SUMMARY
Efficient insulin secretion requires a well-functioning pancreatic islet microvasculature. The dense network of islet capillaries includes the islet pericyte, a cell that has barely been studied. Here we show that islet pericytes help control local blood flow by adjusting islet capillary diameter. Islet pericytes cover 40% of the microvasculature, are contractile, and are innervated by sympathetic axons. Sympathetic adrenergic input increases pericyte activity and reduces capillary diameter and local blood flow. By contrast, activating beta cells by increasing glucose concentration inhibits pericytes, dilates islet capillaries, and increases local blood flow. These effects on pericytes are mediated by endogenous adenosine, which is likely derived from ATP co-released with insulin. Pericyte coverage of islet capillaries drops drastically in type 2 diabetes, suggesting that, under diabetic conditions, islets lose this mechanism to control their own blood supply. This may lead to inadequate insulin release into the circulation, further deteriorating glycemic control.
Keywords: diabetes, pericytes, pancreatic islet, insulin secretion, capillary diameter, blood flow, hyperemia, adenosine, noradrenaline, sympathetic, beta cell, pancreatic slice
eTOC BLURB
XXX et al study pericyte cells, which wrap around capillary endothelial cells, in the islets of the pancreas and show that islet pericytes help control local blood flow by adjusting islet capillary diameter. They further show that this mechanism in lost in diabetes, which may lead to inadequate insulin supply.
INTRODUCTION
The pathogenesis of type 2 diabetes is associated with dysfunction of the pancreatic islet and of its vasculature (Ballian and Brunicardi, 2007; Richards et al., 2010). The microvasculature of the islet originates from few feeding arterioles that branch into a dense network of capillaries that allows efficient insulin release into the circulation (Ballian and Brunicardi, 2007). Capillary tubes are made of a thin layer of endothelial cells covered by pericytes. Pericytes were first characterized by Ebert in the 19th century as mural cells embedded within the endothelium basement membrane (Bergers and Song, 2005). Pericytes interact closely with endothelial cells and are crucial for proper capillary function, giving structural stability, participating in angiogenesis, and controlling vascular permeability and blood flow (Armulik et al., 2005; Armulik et al., 2011).
While a wide body of literature exists on islet endothelial cells, the function of the islet pericyte is largely unknown (Jansson et al., 2016). Studies on islet pericytes focused on the role they play in angiogenesis, fibrosis, vascular stabilization and metastasis in beta cell tumors (Chu et al., 2013; Hayden et al., 2008; Song et al., 2005; Xian et al., 2006), in graft revascularization after transplantation (Juang et al., 2015) and on their mesenchymal stem cell potential (Crisan et al., 2012; Crisan et al., 2008). Previous studies have shown that pericyte ablation in the whole organism impairs glucose-stimulated insulin secretion and leads to beta cell dedifferentiation (Sasson et al., 2016), which could be due to altered platelet-derived growth factor signaling (Chen et al., 2011). Islet pericytes have further been shown to secrete nerve growth factor to potentiate insulin granule exocytosis (Houtz et al., 2016). These studies suggest that pericytes enhance beta cell function and insulin secretion, but it is unclear if these effects are direct or mediated by pericytes impacting vascular function. Surprisingly, the role of pericytes in regulating islet vascular function has not been addressed. Islet blood flow is thought to be regulated at the pre-capillary and capillary levels (Brunicardi et al., 1996; Liu et al., 1993; McCuskey and Chapman, 1969), but control at the capillary level remains controversial (Jansson et al., 2016). Because pericytes regulate capillary diameter in the retina, brain and kidney (Crawford et al., 2013; Hall et al., 2014; Peppiatt et al., 2006), we hypothesized that islet pericytes regulate blood flow in the islet by adjusting capillary diameter.
To test our hypothesis, we conducted immunohistochemical and electron microscopy studies of mouse and human pancreatic sections from non-diabetic and type 2 diabetic individuals to investigate the anatomical properties of islet pericytes. We further performed physiological recordings in living pancreas slices to examine functional responses of islet pericytes and capillaries. We focused on how beta cell activity and sympathetic input affect pericyte function and capillary diameters in the islet. To study the role of islet pericytes in controlling islet perfusion, we performed in vivo imaging of intraocular islet grafts and measured changes in pericyte activity, capillary diameter, and blood flow in response to hyperglycemia and sympathetic agonists. These approaches allowed us to establish the pericyte as an active component of the islet vasculature that mediates vascular responses to increased beta cell activity and autonomic nervous input. Our results further indicate that these pericytic functions are likely compromised in type 2 diabetes.
RESULTS
Pericytes extensively cover the microvasculature in mouse and human islets
The expression of genes and proteins and the location of pericytes overlap with those of vascular smooth muscle cells and other mesenchymal cells (fibroblasts/myofibroblasts) in the periendothelial compartment. A proper identification of pericytes thus requires assessing their location, morphology, and expression of markers (Armulik et al., 2011). We examined the expression of pericytic and endothelial cell markers by immunohistochemistry and ultrastructural features by transmission electron microscopy in pancreatic sections from mice and humans. A subset of vascular cells in mouse and human islets were immunoreactive for two bona fide pericytic markers: chondroitin sulfate proteoglycan 4 (neuron-glial antigen 2, NG2; Figures 1A and 1B) and platelet-derived growth factor receptor-beta (PDGFR-β; Figure 1C). NG2-labeled pericytes constituted ~3% of the human or mouse islet cell population (2.56 ± 0.25 % in mouse and 2.61 ± 0.37 % in human islets). Islet pericytes were closely associated with endothelial cells, extending cytoplasmic processes along the length of the capillaries (Figures 1A–1C). The long cytoplasmic processes spanned several endothelial cells and occasionally bridged neighboring capillary branches (Figures 1A′ and 1C). Many pericyte cell bodies were located at capillary branching points. At the ultrastructural level, pericytes and their processes were found embedded within the vascular basement membrane (Figures 1E and 1F).
Figure 1. Capillaries in mouse and human islets are covered with pericytes.
