Key Points
Question
Which dermoscopic criteria represent potent indicators for the diagnosis of melanoma in situ compared with nevi and seborrheic keratoses, solar lentigines, or lichen planus–like keratoses; pigmented superficial basal cell carcinoma; pigmented intraepidermal carcinoma; and Reed nevi?
Findings
In this diagnostic accuracy study of 1285 lesions from 1285 patients, irregular hyperpigmented areas and prominent skin markings represented potent indicators of melanoma in situ compared with atypical nevi. Atypical network, regression, and angulated lines were additional indicators of melanoma in situ compared with all other tumors.
Meaning
Because the goal of clinicians is to diagnose melanoma at the earliest possible stage, knowing the dermoscopic indicators of melanoma in situ is of paramount importance.
Abstract
Importance
The accuracy of melanoma-specific dermoscopic criteria has been tested mainly in studies including invasive tumors. Scarce evidence exists on the usefulness of these criteria for the diagnosis of melanoma in situ (MIS).
Objective
To investigate the diagnostic accuracy of dermoscopic criteria for the diagnosis of MIS.
Design, Setting, and Participants
A diagnostic accuracy study with retrospective patient enrollment was conducted in 3 centers specializing in skin cancer diagnosis and management. A total of 1285 individuals with histopathologically diagnosed MIS or other flat, pigmented skin tumors that were histopathologically diagnosed or monitored for at least 1 year were included. Dermoscopic images of MIS and other flat, pigmented skin tumors were evaluated by 3 independent investigators for the presence of predefined criteria. Evaluators were blinded to the clinic dermoscopic and histopathologic diagnosis.
Main Outcomes and Measures
Frequencies of dermoscopic criteria per diagnosis were calculated. Crude odds ratios, adjusted odds ratios, and corresponding 95% CIs were calculated by univariate and multivariate logistic regression, respectively.
Results
A total of 1285 patients were included in the study (642 [50%] male); mean age was 45.9 years (range, 9-91 years). Of a total of 1285 lesions obtained from these patients, 325 (25.3%) were MIS; 574 (44.7%) were nevi (312 [24.3%] excised and 262 [20.4%] not excised); 67 (5.2%) were seborrheic keratoses, solar lentigines, or lichen planus–like keratoses; 91 (7.1%) were pigmented superficial basal cell carcinomas; 26 (2.0%) were pigmented intraepithelial carcinomas; 100 (7.8%) were Reed nevi; and 102 (7.9%) were invasive melanomas with a Breslow thickness less than 0.75 mm. The most frequent dermoscopic criteria for MIS were regression (302 [92.9%]), atypical network (278 [85.5%]), and irregular dots and/or globules (163 [50.2%]). The multivariate analysis revealed 5 main positive dermoscopic indicators of MIS: atypical network (3.7-fold; 95% CI, 2.5-5.4), regression (4.7-fold; 95% CI, 2.8-8.1), irregular hyperpigmented areas (5.4-fold; 95% CI, 3.7-8.0), prominent skin markings (3.4-fold; 95% CI, 1.9-6.1), and angulated lines (2.2-fold; 95% CI, 1.2-4.1). When compared only with excised nevi, 2 of these criteria remained potent MIS indicators, namely, irregular hyperpigmented areas (4.3-fold; 95% CI, 2.7-6.8) and prominent skin markings (2.7-fold; 95% CI, 1.3-5.7).
Conclusions and Relevance
Clinicians should take into consideration the aforementioned dermoscopic indicators for the diagnosis of MIS.
This diagnostic accuracy study compares dermoscopic criteria for diagnosis of melanoma in situ.
Introduction
Dermoscopy is nowadays considered an essential tool for melanoma detection because its use significantly increases the ability of clinicians to recognize melanoma earlier. This early detection is because dermoscopy reveals the natural asymmetry of melanoma before it becomes clinically evident.
