Key Points
Question
What were the outcomes in children with common forms of retinitis pigmentosa following supplementation with vitamin A palmitate?
Findings
In this case-control study of children with retinitis pigmentosa, including 55 taking an age-adjusted dose of vitamin A (5000-15 000 IU/d) and 25 not taking vitamin A, vitamin A supplementation was associated with almost 50% slowing of the mean exponential rate of decline of full-field cone electroretinogram amplitude.
Meaning
These findings, while not definitive in the absence of randomized controls, support the hypothesis that vitamin A palmitate can slow loss of cone function in children with common forms of retinitis pigmentosa.
Abstract
Importance
While oral vitamin A supplementation is considered to potentially slow loss of retinal function in adults with retinitis pigmentosa and normal liver function, little data from children with this disease are available.
Objective
To compare disease courses in children with retinitis pigmentosa taking or not taking vitamin A supplementation.
Design, Setting, and Participants
Retrospective, nonrandomized comparison of vitamin A and control cohorts followed up for a mean of 4 to 5 years by the Electroretinography Service of the Massachusetts Eye and Ear Infirmary. The study included children with different genetic types of typical retinitis pigmentosa: 55 taking vitamin A and 25 not taking vitamin A. The dates for patient evaluations ranged from June 1976 to July 2016, and the data analysis occurred in October 2016.
Interventions
Age-adjusted dose of oral vitamin A palmitate (≤15 000 IU/d).
Main Outcomes and Measures
Mean exponential rates of change of full-field cone electroretinogram amplitude to 30-Hz flashes estimated by repeated-measures longitudinal regression without and with adjusting for potential confounders.
Results
Of the 55 children in the vitamin A cohort, 38 (69%) were male; the mean [SD] age was 9.1 [1.9] years; and 48 (87%) were white , 6 (11%) were Asian, and 1 (2%) was black. Of the 25 members of the control cohort, 19 (76%) were male; the mean [SD] age was 9.2 [1.7] years; and 25 (100%) were white. The estimated mean rates of change with the unadjusted model were −0.0713 loge unit/y (−6.9% per year) for the vitamin A cohort and −0.1419 loge unit per year (−13.2% per year) for the control cohort (difference, 0.0706 loge unit per year; 95% CI for the difference, 0.0149-0.1263 loge unit per year; P = .01). The adjusted model confirmed a slower mean rate of decline in the vitamin A cohort (difference, 0.0771 loge-unit per year; 95% CI for the difference, 0.0191-0.1350 loge-unit per year; P = .009). With respect to ocular safety, the mean exponential rates of change of visual field area and visual acuity and the incidences of falling to a visual field diameter of 20° or less or a visual acuity of 20/200 or less in at least 1 eye did not differ by cohort.
Conclusions and Relevance
A vitamin A palmitate supplement was associated with a slower loss of cone electroretinogram amplitude in children with retinitis pigmentosa. Although the relatively small-sample, retrospective, nonrandomized design does not allow a test of causation and is subject to possible biases, these findings support consideration of an age-adjusted dose of vitamin A in the management of most children with the common forms of retinitis pigmentosa.
This case-control study compares disease courses in children with retinitis pigmentosa taking or not taking vitamin A supplementation.