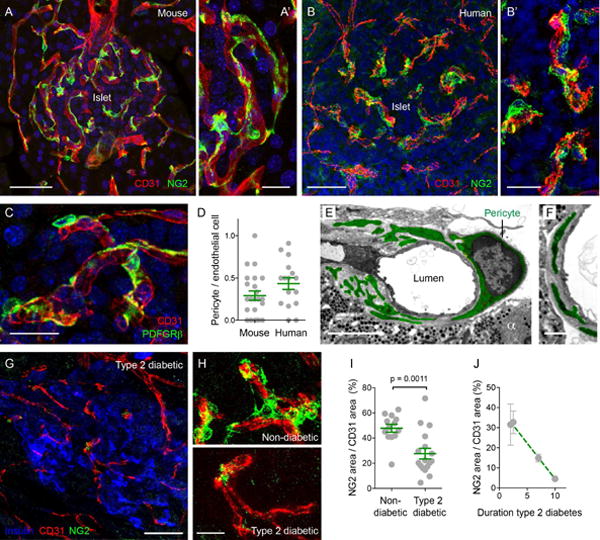
(A–C) Z-stack of confocal images of mouse (A and C) and human islets (B) showing pericytes and endothelial cells respectively immunostained for chondroitin sulfate proteoglycan (NG2, neuron-glial antigen 2, green) and for CD31 (PECAM, red). Nuclei are shown in blue. (A′) and (B′) higher magnifications of (A) and (B). Pericytes in mouse islets also express platelet-derived growth factor receptor-beta (PDGFRβ green) (C). Scale bars, 50 μm (A and B) and 10 μm (A′, B′ and C).
(D) Quantification of the ratio of pericyte number to endothelial cell number in confocal images in mouse and human islets. Dots represent confocal images pooled from > 3 pancreas per group. Average ratios ± SEM are shown in green.
(E and F) Transmission electron microscopic images of a pericyte cell body (E) and cytoplasmic processes wrapping capillaries in mouse islets (E and F). An alpha cell can be seen (α). Pericyte processes are embedded within the endothelial basement membrane (F). The pericyte cytoplasm is shown in green. Scale bars, 5 μm (E) and 2 μm (F).
(G and H) Z-stack of confocal images of an islet from a type 2 diabetic individual (duration of disease = 10 years), showing pericytes (NG2, green), endothelial cells (CD31, red) and beta cells (insulin, blue). (H) Higher magnifications of pericytes covering capillaries in islets from a non-diabetic individual (upper panel) and type 2 diabetic individual (shown in (G), lower panel). Scale bars, 50 μm (G) and 20 μm (H).
(I) Quantification of the ratio of NG2-immunostained area to CD31-immunostained area in human islets from non-diabetic or type 2 diabetic individuals (T2D). Dots represent the ratios of individual islets pooled from > 4 pancreases per group. Average ratios ± SEM are shown in green (unpaired t-test; p-value shown in the graph).
(J) Correlation between pericytic coverage of islet capillaries (ratio as in I) and the duration of type 2 diabetes (r2 = 0.32, p = 0.03).
We calculated that pericytes cover around 40% of capillaries in mouse and human islets (Figures 1D and 1I). In islets from type 2 diabetic donors there was a significant decrease in the number of pericytes (Figures 1G–1I). Pericyte coverage of human islet capillaries was inversely correlated with the duration of the disease (Figure 1J). Islets from an individual that had diabetes for 10 years were nearly devoid of pericytes (Figure 1G). In the obese mouse model of type 2 diabetes (ob/ob mouse), islet blood vessels also had significantly fewer pericytes, which most likely caused the capillary dilation that characterizes the islet vasculature in this mouse strain [Figure S1; (Dai et al., 2013; Hellstrom et al., 2001)].
A subset of islet pericytes expresses contractility markers
Because pericytes have contractile properties, we examined the expression of markers of cell contractility. Alpha smooth muscle actin (αSMA) is a major constituent of the contractile apparatus (Joyce et al., 1985). We found that ~ 50% of islet pericytes surrounding capillaries expressed αSMA (Figures 2A and 2D), in contrast to the paucity of αSMA in pericytes of other capillary beds (Crisan et al., 2012). Vascular smooth muscle cells of the feeding arteriole were also immunoreactive for αSMA (Figure 2A). Most of the αSMA-positive cells in mouse islets were also immunoreactive for NG2 (65 ± 4%) and PDGFRβ (98 ± 1 %). The heterogeneity of pericytes is in line with observations in other microvascular beds that pericytes are a very diverse population (Shepro and Morel, 1993). Furthermore, staining for αSMA was not distributed equally within the individual pericytes (Figures 2B and 2C), suggesting localized contractile function in pericyte processes wrapping capillaries.
Figure 2. A subset of islet pericytes expresses alpha smooth muscle actin.
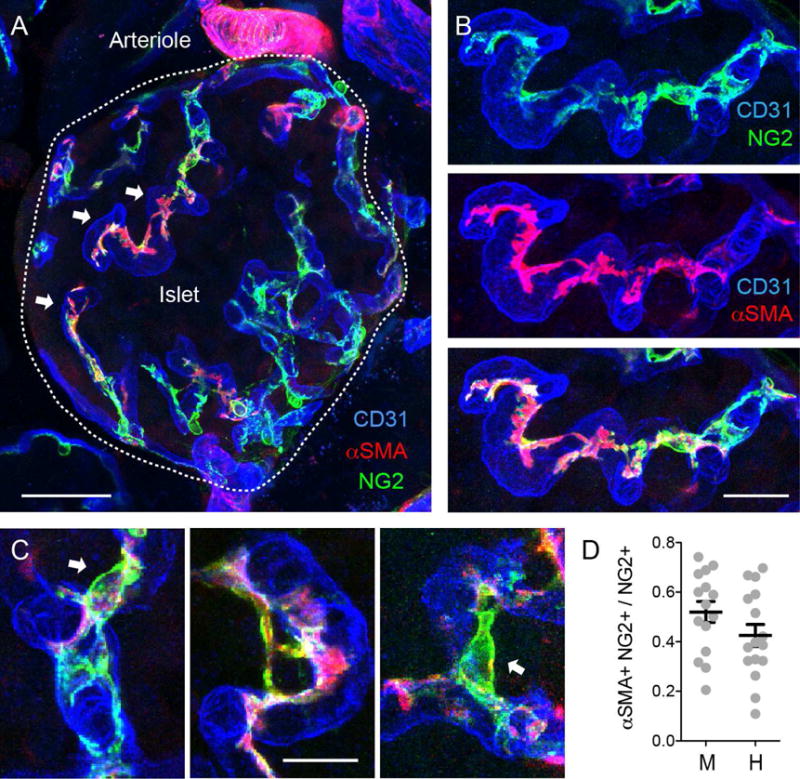
(A and B) Z-stack of confocal images of a mouse islet immunostained for NG2 (pericytes, green), CD31 (endothelial cells, blue), and alpha smooth muscle actin (αSMA, red). White arrows point to pericytes that express both NG2 and αSMA. αSMA expression is not equal throughout the pericytes cytoplasm. Vascular smooth muscle cells with their circular processes around the arteriole show strong αSMA staining. A high magnification of (A) showing islet capillaries is shown in (B).
(C) Pericyte cell bodies (arrows) can be seen extending αSMA-labeled processes between different capillary branches.
(D) Quantification of the fraction of NG2-positive pericytes that expresses αSMA in mouse (M) and human (H) islets. Dots represent individual islets pooled from > 3 mice or human donors. Scale bars, 50 μm (A) and 10 μm (B and C).
