Abstract
The PTEN gene encodes a lipid phosphatase that negatively regulates the phosphatidylinositol 3-kinase pathway and is inactivated in a wide variety of malignant neoplasms. High rates of loss of heterozygosity are observed at the 10q23.3 region containing the human PTEN gene in prostate cancer and other human malignancies, but the demonstrated rate of biallelic inactivation of the PTEN gene by mutation or homozygous deletion is significantly lower than the rate of loss of heterozygosity. The transgenic adenocarcinoma of mouse prostate model is a well characterized animal model of prostate cancer. Analysis of prostate cancer progression in transgenic adenocarcinoma of mouse prostate mice bred to Pten+/− heterozygous mice, coupled with analysis of the Pten gene and protein in the resulting tumors, reveals that haploinsufficiency of the Pten gene promotes the progression of prostate cancer in this model system. This observation provides a potential explanation for the discordance in rates of loss of heterozygosity at 10q23 and biallelic PTEN inactivation observed in prostate cancer and many human malignancies.
The PTEN tumor suppressor gene (also known as MMAC-1) encodes a phosphatase and is inactivated in a wide variety of human malignant neoplasms, including gliomas, melanomas, and carcinomas of the endometrium, kidney, breast, lung, upper respiratory tract, and prostate (1–4). The tumor suppressor activity of PTEN is thought to be primarily due to its ability to dephosphorylate phosphatidylinositol 3,4,5-phosphate at the 3-position and negatively regulate the activity of the phosphatidylinositol 3-kinase pathway (5, 6). A variety of biological effects have been attributed to loss of PTEN activity that are relevant to its role as a tumor suppressor gene, including enhanced cell proliferation (6), decreased apoptosis (5, 6), and increased tumor angiogenesis (7, 8). The PTEN gene is also mutated in Cowden syndrome (9), a hereditary neoplastic syndrome characterized by an increased rate of thyroid cancer and breast cancer in affected females. Thus, the PTEN gene is an important tumor suppressor with a wide range of biological activities relevant to tumor progression.
The PTEN tumor suppressor gene maps to human chromosome 10q23.3, and this region shows high rates of loss of heterozygosity (LOH) in a variety of human malignancies. Such LOH is usually due to the loss of relatively large areas of one copy of chromosome 10. It is generally believed that in the presence of such LOH the tumor suppressor gene present on the retained chromosome is inactivated by smaller deletions, resulting in homozygous deletion or by mutation. However, for the PTEN gene, the rate of LOH at 10q23.3 is often much higher than the apparent rate of inactivation of the retained PTEN allele. For example, LOH at 10q23.3 has been detected in 15–49% of clinically localized human prostate cancers, whereas mutation or homozygous deletion of the PTEN gene is detected in less than 10% of these same cases (4, 10–15). Similarly, LOH at 10q23 is present in more than 50% of metastatic prostate cancers, whereas biallelic PTEN inactivation is present in only 30% of such cases (16). Similar observations have been made in a variety of other human malignancies (3, 4). Several possible explanations for this discordance exist. First, the retained allele may be inactivated by other mechanisms that have not been well evaluated to date, such as methylation or promoter mutation leading to loss of expression. Inactivation of the PTEN gene by methylation has been reported in prostate cancer xenografts (17), but to date there are no reports of methylation of the PTEN promoter in primary or metastatic prostate cancer in vivo. A second potential explanation is that a second tumor suppressor gene is located in the 10q23.3 region, closely linked to the PTEN gene, and inactivation of this gene is driving the LOH in this region in those cases without biallelic PTEN inactivation. Finally, it is possible that loss of a single allele of PTEN (haploinsufficiency) is by itself sufficient to promote tumor progression.
To differentiate between loss of expression of the Pten gene, the presence of a second tumor suppressor gene, and haploinsufficiency of the Pten gene in tumor progression, we bred Pten+/− heterozygous knockout (KO) mice to transgenic adenocarcinoma of mouse prostate (TRAMP) mice, monitored tumor progression in the transgenic male progeny, and analyzed the status of the Pten gene and protein in the resulting prostate cancers. The TRAMP model was generated by microinjection of a construct harboring a probasin regulatory element to direct expression of the simian virus (SV) 40 early genes to prostatic epithelium. The earliest pathology is prostatic intraepithelial neoplasia, and the mice can display well differentiated adenocarcinoma as early as 12 weeks of age. During the next 6 weeks, the TRAMP mice usually display moderately differentiated carcinoma and ultimately develop poorly differentiated carcinoma by the time they reach 24 to 30 weeks of age. Metastasis is detected in many animals by 24 weeks of age (18). Pten+/− heterozygous KO mice develop several proliferative and neoplastic lesions, including hyperplasia and dysplasia of the prostatic epithelium, but invasive prostatic adenocarcinoma is not observed in these mice (19, 20).
