Key Points
Question
Which of 3 fat-processing techniques results in better volume retention in recipient sites in autologous fat grafting to correct facial asymmetry?
Findings
This randomized clinical trial using 3-dimensional volumetric analysis found that the cotton pad filtration technique demonstrated a statistically higher percentage of volume maintenance of grafted fat in comparison with the centrifugation and sedimentation techniques within a 1-year follow-up period.
Meaning
Autologous fat processed by means of a cotton pad filtration technique is an effective method of fat grafting that improves the volume retention of grafted fat.
This randomized clinical trial uses 3-dimensional volumetric analysis to assess which of 3 fat-processing techniques results in better volume retention in recipient sites in autologous fat grafting to correct facial asymmetry.
Abstract
Importance
Autologous fat grafting has revolutionized the field of facial soft-tissue augmentation, despite a lack of standardization. Objective data are needed to arrive at consensus regarding the best technique for optimal volume retention.
Objective
To compare 3 fat-processing techniques with 3-dimensional (3-D) technology to explore the optimal fat-processing technique for improving the volume retention of grafted fat.
Design, Setting, and Participants
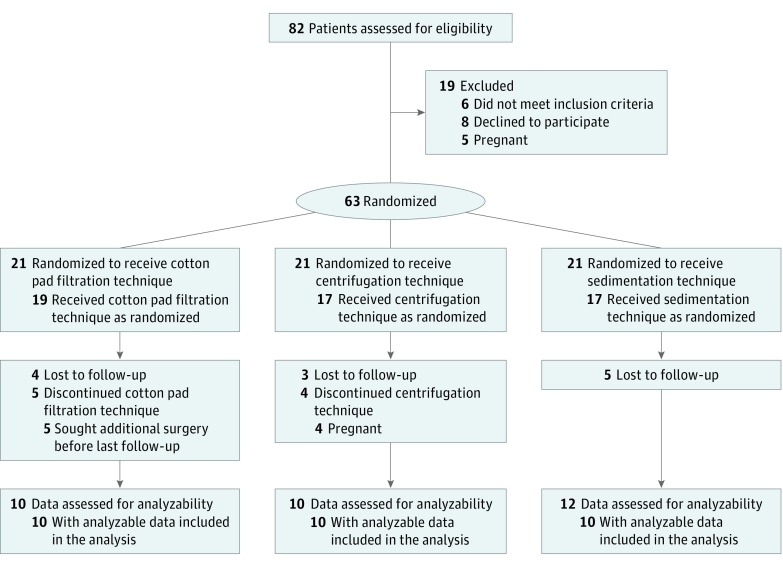
From September 2015 to December 2016, patients with facial asymmetry were treated by initial facial fat grafting at the Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Sixty-three patients (21 per group) were randomized to 1 of 3 fat-processing techniques: sedimentation, centrifugation, and cotton pad filtration. Patients underwent 3-D scanning preoperatively and at 1, 3, 6, and 12 months postoperatively. Patients who did not complete preoperative or postoperative follow-up and 3-D imaging were excluded from the analysis.
Intervention
Autologous fat grafting to correct facial asymmetry.
Main Outcomes and Measures
The percentage volume maintenance of each fat-processing technique was measured with 3-D software and analyzed with variance analysis.
Results
Of the 63 randomized patients, 30 (7 men, 23 women; mean [SD] age at surgery, 22.2 [8.0] years) completed follow-up. The mean (SD) percentage volume maintenance of the 3 groups at 1, 3, 6, and 12 months postoperatively was, respectively, 49% (4%), 45% (3%), 43% (3%), and 41% (3%) for the cotton pad filtration group; 41% (3%), 38% (4%), 36% (4%), and 34% (3%) for the centrifugation group; and 37% (4%), 34% (4%), 31% (3%), and 31% (3%) for sedimentation group. The variance analysis showed that the cotton pad filtration group demonstrated a statistically significant higher percentage volume maintenance in comparison with the centrifugation and sedimentation groups.
