Abstract
This cohort study assesses utilization patterns of continuous renal replacement therapy and attempts to establish evidence-based guidelines to standardize the process flow and to promote meaningful use.
Continuous renal replacement therapy (CRRT) is an essential tool in the management of renal failure in patients who are critically ill. Though its utilization has increased globally, it is a resource-intensive, costlier modality of dialysis. Furthermore, its usage is highly variable owing to the heterogeneity of patients and physicians, as well as the paucity of evidence to guide practice. These characteristics make it a prime target for high-value care through standardization of practice. At our institution, we assessed utilization patterns of CRRT and established evidence-based guidelines to standardize process flow and promote meaningful use.
Methods
A multidisciplinary task force was organized in October 2015 to assess CRRT utilization patterns. Interventions were implemented throughout fiscal year (FY) 2016, including the creation of evidence-based guidelines that: (1) clarified each physician’s role in the initiation, maintenance, and cessation of CRRT; (2) defined indications to start therapy with a focus on patient goals of care; (3) described situations where CRRT would be medically inappropriate; (4) mandated daily cross-disciplinary communication between medical teams and key stakeholders; and (5) provided guidance on discontinuing CRRT. Additional measures to minimize excess laboratory tests and promote awareness of CRRT were also implemented. Comparisons between preintervention (FY 2014-2015) and postintervention (FY 2016-2017) cohorts were made with the independent samples t test for continuous variables, and Pearson χ2 test for categorical variables. Institutional board approval was waived, and patient consent was not necessary because all data was based on deidentified records.
Results
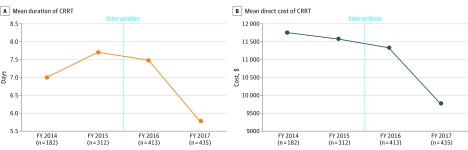
A total of 1342 patients received CRRT from 2014 to 2017 (Figure). The number of patients on CRRT increased from 182 in 2014 to 435 in 2017, while the total number of days on CRRT increased from 1272 days to 2505 days. The majority were men (n = 842 [62.7%]) and surgical patients (n = 726 [54.1%]), while the rest were medical (n = 616 [45.9%]).
Figure. Improvement in CRRT Utilization After a Series of Interventions.
While the number of patients on CRRT increased over time, the mean duration of CRRT decreased by 11.3% from the preintervention period to the postintervention period (7.43 vs 6.59 days; P = .03). The average direct cost of CRRT decreased by 9.8% ($11 642 vs $10 506; P < .001). CRRT indicates continuous renal replacement therapy; FY, fiscal year.
The mean duration of CRRT decreased by 11.3% from the preintervention period to the postintervention period (7.43 vs 6.59 days; P = .03). Similarly, the average direct cost of CRRT decreased by 9.8% ($11 642 vs $10 506; P < .001). This led to a savings of $1136 per patient, or an estimated annual savings of $481 664 after the intervention.
Discharge disposition of patients on CRRT changed from the preintervention period to the postintervention period (Table). The proportion of patients expiring on CRRT decreased from 60.3% to 47.5%, while the proportion of patients transitioning to comfort care increased from 3% to 8.6% (P < .001).
Table. Discharge Disposition of Patients on CRRT by Time and Age.
| Characteristic | Discharge Disposition, No. (%) | P Value | ||||
|---|---|---|---|---|---|---|
| Death | Comfort Care | Home | Skilled Nursing Facility | Transfer | ||
| Intervention | ||||||
| Pre- (n = 494) | 298 (60.3) | 15 (3.0) | 89 (18.0) | 75 (15.2) | 17 (3.4) | <.001 |
| Post- (n = 848) | 403 (47.5) | 73 (8.6) | 190 (22.4) | 156 (18.9) | 26 (3.1) | |
| Age, y | ||||||
| 18-45 (n = 260) | 114 (43.8) | 3 (1.2) | 89 (34.2) | 46 (17.7) | 8 (3.1) | <.001 |
| 46-65 (n = 637) | 319 (50.1) | 47 (7.4) | 135 (21.2) | 112 (17.6) | 24 (3.8) | |
| 65-80 (n = 337) | 199 (59.1) | 27 (8.0) | 50 (14.8) | 51 (15.1) | 10 (3.0) | |
| >80 (n = 108) | 69 (63.9) | 11 (10.2) | 5 (4.6) | 22 (20.4) | 1 (0.9) | |
Abbreviation: CRRT, continuous renal replacement therapy.
Discharge disposition of patients on CRRT also varied by age. Compared with patients ages 18 to 45 years, those over the age of 80 years had the highest mortality rate (63.9% vs 43.8%), and the lowest rate of discharge to home (4.6% vs 34.2%; P < .001).
Discussion
Our institution targeted high-value care in CRRT with a series of interventions that standardized its process flow. These interventions included a set of evidence-based guidelines that established physician roles, mandated daily cross-disciplinary communication, encouraged appropriate patient selection for therapy, and emphasized patient goals of care. While the volume of patients and total number of CRRT days continued to grow after our intervention in FY 2016, we decreased the average duration of treatment from 7.43 days to 6.59 days per patient, and decreased the average direct cost of CRRT from $11 642 to $10 506. Furthermore, the interventions were associated with metrics of appropriate use, including a decrease in the proportion of patients who died while on CRRT, and an increase in the proportion of patients transitioning to comfort care. These results reflect our institution’s goal to facilitate meaningful use of continuous dialysis in our intensive care units.
References
- 1.Afshinnia F, Straight A, Li Q, Slinin Y, Foley RN, Ishani A. Trends in dialysis modality for individuals with acute kidney injury. Ren Fail. 2009;31(8):647-654. [DOI] [PubMed] [Google Scholar]
- 2.Iwagami M, Yasunaga H, Noiri E, et al. Choice of renal replacement therapy modality in intensive care units: data from a Japanese Nationwide Administrative Claim Database. J Crit Care. 2015;30(2):381-385. [DOI] [PubMed] [Google Scholar]
- 3.Manns B, Doig CJ, Lee H, et al. Cost of acute renal failure requiring dialysis in the intensive care unit: clinical and resource implications of renal recovery. Crit Care Med. 2003;31(2):449-455. [DOI] [PubMed] [Google Scholar]
- 4.Srisawat N, Lawsin L, Uchino S, Bellomo R, Kellum JA; BEST Kidney Investigators . Cost of acute renal replacement therapy in the intensive care unit: results from The Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) study. Crit Care. 2010;14(2):R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rewa O, Villeneuve PM, Eurich DT, et al. Quality indicators in continuous renal replacement therapy (CRRT) care in critically ill patients: protocol for a systematic review. Syst Rev. 2015;4:102. [DOI] [PMC free article] [PubMed] [Google Scholar]