We have found that the loss of a single Pten allele in the tumor dramatically increases the rate of progression of prostate cancer in TRAMP mice. Thus, haploinsufficiency of the Pten gene promotes progression of prostate cancer in this mouse model. This observation provides a potential explanation for the discordance between observed rates of LOH at the PTEN locus and the much lower rates of PTEN inactivation in human prostate cancer and a variety of other malignant neoplasms.
Materials and Methods
Breeding of Mice, Necropsy, and Survival Analysis.
Pten+/− heterozygous KO mice (SV129/J background) and TRAMP transgenic mice (C57BL/6 background) have been described (18, 19). Mice were mated, and male progeny were screened for the presence of the SV40 large T antigen by PCR as described at the TRAMP model website (www-tramp-model.cellb.bcm.tmc.edu). Transgenic male mice were monitored until they met criteria for euthanasia, including large palpable tumor, huddled posture, immobility, or an obviously moribund appearance. Some mice died without displaying criteria for euthanasia. Only tumors from euthanized animals were used for DNA, RNA, and protein extraction. A full autopsy was performed on all animals including microscopic examination of all thoracic, abdominal and pelvic organs, and cervical lymph nodes if enlarged. The central nervous system, spine, skull, and pelvis from 10 mice with metastatic disease were examined in a pilot study, which did not reveal metastasis to these sites at a high rate; thus, they were not subsequently examined routinely. Kaplan–Meier analysis of survival was performed with the assistance of SPSS statistical analysis software with use of a log ranks pairwise comparison.
Southern Blot Analysis.
DNA was extracted as described (13). Tail or tumor tissue DNA (10 μg) was digested with 50 units of SacI restriction endonuclease (Life Technologies, Grand Island, NY) in a 100-μl volume of reaction buffer containing 50 M Tris⋅HCl (pH 8.0), 10 mM MgCl2, and 50 mM NaCl at 37°C for 16 h. The digested DNA was fractionated on a 0.7% agarose gel with electrophoresis and transferred to a positively charged nylon membrane (Roche Molecular Biochemicals). Southern hybridization was performed at 68°C in 10 ml of PerfectHyb Plus hybridization solution (Sigma). Nylon membrane was prehybridized in the aforementioned buffer for 15 min. Hybridization was performed for 3 h by adding 50 ng of Pten probe B fragment (19) that was radioactively labeled with [α-32P]dCTP [3,000 Ci/mmol (1 Ci = 37 GBq); NEN Life Sciences] by using RadPrime Labeling Kit (Life Technologies) and included at a concentration of 1 × 109 cpm/μg. Blots were washed according to the manufacturer's protocol, and signals were visualized by autoradiography.
LOH Analysis.
LOH was determined by PCR of microsatellite markers, with tail and tumor DNA. Map position, sequence information, and strain polymorphisms for microsatellite markers were determined from the Whitehead/MIT Genome Database and the Jackson Laboratories Database. The primers for analysis of the microsatellite markers D19Mit119 and D19Mit33 were obtained from Research Genetics. PCR was performed in a 50-μl reaction volume containing 100 ng of genomic DNA, 1× PCR buffer (Takara Shuzo, Kyoto, Japan), 0.264 μM each forward and reverse primer, and 200 μM each dNTP on a Mastercycler gradient PCR instrument (Eppendorf). The PCR consisted of an initial denaturing step (3 min at 94°C); the subsequent 35-cycle amplification consisted of the following three steps: DNA denaturing (30 s at 94°C), primer annealing [30 s at 52 oC (D19Mit119) or 55.5°C (D19Mit33)], and primer extension (1 min at 72°C). A final primer extension (7 min at 72°C) concluded the PCR amplification. PCR products were analyzed by fractionation on a 2% agarose gel and visualized by ethidium bromide staining.
Mutational Analysis of the Pten Coding Region.
Total RNA was extracted from tumor tissue samples with the Qiagen RNeasy Maxi prep kit (Qiagen, Chatsworth, CA). Reverse transcription–PCR and sequencing analysis were performed as described (21). Mutation analysis of exon 5 of the Pten gene was performed by PCR amplification of genomic DNA from primary prostate cancers by using primers corresponding to the 5′ region of exon 4 (4F) and the 3" portion of exon 5 (5R). The resulting 2-kb fragment was isolated and sequenced as described (21).
Western Blotting.
PC3 prostate cancer cells were grown in RPMI 1640 medium with 10% FBS. Total protein extracts were prepared from tumor tissue samples and cell lines as described (22). For Western blots, 30–40 μg of protein extract per lane was electrophoresed, transferred to nitrocellulose membrane (Hybond ECL, Amersham Pharmacia), and immunoblotted overnight with a 1:1,000 dilution of anti-PTEN antibody (Cascade Biosciences, Winchester, MA) and a 1:5,000 dilution of anti-β-actin antibody (Sigma). Membranes were washed and treated with rat anti-mouse IgG κ-chain secondary antibody (1:2,000) conjugated to horseradish peroxidase (Southern Biotechnology Associates). The antigen–antibody reaction was visualized by using an enhanced chemiluminesence assay (Amersham Pharmacia) and exposure to enhanced chemiluminesence film.