Functional characterization of islet pericytes
To characterize islet pericytes physiologically, we adapted the pancreatic slice technique (Marciniak et al., 2014) to study responses to vasoactive substances in pericytes in their native environment. In living pancreatic slices, the different tissue components are preserved allowing to study interactions between endocrine cells and the vasculature, nerves, immune cells, and surrounding exocrine acini (Figure 3). Pericytes are electrically excitable cells and their contractile activity is controlled by changes in cytosolic free Ca2+ concentration (Burdyga and Borysova, 2014). We used transgenic mice that express the genetically encoded Ca2+ indicator (GCaMP3) in an inducible Cre recombinase dependent manner. Cre recombinase expression was driven by the NG2 promoter and thus, besides NG2-expressing oligodendrocyte precursor glia in the central nervous system, only vascular smooth muscle cells and pericytes express the Ca2+ sensor [Figures 3A, 3A′, and 3B; (Zhu et al., 2008)]. By confocal time-lapse imaging of living pancreatic slices of NG2-GCaMP3 mice, we tested the effects of different neural, hormonal, and local paracrine signaling molecules on cytosolic Ca2+ responses in islet pericytes. We compared these responses to those of pericytes in the acinar tissue and vascular smooth muscle cells in arteries.
Figure 3. Recording Ca2+ responses in living pancreatic slices reveals functional differences between mural cell populations.
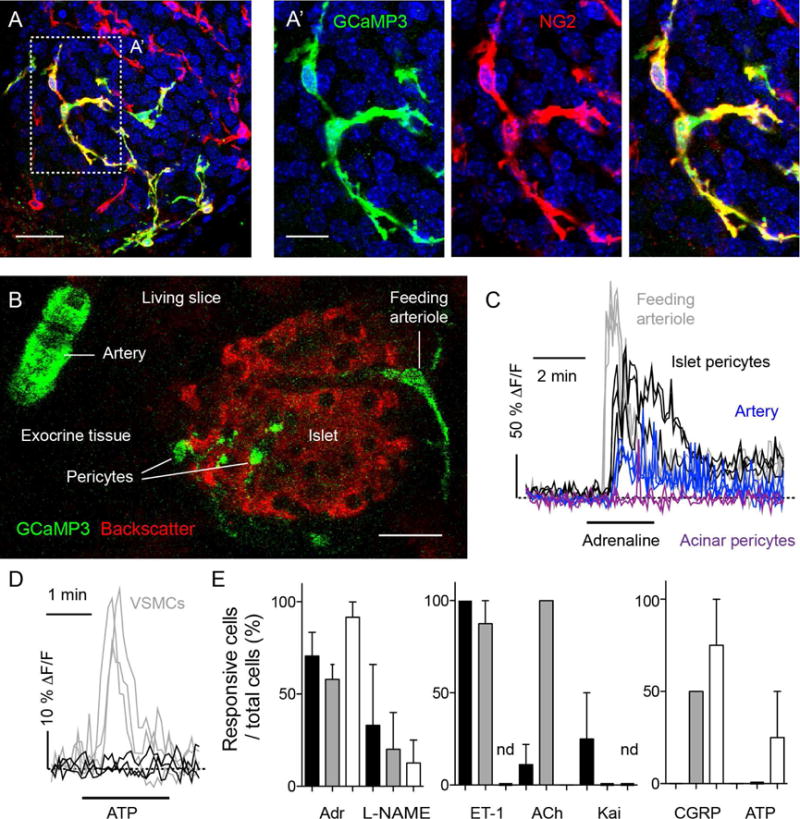
(A and A′) Z-stack of confocal images of a pancreatic slice from an NG2-GCaMP3 transgenic mouse processed for immunohistochemistry after a physiological experiment. Shown is an islet immunostained for GFP (GCaMP3, green) and NG2 (red). The Ca2+ sensor GCaMP3 is expressed in pericytes (colocalization appears yellow in merged images). Cell nuclei are shown in blue. Scale bars, 20 μm (A) and 10 μm (A′).
(B) Confocal image of a pancreatic slice from an NG2-GCaMP3 transgenic mouse showing mural cells expressing GCaMP3 (green) and islet endocrine cells (backscatter signal, red). GCaMP3 is expressed by different mural cells located around arteries, arterioles and within the islet parenchyma. Scale bar, 20 μm.
(C) Representative traces showing changes in mean GCaMP3 fluorescence intensity induced by adrenaline (100 μM) in islet pericytes (black), acinar pericytes (purple) and mural cells on the feeding arteriole (gray) or around the artery (blue). Increases in GCaMP3 fluorescence indicate increases in cytosolic Ca2+ levels and are expressed as ΔF/F. Dashed horizontal line indicates the zero value. Each trace corresponds to one cell.
(D) Representative traces showing changes in cytosolic Ca2+ levels in islet pericytes (black) and smooth muscle cells (VSMCs, gray) induced by ATP (100 μM). Dashed horizontal line indicates the zero value.
(E) Quantification of the percentage of islet pericytes (black bars), acinar pericytes (white bars) and smooth muscle cells (gray bars) that respond to different vasoactive substances: adrenaline (Adr, 50–100 μM), l-nitro-arginine methyl ester (L-NAME, 10 μM), endothelin-1 (ET-1, 10 nM), acetylcholine (ACh, 100 μM), kainate (Kai, 10 μM), calcitonin gene-related peptide (CGRP, 10 μM) and ATP (100 μM). (nd, not determined; N = 3 – 22 cells were examined per substance).
The catecholamines noradrenaline and adrenaline are vasoactive substances that induce changes in cytosolic Ca2+ levels in pericytes (Borysova et al., 2013; Burdyga and Borysova, 2014; Peppiatt et al., 2006). Applying adrenaline (100 μM) elicited increases in cytosolic Ca2+ in capillary and feeding arteriole mural cells (Figure 3C, Movie S1). Compared to Ca2+ responses in mural cells of the feeding arteriole, those in islet pericytes were more prolonged and showed occasional Ca2+ spikes (Figure 3C, Movie S1). In vascular smooth muscle cells of arteries, adrenaline induced a smaller cytosolic Ca2+ response, which, after a transient increase, was followed by repetitive, propagating Ca2+ spikes (Figure 3C). Adrenaline induced a small and transient response only in a subset of acinar pericytes (~ 50%).