Conclusions and Relevance
The use of 3-D technology provides an objective and accurate way to evaluate different fat-processing techniques. Autologous fat processed by cotton pad filtration had a significant higher volume retention than did that processed by centrifugation and sedimentation technique.
Trial Registration
chictr.org.cn Identifier: ChiCTR-IOR-14005599
Level of Evidence
1.
Introduction
Autologous fat grafting has long been considered a well-established and frequently applied option for facial volume augmentation in plastic surgery.1,2 Despite its increasing popularity, the volume of transplanted fat has been limited by a variable and unpredictable rate of fat absorption (20%-90%).3,4,5 To minimize this drawback, a wealth of publications in the literature have proposed ways to optimize each step of the fat grafting, including the techniques of fat harvesting, fat processing, and fat injecting. Unfortunately, no standardized protocol has been adopted.6,7,8,9 The optimal fat-processing technique remains one of the most controversial issues.10,11 To our knowledge, several common fat-processing techniques have been widely applied in the clinic, primarily based on sedimentation,12 centrifugation,13 and filtration principles.14,15 Many studies published in recent decades have demonstrated clinical results with a certain fat-processing technique; however, it is hard to reach a consensus on which fat-processing technique would reveal better volume retention without objective and comparative evidence.
Previous evaluations of fat grafting mainly relied on the subjective assessment of 2-dimensional (2D) photographs or questionnaires for patients.16 Although some measurement methods existed that could provide objective values such as the anthropometric method, water displacement, thermoplastic casting, computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography, these can be inaccurate, cumbersome, and time-consuming.17,18,19,20,21 Currently, 3-dimensional (3-D) technology has become more important in plastic surgery. Its accuracy and validation in evaluation of facial fat grafting has been proved by our group.22 Moreover, the term percentage volume maintenance has emerged as a promising indicator in evaluating the effect of fat grafting with 3-D technology.23 However, previous articles about 3-D techniques have not gone beyond emphasizing the importance of a certain fat-processing technique. Thus, the aim of this study was to compare different fat-processing techniques (sedimentation, centrifugation, and cotton pad filtration) with 3-D volumetric analysis and then to objectively determine the optimal fat-processing technique.
Methods
Patients
From September 2015 to December 2016, 63 patients with facial asymmetry undergoing initial facial fat grafting at the Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, were enrolled in this study. They were randomly allocated to 1 of 3 fat-processing groups: sedimentation, centrifugation, or cotton pad filtration (Figure 1). Patients underwent 3-D facial scanning preoperatively and postoperatively at 1-, 3-, 6-, and 12-month follow-up with the Artec Spider 3D Scanner (Artec 3D). The protocol (Supplement 1) was approved by the Ethical Institutional Committee of the Plastic Surgery Hospital, Chinese Academy of Medical Sciences and registered with ChiCTR (ChiCTR-IOR-14005599). After written informed consent was obtained, patients who did not complete preoperative or postoperative follow-up and 3-D imaging were excluded.
Figure 1. CONSORT Flowchart.
Surgical Techniques
The procedure was performed under sedative anesthesia in clinic operating rooms. Apart from different fat-processing techniques, the fat-harvesting and fat-injecting techniques applied were the same for patients. The lower abdomen was chosen to be the donor site for all patients. After infiltration with tumescent solution, fat tissue aspiration was manually performed using a blunt-tipped cannula (2.5 mm in diameter) connected to a 20-mL syringe. Next, the harvested fat was washed with normal saline twice and then processed with one of the following fat-processing techniques: (1) For the sedimentation technique, the syringes filled with lipoaspirates were held upside down statically for 15 minutes to let gravity separate the contents into distinct layers. The top layer of fatty oil was decanted, and the bottom aqueous layer was drained away through the syringe’s outlet. The remaining middle layer of fat tissue was then transferred for fat grafting (eFigure 1A and B in Supplement 2). (2) For the centrifugation technique, the syringes were placed in a centrifuge and spun at 1000 rpm (161 g) for 3 minutes. Similarly, the top free lipid and lower mixture of fluids, blood cells, and cellular debris in the syringes were discarded. Similarly, the middle layer of refined fat tissue was reserved for fat injection (eFigure 1C and D in Supplement 2). (3) For the cotton pad filtering technique, harvested fat was directly poured from each syringe onto a sterilized cotton pad (30 × 20 × 1.5 cm), which was wrapped with multiple pieces of gauze. The aspirated fat was gently rolled and kneaded along the cotton pad using a sterile scalpel handle for 5 minutes, allowing the oil and aqueous portions to be absorbed by the cotton pad. Finally, the residual fat tissue left on the cotton pad was collected for implantation (eFigure 1E and F in Supplement 2). The volume of injected fat was recorded in milliliters.