Results
Survival Analysis of Wild-Type (WT) and PTEN+/− TRAMP Mice.
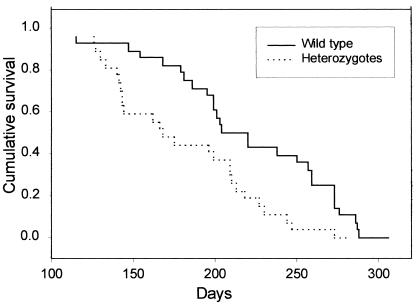
To determine whether loss of the Pten tumor suppressor gene was associated with increased rates of prostate cancer progression, we bred TRAMP mice with heterozygous Pten KO mice (Pten+/−) and analyzed the survival of the transgenic male progeny. Half of all progeny would be predicted to be WT at the Pten locus, whereas half would have one inactivated (KO) allele. Mice were monitored until they reached criteria for euthanasia, including the presence of a large palpable tumor exceeding 10% of body weight, difficulty ambulating, huddled posture, or an obviously moribund appearance. In some cases, animals died unexpectedly before euthanasia. In all cases, necropsy was performed with full gross and microscopic examination. The genotype of the animal was not determined until after necropsy to prevent bias in selection for euthanasia or in histopathological examination. Given that Pten+/− animals develop several spontaneous pathologies, including a lymphoproliferative disorder (23), a meaningful comparison of survival with WT animals requires that animals dying or requiring euthanasia be analyzed by necropsy. Transgenic animals (n = 70) were monitored and included litters born during a 7-month period. Seven animals were excluded because of the presence of lymphoproliferative disorder or the absence of invasive prostate carcinoma at the time of necropsy. On the basis of the necropsy findings, the remaining 63 mice had three basic findings that led to death. In ≈30% of cases, animals had local disease and required euthanasia for large primary tumors and/or bladder obstruction leading to obvious morbidity. In the remaining cases, animals had metastatic disease, often to multiple organs, including lung (28 of 63 cases), abdominal lymph nodes (26 of 63 cases), liver (22 of 63 cases), and kidney (4 of 63 cases). Kaplan–Meier analysis of the survival of the Pten+/− TRAMP animals in comparison with WT TRAMP littermate controls revealed that the Pten+/− TRAMP mice had a significantly decreased survival (P < 0.002) compared with WT TRAMP littermate controls (Fig. 1). The Pten +/− TRAMP animals died at a mean age of 185 ± 9 days (SEM, n =35) as compared with a mean age of 226 ± 10 days (SEM, n =28) for WT TRAMP littermate controls. Analysis of the pathology at necropsy revealed that the Pten+/− and WT TRAMP animals had quite similar pathological findings. In both cases, ≈30% of animals died of local disease rather than metastasis (32% for Pten+/− vs. 30% for WT). Thus, inactivation of the Pten gene seems to be promoting aggressive tumor growth rather than metastasis per se. In addition, the pattern of metastatic spread to various organs was not altered in the Pten+/− TRAMP mice when compared with WT TRAMP controls (data not shown). One possible explanation for decreased survival in the Pten+/− TRAMP animals was that they may be less healthy than WT animals because of pathology related to loss of one Pten allele and thus died more easily than control animals. However, we found that the mean size of the primary tumors in the Pten+/− TRAMP mice (4.3 ± 1.0 g, SEM, n =34) was actually greater than WT TRAMP controls (3.0 ± 0.28 g, SEM, n =28), which is more consistent with increased rates of tumor progression in these mice.
Figure 1.
Kaplan–Meier analysis of survival for Pten+/− and WT TRAMP mice. Male TRAMP mice that had a germ-line inactivation of one allele of the Pten gene or were WT at the Pten locus were monitored until they reached criteria for euthanasia or died. Kaplan–Meier analysis of survival is shown. Solid line, WT mice; hatched line, Pten+/− mice. The difference between the two groups is statistically significant (P = 0.002).
Analysis of the PTEN Gene and Protein in Prostate Cancer from PTEN+/− and WT+/+ Mice.