Introduction
In 1993, we reported the results of a randomized clinical trial for 601 adults (aged 18-49 years) with the common forms of retinitis pigmentosa treated with vitamin A and/or vitamin E for 4 to 6 years.1 Oral vitamin A palmitate, 15 000 IU/d, on average, slowed the course of retinal degeneration, while oral vitamin E (dl-α tocopherol acetate), 400 IU/d, on average, appeared to hasten the course of retinal degeneration as monitored by the full-field cone electroretinogram (ERG) to 30-Hz flashes. With the full-field ERG to 0.5-Hz flashes, a significant benefit was found for vitamin A, but no significant detriment was found for vitamin E.1 Although 1 member of an independent Data and Safety Monitoring Committee questioned the 2 × 2 factorial design used in the study2 and 2 members questioned whether the sixth year of follow-up based on a subset should have been included in the analyses,2,3 the Data and Safety Monitoring Committee as a body concluded that most adults with retinitis pigmentosa and normal liver function should take vitamin A palmitate and avoid a high dose of vitamin E. Women who were pregnant or planning to become pregnant were advised not to take this dose of vitamin A because of an increased risk of having children with birth defects. No significant toxic adverse effect was noted during that clinical trial, and none was reported by the study patients taking this dose of vitamin A for up to 12 years.4 The National Eye Institute sent an information alert to thousands of ophthalmologists across North America endorsing this recommendation, followed by a confirmatory press release in 2008.5 In addition, a diet rich in vitamin A palmitate was found to reduce the rate of ERG loss and result in longer, more numerous photoreceptors in 1 of 2 murine models of dominantly inherited retinitis pigmentosa.6
Because patients younger than 18 years with retinitis pigmentosa had not been included in the clinical trial, no formal recommendation was possible for them. However, after publication of the clinical trial, families asked us if their affected children could take vitamin A. We consulted with pediatricians, who suggested an oral dose of 5000 IU/d for ages 6 to 10 years, 10 000 IU/d for ages 10 to 15 years, and 15 000 IU/d for ages 15 years and older, provided that the children had normal serum liver function test results. Parents were advised that their children taking vitamin A should eat a regular diet, avoid a high-dose vitamin E supplement, monitor their serum liver function annually, and return to our clinic for follow-up assessment and possible dose adjustment every 2 years.
Of the families offered treatment for their affected children, a minority did not pursue treatment on advice of their pediatrician. Nevertheless, they also were encouraged to have their children return for reassessment every 2 years. This resulted in 1 group of children who took vitamin A consistently during some interval (vitamin A cohort) and another group not receiving vitamin A, including children who were ascertained before publication of the clinical trial or who opted not to take vitamin A after publication (control cohort). This study addresses whether the mean disease course monitored by the ERG in the vitamin A cohort differed from that in the control cohort.
Methods
Outcome Measures
The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review boards of the Massachusetts Eye and Ear Infirmary and Harvard Medical School. Patients and families seen in the Electroretinography Service were routinely advised that their deidentified clinical records might be used for future research and gave oral consent. The dates for patient evaluations ranged from June 1976 to July 2016, and the data analysis occurred in October 2016. We used the annual rate of change in cone ERG amplitude to 30-Hz full-field white flashes (0.2 cd s/m2) to assess vitamin A treatment efficacy. This measure had afforded us the most power to show a vitamin A benefit in the adult clinical trial, and cone ERG amplitude was significantly correlated with vision-related quality of life in those patients.1 Responses to 30-Hz flashes have been routinely recorded by us with band-pass filtering and signal averaging if amplitudes are less than 10 μV, allowing quantification of amplitudes of at least .05 μV.7,8,9 A review of our database identified 80 children from 72 families with typical forms of retinitis pigmentosa based on clinical examination who had a cone ERG amplitude to 30-Hz flashes of at least 0.68 μV in at least 1 eye at their baseline visit and cone ERGs recorded on at least 1 succeeding visit spanning at least 3 years, our eligibility criteria for inclusion in a longitudinal study to compare mean rates of cone ERG amplitude decline.7,8,9 We had also routinely recorded responses to 0.5-Hz flashes of the same white light with signal averaging if amplitudes were less than 10 μV, but this allowed quantification only of amplitudes of at least 1 μV, a lower sensitivity than that for the cone ERG to 30-Hz flashes. Because review of our database revealed that only 31 of 80 children (38.8%) could be followed up over time by the ERG to 0.5-Hz flashes, we did not use this response as an outcome measure owing to reduced statistical power and a concern about sampling error.
We also considered comparing decline of visual field area contiguous to the center by cohort because we had reported a vitamin A treatment benefit by that measure based on kinetic perimetry in the adult clinical trial10; however, that analysis had been limited to patients with the most reproducible visual field areas on repeated pretreatment testing within 6 weeks,10 a criterion that was not available to us in this study. Furthermore, preliminary analyses on affected children with retinitis pigmentosa failed to reveal, on average, a significant decline in total visual field area or in visual field area contiguous to the center during follow-up, invalidating its use by us as a measure of disease progression. Decline in visual acuity was ruled out as an outcome measure a priori because it had not shown a vitamin A benefit in adults.1 Nevertheless, we analyzed visual field area and visual acuity longitudinal data from these cohorts for ocular evidence of supplement safety.