3-D Volumetric Analysis
The facial scanning was obtained preoperatively and postoperatively at 1-, 3-, 6-, and 12-month follow-up through the Artec Spider 3D Scanner, with high resolution (up to 0.01 mm) and superior accuracy (up to 0.03 mm). During image acquisition, patients were instructed to close their eyes and sit upright, with the Frankfurt horizontal plane parallel to the ground. A skilled technician held the scanner to capture facial images with an optimum object-to-scanner distance of 450 to 700 mm. The total scanning time was approximately 10 seconds. If the patient moved during the scan, the procedure was repeated.
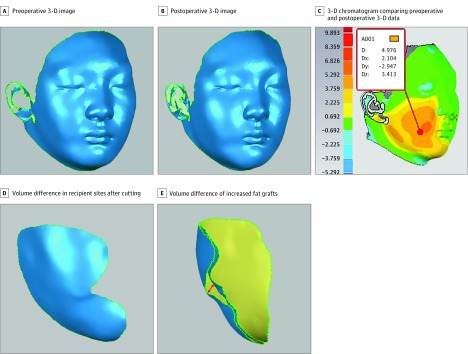
After image acquisition, the preoperative and postoperative 3-D data were converted into reverse-engineering software Geomagic Qualify 12.0 (Raindrop Geomagic) for image registration. The preoperative image (Figure 2A) was defined as the reference, and the postoperative image (Figure 2B) was automatically matched with the reference to integrate 3-D superimposed data by a best-fit algorithm. The 3-D superimposition was an integral image showing the difference between preoperative and postoperative data, which can be manifested by a 3-D chromatogram (Figure 2C). From this colored hypsography, the zone of facial discrepancy was highlighted and used to select the recipient sites. Moreover, the depth of morphological changes was also noted. Next, the software’s cutting tool was used to eliminate irrelevant areas of preoperative and postoperative data, generating 2 sectional models in the recipient sites (Figure 2D). The spatial region (arrowhead) between the preoperative and postoperative models can be regarded as the increased area of soft-tissue augmentation (Figure 2E). The volume difference was precisely calculated with a Boolean operation by means of 3-matic 7.0 (Materialise) software, with the result measured in cubic millimeters. Finally, the percentage volume maintenance of grafted fat was obtained using the following equation: volume maintenance (%) = (3-D volume difference/injected fat amount) × 100.
Figure 2. Three-Dimensional (3-D) Volumetric Analysis.
The arrowhead in E indicates the spatial region between the preoperative and postoperative models, which can be regarded as the volume difference of the increased fat grafts. D indicates distance; Dx, x-axis distance; Dy, y-axis distance; Dz, z-axis distance.
Statistical Analysis
Statistical analysis was performed with SPSS, version 17.0. The percentage volume maintenance in the 3 fat-processing groups was calculated with 3-D technology and was analyzed with variance analysis, which was expressed as mean (SD). A value of P < .05 was considered statistically significant. In other studies, patient sex, age, fat-processing group, and body mass index (BMI) at 1-, 3-, 6-, and 12-month follow-up have been associated with the percentage volume maintenance, so these variables were recorded and analyzed with covariance analysis to estimate the reliability of the study.