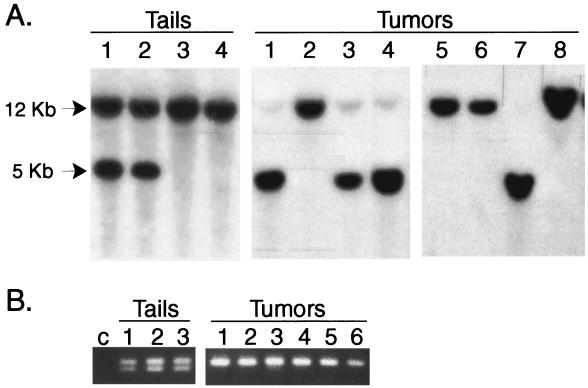
To determine the basis for the decreased survival seen in the Pten+/− mice we determined the status of the Pten gene and protein in the subset of the primary prostate cancers for which sufficient tumor was available. This analysis was greatly facilitated, because the large, poorly differentiated tumors that were the most common finding at necropsy were greater than 90% carcinoma by histopathology. This is a significant advantage is significant in working with this model system and contrasts with human prostate cancer specimens, in which such pure tumors are unusual. We performed Southern blot analysis of DNAs extracted from primary prostate cancers from 19 Pten+/− TRAMP and 19 WT TRAMP mice by using a probe that distinguishes the KO and WT alleles (19). After SacI digestion, the KO allele gives a 5-kb band, whereas the WT allele gives a 12-kb band (Fig. 2A). As seen in tumor DNAs 1, 3, 4, and 7, loss of the WT allele was common in the tumors derived from Pten+/− TRAMP mice. The small amount of residual WT allele is due to the small amount of contaminating normal tissue in the tumor, consistent with the high purity of the tumor samples as described above. Of the primary tumors analyzed from the Pten+/− TRAMP mice, 13 of 19 (68%) had loss of the WT allele, resulting in complete loss of functional Pten gene.
Figure 2.
Analysis of prostate cancers from Pten+/− TRAMP mice with loss of the WT allele. (A) DNAs from tails or primary prostate cancers were extracted, digested with SacI, and analyzed by Southern blotting with a Pten probe that distinguishes the WT (12 kb) and KO (5 kb) alleles. Tails 1 and 2 and tumors 1, 3, 4, and 7 were from Pten+/− TRAMP mice, whereas tails 3 and 4 and tumors 2, 5, 6, and 8 were from WT TRAMP mice. (B) DNAs were extracted from tails or primary prostate cancers, then analyzed by PCR with primers amplifying the D19Mit119 polymorphic locus, followed by agarose gel electrophoresis. The allele derived from the SV129 chromosome gives a 281-bp band, whereas the allele from the C57BL/6 chromosome gives a 265-bp band. c, water control; Tails 1–3, tail DNAs: Tumors 1–6, prostate cancers with loss of the WT Pten allele by Southern blotting.
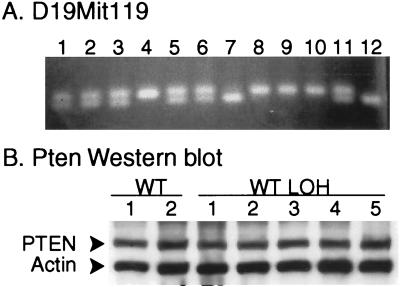
The mouse Pten gene has been mapped to 24 centimorgans (cM) on chromosome 19 (24), a region syntenic to human 10q23. To determine the extent of loss on mouse chromosome 19 in tumors with loss of the WT allele, we analyzed a subset of six tumors that had lost the WT Pten allele for LOH on chromosome 19 by using PCR of polymorphic repeats. These repeats differ between the chromosome 19 derived from the SV129 strain (carrying the KO allele) and that derived from the C57BL/6 strain (carrying the WT allele). Analysis of the closely linked D19Mit119 polymorphic marker, which is located at 27.5 cM on chromosome 19, reveals loss of the 265-bp band derived from the C57BL/6 allele with retention of the 281-bp band derived from the SV129 allele in all cases, consistent with the Southern blot analysis (Fig. 2B). Analysis of the D19Mit33 polymorphic repeat, located at 53cM on chromosome 19 in these same six cases, again showed loss of the C57BL/6 allele in all cases (data not shown). Thus, in approximately two-thirds of primary prostate cancers from the Pten+/− TRAMP mice, there is loss of the WT allele because of loss of a large region (from 24 to 53 cM) of the WT chromosome 19. Loss of the entire WT chromosome 19 cannot be excluded.
In 6 of 19 prostate cancers from the Pten+/− TRAMP mice, there was retention of the WT Pten allele. As determined by Southern blotting, there was retention of both WT and KO alleles in five cases, whereas in one case there was loss of the KO allele. Analysis of the closely linked D19Mit119 marker was concordant with the Southern blot results (Fig. 3A). To determine whether the retained WT Pten allele was inactivated by methylation or promoter mutations leading to loss of expression, we analyzed the five tumors for which protein extracts were available by Western blotting for Pten protein expression. In all cases, Pten protein expression was still present in substantial amounts, similar to the level in tumors from WT TRAMP mice (Fig. 3B). More than 90% of mutations in the PTEN gene that have been detected in human prostate cancer specimens, cell lines, and xenografts (1–4, 10–16, 25) have been frameshift, nonsense, or splice site mutations that lead to the generation of truncated proteins. The rare missense mutations reported have not actually been shown to inactivate the PTEN gene. We found no evidence of truncated protein in any sample used for Western blotting. We also performed mutation analysis by reverse transcription–PCR and sequencing by using mRNAs derived from two tumors for which material was available and found no evidence of point mutation in the coding region of the Pten gene. In addition, we analyzed genomic DNAs for mutations in the phosphatase domain in exon 5 and found no mutations in any of the six tumors that retained the WT Pten allele.