Differences in Ca2+ responses between islet pericytes, vascular smooth muscle cells, and acinar pericytes were also observed with other vasoactive substances (Figures 3D and 3E). While some stimuli elicited responses in all types of mural cells (adrenaline and L-NAME; Figure 3E, left panel), other stimuli activated selectively pericytes (Figure 3E, middle panel) or smooth muscle cells (Figure 3E, right panel). Inhibiting nitric oxide synthase with L-NAME raised cytosolic Ca2+ in all types of mural cells (Figure 3E, left panel), likely by reversing nitric oxide decreases in IP3-stimulated Ca2+ release from intracellular stores that result from increasing cGMP levels and activating cGMP-dependent protein kinase I (Borysova and Burdyga, 2015; Ruth et al., 1993) present in pericytes (Tian et al., 1999) and smooth muscle cells (Feil et al., 2002). Islet and exocrine pericytes differed in their responses to calcitonin gene related peptide (CGRP) and acetylcholine (ACh; Figure 3E), reflecting the known heterogeneity of pericyte populations (Rucker et al., 2000; Shepro and Morel, 1993).
Pericyte activation is associated with changes in vascular diameter
Pericytes’ potential for contractility has been demonstrated ex vivo in brain and retinal slices (Peppiatt et al., 2006). Because vasoactive substances elicited Ca2+ responses in islet pericytes (Figure 3), we sought to determine whether pericyte activation changes blood vessel diameter in the islet. To correlate changes in cytosolic Ca2+ levels in pericytes with changes in vessel diameter, we labeled blood vessels in NG2-GCaMP3 mice with an intravascular injection of fluorescent Lycopersicon esculentum lectin (Figures 4A and 4B).
Figure 4. Simultaneous recording of pericyte activation and capillary constriction.
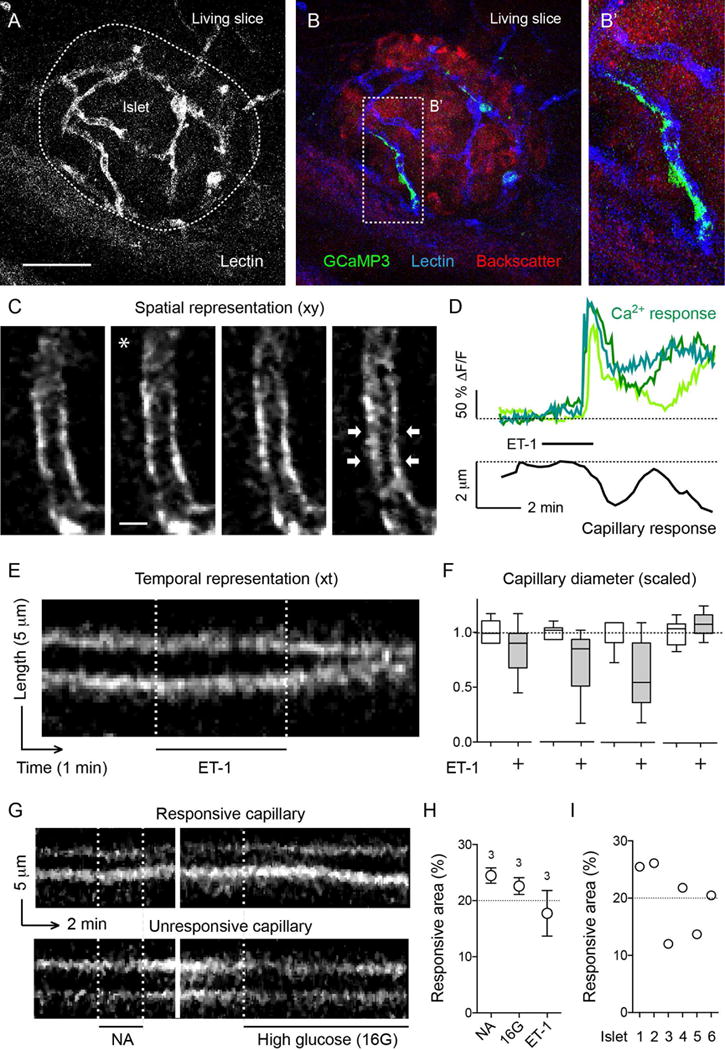
(A) Z-stack of confocal images of a pancreatic slice from an NG2-GCaMP3 transgenic mouse after intravascular injection of fluorescent Lycopersicon esculentum lectin to label the blood vessels (white).
(B and B′) In the slice shown in (A), GCaMP3 fluorescence (Ca2+ levels) in pericytes (green) and capillaries (labeled with lectin, blue) in islets (red, backscatter signal) can be visualized simultaneously. Zoomed image of (B) is shown in (B′).
(C) Sequential confocal images of an islet capillary before (left panel), during (middle panels; * indicates drug application) and after exposure (right panel) of a slice to endothelin 1 (ET-1, 10 nM). White arrows point at a constricting capillary region.
(D) Representative traces showing simultaneous changes in cytosolic Ca2+ levels in islet pericytes (green traces) and capillary diameter (black trace) induced by endothelin 1 (10 nM). Dashed line on Ca2+ traces shows the zero value.
(E) Temporal projection of a line scan perpendicular to the vessel axis shows the temporal pattern of changes in vessel diameter (reslice image, see STAR Methods and Figure S5). Capillary borders can be seen in white. Endothelin 1 (ET-1) induced a strong constriction of the islet capillary.
(F) Quantification of changes in diameter induced by endothelin 1 based on temporal projections as shown in (E). Values were scaled to the initial diameter (before drug application). Changes in diameter are shown for four different capillaries of the same islet. Not all islet capillaries constricted upon stimulation.
(G) Temporal projection of line scans of two different islet capillaries stimulated with noradrenaline (NA, 10 μM, left) and high glucose (16G, 16 mM, right). The capillary shown in the top panel is the same as the one shown in (E) that had responded to endothelin 1. The same vessel constricted upon noradrenaline and dilated upon high glucose (responsive capillary). The one shown in the bottom panel does not respond to the stimuli applied (unresponsive capillary).
(H and I) Quantification of the percentage of the islet microvasculature that responded to vasoactive stimuli such as noradrenaline (NA), high glucose (16G) or ET-1, plotted for each stimulus (H), or for different islets independently of the stimulus used (I).
Scale bars, 50 μm (A, applies to B) and 5 μm (C).
Applying the vasoconstrictor endothelin 1 elicited a strong and prolonged contraction of islet capillaries (average reduction in capillary diameter of 29 ± 4 %, N = 5 vessels / 3 slice preparations; Figures 4C–4F), which was preceded by robust, long-lasting increases in cytosolic Ca2+ levels in pericytes (Figure 4D). We exposed slices to three different stimuli and found that each time the same subset of islet capillaries responded (Figures 4E, 4G–4I). On average, only 20% of the microvasculature responded to a vasoactive stimulus (19.9 ± 2.4%, N = 6 islets / 4 slice preparations; Figures 4H and 4I), which correlates with the proportion of capillaries covered by pericytes expressing αSMA (Figures 1 and 2).