Results
Of the 63 randomized patients with a diagnosis of facial asymmetry, 30 (7 men, 23 women) completed follow-up after undergoing initial facial fat grafting. The mean (SD) age of these patients at surgery was 22.2 (8.0) years. In the lipotransfer, all fat used was harvested from the abdomen, and the mean volume of fat injected in the face was 27.5 mL. Patients obtained excellent outcomes, and no early or late complications were recorded. However, among the patients who did not complete follow-up were 5 in the cotton pad filtration group who were too impatient to wait until 12-month follow-up before undergoing further facial plastic surgery procedures. Other patients in the centrifugation and sedimentation groups completed the first 3 follow-up visits but did not do the 3-D scanning at 12-month follow-up. Thus, 8 cases were excluded at 12-month follow-up.
The operation of the 3-D scanner and the volumetric calculation using the 3-D software was performed by the same technician (B.L.). The percentage volume maintenance in each fat-processing group in the 30 cases was recorded (eTable 1 in Supplement 2). The mean (SD) percentage volume maintenance at 1-, 3-, 6-, and 12-month follow-up was, respectively, 49% (4%), 45% (3%), 43% (3%), and 41% (3%) for the cotton pad filtering group; 41% (3%), 38% (4%), 36% (4%), and 34% (3%) for the centrifugation group; and 37% (4%), 34% (4%), 31% (3%), and 31% (3%) for the sedimentation group. These data showed that grafts processed by means of cotton pad filtration had the highest percentage volume maintenance, followed by the centrifugation and sedimentation methods. Variance analysis revealed that the cotton pad filtering group demonstrated a statistically significant higher percentage volume maintenance in comparison with the centrifugation group and the sedimentation group at 1-, 3-, 6-, and 12-month follow-up (eTable 2 in Supplement 2).
Covariance analysis revealed that the fat-processing method was associated with the percentage volume maintenance (eTables 3-6 in Supplement 2). Factors such as sex, age, and BMI at 1-, 3-, 6-, and 12-month follow-up had no association with the percentage volume maintenance (eTables 3-6 in Supplement 2). These results confirmed that the superiority of cotton pad filtration was not confounded by other factors.
Case Reports
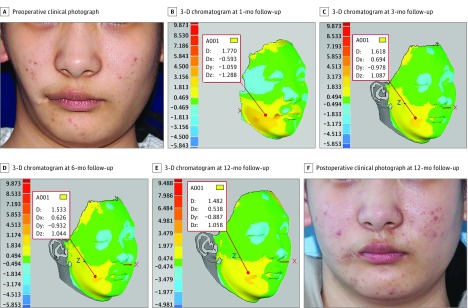
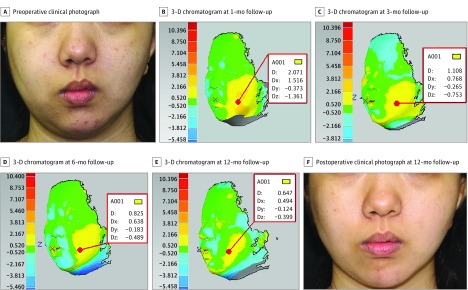
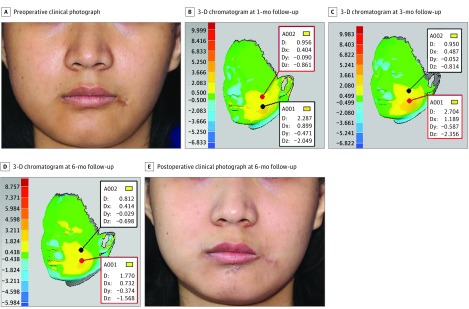
Facial asymmetry is a group of complex deformities that feature differences in the location of anatomic landmarks, and in the volume of tridimensional surfaces. Herein we summarize the outcomes of 1 patient with a diagnosis of hemifacial microsomia in each fat-processing group. Using the cotton pad filtering technique, 35 mL of purified fat was injected into the right side of the face of a 19-year-old woman. With 3-D volumetric analysis, the zone of facial discrepancy was clearly highlighted and the depth of morphological changes was noted at 1-, 3-, 6-, and 12-month follow-up (Figure 3B-E). The facial volumetric difference for this patient at 1-, 3-, 6-, and 12-month follow-up was, respectively, 15.770 mL (eFigure 2 in Supplement 2), 15.484 mL (eFigure 3 in Supplement 2), 15.227 mL (eFigure 4 in Supplement 2), and 14.551 mL (eFigure 5 in Supplement 2). The percentage volume maintenance was 45%, 44%, 44%, and 42%. Using the centrifugation technique, a 19-year-old woman was treated by facial fat grafting on the left side of the face. The injected fat volume was 29 mL; postoperative 3-D chromatograms at 1-, 3-, 6-, and 12-month follow-up (Figure 4B-E) showed percentage volume maintenance of, respectively, 42%, 39%, 38%, and 36%. Using the sedimentation technique, 25 mL of purified fat was implanted into the left side of the face to obtain facial symmetry for a 20-year-old woman. Results from 3-D colored hypsography were presented at 1-, 3-, and 6-month follow-up (Figure 5B-D). The percentage volume maintenance was, respectively, 37%, 33%, and 32%.