Several dermoscopic criteria have been associated with melanoma diagnosis. These criteria were tested in appropriately designed studies comparing melanomas with other tumors included in the differential diagnosis and were proven valid melanoma indicators. However, in most of these studies, the included melanomas were invasive and, thus, after a certain point of biologic and morphologic evolution. As a result, the established criteria were later proven to be insufficient to diagnose melanoma at an earlier stage, especially when restricted within the epidermis (melanoma in situ [MIS]). This finding is reasonable because some of these criteria correspond to histopathologic alterations occurring in the dermis and, thus, should not be expected to be found in in situ tumors. Many studies concluded that, among the well-known melanoma-specific criteria, only atypical network and regression are frequently seen in MIS.
The significant advances in diagnostics achieved by dermoscopy and newer imaging techniques, in conjunction with the overall increase of melanoma awareness, changed the everyday goal of clinicians dealing with melanoma screening. Our goal today is to detect melanoma, if possible, before it becomes invasive. To meet this goal, several investigators attempted to re-examine melanoma criteria and decrease the threshold of excision. As expected, this strategy improves the recognition of early tumors but sacrifices specificity, because numerous atypical nevi display 1 or more of the subtle melanoma criteria.
Appropriately designed diagnostic accuracy studies investigating the validity of dermoscopic criteria for the diagnosis of MIS are lacking. The aim of our study was to assess the accuracy of known melanoma criteria to diagnose MIS compared with benign lesions included in its differential diagnosis.
Methods
This was a multicenter study conducted in 3 skin cancer centers in Greece and Italy. Our databases were screened to identify eligible cases, namely, flat pigmented lesions that clinicians decided to excise or monitor to rule out melanoma. All cases with a definite histopathologic diagnosis of MIS; nevus; or seborrheic keratosis, solar lentigines, or lichen planus–like keratosis (SK/SL/LPLK), superficial basal cell carcinoma, intraepidermal carcinoma, and Reed nevus were included in the study. Randomly selected cases of nonexcised nevi with an available dermoscopic follow-up of at least 1 year were also added to the control group. A group of randomly selected invasive melanomas with a Breslow thickness less than 0.75 mm was also included for comparison purposes. Lesions lacking dermoscopic pigmentation and lesions located on the scalp, face, palms, soles, mucosa, and nails were excluded from the study. Patients’ age and sex and the lesion’s location were recorded.
Ethics committee approval was waived by the Arcispedale Santa Maria Nuova of Reggio Emilia and Aristotle University of Thessaloniki. Patient data from the database were deidentified.
Dermoscopic Evaluation
Dermoscopic images were evaluated by 3 of us independently (E.B., G.B., C.C.), blinded to the histopathologic and clinicodermoscopic diagnoses. The investigators were asked to assess the presence or absence of predefined dermoscopic structures. The selection of dermoscopic criteria was based on the available literature and was a result of consensus among us. We included the traditional melanoma-specific criteria, SK-related criteria, basal cell carcinoma–associated criteria, intraepidermal carcinoma–related criteria, and Reed nevus–associated criteria, as well as 3 additional features: irregular hyperpigmented areas, prominent skin markings, and angulated lines.
Statistical Analysis
Intraobserver agreement was examined with Cohen κ and intraclass correlation coefficient. All separate dermoscopic variables were included in the analysis. Relative risks were calculated for all dichotomous variables. Crude and adjusted odds ratios and corresponding 95% CIs were calculated by univariate and conditional multivariate logistic regression, respectively. Conditional backward elimination proved to be more parsimonious.
The α level was set at .05 and an α level of .10 was used as the cutoff for variable removal in the automated model selection for multivariate logistic regression using 2-tailed, paired testing. The type I error probability associated with all tests in this study was set to .05.
Statistical analyses were performed using SPSS Statistics for Windows, version 22.0 (IBM Corp).
Results
Overall, 1285 index lesions from 1285 patients were included in the study. The epidemiologic characteristics of the patients and the main characteristics of the lesions are given in Table 1. Of 67 SK/SL/LPLK lesions included, 43 were diagnosed as LPLK. The mean Breslow thickness in the invasive melanoma group was 0.49 mm.