Figure 3.
Analysis of prostate cancers from Pten+/− TRAMP mice that retain the WT Pten allele. (A) DNAs were extracted from primary prostate cancers from Pten+/− TRAMP mice and then analyzed by PCR with primers amplifying the D19Mit119 polymorphic locus followed by agarose gel electrophoresis. All tumors retained the WT Pten allele as determined by Southern blotting. The allele derived from the SV129 chromosome gives a 281-bp band, whereas the allele from the C57BL/6 (WT) chromosome gives a 265-bp band. (B) Western blot analysis with anti-Pten antibody and anti-β-actin (control) antibody was performed on protein extracts from primary prostate cancers arising in the Pten+/− TRAMP mice with retention of the WT Pten allele as determined by Southern blotting (Het tumors 1–5), a primary prostate cancer from a WT TRAMP mouse, and the human prostate cancer cell lines PC3, known to have a homozygous deletion of PTEN (PC).
Given that loss of the Pten gene enhances tumor progression in the Pten+/− TRAMP mice, we examined the primary tumors from the WT TRAMP animals for evidence of Pten inactivation. Southern blotting of 19 primary tumors from WT TRAMP mice revealed no evidence of homozygous deletion of the Pten gene (Fig. 2A). Such homozygous deletion should be easily observable given the purity of the tumors. Loss of a single allele is difficult to detect with certainty on Southern blots. However, analysis of the D19Mit119 polymorphic locus, which is 3.5 cM telomeric to the Pten gene, revealed LOH at this locus in 9 of 19 WT tumors (Fig. 4A). Given the fact that LOH typically involves large areas of the affected chromosome, there is almost certainly loss of a single Pten allele in most, if not all, of these cases. Western blot analysis of protein extracts from 14 WT tumors, including all 9 with LOH at the D19Mit119 locus, revealed expression of the Pten protein at substantial levels in all cases (Fig. 4B). We found no evidence of truncated Pten protein. We performed mutation analysis on RNAs from three WT TRAMP tumors with LOH at D19Mit119 for which material was available, and again no mutation was detected in the Pten coding region. Thus, there is LOH near the Pten locus in almost half of the tumors from WT animals but no evidence of inactivation of the retained Pten allele.
Figure 4.
Analysis of prostate cancers from WT TRAMP mice. (A) DNAs were extracted from primary prostate cancers arising in WT TRAMP mice, then analyzed by PCR with primers amplifying the D19Mit119 polymorphic locus, followed by agarose gel electrophoresis. The allele derived from the SV129 chromosome gives a 281-bp band, whereas the allele from the C57BL/6 chromosome gives a 265- bp band. (B) Western blot analysis with anti-Pten antibody and anti-β- actin (control) antibody was performed on protein extracts from primary prostate cancers arising in WT TRAMP mice. WT 1 and 2 were from tumors without LOH at the D19Mit119 polymorphic locus, whereas WT LOH 1–5 were from tumors with LOH at this locus.
Survival Analysis Based on the Status of the Pten Gene in Primary Tumors.
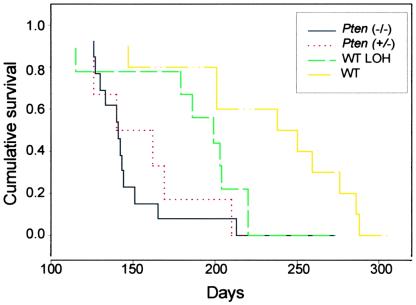
We divided the mice into four groups on the basis of the analysis of the Pten gene in the primary tumors for comparison of survival by Kaplan–Meier analysis (Fig. 5). Group 1 consisted of 13 Pten+/− TRAMP mice that had loss of the WT Pten allele in their primary tumors (Pten−/−). Group 2 consisted of Pten+/− TRAMP mice that retained the WT Pten allele in the primary tumor (Pten+/−) and thus had one fully active Pten allele. Group 3 consisted of the nine WT TRAMP mice with LOH at the D19Mit119 locus and presumably at the adjacent Pten locus (WT LOH). The 10 WT TRAMP mice without evidence of LOH at D19Mit119 (WT) were placed in group 4. The WT TRAMP mice without LOH had a mean survival of 245 ± 16 days, whereas the WT TRAMP mice with LOH had a mean survival of 206 ± 16 days, the Pten+/− TRAMP mice with retention of the WT allele had a mean survival of 171 ± 15 days, and the Pten+/− TRAMP mice with loss of the WT Pten allele had a mean survival of 159 ± 11 days (SEM). The differences between the WT TRAMP animals (group 4) and groups 1–3 were all statistically significant by Kaplan–Meier analysis (P = 0.0006, P = 0.003, and P = 0.049, respectively). The only other pairwise comparison between groups that revealed a statistically significant difference in survival was the comparison of the Pten+/− TRAMP mice with loss of the WT Pten allele with the WT TRAMP mice with LOH at D19Mit119 (P = 0.041). If we exclude the one Pten+/− TRAMP mouse with retention of the WT Pten gene in its tumor for which we do not have Western blotting data, we still find a significant decrease in survival of these mice as compared with WT TRAMP animals without LOH (P = 0.002). Thus, loss of a single Pten allele leads to a statistically significant increase in the rate of progression of prostate cancer in TRAMP mice, and loss of both alleles apparently further accelerates tumor progression.