Figure 3. Three-Dimensional (3-D) Volumetric Analysis of Cotton Pad Filtration Technique.
Figure 4. Three-Dimensional (3-D) Volumetric Analysis of Centrifugation Technique.
Figure 5. Three-Dimensional (3-D) Volumetric Analysis of Sedimentation Technique.
Discussion
Autologous fat has already been accepted as an excellent soft-tissue filler in correcting facial asymmetry, given its abundance, ease of harvest, and natural appearance. However, many patients have needed repeated fat grafting because of the variable rate of fat absorption. Thus, surgeons go to great lengths to carefully process the harvested fat in the hope of improving the volume retention of the grafts. The proper fat-processing technique should maximize the volume of viable and intact fat granule while minimizing trauma and removing nonviable components of lipoaspirates such as free lipids from ruptured adipocytes, tumescent solution, blood cells, and debris.11,24,25 In evaluating the efficacy of fat grafting, some surgeons have used 2-D photographs or questionnaires before and after surgery.16 However, these methods are subjective. Some investigators have advocated measuring tools such as the anthropometric method, animal experimentation, or water displacement; however, these have been criticized as being inaccurate and impractical. Other scholars have promoted objective measurements such as CT, MRI, and ultrasonography.17,18,19,20 Although CT has long been successful in capturing details of hard tissues, it cannot image soft tissues precisely. Moreover, exposure to multiple CT scans is associated with potential radiation exposure and high cost. Although MRI and ultrasonography are adaptable for imaging soft tissues, these techniques cannot distinguish the transferred fat from fat tissues previously at the implantation sites. In addition, patients must stay in a supine position when examined by CT or MRI, and any position changes will affect the volumetric calculation of grafts.26 On the basis of a thorough review of the present scientific literature and clinical experience, the American Society of Plastic Surgeons Fat Graft Task Force proposed that no consensus statement be issued on which fat-processing technique is superior to the others unless there is evidence-based and comparative study.27
Recently, 3-D technology has been introduced to evaluate the efficiency of fat grafting with the metric percentage volume maintenance.23 Although metrics such as graft take or percentage survival28 also suggest the ability to quantify the volume of grafted fat, this is unscientific in clinical practice because it is the volume that we can measure objectively and not the adipocyte survival. Therefore, we do not speak of percentage survival in evaluating fat grafting but recommend use of the term percentage volume maintenance. The structure of the face is tridimensional, and 3-D technology serves as a valuable tool to capture photorealistic images of facial soft tissues. The Artec Spider 3D scanner used in the present study weighs only 850 g, making it portable and convenient to use. There is no need for markers to be placed on patients’ faces, and there are no complicated post-processing procedures. The scanner can capture up to 7.5 frames per second and ultimately obtain brilliant color, as well as high-resolution, accurate 3-D images. After scanning, the preoperative and postoperative data were analyzed with 3-D software for volumetric analysis. To acquire precise volumetric changes in the recipient sites, we used the best-fit module to automatically compare the preoperative and postoperative images. Based on this accurate registration, volumetric discrepancies of facial asymmetry can be visually manifested with a 3-D chromatogram. Then, detailed information including the depth and the volume difference of facial discrepancy can be objectively quantified.