Table 1. Epidemiologic Characteristics of Included Patients and Characteristics of Lesions.
| Characteristic | No. (%) |
|---|---|
| Study population | |
| Patients | 1285 |
| Lesions | 1285 |
| Age, mean (range), y | 45.9 (9-91) |
| Sex | |
| Male | 642 (50.0) |
| Female | 643 (50.0) |
| Race | |
| White | 1285 (100) |
| Anatomic site | |
| Thorax | 134 (10.4) |
| Abdomen | 157 (12.2) |
| Upper back | 352 (27.4) |
| Lower back | 192 (14.9) |
| Upper extremities | 153 (11.9) |
| Lower extremities | 297 (23.1) |
| Diagnosis | |
| Melanoma in situ | 325 (25.3) |
| Nevus (atypical) | 312 (24.3) |
| Nevus (typical) | 262 (20.4) |
| Seborrheic keratosis, solar lentigines, or lichen planus–like keratosis | 67 (5.2) |
| Basal cell carcinoma | 91 (7.1) |
| Intraepidermal carcinoma (Bowen disease) | 26 (2.0) |
| Reed nevus | 100 (7.8) |
| Invasive melanoma | 102 (7.9) |
Detailed results of the dermoscopic analysis are reported in Table 2. As given in the table, regression, atypical network, and irregular dots/globules were the prevailing features of MIS and atypical nevi with similar frequencies in the 2 groups. In contrast, irregular hyperpigmented areas, prominent skin markings, and angulated lines were more frequent in MIS compared with atypical nevi.
Table 2. Results of the Dermoscopic Analysis: Global Pattern and Local Features.
| Dermoscopic Criteria | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Melanoma In Situ (n = 325) | Nevi Excised (n = 312) | Nevi Not Excised (n = 262) | Seborrheic Keratosis (n = 67) | Basal Cell Carcinoma (n = 91) | Bowen Disease (n = 26) | Reed Nevi (n = 100) | Invasive Melanoma (n = 102) | |
| Global dermoscopic pattern | ||||||||
| Reticular | 199 (61.2) | 188 (60.3) | 130 (49.6) | 6 (9.0) | 2 (2.2) | 0 | 5 (5.0) | 29 (28.4) |
| Globular | 10 (3.1) | 21 (6.7) | 61 (23.3) | 3 (4.5) | 7 (7.7) | 4 (15.4) | 18 (18.0) | 4 (3.9) |
| Homogeneous | 54 (16.6) | 58 (18.6) | 55 (21.0) | 21 (31.3) | 0 | 10 (38.5) | 8 (8.0) | 19 (18.6) |
| Starburst | 0 | 0 | 0 | 0 | 2 (2.2) | 0 | 62 (62.0) | 0 |
| Multicomponent | 46 (14.2) | 33 (10.6) | 8 (3.1) | 2 (3.0) | 9 (9.9) | 7 (26.9) | 5 (5.0) | 30 (29.4) |
| Nonspecific | 16 (4.9) | 12 (3.8) | 8 (3.1) | 35 (52.2) | 71 (78.0) | 5 (19.2) | 2 (2.0) | 20 (19.6) |
| Atypical network | 278 (85.5) | 258 (82.7) | 144 (55.0) | 18 (26.9) | 2 (2.2) | 2 (7.7) | 13 (13.0) | 67 (65.7) |
| Irregular dots or globules | 163 (50.2) | 170 (54.5) | 141 (53.8) | 30 (44.8) | 18 (19.8) | 13 (50.0) | 25 (25.0) | 70 (68.6) |
| Irregular streaks | 90 (27.7) | 88 (28.2) | 18 (6.9) | 3 (4.5) | 7 (7.7) | 3 (11.5) | 31 (31.0) | 27 (26.5) |
| Irregular blotch | 59 (18.2) | 43 (13.8) | 12 (4.6) | 5 (7.5) | 3 (3.3) | 1 (3.8) | 15 (15.0) | 42 (41.2) |
| Blue-white veil | 34 (10.5) | 50 (16.0) | 10 (3.8) | 4 (6.0) | 5 (5.5) | 2 (7.7) | 24 (24.0) | 25 (24.5) |