Patients
The vitamin A cohort included 43 children who began supplementation after their baseline ERGs but preceding their first follow-up ERGs and 12 children who began supplementation preceding their baseline ERGs, totaling 55 children. We found that 5000 IU/d was taken by children aged 7 to 15 years, 7500 IU/d was taken by a child aged 14 years, 10 000 IU/d was taken by children aged 6 to 15 years, and 15 000 IU/d was taken by children aged 12 to 15 years. Despite cases where the age-adjusted recommendation was exceeded, no families with children taking vitamin A reported an adverse response to the supplement. The control cohort included 25 children who did not report vitamin A supplementation.
Statistical Analyses
Cone ERG amplitudes were converted to natural logarithms to better normalize their distributions and to fit linear models to time because cone ERG amplitude was found to decline exponentially in patients with retinitis pigmentosa7,11 and a logarithmic transform has been used to quantify full-field cone ERG decline in retinitis pigmentosa by our laboratory1,7,8,9,11,12,13,14 and another.15,16 The MIXED procedure of SAS, version 9.4 (SAS Institute Inc) was then used to perform repeated-measures longitudinal regression with the eye as the unit of analysis and an unstructured@compound symmetry correlation structure, which allows for correlation over time in the same eye, correlation between eyes at 1 time, and correlation between 1 eye at 1 time and the fellow eye at a different time. This procedure permitted us to use eligible data from all available eyes and times.
Model 1 fitted the following equation to the data:
| loge amplitude = b0 + b1(cohort) + b2(time) + b3(time)(cohort) |
where b0 was the y-intercept for cohort = 0 (ie, control cohort), b1 was the change in intercept for cohort = 1 (ie, vitamin A cohort), b2 was the coefficient for the effect of time on amplitude (ie, the slope) for cohort = 0, and b3 was the coefficient for the change in the effect of time on amplitude for cohort = 1. Thus, for the vitamin A cohort the intercept equaled b0 + b1 and the effect of time on amplitude equaled b2 + b3.
Model 2 additionally adjusted for eye and its cross-product with time, the cross-product of loge baseline amplitude with time, baseline cone ERG implicit time and its cross-product with time, and genetic type (dominant, recessive, X-linked, or unknown) and its cross-product with time to try to reduce the unexplained variance and to control for possible confounders. Model 2 included eye because the right eye was always tested before the left eye; it included the cross-product of loge baseline amplitude with time because a preliminary analysis revealed that loge baseline amplitude was a strong predictor of rate of progression in the children; it included the cross-product of baseline cone ERG implicit time with time because baseline cone ERG implicit time has been reported to be a biomarker for the rate of cone ERG amplitude decline in patients with RHO mutations17; it included the cross-product of genetic type with time because the rate of cone ERG amplitude decline in patients with retinitis pigmentosa can vary by genetic type.9 Because only 14 of 80 patients with eligible cone ERG data had a known genotype, we did not consider replacing genetic type with genotype.
For ocular safety analyses, loge visual field area or loge visual acuity was regressed on time, vitamin A, and the cross-product of time with vitamin A with Model 1 and by also including eye, genetic type, and their cross-products with time for adjusted results with Model 3. We also compared the incidences of falling to a visual field diameter of 20° or less or to a visual acuity of 20/200 or less in at least 1 eye at follow-up by cohort.
In all of these models, continuous predictor variables (ie, time, loge baseline cone ERG amplitude, and baseline cone ERG implicit time) were mean-centered. JMP Pro, version 13 (SAS Institute), was used for analyses not involving a repeated variable including t test for continuous variables and either the χ2 test or Fisher Exact test (when expected cell frequencies were <5) for proportions. Statistical significance was considered to be a 2-sided P less than .05.
Results
Table 1 lists the baseline demographic and cone ERG characteristics of the children with retinitis pigmentosa. The cohorts were comparable except for borderline differences in their distributions by genetic type and in their mean cone ERG implicit (peak) times. Length of follow-up and number of follow-up visits (mean [SE]) were 5.0 (0.2) years and 3.7 (0.2) visits for the vitamin A cohort and 4.5 (0.4) years and 3.4 (0.2) visits for the control cohort; these pairs of means were not different. From these means, the mean interval between follow-up visits was 1.35 years for the vitamin A cohort and 1.32 years for the control cohort. On the other hand, the 2 cohorts differed with respect to the timing of their first follow-up examination: 37 (67%) of those in the vitamin A cohort compared with only 10 (40%) in the control cohort, returned within the recommended 2 years.