Previous 3-D research has emphasized the importance of one fat-processing technique or another without reporting any data that objectively compare techniques with 3-D technology. For the sedimentation technique, no evidence has been provided to quantify the 3-D volume retention. For the centrifugation technique, no consensus has been accepted because various centrifuge speeds in surgery produced different outcomes. Guibert et al29 reported that the volume retention for 11 children treated with centrifuged fat (3000 rpm, 2 minutes) was 41% at 3-month follow-up and 36% at 6-month follow-up. Meier et al3 found a mean percentage volume maintenance in aesthetic surgery of 31.8%, without centrifugation parameters mentioned. For the cotton pad filtration technique, few 3-D volumetric analyses have been investigated. Use of filter media such as cotton gauze or cotton towel has been reported in animal experiments, but weight or volume of grafts has been measured with rough tools.14 The sole finding of adequate precision is that of Gerth et al,30 who reported a 3-D volumetric retention with closed-membrane filtration of 41.2%. Moreover, this finding was significantly higher than the 31.8% noted in their prior study with centrifugation. In addition, they supposed that purer grafted fat with less contamination led to the increased graft volume. Our findings coincide with their conclusion. The cotton pad filtration method demonstrated a statistically significant higher percentage volume maintenance in comparison with the centrifugation and sedimentation techniques during the entire follow-up period. A higher proportion of oil and aqueous contents were filtered out and a higher concentration of fat contents was reserved for implantation. In contrast, the oil and aqueous portions were not drained adequately in the sedimentation method. Additionally, more manipulation was used in centrifugation, which may result in higher fat absorption than cotton pad filtration. Moreover, factors such as sex, age, and BMI at 1-, 3-, 6-, and 12-month follow-up were not associated with the percentage volume maintenance, which helps to eliminate bias and increase the reliability of this study.
Limitations
This study has some limitations. Patients underwent 3-D scanning preoperatively and at 1, 3, 6, and 12 months postoperatively. The poor adherence of patients was the main concern. The cost of several follow-up visits was high and troublesome. Some patients were too impatient to wait until 12-month follow-up and sought other facial esthetic surgical procedures. Other patients, although they completed the first 2 or 3 follow-up visits, did not do the subsequent 3-D scanning. The number of follow-up cases needs to be increased and emphasized in further study.
Conclusions
Three-dimensional technology is an objective and effective way to quantify facial volumetric differences in fat grafting. In this comparative study, cotton pad filtration demonstrated a statistically significant higher volume retention of grafted fat in comparison with the centrifugation and sedimentation techniques. The superiority of the cotton pad filtration makes it worthwhile for clinical application. The long-term volumetric retention of transplanted fat needs to be elucidated further, and these studies are under way.