Figure 5.
Kaplan–Meier analysis of survival in Pten+/− and WT TRAMP mice by tumor genotype. Male TRAMP mice that had a germ-line inactivation of one allele of the Pten gene or were WT at the Pten locus were monitored until they reached criteria for euthanasia or died. Tumors were then analyzed at the Pten locus by Southern blotting and at the adjacent D19Mit119 polymorphic locus by PCR, and four groups were established. Group 1, Pten+/− TRAMP mice that had loss of the WT Pten allele in their primary tumor (Pten−/−), black line; group 2, Pten+/− TRAMP mice that retained the WT Pten allele in the primary tumor (Pten+/−), red line; group 3, WT TRAMP mice with LOH at the D19Mit119 locus in their primary tumors (WT LOH), green line; group 4, WT TRAMP mice without evidence of LOH at D19Mit119 (WT), yellow line. Survival was compared by Kaplan-Meier analysis with SPSS statistical software (SPSS, Chicago).
Discussion
Our results strongly support the hypothesis that inactivation of the Pten gene promotes progression of prostate cancer in the TRAMP mouse model system. The key observation in this study is that the Pten+/− TRAMP mice that retain one Pten allele in their prostate cancers show a rate of tumor progression that is significantly faster than WT TRAMP animals with retention of both Pten alleles. Although such tumors seem to progress somewhat more slowly than tumors with biallelic inactivation of the Pten gene, this difference was not statistically significant. Therefore, haploinsufficiency of the Pten gene leads to increased rates of tumor progression in the TRAMP mouse model of prostate cancer.
The finding of LOH near the Pten locus in the WT TRAMP mice without evidence of mutation of the retained Pten allele is similar to the situation observed in a significant fraction of human prostate cancers. We have been able to rule out loss of Pten protein expression in these tumors due to methylation or promoter mutation by Western blotting. Another possible explanation for this finding is the presence of a tumor suppressor gene near the Pten locus. However, analysis of the Pten/D19Mit119 region in tumors from the Pten+/− TRAMP mice shows that in 13 cases there was loss of the WT (C57BL/6) allele as compared with a single case of loss of the KO (SV129) allele. If a second strong tumor suppressor gene was present near the Pten locus we would expect to see a more even distribution of loss, because the putative second tumor suppressor should be equally likely to show LOH on either chromosome. However, we cannot rule out the possibility that there is a second tumor suppressor gene on mouse chromosome 19 not adjacent to the Pten locus that may account for some of the LOH in this region and/or decreased survival in mice with LOH at the D19Mit119 marker. Thus, the loss of the D19Mit119 marker in the WT TRAMP tumors, the absence of alterations of the Pten gene in these tumors, and the decreased survival in these mice is consistent with haploinsufficiency of the Pten gene promoting progression of prostate cancer , but does not constitute proof of this hypothesis.
If haploinsufficiency of the PTEN gene is sufficient to promote progression in human prostate cancer, as it is in the TRAMP system, this would provide an explanation for the observed discordance between the low rate of biallelic PTEN inactivation and the higher rate of LOH at the PTEN locus that has been found in all investigations of human prostate cancer to date. Certainly inactivation of both PTEN alleles does occur in human prostate cancers. It is likely that such biallelic inactivation is even more effective at promoting cancer progression than loss of one allele, on the basis of our survival data in the Pten+/− TRAMP mice, in which mice with inactivation of both Pten alleles in their prostate cancers tend to have the shortest survival. The discordance between rates of LOH and biallelic loss of the PTEN gene is seen in most of the human tumors in which PTEN is inactivated and is particularly striking in breast cancer. LOH at 10q23 is seen in 30–40% of invasive breast cancers, but mutations of PTEN are found in less than 5% of such cancers (3, 4, 26–28). Loss of PTEN expression has been detected in 15% of ductal breast carcinomas, but the mechanism for this loss is unclear and only half the cases with LOH at the PTEN locus have complete loss of PTEN expression (29). In addition, female patients with Cowden disease have high rates of breast cancer. Haploinsufficiency of the PTEN gene, which is sufficient to promote tumorigenesis in human breast cancer, would provide an explanation for this discordance. Li et al. (30) have shown that transgenic MMTV-Wnt-1 Pten+/− mice develop breast cancer significantly more rapidly than control mice and that 70% of breast cancers from these mice had lost the WT Pten allele. These findings are very similar to our results and strongly support the idea that Pten inactivation accelerates the progression of breast cancer. In some human malignancies, such as gliomas and endometrial carcinomas, there is a better concordance between rates of LOH at 10q23 and biallelic PTEN inactivation (4) so that the occurrence of haploinsufficiency may depend on the biology of each tumor type.