Beta cell activation inhibits islet pericytes and induces vascular dilation
Functional hyperemia is an increase in tissue blood perfusion during periods of heightened cellular metabolism. Whether or not this process involves pericytes of the microcirculation remains controversial. In pancreatic islets there is a positive correlation between endocrine cell activity and blood flow (Hellerstrom et al., 1960; Jansson et al., 2016). Hyperglycemia and its associated beta cell activation increase islet capillary blood pressure (Carlsson et al., 1997), dilate islet arterioles (Lai et al., 2007a), accelerate blood flow and increase blood volume (Nyman et al., 2010; Short et al., 2014). We hypothesized that activating beta cells with high glucose concentrations decreases islet pericyte activity, which relaxes pericytes and dilates capillaries. We found that exposing pancreatic slices to an increase in glucose concentration from 3 mM to 16 mM decreased cytosolic Ca2+ levels in pericytes and simultaneously dilated islet capillaries (Figures 5A–5C, 5F–5H, Figure S2 and Movie S2). Diameters increased by 36 ± 6% (N = 10 vessels from 4 slice preparations, Figure 5G) in a subset of islet capillaries (Figures 4G and 4H, Movies S2 and S3). High glucose did not decrease cytosolic Ca2+ levels in vascular smooth muscle cells or in acinar pericytes (Figure S2).
Figure 5. High glucose stimulation of beta cells inhibits pericytes and dilates capillaries through adenosine and A1 receptors.
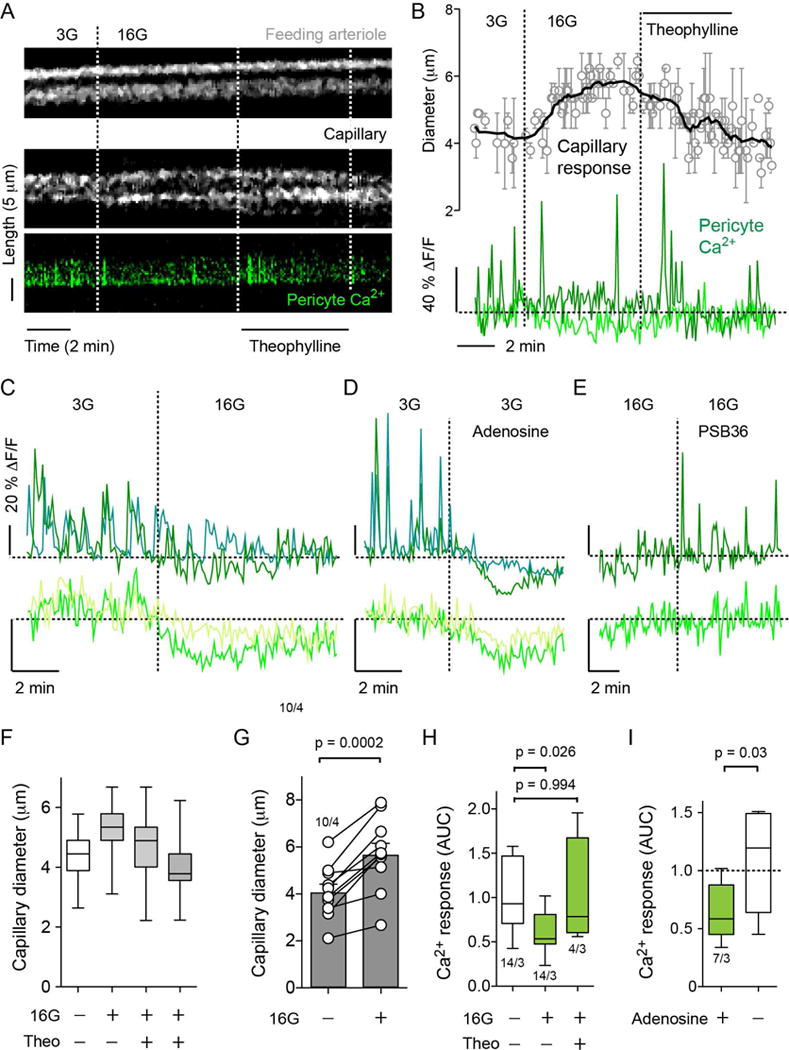
(A) Temporal projections of line scans showing changes in vessel diameter (upper and middle panels) of a feeding arteriole (upper panel) and an islet capillary (middle panel) and of cytosolic Ca2+ levels in a nearby capillary pericyte (green, lower panel) induced by increasing extracellular glucose concentration from 3 mM (3G) to 16 mM (16G) in a living pancreatic slice. High glucose increased capillary, but not arteriole, diameter and simultaneously decreased cytosolic Ca2+ in the pericyte. Theophylline (20 μM, in 16G), a non-specific antagonist of adenosine receptors, reversed the effects of high glucose.
(B) Traces of responses as in (A) show the average change in vessel diameter (upper panel, N = 3 capillaries) and cytosolic Ca2+ levels in pericytes (lower panel). Dashed line on Ca2+ traces shows the zero value. Each trace corresponds to one pericyte.
(C–E) Traces showing changes in cytosolic Ca2+ levels in islet pericytes induced by (C) high glucose (16 mM, 16G), (D) adenosine (50 μM) in 3 mM glucose and (E) A1 adenosine receptor antagonist (PSB36, 100 nM) in 16 mM glucose. Changes in baseline cytosolic Ca2+ levels (lower panel) are shown at a higher gain. Y-axis bars correspond to 20% change (ΔF/F). Each trace corresponds to one pericyte. Horizontal dashed lines show the zero value, and vertical dashed lines when stimuli were applied.
(F) Quantification of the changes in capillary diameter of a responsive capillary induced by high glucose (16G) and the reversal by theophylline (Theo; in 16G) 2 min and 5 min after application of the antagonist.
(G) Quantification of the changes in capillary diameter induced by 16 mM glucose. Each pair of symbols is one capillary (N = 10 capillaries pooled from 4 slice preparations, paired t-test).
(H) Quantification of changes in cytosolic Ca2+ levels in pericytes induced by high glucose (16G) and high glucose plus theophylline (Theo, 20 μM). The area under the curve (AUC) was quantified in a 4-5 min recording in each condition. N = 4–14 pericytes from 3 slice preparations, one-way ANOVA corrected for multiple comparisons.
(I) Quantification of the changes in cytosolic Ca2+ levels in pericytes induced by adenosine (50 μM). Area under the curve (AUC) was quantified in 3 min recordings before, during and after adenosine. Data are scaled to AUC values before adenosine application (dashed horizontal line). N = 7 pericytes from 3 slices preparations, paired t-test.
