE regimen is commonly used in clinical practice. However, because of the widths of the confidence intervals on the visual acuity differences between the monthly and TAE arms, caution is warranted before concluding that the 2 dosing regimens are associated with similar vision outcomes.
Similarly, no clinically meaningful differences were noted for CST outcomes between the monthly and TAE schedules in the aflibercept and bevacizumab arms, although the proportion of participants with resolution of their macular edema between months 7 to 12 was significantly higher with a monthly regimen compared with a TAE regimen in the aflibercept arm. This finding may be associated with the monthly aflibercept group having a mean of 2 additional injections in the period between month 6 and month 12 compared with the TAE aflibercept group.
While the mean number of injections administered from month 6 to month 12 was significantly lower in the TAE groups than in monthly injection groups in both the aflibercept and the bevacizumab arms (eTable 2 in the Supplement), the difference in the mean number of injections between TAE and monthly regimens is larger, and the proportion of visits extended was higher, in the aflibercept arm than in the bevacizumab arm. These findings are consistent with the better anatomic outcomes observed with aflibercept compared to bevacizumab reported previously.
Although this study was not powered to identify differences between the monthly and TAE groups in rare adverse events, no new safety concerns associated with anti-VEGF therapy were identified. Rates of adverse events in the current study are consistent with those observed in the first 6 months of SCORE2 and those reported in other phase 3 trials evaluating anti-VEGF therapy for retinal vein occlusion, neovascular age-related macular degeneration, and diabetic macular edema.
Strengths of the current study include a comparison, based on a secondary randomization at month 6, of a monthly schedule with a TAE regimen that is consistent with clinical practice. In addition, the study had limited loss to follow-up and excellent adherence to the protocol.
Limitations
Study limitations include the inability to make comparisons of VALS and CST outcomes across study drug groups, because more participants in the aflibercept group than those in the bevacizumab group were good responders at month 6, so aflibercept-bevacizumab comparisons would be based on a nonrandomized subset of the SCORE2 population. Another limitation is that the month 6 to 12 period may be too short to fully implement a TAE dosing schedule and thus too short to expect major differences between monthly and TAE groups. Longer follow-up may have found increased differences between the monthly and TAE groups. In addition, the study compared monthly dosing only with TAE dosing and not with other flexible dosing regimens, such as a pro re nata (as needed) schedule. Lastly, the sample sizes for the monthly with TAE comparisons were determined by the number of SCORE2 participants classified as good responders at month 6, which impacted precision and statistical power. Thus, there is lack of evidence to conclude that there is no difference between the monthly and TAE groups in the bevacizumab group, as the 97.5% confidence limits around the estimate of difference in VALS at month 12 included a clinically meaningful difference of 5 letters.
Conclusions
In sum, an average of 1 to 2 fewer injections were administered to the TAE compared with the monthly groups during the 6 months following administration of 6 monthly injections. Because of the widths of the confidence intervals on the visual acuity differences between the monthly and TAE dosing groups, caution is warranted before concluding that the 2 regimens are associated with similar vision outcomes at month 12.
eFigure 1. SCORE2 Participant Flow into Month 6 to 12 Secondary Randomization Phase.
eFigure 2. Percent of Participants with Resolution of Macular Edema by SD-OCT.
eTable 1. Characteristics in Good Responders at Month 0 or Prior to Month 6 Randomization
eTable 2. Description of Anti-VEGF Treatments between Months 6 and before Month 12 Visit
eTable 3. VALS Change from Month 6 through Month 12: Monthly vs TAE
eTable 4. SD-OCT Central Subfield Thickness Change from Month 6 through Month 12: Monthly vs TAE
eTable 5. Safety Outcomes Reports between Month 6 and Month 12: Monthly vs TAE
References
- 1.Brown DM, Campochiaro PA, Singh RP, et al. ; CRUISE Investigators . Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124-1133.e1. [DOI] [PubMed] [Google Scholar]
- 2.Boyer D, Heier J, Brown DM, et al. . Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119(5):1024-1032. [DOI] [PubMed] [Google Scholar]
- 3.Holz FG, Roider J, Ogura Y, et al. . VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol. 2013;97(3):278-284. [DOI] [PubMed] [Google Scholar]
- 4.Scott IU, VanVeldhuisen PC, Ip MS, et al. ; SCORE2 Investigator Group . Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: the SCORE2 randomized clinical trial. JAMA. 2017;317(20):2072-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott IU, VanVeldhuisen PC, Ip MS, et al. ; SCORE2 Investigator Group . SCORE2 Report 2: study design and baseline characteristics. Ophthalmology. 2017;124(2):245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 7.Beck RW, Moke PS, Turpin AH, et al. . A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194-205. [DOI] [PubMed] [Google Scholar]
- 8.Kitchens JW, Do DV, Boyer DS, et al. . Comprehensive review of ocular and systemic safety events with intravitreal aflibercept injection in randomized controlled trials. Ophthalmology. 2016;123(7):1511-1520. [DOI] [PubMed] [Google Scholar]
- 9.Heier JS, Clark WL, Boyer DS, et al. . Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology. 2014;121(7):1414-1420.e1. [DOI] [PubMed] [Google Scholar]
- 10.Campochiaro PA, Brown DM, Awh CC, et al. . Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118(10):2041-2049. [DOI] [PubMed] [Google Scholar]
- 11.Brown DM, Campochiaro PA, Bhisitkul RB, et al. . Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118(8):1594-1602. [DOI] [PubMed] [Google Scholar]
- 12.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ; CATT Research Group . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. SCORE2 Participant Flow into Month 6 to 12 Secondary Randomization Phase.
eFigure 2. Percent of Participants with Resolution of Macular Edema by SD-OCT.
eTable 1. Characteristics in Good Responders at Month 0 or Prior to Month 6 Randomization
eTable 2. Description of Anti-VEGF Treatments between Months 6 and before Month 12 Visit
eTable 3. VALS Change from Month 6 through Month 12: Monthly vs TAE
eTable 4. SD-OCT Central Subfield Thickness Change from Month 6 through Month 12: Monthly vs TAE
eTable 5. Safety Outcomes Reports between Month 6 and Month 12: Monthly vs TAE