Trial Protocol
eTable 1. Percentage volume maintenance of different fat-processing groups in 30 cases
eTable 2. Percentage Volume Maintenance of the Fat-Processing Groups at 1-, 3-, 6-, and 12-Month Follow-up
eTable 3. Covariance analysis (grouping, gender, age, BMI-1m factor)
eTable 4. Covariance analysis (grouping, gender, age, BMI-3m factor)
eTable 5. Covariance analysis (grouping, gender, age, BMI-6m factor)
eTable 6. Covariance analysis (grouping, gender, age, BMI-12m factor)
eFigure 1A. pre-sedimentation
eFigure 1B. post-sedimentation
eFigure 1C. pre-centrifugation
eFigure 1D. post-centrifugation
eFigure 1E. the cotton pad filtering
eFigure 1F. post-filtration (the residual fat tissue left on the cotton pad was collected again for implantation)
eFigure 2. facial volumetric differences of the patient at post-1m
eFigure 3. facial volumetric differences of the patient at post-3m
eFigure 4. facial volumetric differences of the patient at post-6m
eFigure 5. facial volumetric differences of the patient at post-12m
References
- 1.Tocco I, Widgerow AD, Lalezari S, Banyard D, Shaterian A, Evans GR. Lipotransfer: the potential from bench to bedside. Ann Plast Surg. 2014;72(5):599-609. [DOI] [PubMed] [Google Scholar]
- 2.Zielins ER, Brett EA, Longaker MT, Wan DC. Autologous fat grafting: the science behind the surgery. Aesthet Surg J. 2016;36(4):488-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier JD, Glasgold RA, Glasgold MJ. Autologous fat grafting: long-term evidence of its efficacy in midfacial rejuvenation. Arch Facial Plast Surg. 2009;11(1):24-28. [DOI] [PubMed] [Google Scholar]
- 4.Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130(1):249-258. [DOI] [PubMed] [Google Scholar]
- 5.Rohrich RJ, Sorokin ES, Brown SA. In search of improved fat transfer viability: a quantitative analysis of the role of centrifugation and harvest site. Plast Reconstr Surg. 2004;113(1):391-395. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Brace M, Taylor SM, Bezuhly M, Hong P. In search of the optimal processing technique for fat grafting. J Craniofac Surg. 2015;26(1):94-99. [DOI] [PubMed] [Google Scholar]
- 7.Smith P, Adams WP Jr, Lipschitz AH, et al. Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast Reconstr Surg. 2006;117(6):1836-1844. [DOI] [PubMed] [Google Scholar]
- 8.Hsu VM, Stransky CA, Bucky LP, Percec I. Fat grafting’s past, present, and future: why adipose tissue is emerging as a critical link to the advancement of regenerative medicine. Aesthet Surg J. 2012;32(7):892-899. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman MR, Bradley JP, Dickinson B, et al. Autologous fat transfer national consensus survey: trends in techniques for harvest, preparation, and application, and perception of short- and long-term results. Plast Reconstr Surg. 2007;119(1):323-331. [DOI] [PubMed] [Google Scholar]
- 10.Tuin AJ, Domerchie PN, Schepers RH, et al. What is the current optimal fat grafting processing technique? a systematic review. J Craniomaxillofac Surg. 2016;44(1):45-55. [DOI] [PubMed] [Google Scholar]
- 11.Pu LL. Towards more rationalized approach to autologous fat grafting. J Plast Reconstr Aesthet Surg. 2012;65(4):413-419. [DOI] [PubMed] [Google Scholar]
- 12.Condé-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63(8):1375-1381. [DOI] [PubMed] [Google Scholar]
- 13.Coleman SR. Hand rejuvenation with structural fat grafting. Plast Reconstr Surg. 2002;110(7):1731-1744. [DOI] [PubMed] [Google Scholar]
- 14.Ramon Y, Shoshani O, Peled IJ, et al. Enhancing the take of injected adipose tissue by a simple method for concentrating fat cells. Plast Reconstr Surg. 2005;115(1):197-201. [PubMed] [Google Scholar]
- 15.Minn KW, Min KH, Chang H, Kim S, Heo EJ. Effects of fat preparation methods on the viabilities of autologous fat grafts. Aesthetic Plast Surg. 2010;34(5):626-631. [DOI] [PubMed] [Google Scholar]
- 16.Lee MS, Chung DH, Lee JW, Cha KS. Assessing soft-tissue characteristics of facial asymmetry with photographs. Am J Orthod Dentofacial Orthop. 2010;138(1):23-31. [DOI] [PubMed] [Google Scholar]