| Atypical vessels | 98 (30.2) | 107 (34.3) | 25 (9.5) | 34 (50.7) | 27 (29.7) | 9 (34.6) | 10 (10.0) | 36 (35.3) |
| Regression (any type) | 302 (92.9) | 296 (94.9) | 152 (58.0) | 49 (73.1) | 55 (60.4) | 8 (30.8) | 11 (11.0) | 84 (82.4) |
| Regression type | ||||||||
| No | 23 (7.1) | 16 (5.1) | 110 (42.0) | 17 (25.4) | 36 (39.6) | 18 (69.2) | 89 (89.0) | 18 (17.6) |
| Blue-gray | 109 (33.5) | 112 (36.0) | 100 (38.2) | 13 (19.4) | 17 (18.7) | 2 (7.7) | 4 (4.0) | 21 (20.6) |
| White | 38 (11.7) | 47 (15.1) | 24 (9.2) | 16 (23.9) | 18 (19.8) | 4 (15.4) | 4 (4.0) | 17 (16.7) |
| Both | 155 (47.7) | 137 (43.9) | 28 (10.7) | 21 (31.3) | 20 (22.0) | 2 (7.7) | 3 (3.0) | 46 (45.1) |
| Regression quantity | ||||||||
| 0 | 23 (7.1) | 16 (5.1) | 110 (42.0) | 17 (25.4) | 36 (39.6) | 18 (69.2) | 89 (89.0) | 18 (17.6) |
| <10 | 26 (8.0) | 25 (8.0) | 40 (15.3) | 6 (9.0) | 13 (14.3) | 4 (15.4) | 6 (6.0) | 17 (16.7) |
| 10-50 | 111 (34.2) | 112 (36.0) | 59 (22.5) | 25 (37.3) | 20 (22.0) | 2 (7.7) | 5 (5.0) | 30 (29.4) |
| >50 | 165 (50.8) | 159 (51.0) | 53 (20.2) | 19 (28.4) | 22 (24.2) | 2 (7.7) | 0 | 37 (36.3) |
| Milia-like cysts | 22 (6.8) | 21 (6.7) | 12 (4.6) | 16 (23.9) | 2 (2.2) | 0 | 2 (2.0) | 8 (7.8) |
| Comedo-like openings | 1 (0.3) | 0 | 3 (1.1) | 15 (22.4) | 4 (4.4) | 1 (3.8) | 4 (4.0) | 3 (2.9) |
| Abrupt border | 18 (5.5) | 8 (2.6) | 3 (1.1) | 42 (62.7) | 7 (7.7) | 2 (7.7) | 5 (5.0) | 16 (15.7) |
| Fingerprinting | 6 (1.8) | 5 (1.6) | 0 | 16 (23.9) | 2 (2.2) | 0 | 2 (2.0) | 7 (6.9) |
| Cerebriform structures | 3 (0.9) | 5 (1.6) | 0 | 20 (29.9) | 1 (1.1) | 0 | 1 (1.0) | 8 (7.8) |
| Irregular hyperpigmented areas | 113 (34.8) | 31 (9.9) | 8 (3.1) | 3 (4.5) | 8 (8.8) | 1 (3.8) | 4 (4.0) | 25 (24.5) |
| Prominent skin markings | 36 (11.1) | 13 (4.2) | 13 (5.0) | 3 (4.5) | 3 (3.3) | 1 (3.8) | 6 (6.0) | 10 (9.8) |
| Angulated lines | 35 (10.8) | 15 (4.8) | 4 (1.5) | 0 | 5 (5.5) | 2 (7.7) | 5 (5.0) | 20 (19.6) |
Intraobserver Agreement
Agreement among raters was moderate to substantial. Cohen κ values ranged from 0.393 (for atypical network) to 0.767 (for prominent skin markings).
Univariate and Multivariable Analyses
After the multivariable regression analysis, 5 variables remained significant indicators of MIS, adjusted for the effect of the remaining variables (Table 3). Among them, irregular hyperpigmented areas represented the strongest MIS indicator, posing a 5.4-fold probability of MIS when present, followed by extensive regression (4.7-fold probability of MIS). In addition, we aimed to investigate whether melanoma indicators are modified when the group of early invasive melanomas is added to the MIS group and all melanomas are compared with all other diagnoses. The latter analysis revealed 1 additional melanoma indicator: irregular blotches (Table 3). Furthermore, we performed several subgroup analyses among all study groups; some of these are reported in Table 3.