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Control Cohort | Vitamin A Cohort | |
| No. | 25 | 55 |
| Male sex | 19 (76) | 38 (69) |
| Ethnicity | ||
| Hispanic | 0 | 2 (4) |
| Nonhispanic | 25 (100) | 53 (96) |
| Race | ||
| Asian | 0 | 6 (11) |
| Black | 0 | 1 (2) |
| White | 25 (100) | 48 (87) |
| Genetic type | ||
| Dominant | 6 (24) | 10 (18) |
| Recessivea | 9 (36) | 22 (40) |
| X-linked | 9 (36) | 9 (16) |
| Unknown | 1 (4) | 14 (25) |
| Age, mean (SE), y | 9.2 (0.3) | 9.1 (0.3) |
| Cone ERG amplitude, mean (SE), Loge, μVb | 1.98 (0.25) | 1.87 (0.15) |
| Cone ERG amplitude, geometric mean | 7.2 | 6.5 |
| Cone ERG implicit time, mean (SE), msb | 39.9 (0.9) | 42.0 (0.6) |
Abbreviation: ERG, electroretinogram.
Includes isolate.
Mean of right eye and left eye (or single eye if right eye or left eye unavailable).
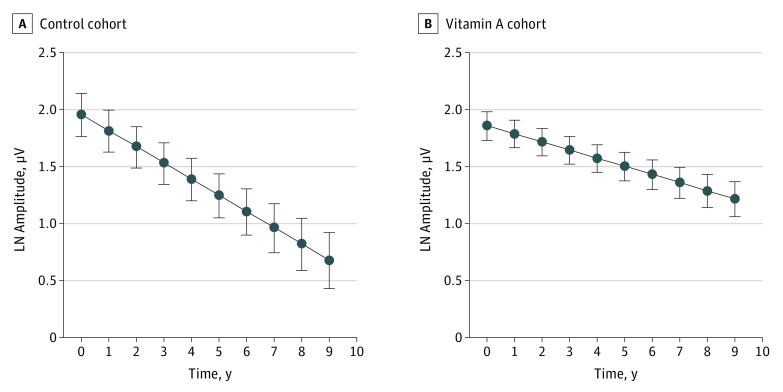
The estimated mean rates of change with Model 1 were −0.1419 loge-unit per year (−13.2% per year) for the control cohort (Table 2, b2) and −0.0713 loge-unit per year (−6.9% per year) for the vitamin A cohort (b2 + b3); the difference (b3) was 0.0706 loge-unit per year (95% CI, 0.0149-0.1263 loge-unit per year; P = .01). Plots from the Figure predicted loge amplitude (SE) by cohort and actual (not mean-centered) year. The fitted line for the vitamin A cohort has only a slightly lower y-intercept (amplitude at year 0) but a markedly shallower slope than the fitted line for the control cohort.
Table 2. Repeated-Measures Longitudinal Regression of Loge Cone ERG Amplitude to 30-Hz Flashes on Model 1 Variablesa.
| Coefficient | Estimate (SE) | 95% CI | Degrees of Freedom | t Value | Probability >|t| |
|---|---|---|---|---|---|
| b0b | 1.5874 (0.1816) | 1.2258 to 1.9490 | 78 | 8.74 | <.001 |
| b1c | 0.0850 (0.2176) | −0.3483 to 0.5183 | 78 | 0.39 | .70 |
| b2d | −0.1419 (0.0244) | −0.1898 to −0.0940 | 393 | −5.82 | <.001 |
| b3e | 0.0706 (0.0283) | 0.0149 to 0.1263 | 393 | 2.49 | .013 |
Abbreviation: ERG, electroretinogram.
Based on 80 patients and 475 observations analyzed by the MIXED procedure of SAS.
b0 indicates intercept for the control cohort at mean-centered time.
b1 indicates change in intercept at mean-centered time for the vitamin A cohort.
b2 indicates annual change in loge amplitude for the control cohort.
b3 indicates change in annual change in loge amplitude for the vitamin A cohort.
Figure. Plot of Predicted Loge Cone Electroretinogram (ERG) Amplitude .