Among the signaling molecules released from beta cells that could mediate the effects on pericyte activity, we focused on ATP because ATP and its breakdown products ADP and adenosine have strong vascular effects (Burnstock and Ralevic, 2014). Of these, adenosine is a potent vasodilator (Berne et al., 1983; Collis, 1989; O’Farrell et al., 2017). In islet pericytes, both adenosine and NECA (a non-specific agonist of adenosine receptors) decreased cytosolic Ca2+ levels (Figures 5D and 5I; Figure S3). The effect of exogenous adenosine mimicked that of high glucose; both inhibited cytosolic Ca2+ transients and baseline Ca2+ levels in islet pericytes (Figures 5C and 5D). Adenosine also inhibited cytosolic Ca2+ in smooth muscle cells in arterioles but not acinar pericytes (Figure S3).
To test if endogenous adenosine mediated the inhibitory effects of high glucose concentration on pericytes, we stimulated beta cells with 16 mM glucose concentration and applied theophylline, a non-specific antagonist of adenosine receptors reported to prevent islet blood flow increases after in vivo glucose administration (Carlsson et al., 2002). Theophylline abolished high glucose-induced capillary dilation (Figures 5A, 5B and 5F) and reversed the inhibition of cytosolic Ca2+ in islet pericytes (Figures 5A, 5B, and 5H), as did a selective adenosine A1 receptor antagonist [PSB36; Figure 5E]. These results indicate that, upon high glucose stimulation, adenosine accumulates in the islet and activates A1 receptors in islet pericytes, relaxing these cells and dilating capillaries.
In vivo imaging reveals functional hyperemia in pancreatic islets
Our results suggest that stimulated beta cells inhibit pericytes and thus dilate capillaries in the islet. To determine if high glucose-induced capillary dilation affects islet blood flow, we transplanted islets into the anterior chamber of the eye for in vivo imaging (Speier et al., 2008). Intraocular islet grafts are fully vascularized one month after transplantation in a pattern that closely resembles that of islets in the pancreas. Importantly, pericytic coverage of capillaries in islet grafts (Figures 6A and 6B) was similar to that found in the pancreas (Figure 1).
Figure 6. Raising glycemia dilates islet capillaries in vivo and increases blood flow.
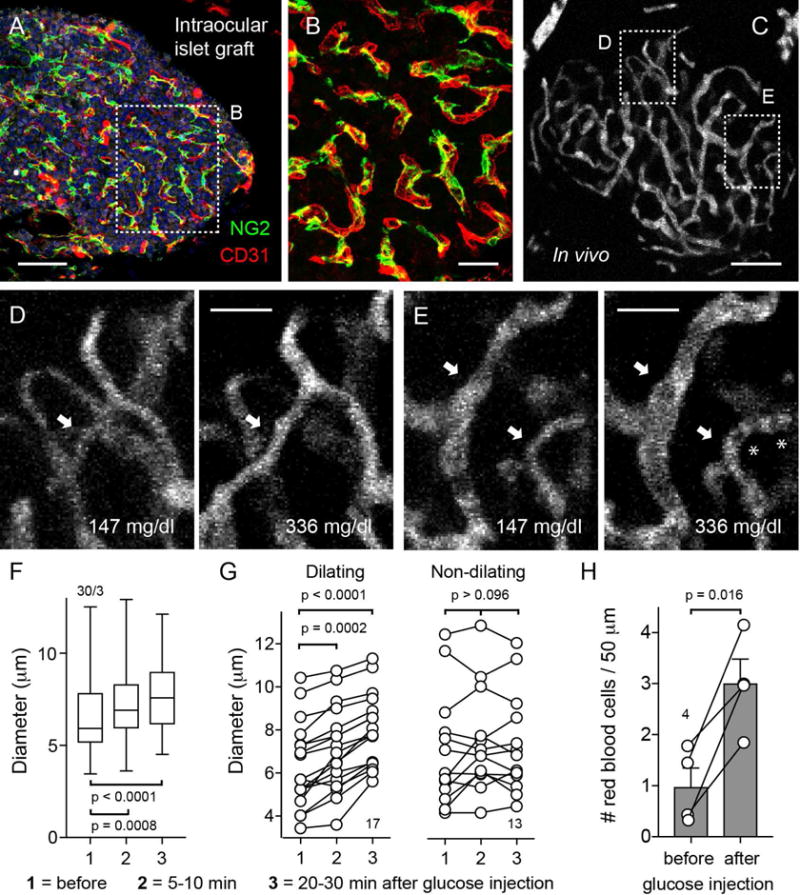
(A and B) Z-stack of confocal images of an islet graft 6 months after transplantation into the eye immunostained for NG2 (pericytes, green), CD31 (endothelial cells, red), and insulin (beta cells, gray). Cell nuclei are shown in blue. Pericytes cover capillaries in transplanted islets as they do in the pancreas. (B) Zoomed image of (A).
(C–E) Z-stack of confocal images of the graft vasculature visualized with an i.v. injection of FITC-dextran.
(D and E) Islet capillaries were imaged before and after an i.p. injection of 20% glucose (2 g/Kg body weight). Glycemia was measured at different time points. A rise in glycemia caused regions of the islet to become perfused (arrow in D) or dilated islet capillaries (arrows in E). Asterisks point at red blood cells.
(F) Quantification of changes in capillary diameter in the islet before (glycemia = 147 mg/dL), 5-10 min (glycemia = 279 mg/dL) or 20-30 min (glycemia = 336 mg/dL) after injection of glucose. N = 30 capillaries from 3 islet grafts, one-way ANOVA corrected for multiple comparisons.
(G) Capillaries were grouped according to their responses to a rise in glycemia: vessels that showed a progressive dilation (dilating capillaries) or vessels that did not change or exhibited a transient non-significant dilation (non-dilating). N = 13-17 capillaries from 3 islet grafts, one-way ANOVA corrected for multiple comparisons.
(H) Quantification of the number of red blood cells, identified as black shadows in the vessel lumen, in different islet capillaries before (glycemia = 147 mg/dL) and after injection of glucose (glycemia 336 mg/dL). N = 4 capillaries, paired t-test.
Scale bars, 50 μm (A and C), 20 μm (B, D, and E).
For in vivo imaging, blood vessels in islet grafts were visualized with an intravenous injection of a fluorescent dextran (Figure 6C). Capillary blood flow was intermittent and varied from capillary to capillary, as previously described (Liu et al., 1993). We found that raising glycemia increased blood perfusion of islet grafts (Figures 6D and 6E). Blood started flowing through islet regions that were not perfused at lower glycemic levels (Figure 6D). Simultaneously, we observed a significant dilation of islet capillaries (Figures 6E–6G, Movies S4–S6). Not all blood vessels in the islet graft dilated, in line with our ex vivo findings (Figure 6G). The vascular dilation was similar to that elicited by high glucose concentrations in pancreas slices (Figure 5). We also quantified blood flow by counting red blood cells in the vascular lumen and found that blood flow increased in blood vessels (Figure 6H, Movies S4–S6), similarly to what has been reported for islets in the pancreas (Nyman et al., 2010; Short et al., 2014). These results indicate that raising glycemia increases islet blood flow through local dilation of capillaries in the islet microcirculation.