Table 3. Melanoma Indicators After Multivariable Analysis.
| Dermoscopic Indicator | OR (95% CI) | P Value |
|---|---|---|
| Melanoma in situ vs all others (invasive melanoma excluded) | ||
| Atypical network | 3.7 (2.5-5.4) | <.001 |
| Regression >50% | 4.7 (2.8-8.1) | <.001 |
| Irregular hyperpigmented areas | 5.4 (3.7-8.0) | <.001 |
| Angulated lines | 2.2 (1.2-4.1) | .01 |
| Prominent skin markings | 3.4 (1.9-6.1) | <.001 |
| All melanomas (in situ plus invasive) vs all others | ||
| Atypical network | 3.3 (2.4-4.7) | <.001 |
| Regression >50% | 2.8 (1.8-4.4) | <.001 |
| Irregular blotches | 2.8 (1.9-4.2) | <.001 |
| Irregular hyperpigmented areas | 4.5 (3.0-6.8) | <.001 |
| Angulated lines | 3.1 (1.7-5.7) | <.001 |
| Prominent skin markings | 2.8 (1.5-5.0) | .001 |
| Melanoma in situ vs atypical nevi | ||
| Irregular hyperpigmented areas | 4.3 (2.7-6.8) | <.001 |
| Prominent skin markings | 2.7 (1.3-5.7) | .01 |
| Indicators of melanoma in situ vs invasive melanoma | ||
| Multicomponent global pattern | 1.5 (1.3-1.7) | <.001 |
| Blue-white veil | 4.8 (2.4-9.5) | <.001 |
| Regression >50% | 0.2 (0.1-0.5) | <.001 |
Abbreviation: OR, odds ratio.
MIS vs Atypical Nevi
Melanoma in situ was compared with excised nevi only, which represented the most diagnostically challenging group of benign tumors of the study. As demonstrated in Table 3, only 2 dermoscopic indicators retained their significance to differentiate MIS from excised nevi, namely, irregular hyperpigmented areas and prominent skin markings, associated with a 4.3-fold and 2.7-fold probability of MIS, respectively.
MIS vs Invasive Melanoma
A comparison between MIS and invasive melanoma was performed. In addition to the differences in the frequency of some criteria given in Table 2, this analysis suggested multicomponent global pattern and blue-white veil as indicators of invasive melanoma, whereas extensive regression was the only indicator of MIS.
Sun Exposed vs Non–Sun-Exposed Skin
We intended to investigate possible differences in melanoma indicators according to the location of the tumor on a sun-exposed or non–sun-exposed anatomic site. To do so, we created a variable (photodamaged skin) by combining location and age, assuming that photodamage would be present in the extremities of individuals older than 50 years. According to the results of this analysis, 3 dermoscopic criteria were more frequent in melanomas on sun-damaged skin compared with melanomas on non–sun-damaged skin, respectively: angulated lines (14.5% vs 9.6%), prominent skin markings (14.5% vs 10.0%), and irregular hyperpigmented areas (39.5% vs 33.3%). However, the differences were not statistically significant. All other dermoscopic criteria were present in nearly identical frequencies in both groups.
Discussion
Our study suggests that irregular hyperpigmented areas and prominent skin markings represent potent dermoscopic indicators of MIS, compared with atypical nevi (Figure 1 and Figure 2). The clinical relevance of the latter finding is significant, considering the clinical and dermoscopic similarity between the 2 groups. Indeed, the frequencies of the main dermoscopic criteria of MIS and atypical nevi were nearly identical and the subgroup analysis suggested irregular hyperpigmented areas and prominent skin markings as the only robust features that help in the diagnosis of MIS over atypical nevi. When compared with all other diagnoses, 3 additional MIS indicators were identified, namely, atypical network, extensive regression, and angulated lines.