Plot of predicted loge cone ERG amplitude (mean [SE]) over 9 years of follow-up by cohort based on Model 1 estimates using the MIXED procedure of SAS and the outpred option. Estimates derive from data of 25 children (control cohort) and 55 children (vitamin A cohort). Note that the abscissa plots actual (not mean-centered) time; therefore, the plotted intercepts differ from the intercepts listed in Table 2. LN indicates natural logarithm.
The effect of vitamin A supplementation on the rate of amplitude decline with Model 2 (eTable 1 in the Supplement; b3, 0.0771; b3, 95% CI, 0.0191-0.1350; P = .009) was comparable with that with Model 1. In addition, Model 2 showed effects of loge baseline amplitude and baseline cone ERG implicit time on time, primarily responsible for reducing the unexplained variance of Model 1 by 20%. A 1 loge-unit (2.7-fold) higher baseline amplitude was associated with a 0.0370 loge-unit per year (3.6% per year) faster mean amplitude decline (P < .001). A 1-ms longer baseline implicit time was associated with a 0.0112 loge-unit per year (1.1% per year) faster mean amplitude decline (P < .001). In contrast, the rate of amplitude decline was not associated with eye or genetic type.
With respect to supplement ocular safety, mean visual field area increased by means of 0.0037 loge-unit per year (0.37% per year) in the control cohort and 0.0241 loge-unit per year (2.4% per year) in the vitamin A cohort with an unadjusted analysis (Table 3); the difference was not statistically significant.The adjusted analysis also found comparable mean rates (eTable 2 in the Supplement). Incidences of falling to a visual field diameter of 20° or less in at least 1 eye during follow-up were 0% (0 of 24 patients) in the control cohort and 4% (2 of 50 patients) in the vitamin A cohort; the difference in percentages (4%) was not statistically significant (P = .45).
Table 3. Repeated-Measures Longitudinal Regression of Loge Visual Field Area on Model 1 Variablesa.
| Coefficient | Estimate (SE) | 95% CI | Degrees of Freedom | t Value | Probability >|t| |
|---|---|---|---|---|---|
| b0b | 9.0416 (0.0987) | 8.8449 to 9.2383 | 72 | 91.61 | <.001 |
| b1c | 0.1282 (0.1196) | −0.1102 to 0.3667 | 72 | 1.07 | .29 |
| b2d | 0.0037 (0.0241) | −0.0436 to 0.0511 | 424 | 0.15 | .88 |
| b3e | 0.0204 (0.0283) | −0.0353 to 0.0760 | 424 | 0.72 | .47 |
Kinetic visual fields measured with the V4e white test light of the Goldmann perimeter on a white background of 31.5 apostilbs, scanned, and then converted to areas by custom software. Based on 74 patients and 500 observations analyzed by the MIXED procedure of SAS.
b0 indicates intercept for the control cohort at mean-centered time.
b1 indicates change in intercept at mean-centered time for the vitamin A cohort.
b2 indicates annual change in loge visual field area for the control cohort.
b3 indicates change in annual change in loge visual field area for the vitamin A cohort.
Mean visual acuity increased by 0.0012 loge-unit per year (0.1% per year) in the control cohort and by 0.0151 loge-unit per year (1.5% per year) in the vitamin A cohort with an unadjusted analysis (Table 4); the difference in rates was not statistically significant. The adjusted analysis also found comparable mean rates (eTable 3 in the Supplement). Incidences of falling to a visual acuity of 20/200 or less in at least 1 eye during follow-up were 4.2% (1 of 24 patients) in the control cohort and 5.5% (3 of 55 patients) in the vitamin A cohort; the difference in percentages (1.3%) was not statistically significant (P = .65).
Table 4. Repeated-Measures Longitudinal Regression of Loge Visual Acuity on Model 1 Variablesa.
| Coefficient | Estimate (SE) | 95% CI | Degrees of Freedom | t Value | Probability>|t| |
|---|---|---|---|---|---|
| b0b | −0.5929 (0.0576) | −0.7079 to −0.4779 | 67 | −10.29 | <.001 |
| b1c | 0.0875 (0.0698) | −0.0517 to 0.2268 | 67 | 1.25 | .21 |
| b2d | 0.0012 (0.0146) | −0.0274 to 0.0299 | 321 | 0.08 | .93 |
| b3e | 0.0139 (0.0168) | −0.0192 to 0.0469 | 321 | 0.82 | .41 |
Best-corrected visual acuities measured with a projected Snellen chart and converted to decimals. Based on 69 patients and 392 observations analyzed by the MIXED procedure of SAS, after censoring initial visual acuities of 20/20 to rule out a ceiling effect, initial visual acuities of 20/100 or less to rule out a floor effect, and follow-up data after a visual acuity of 20/100 or less also to rule out a floor effect.7
b0 indicates intercept for the control cohort at mean-centered time.
b1 indicates change in intercept at mean-centered time for the vitamin A cohort.
b2 indicates annual change in loge visual acuity for the control cohort.
b3 indicates change in annual change in loge visual acuity for the vitamin A cohort.