Alternative explanations of our finding of increased rates of tumor progression in Pten+/− TRAMP mice need to be considered. Although the decreased survival in the Pten+/− TRAMP mice might be attributed to a constitutional weakening and not to loss of the Pten gene in the tumors, this is unlikely for several reasons. First, male Pten+/− mice are not affected by the lymphoproliferative disorder associated with loss of one Pten allele to the same extent as females are, and they do not develop significant problems in most cases until 8 months of age (23), and in our experiment, almost all the Pten+/− TRAMP mice had died of prostate cancer before this time. In addition, animals with lymphoproliferative disorder or without invasive prostate cancer were excluded by autopsy examination. Second, the sizes of the primary tumors were actually larger in Pten+/− TRAMP mice than in WT TRAMP animals, and the extent and pattern of metastatic spread in Pten+/− and WT TRAMP mice was indistinguishable. Third, it is hard to reconcile the finding of extensive biallelic loss of the Pten gene with the decreased survival being caused by a weakened host. Finally, the loss of Pten locus in WT TRAMP mice and the decreased survival in such animals further supports the notion that the loss of Pten directly increases the rate of tumor progression. Another alternative hypothesis is that loss of one copy of the Pten gene in stromal cells or in other nontumor cells might be responsible for the increased rate of tumor progression in the Pten+/− mice. Again it is hard to explain the biallelic loss of the Pten gene in the tumors from Pten+/− TRAMP mice and the loss of one copy of mouse chromosome 19 near the Pten allele in WT TRAMP mice on the basis of a stromal effect on tumorigenesis in the Pten+/− mice.
We have demonstrated that loss of one or both Pten alleles accelerates progression of prostate cancer in the TRAMP model, but whether this acceleration is due to enhanced proliferation, decreased apoptosis, increased angiogenesis, or some combination of these is unclear. The model we used has many advantages in trying to sort out this question. First, tumor representing all stages of progression is readily available, including androgen-independent disease (31). Second, the late-stage tumors are almost pure carcinoma, which facilitates molecular analysis. Third, cell cultures can be established from tumors, and we have already established numerous clonal lines that express or completely lack Pten protein. Fourth, animals can be readily manipulated, so that the impact of Pten loss on the efficacy of drug treatments or genetic manipulations having an impact on pathways essential for progression of prostate cancer can be determined. For example, when Pten +/− heterozygous KO mice are crossed with p27kip1 KO mice, the Pten+/− p27−/− mice develop invasive prostate cancers that do not occur in Pten+/− animals (32). These findings reveal a potential synergy between loss of expression of these two proteins in the progression of prostate cancer. However, we have analyzed both tumor extracts and cell lines from primary prostate cancers with WT Pten and complete loss of Pten and have observed no difference in the level of p27kip1 protein expression by Western blotting (data not shown). Our observations are in agreement with recently published studies that show no correlation between PTEN inactivation and p27kip1 expression in human ovarian epithelial carcinomas (33). Although the total level of p27kip1 is similar in the tumors and cell lines with and without Pten inactivation, it is possible that p27kip1 is inactivated by other mechanisms in the cancers with loss of Pten, for example, by formation of complexes with D-type cyclins in the cytoplasm. Further studies are needed to define the role of p27kip1 in prostate cancers with loss of Pten activity. Our model system will allow such studies to be conducted in a relatively straightforward manner. Further studies evaluating the role of other pathways modified by loss of Pten activity are also underway and should help answer the question of how Pten inactivation facilitates the progression of prostate cancer.
Acknowledgments
This work is supported by Department of Defense Prostate Cancer Research Program Grant DAMD17-98-1-8576.