Control of pericyte activity by adrenergic input ex vivo and in vivo
In mouse and human islets, arterioles and capillaries are innervated by sympathetic axons (Rodriguez-Diaz et al., 2011; Tang et al., 2014). We conducted immunohistochemical studies on mouse pancreatic sections and found that tyrosine hydroxylase-positive sympathetic axons were in close contact with αSMA-positive pericytes [Figure 7A; see also (Rodriguez-Diaz et al., 2011)]. In slices, islet pericytes responded to application of the sympathetic neurotransmitters adrenaline and noradrenaline with strong increases in cytosolic Ca2+ (Figures 7B and 7C, see also Figure 3). To determine if islet pericytes respond to endogenous, locally released noradrenaline, we applied tyramine, a sympathomimetic that stimulates the release of neurotransmitters from nerve terminals (Gilliam et al., 2007; Graefe et al., 1999). Tyramine (50 μM) elicited an increase in cytosolic Ca2+ in islet pericytes (Figures 7B and 7C), indicating that islet pericytes responded to sympathetic nervous input. We investigated the effects of sympathetic neurotransmitters on islet blood vessel diameter and found that noradrenaline (10 μM) induced a strong contraction of a subset of islet capillaries (29 ± 6% reduction in capillary diameter, N = 5 vessels from 3 slice preparations; Figure 7F and Movie S7). Adrenaline (50–100 μM) also constricted islet capillaries (average reduction of capillary diameter of 12 ± 2%, N = 4 vessels from 3 slice preparations) but had a stronger effect on feeding arterioles (21 ± 4% reduction of arteriole diameter, N = 4 arterioles from 4 slice preparations; Figures 7D–7F).
Figure 7. Sympathetic activation of islet pericytes leads to capillary constriction ex vivo and in vivo.
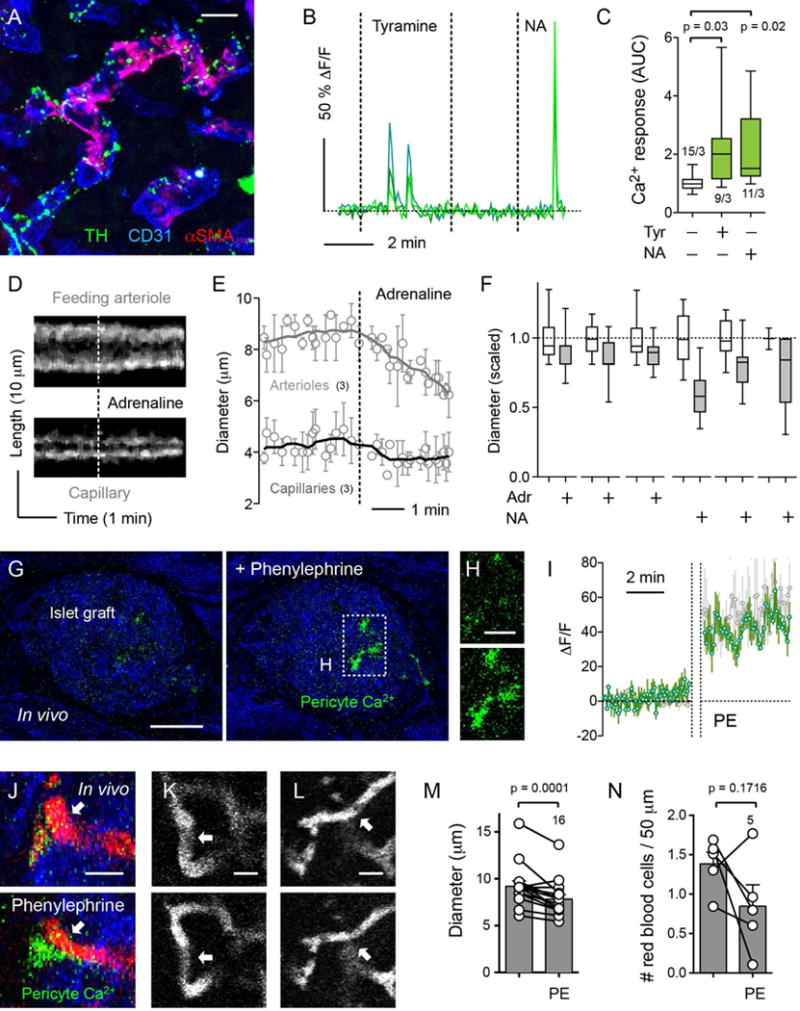
(A) Z-stack of confocal images of a mouse islet immunostained for the sympathetic nerve marker tyrosine hydroxylase (TH, green), for αSMA (pericytes, red) and for CD31 (endothelial cells, blue). TH-labeled axons and varicosities can be seen in close contact with endothelial cells and pericytes in islet blood vessels.
(B) Representative traces showing changes in cytosolic Ca2+ levels in islet pericytes induced by tyramine (50 μM) and noradrenaline (NA, 50 μM). Values are expressed as ΔF/F. Each trace corresponds to one pericyte. Dashed horizontal line indicates the zero value.
(C) Quantification of changes in cytosolic Ca2+ levels in pericytes induced by tyramine (Tyr, 50 μM) and noradrenaline (NA, 50 μM). AUC was quantified in a 2 min recording in each condition and scaled to control values (in 3 mM glucose). N = 9–15 cells per group from 3 slice preparations; one-way ANOVA corrected for multiple comparisons.
(D) Temporal projections of line scans through a feeding arteriole (upper panel) and a capillary (lower panel) show a reduction in vessel diameter induced by adrenaline (50 μM) in a living pancreatic slice.
(E) Traces showing the average change in vessel diameter induced by adrenaline (black = capillaries, N = 3; gray = arterioles, N = 3).
(F) Quantification of changes in vessel diameter for individual capillaries induced by adrenaline (50 μM) and noradrenaline (NA, 10 μM), scaled to the initial diameter (before catecholamine application, in 3G).
(G and H) In vivo imaging of islets from NG2-GCaMP3 mice transplanted into the eye before and 5 min after administration of phenylephrine as eye drops. Backscattered light is shown in blue. (H) Increases in cytosolic Ca2+ levels in pericytes are evident in the zoomed images (upper panel, before; lower panel, 5 min after phenylephrine).