- 17.Herold C, Ueberreiter K, Busche MN, Vogt PM. Autologous fat transplantation: volumetric tools for estimation of volume survival: a systematic review. Aesthetic Plast Surg. 2013;37(2):380-387. [DOI] [PubMed] [Google Scholar]
- 18.Choi M, Small K, Levovitz C, Lee C, Fadl A, Karp NS. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg. 2013;131(2):185-191. [DOI] [PubMed] [Google Scholar]
- 19.Hörl HW, Feller AM, Biemer E. Technique for liposuction fat reimplantation and long-term volume evaluation by magnetic resonance imaging. Ann Plast Surg. 1991;26(3):248-258. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Jiang Y, Meng H, Yu Y, Qi K. Sonographic assessment on breast augmentation after autologous fat graft. Plast Reconstr Surg. 2008;122(1):36e-38e. [DOI] [PubMed] [Google Scholar]
- 21.Wu R, Jiang H, Chen W, et al. Three-dimensional chest computed tomography analysis of thoracic deformities in patients with microtia. J Plast Reconstr Aesthet Surg. 2015;68(4):498-504. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Wu R, Bi H, et al. Autologous fat grafting with combined three-dimensional and mirror-image analyses for progressive hemifacial atrophy. Ann Plast Surg. 2016;77(3):308-313. [DOI] [PubMed] [Google Scholar]
- 23.Del Vecchio DA, Del Vecchio SJ. The graft-to-capacity ratio: volumetric planning in large-volume fat transplantation. Plast Reconstr Surg. 2014;133(3):561-569. [DOI] [PubMed] [Google Scholar]
- 24.Fisher C, Grahovac TL, Schafer ME, Shippert RD, Marra KG, Rubin JP. Comparison of harvest and processing techniques for fat grafting and adipose stem cell isolation. Plast Reconstr Surg. 2013;132(2):351-361. [DOI] [PubMed] [Google Scholar]
- 25.Zhu M, Cohen SR, Hicok KC, et al. Comparison of three different fat graft preparation methods: gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast Reconstr Surg. 2013;131(4):873-880. [DOI] [PubMed] [Google Scholar]
- 26.Kau CH, Richmond S, Incrapera A, English J, Xia JJ. Three-dimensional surface acquisition systems for the study of facial morphology and their application to maxillofacial surgery. Int J Med Robot. 2007;3(2):97-110. [DOI] [PubMed] [Google Scholar]
- 27.Gutowski KA; ASPS Fat Graft Task Force . Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg. 2009;124(1):272-280. [DOI] [PubMed] [Google Scholar]
- 28.Ueberreiter K, von Finckenstein JG, Cromme F, Herold C, Tanzella U, Vogt PM. BEAULI™—a new and easy method for large-volume fat grafts [in German]. Handchir Mikrochir Plast Chir. 2010;42(6):379-385. [DOI] [PubMed] [Google Scholar]
- 29.Guibert M, Franchi G, Ansari E, et al. Fat graft transfer in children’s facial malformations: a prospective three-dimensional evaluation. J Plast Reconstr Aesthet Surg. 2013;66(6):799-804. [DOI] [PubMed] [Google Scholar]
- 30.Gerth DJ, King B, Rabach L, Glasgold RA, Glasgold MJ. Long-term volumetric retention of autologous fat grafting processed with closed-membrane filtration. Aesthet Surg J. 2014;34(7):985-994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Percentage volume maintenance of different fat-processing groups in 30 cases
eTable 2. Percentage Volume Maintenance of the Fat-Processing Groups at 1-, 3-, 6-, and 12-Month Follow-up
eTable 3. Covariance analysis (grouping, gender, age, BMI-1m factor)
eTable 4. Covariance analysis (grouping, gender, age, BMI-3m factor)
eTable 5. Covariance analysis (grouping, gender, age, BMI-6m factor)
eTable 6. Covariance analysis (grouping, gender, age, BMI-12m factor)
eFigure 1A. pre-sedimentation
eFigure 1B. post-sedimentation
eFigure 1C. pre-centrifugation
eFigure 1D. post-centrifugation
eFigure 1E. the cotton pad filtering
eFigure 1F. post-filtration (the residual fat tissue left on the cotton pad was collected again for implantation)
eFigure 2. facial volumetric differences of the patient at post-1m
eFigure 3. facial volumetric differences of the patient at post-3m
eFigure 4. facial volumetric differences of the patient at post-6m
eFigure 5. facial volumetric differences of the patient at post-12m