Discussion
The results show that the vitamin A cohort experienced a statistically significant slower exponential rate of cone ERG amplitude decline during follow-up than the control cohort. The unadjusted analysis permitted estimation of the mean rates of decline to have been 13.2% per year in the control cohort and 6.9% per year in the vitamin A cohort, and the analysis adjusting for covariates confirmed that the slowing with vitamin A was at least as large as that with the unadjusted model. Based on these estimates, falling to half baseline amplitude occurred, on average, in 4.9 years in the control cohort and in 9.7 years in the vitamin A cohort, a nearly 2-fold difference. The size of this vitamin A benefit in children exceeded that from the adult clinical trial (ie, 10% per year for the trace cohort vs 8.3% per year for the vitamin A cohort based on the same amplitude eligibility criteria),1 raising the possibility that a vitamin A supplement for patients with retinitis pigmentosa is more effective in childhood. We also did not find any safety concerns linked to vitamin A supplementation reported by families or with respect to mean rates of change of visual field or visual acuity or by the proportions of children whose visual field diameter fell to 20° or less or whose visual acuity fell to 20/200 or less in at least 1 eye during follow-up.
Two ancillary findings are noteworthy. First, the exponential rate of cone ERG amplitude loss increased with increasing baseline amplitude, a mean 3.6% per year increase in the rate of amplitude decline for a 2.7-fold increase in baseline amplitude, cautioning that a larger response in children should not rule out a fast future disease course. Second, the rate of cone ERG amplitude loss increased with increasing baseline cone ERG implicit time, a mean 1.1% increase in the rate of amplitude decline for a 1-ms increase in baseline implicit time, independent of baseline amplitude. Because only 16 children (20%) had dominantly inherited disease, this finding broadens a similar observation reported for patients with retinitis pigmentosa and dominant RHO mutations.17 If cone ERG implicit time is a biomarker for rate of disease progression in retinitis pigmentosa in general, then it could be used to help predict any given patient’s prognosis in the absence of intervention and for patient selection to minimize the duration of clinical trials when cone ERG amplitude decline is an outcome measure. Unfortunately, to our knowledge, rates of full-field cone ERG amplitude decline specific to children with different genetic types of typical retinitis pigmentosa have not been reported previously to corroborate these additional findings.
Limitations
Because of its smaller sample size and retrospective, observational design, this study is more limited in its implications than the randomized masked clinical trial in adults1 that prompted it. For example, without analyzing data from other centers, we cannot be certain that the mean course of disease in our control group was representative of that of untreated children with retinitis pigmentosa from the general population who return for reassessment. Moreover, although the 2 cohorts did not differ with respect to baseline disease stage, follow-up time, and number of visits, a higher percentage of the vitamin A cohort than the control cohort returned within 2 years for their first follow-up examination, suggesting additional motivation on the part of those families and, therefore, a possible placebo effect (assuming that a placebo effect would develop in children routinely taking a capsule each day). Furthermore, we cannot exclude residual confounding with our relatively small-sample, nonrandomized design. These potential biases could have influenced estimation of the vitamin A effect size and rule out any determination of causality.