Abbreviations
- LOH
loss of heterozygosity
- TRAMP
transgenic adenocarcinoma of mouse prostate
- WT
wild type
- KO
knockout
- cM
centimorgan
- SV
simian virus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 2.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 3.Teng D H, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen K L, Vinson V L, Gumpper K L, et al. Cancer Res. 1997;57:5221–5225. [PubMed] [Google Scholar]
- 4.Ali I U, Schriml L M, Dean M. J Natl Cancer Inst. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 5.Di Cristofano A, Pandolfi P P. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 6.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 7.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu M M, Simons J W, Semenza G L. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 8.Giri D, Ittmann M. Hum Pathol. 1999;30:419–424. doi: 10.1016/s0046-8177(99)90117-x. [DOI] [PubMed] [Google Scholar]
- 9.Liaw D, Marsh D J, Li J, Dahia P L, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, et al. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 10.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman J G, Jen J, Isaacs W B, Bova G S, Sidransky D. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- 11.Pesche S, Latil A, Muzeau F, Cussenot O, Fournier G, Longy M, Eng C, Lidereau R. Oncogene. 1998;16:2879–2883. doi: 10.1038/sj.onc.1202081. [DOI] [PubMed] [Google Scholar]
- 12.Feilotter H E, Nagai M A, Boag A H, Eng C, Mulligan L M. Oncogene. 1998;16:1743–1748. doi: 10.1038/sj.onc.1200205. [DOI] [PubMed] [Google Scholar]
- 13.Wang S I, Parsons R, Ittmann M. Clin Cancer Res. 1998;4:811–815. [PubMed] [Google Scholar]
- 14.Facher E, Law J. J Med Genet. 1998;35:790. doi: 10.1136/jmg.35.9.790-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ittmann M M. Oncol Rep. 1998;5:1329–1335. doi: 10.3892/or.5.6.1329. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Freije D, Nusskern D R, Okami K, Cairns P, Sidransky D, Isaacs W B, Bova G S. Cancer Res. 1998;58:204–209. [PubMed] [Google Scholar]
- 17.Whang Y E, Wu X, Suzuki H, Reiter R E, Tran C, Vessella R L, Said J W, Isaacs W B, Sawyers C L. Proc Natl Acad Sci USA. 1998;95:5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gingrich J R, Barrios R J, Morton R A, Boyce B F, DeMayo F J, Finegold M J, Angelopoulou R, Rosen J M, Greenberg N M. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 19.Podsypanina K, Ellenson L H, Nemes A, Gu J, Tamura M, Yamada K M, Cordon-Cardo C, Catoretti G, Fisher P E, Parsons R. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi P P. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 21.Kwabi-Addo B, Thompson T C, Ittmann M. DNA Cell Biol. 2000;19:301–305. doi: 10.1089/10445490050021212. [DOI] [PubMed] [Google Scholar]
- 22.Giri D, Ropiquet F, Ittmann M. J Cell Physiol. 1999;180:53–60. doi: 10.1002/(SICI)1097-4652(199907)180:1<53::AID-JCP6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Di Cristofano A, Kotsi P, Peng Y F, Cordon-Cardo C, Elkon K B, Pandolfi P P. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 24.Hansen G M, Justice M J. Mamm Genome. 1998;9:88–90. doi: 10.1007/s003359900690. [DOI] [PubMed] [Google Scholar]
- 25.Vlietstra R J, van Alewijk D C, Hermans K G, van Steenbrugge G J, Trapman J. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 26.Singh B, Ittmann M M, Krolewski J J. Genes Chromosomes Cancer. 1998;21:166–171. [PubMed] [Google Scholar]
- 27.Bose S, Wang S I, Terry M B, Hibshoosh H, Parsons R. Oncogene. 1998;17:123–127. doi: 10.1038/sj.onc.1201940. [DOI] [PubMed] [Google Scholar]
- 28.Feilotter H E, Coulon V, McVeigh J L, Boag A H, Dorion-Bonnet F, Duboue B, Latham W C, Eng C, Mulligan L M, Longy M. Br J Cancer. 1999;79:718–723. doi: 10.1038/sj.bjc.6690115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perren A, Weng L P, Boag A H, Ziebold U, Thakore K, Dahia P L, Komminoth P, Lees J A, Mulligan L M, Mutter G L, Eng C. Am J Pathol. 1999;155:1253–1260. doi: 10.1016/S0002-9440(10)65227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Podsypanina K, Liu X, Crane A, Tan L K, Parsons R, Varmus H E. BMC Mol Biol. 2001;2:2. doi: 10.1186/1471-2199-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gingrich J R, Barrios R J, Kattan M W, Nahm H S, Finegold M J, Greenberg N M. Cancer Res. 1997;57:4687–4691. [PubMed] [Google Scholar]
- 32.Di Cristofano A, Acetis M, Koff A, Cordon-Cardo C, Pandolfi P P. Nat Genet. 2001;27:1–3. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- 33.Kurose K, Zhou X, Araki T, Cannistra S, Maher E, Eng C. Am J Pathol. 2001;158:2097–2106. doi: 10.1016/S0002-9440(10)64681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]