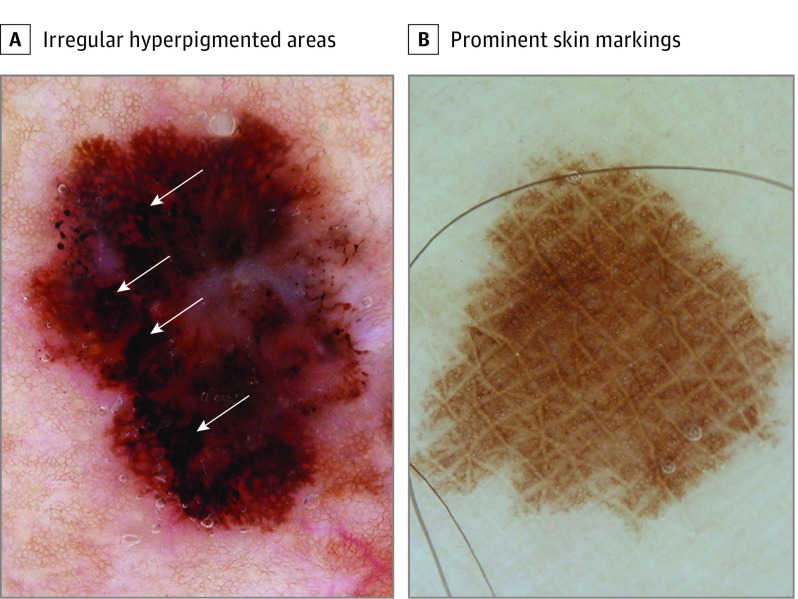
Figure 1. The 2 Potent Dermoscopic Indicators of Melanoma In Situ.
A, Irregular hyperpigmented areas (arrows) irregularly shaped and outlined. B, Prominent skin markings are linear intersecting furrows, clearly hypopigmented compared with the lesion’s overall pigmentation.
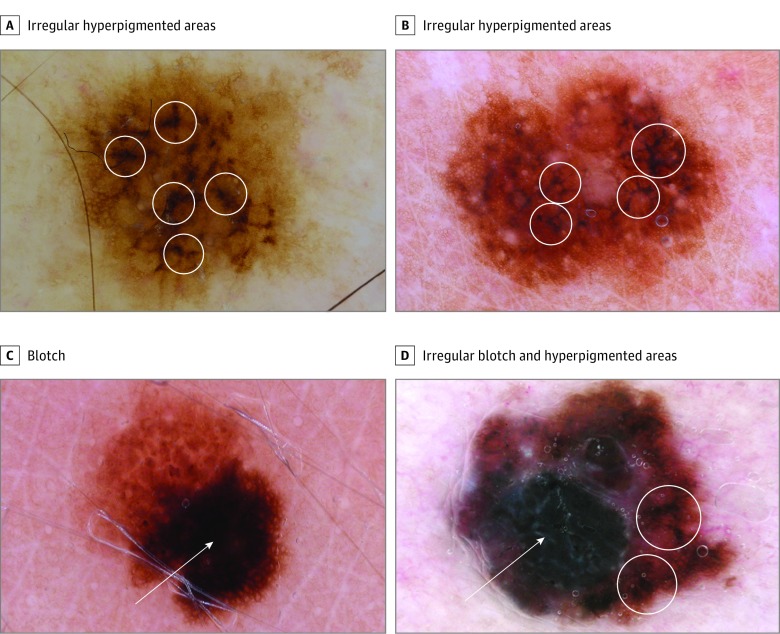
Figure 2. Irregular Hyperpigmented Areas and Blotches.
A and B, Irregular hyperpigmented areas are multiple, small, irregularly shaped and bizarrely outlined dark areas (circles). C, A blotch is a relatively large, roundish, hyperpigmented, structureless zone. When eccentric, a blotch is considered irregular (arrow). D, A melanoma displaying an irregular blotch (arrow) and irregular hyperpigmented areas (circles).
Several studies have focused on the frequency of dermoscopic criteria of MIS. In line with our results, atypical network and regression have been suggested as the most frequently seen dermoscopic features. Although representing 1 of the most common melanoma-specific criteria, atypical network has been associated with low intraobserver agreement, which was also confirmed by our study. Two types of dermoscopic regression exist: blue-gray granules (peppering) and white depigmentation. Zalaudek et al in 2004 suggested that the quality and quantity of regression differ between melanoma and nevi, with melanoma more often displaying extensive regression of both types (blue-gray and white). In the present study, the quality of regression was not shown to be a significant melanoma indicator. In contrast, the quantity of regression was found to be much more significant because the presence of regression in more than 50% of the lesion’s surface was associated with a 4.7-fold probability of melanoma.