Conclusions
Taking into account the positive ERG results, the evidence of supplement safety, and the possible biases from the relatively small-sample, retrospective, nonrandomized study design, we suggest that children with typical retinitis pigmentosa and normal liver function consider oral supplementation with an age-adjusted dose of vitamin A palmitate under a pediatrician’s care to try to slow loss of cone function. This advice could be particularly meaningful to children with a longer cone ERG implicit time who are at higher risk for aggressive disease. Such supplementation may favor retention of visual field,18 mobility,1 and vision-related quality of life1 and maximize the efficacy of other treatment approaches (eg, gene therapy) that depend on the number and health of remaining cone photoreceptors.
eTable 1. Repeated-Measures Longitudinal Regression of Loge Cone ERG Amplitude to 30-Hz Flashes on Model 2 Variables
eTable 2. Repeated-Measures Longitudinal Regression of Loge Visual Field Area on Model 3 Variables
eTable 3. Repeated-Measures Longitudinal Regression of Loge Visual Acuity on Model 3 Variables
References
- 1.Berson EL, Rosner B, Sandberg MA, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111(6):761-772. [DOI] [PubMed] [Google Scholar]
- 2.Norton EW. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111(11):1460. [DOI] [PubMed] [Google Scholar]
- 3.Marmor MF. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111(11):1460-1461. [DOI] [PubMed] [Google Scholar]
- 4.Sibulesky L, Hayes KC, Pronczuk A, Weigel-DiFranco C, Rosner B, Berson EL. Safety of <7500 RE (<25000 IU) vitamin A daily in adults with retinitis pigmentosa. Am J Clin Nutr. 1999;69(4):656-663. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health. Update on vitamin A as a treatment for retinitis pigmentosa. https://nei.nih.gov/news/statements/pigmentosa. Accessed December 19, 2017.
- 6.Li T, Sandberg MA, Pawlyk BS, et al. Effect of vitamin A supplementation on rhodopsin mutants threonine-17–> methionine and proline-347–> serine in transgenic mice and in cell cultures. Proc Natl Acad Sci U S A. 1998;95(20):11933-11938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berson EL, Rosner B, Weigel-DiFranco C, Dryja TP, Sandberg MA. Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest Ophthalmol Vis Sci. 2002;43(9):3027-3036. [PubMed] [Google Scholar]
- 8.Sandberg MA, Rosner B, Weigel-DiFranco C, Dryja TP, Berson EL. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci. 2007;48(3):1298-1304. [DOI] [PubMed] [Google Scholar]
- 9.Sandberg MA, Rosner B, Weigel-DiFranco C, McGee TL, Dryja TP, Berson EL. Disease course in patients with autosomal recessive retinitis pigmentosa due to the USH2A gene. Invest Ophthalmol Vis Sci. 2008;49(12):5532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berson EL, Rosner B, Sandberg MA, et al. Vitamin A supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111(11):1456-1459. [DOI] [PubMed] [Google Scholar]
- 11.Berson EL. Long-term visual prognoses in patients with retinitis pigmentosa: the Ludwig von Sallmann lecture. Exp Eye Res. 2007;85(1):7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berson EL, Sandberg MA, Rosner B, Birch DG, Hanson AH. Natural course of retinitis pigmentosa over a three-year interval. Am J Ophthalmol. 1985;99(3):240-251. [DOI] [PubMed] [Google Scholar]
- 13.Berson EL, Rosner B, Sandberg MA, et al. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Arch Ophthalmol. 2004;122(9):1297-1305. [DOI] [PubMed] [Google Scholar]
- 14.Berson EL, Rosner B, Sandberg MA, et al. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol. 2010;128(4):403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birch DG, Anderson JL, Fish GE. Yearly rates of rod and cone functional loss in retinitis pigmentosa and cone-rod dystrophy. Ophthalmology. 1999;106(2):258-268. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman DR, Hughbanks-Wheaton DK, Pearson NS, et al. Four-year placebo-controlled trial of docosahexaenoic acid in X-linked retinitis pigmentosa (DHAX trial): a randomized clinical trial. JAMA Ophthalmol. 2014;132(7):866-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahmani S, Comander J, DiFranco CW, Rosner B, Sandberg MA. A biomarker for disease course in patients with autosomal dominant retinitis pigmentosa due to RHO mutations. Invest Ophthalmol Vis Sci. 2016;57(12):134. [Google Scholar]
- 18.Sandberg MA, Weigel-DiFranco C, Rosner B, Berson EL. The relationship between visual field size and electroretinogram amplitude in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1996;37(8):1693-1698. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Repeated-Measures Longitudinal Regression of Loge Cone ERG Amplitude to 30-Hz Flashes on Model 2 Variables
eTable 2. Repeated-Measures Longitudinal Regression of Loge Visual Field Area on Model 3 Variables
eTable 3. Repeated-Measures Longitudinal Regression of Loge Visual Acuity on Model 3 Variables