In the present study, we introduce a new dermoscopic feature, namely, irregular hyperpigmented areas, that our analysis suggests as the most potent dermoscopic indicator of MIS, even when compared with atypical nevi only. These hyperpigmented areas are dark brown or black small areas seen in the central parts of a lesion. Their shape is irregular and does not fit with any known geometric shape (eg, triangle, line, square); therefore, they cannot be classified as any other previously known feature (eg, dots, globules, blotches, lines). In particular, blotch is usually a solitary, roundish, large, hyperpigmented area that might be central (regular blotch) or eccentric (irregular blotch). In contrast, irregular hyperpigmented areas are typically smaller, multiple, and bizarrely outlined (Figure 2).
Prominent skin markings represented the second potent indicator of MIS over atypical nevi. This term prominent skin markings was recently introduced to describe the presence of linear intersecting furrows, lighter than the lesion’s overall pigmentation, mainly seen in melanoma located on the extremities. In the present study, prominent skin markings were more frequently seen on the lower extremities compared with all other anatomic sites, but the differences were not statistically significant. Finally, angulated lines have been described as a feature seen in melanomas with a lentiginous growth pattern, usually on sun-damaged skin. In our study, angulated lines were more frequent on lesions located on sun-damaged skin. The presence of angulated lines was associated with a 2.2-fold probability of melanoma and the fact that the criterion lost its probability value when MIS was compared with excised nevi might be a result of data dilution, because angulated lines are rather a site-specific feature (present on sun-damaged areas).
Limitations
Our study has some limitations. First, the retrospective design is subject to recall and observation biases, which were addressed by involving 3 independent evaluators blinded to the clinical and histopathologic diagnosis. Second, retrospective design is also subject to a selection bias, mainly concerning the control group. In detail, in this study, we included only cases with a definite diagnosis, established either by histopathology or by a minimum follow-up of 1 year (for nevi). However, this sample is far from representative of the incidence of each tumor in the real clinical setting, where “ordinary” nevi and SL or SK with typical clinical and dermoscopic features are more frequent. As a result of this bias, the value of melanoma indicators might be underestimated by the present study. Third, the histopathologic differentiation between MIS and atypical nevi might be challenging because early melanoma might not display diagnostic criteria, while some nevi might display criteria suggestive of melanoma (dysplastic nevi). Therefore, we cannot rule out the possibility that some tumors were misclassified. Fourth, we included nonexcised nevi with a minimum follow-up of 1 year. Although unlikely, we cannot exclude the possibility that a lesion assessed as a nevus and remaining stable after 1 year is a very slow-growing melanoma. Fifth, we did not include in our analysis dermoscopic features seen only with polarized light because most of the images evaluated had been captured with nonpolarized dermoscopy. White shiny lines have been suggested as an additional melanoma-specific criterion, and their usefulness for the diagnosis of MIS was not assessed by our study. Sixth, we propose 2 new melanoma indicators (irregular hyperpigmented areas and prominent skin markings). To our knowledge, no previous data exist on the usefulness of these criteria; they need to be validated by future studies. Finally, we aimed to assess the accuracy of dermoscopic criteria, independent of clinical and epidemiologic factors. However, clinicians should take into consideration that the risk of melanoma increases with a patient’s age, as shown by the results of the present and previous studies. Furthermore, clinicians should remember that clinical and dermoscopic morphologic findings should be interpreted within the context of a given patient, integrating morphology with known risk factors of melanoma.
Conclusions
In the present study we suggest 5 dermoscopic features suggestive of MIS compared with all other diagnoses. In addition, we introduce 2 dermoscopic features that are indicative of MIS over atypical nevi, which represent the most challenging-to-differentiate group of tumors. These findings might enhance the ability of clinicians to improve their accuracy in recognizing melanoma at the earliest possible stage, lowering the risk of diagnostic delays and the subsequent burden for the patients, clinicians, and health systems.
References
- 1.Argenziano G, Cerroni L, Zalaudek I, et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67(1):54-59. [DOI] [PubMed] [Google Scholar]
- 2.Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159(3):669-676. [DOI] [PubMed] [Google Scholar]
- 3.Argenziano G, Catricalà C, Ardigo M, et al. Seven-point checklist of dermoscopy revisited. Br J Dermatol. 2011;164(4):785-790. [DOI] [PubMed] [Google Scholar]
- 4.Seidenari S, Bassoli S, Borsari S, et al. Variegated dermoscopy of in situ melanoma. Dermatology. 2012;224(3):262-270. [DOI] [PubMed] [Google Scholar]
- 5.Argenziano G, Kittler H, Ferrara G, et al. Slow-growing melanoma: a dermoscopy follow-up study. Br J Dermatol. 2010;162(2):267-273. [DOI] [PubMed] [Google Scholar]
- 6.Pellacani G, Longo C, Malvehy J, et al. In vivo confocal microscopic and histopathologic correlations of dermoscopic features in 202 melanocytic lesions. Arch Dermatol. 2008;144(12):1597-1608. [DOI] [PubMed] [Google Scholar]
- 7.Seidenari S, Ferrari C, Borsari S, et al. Reticular grey-blue areas of regression as a dermoscopic marker of melanoma in situ. Br J Dermatol. 2010;163(2):302-309. [DOI] [PubMed] [Google Scholar]
- 8.Borsari S, Longo C, Ferrari C, et al. Dermoscopic island: a new descriptor for thin melanoma. Arch Dermatol. 2010;146(11):1257-1262. [DOI] [PubMed] [Google Scholar]
- 9.Bassoli S, Borsari S, Ferrari C, et al. Grey-blue regression in melanoma in situ-evaluation on 111 cases. J Skin Cancer. 2011;2011(4):180980-180985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lallas A, Zalaudek I, Apalla Z, et al. Management rules to detect melanoma. Dermatology. 2013;226(1):52-60. [DOI] [PubMed] [Google Scholar]
- 11.Pizzichetta MA, Argenziano G, Talamini R, et al. Dermoscopic criteria for melanoma in situ are similar to those for early invasive melanoma. Cancer. 2001;91(5):992-997. [PubMed] [Google Scholar]
- 12.Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48(5):679-693. [DOI] [PubMed] [Google Scholar]
- 13.Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74(6):1093-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrera C, Marchetti MA, Dusza SW, et al. Validity and reliability of dermoscopic criteria used to differentiate nevi from melanoma: a web-based International Dermoscopy Society study. JAMA Dermatol. 2016;152(7):798-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribero S, Moscarella E, Ferrara G, Piana S, Argenziano G, Longo C. Regression in cutaneous melanoma: a comprehensive review from diagnosis to prognosis. J Eur Acad Dermatol Venereol. 2016;30(12):2030-2037. [DOI] [PubMed] [Google Scholar]
- 16.Zalaudek I, Argenziano G, Ferrara G, et al. Clinically equivocal melanocytic skin lesions with features of regression: a dermoscopic-pathological study. Br J Dermatol. 2004;150(1):64-71. [DOI] [PubMed] [Google Scholar]
- 17.Braun RP, Gaide O, Oliviero M, et al. The significance of multiple blue-grey dots (granularity) for the dermoscopic diagnosis of melanoma. Br J Dermatol. 2007;157(5):907-913. [DOI] [PubMed] [Google Scholar]
- 18.Keir J. Dermatoscopic features of cutaneous non-facial non-acral lentiginous growth pattern melanomas. Dermatol Pract Concept. 2014;4(1):77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaimes N, Marghoob AA, Rabinovitz H, et al. Clinical and dermoscopic characteristics of melanomas on nonfacial chronically sun-damaged skin. J Am Acad Dermatol. 2015;72(6):1027-1035. [DOI] [PubMed] [Google Scholar]
- 20.Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. [DOI] [PMC free article] [PubMed] [Google Scholar]