(I) Trace (green) showing changes in cytosolic Ca2+ levels in islet pericytes in vivo before (left) and 5 min after application of phenylephrine (PE). Vertical dashed lines correspond to the 5 min period that the eye was exposed to eye drops before rinsing with imaging buffer. Values are expressed as ΔF/F. A sustained response to phenylephrine was observed in islet pericytes in vivo, which was similar to the response to adrenaline of islet pericytes in slices (gray trace shown behind the in vivo trace). Average traces ± SEM are shown (N = 3 pericytes in vivo and in slices).
(J–L) In vivo imaging of NG2-GCaMP3 islet grafts in the eye before (upper panels) and 5 min after (lower panels) phenylephrine. Phenylephrine increased cytosolic Ca2+ levels in a pericyte (green) wrapping a constricting islet blood vessel (J). Arrows in (J), (K) and (L) point to constricting islet capillaries. Blood vessels were visualized with an i.v. of TRITC-dextran (red or grey) and islet tissue by backscatter (blue in J).
(M) Quantification of changes in blood vessel diameter in the islet before and after phenylephrine (N = 16 capillaries from 5 islet grafts in 3 mice; paired t-test).
(N) Quantification of the number of red blood cells, identified as black shadows in the vessel lumen, in different islet blood vessels before and after phenylephrine (N = 5 capillaries).
Scale bars, 10 μm (A), 50 μm (G), 20 μm (H), and 10 μm (J, K, and L).
To determine if sympathetic activation of pericytes affects islet blood perfusion, we transplanted islets from NG2-GCaMP3 mice into the eyes of NG2-GCaMP3 mice for in vivo imaging. Three months after transplantation, islets were well engrafted and pericytes expressing GCaMP3 could be visualized. Similar to what we observed ex vivo, spontaneous Ca2+ spikes could be seen in some islet pericytes before stimulation. Eye drops containing the α1-adrenergic receptor agonist phenylephrine applied topically increased cytosolic Ca2+ levels in islet pericytes (Figures 7G–7J), induced contraction of islet capillaries (Figures 7J–7M) and decreased capillary blood flow in most vessels (Figure 7N). These results indicate that sympathetic agents activate islet pericytes in vivo, leading to capillary constriction and reducing blood flow.
DISCUSSION
Our study establishes that pericytes play an essential role in islet microvascular function. Pericytes extensively cover capillaries in mouse and human islets and respond to vasoactive substances released by the neighboring endothelium, sympathetic nerves, and beta cells. By changing cytosolic Ca2+ levels, these vasoactive substances alter pericytic contractile activity, thus regulating islet capillary diameter. Pericyte coverage of islet capillaries is strongly reduced in diabetic humans and mice, suggesting that under diabetic conditions islets lose pericyte control of vascular diameter and with it their ability to determine their own blood supply.
Although they constitute only 3% of the human or mouse islet cell population, pericytes contact several endothelial cells through long cytoplasmic processes, covering approximately 40% of the islet vasculature. Pericyte density or coverage correlates with endothelial barrier properties (Shepro and Morel, 1993). Our data suggest that the islet endothelial barrier has an intermediate leakiness (Richards et al., 2010): not as tight as the microvasculature of the retina or brain (pericyte: endothelial cell ratio of 1:1) but tighter than that of the lungs (ratio of 1:10). Our results challenge the idea that islet pericytes are few scattered vascular smooth muscle-like cells (Chu et al., 2013; Jansson et al., 2016; Lukinius et al., 1995). Instead, islet pericytes form a well-structured network around capillaries that may communicate with mural cells of the feeding arteriole and collecting veins. Islet pericytes express alpha smooth muscle actin (αSMA) and tropomyosin (Joyce et al., 1985), both essential proteins for contractile function. These findings indicate islet pericytes play an active role in the regulation of blood vessel diameter in the islet, as they do in other microvascular beds (Attwell et al., 2016; Hall et al., 2014; Peppiatt et al., 2006).
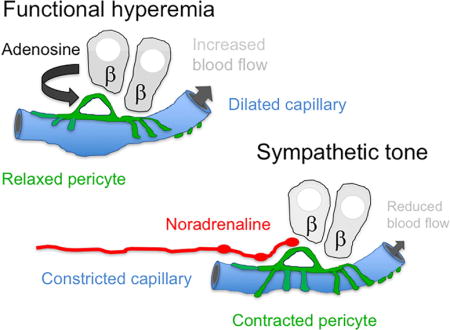
Dilation of pre-capillary arterioles is considered to be the main mechanism that increases islet blood flow during heightened cellular metabolism (Guyton and Hall, 2006; Jansson et al., 2016). Yet we found that stimulating beta cells diminishes pericyte activity and leads to capillary dilation. That this happens ex vivo in pancreas slices indicates that islet capillaries can dilate actively and independently of the increase in blood pressure that could result from an opening of the feeding arteriole. Moreover, reversing glucose-induced pericyte relaxation reversed capillary dilation, demonstrating that local capillary dilation is a regulated process linked to a reduction in pericyte activity. Vasoactive substances known to dilate feeding arterioles also decreased pericyte activity and dilated islet capillaries [e.g. adenosine analogs; Figure 5D; (Lai et al., 2007a; Olsson et al., 2000)]. Based on our results, we propose a mechanism for functional hyperemia where beta cell-derived signals inhibit islet pericytes, causing intra-islet capillaries to dilate.
What makes islet pericytes relax when beta cells are active? In beta cells, cytoplasmic ATP is transported into insulin granules by the vesicular nucleotide transporter (VNUT) and secreted together with insulin upon stimulation with increases in glucose concentration (Detimary et al., 1996; Geisler et al., 2013; Hazama et al., 1998). Once secreted, different ectonucleotidases rapidly degrade extracellular ATP to ADP, AMP, and adenosine, all of which play pivotal roles in the control of vascular tone (Burnstock and Ralevic, 2014; Yegutkin, 2008). Adenosine accumulates in metabolically active tissues, where it has potent vasodilator effects (Berne et al., 1983; Collis, 1989; O’Farrell et al., 2017; Tabrizchi and Bedi, 2001). Mouse pancreatic islets display broad nucleotidase activity in endocrine cells (e.g. NTPDase3) or in capillaries inside the islets [e.g. ecto-5′-nucleotidase; (Lavoie et al., 2010)]. It is therefore likely that ATP released from beta cells is eventually degraded to adenosine in the islet.
Endogenous adenosine may relax islet pericytes by activating potassium channels and inducing hyperpolarization (Hamilton et al., 2010). Adenosine has been shown to open KATP channels on retinal pericytes (Li and Puro, 2001) by binding to A1 receptors and activating Gαi, which antagonizes the inhibitory effect that ATP has on channel conductance (Terzic et al., 1994). Our results suggest that endogenous adenosine acts on Gαi-coupled A1 receptors (Figure 5E). As shown for retinal pericytes, activating A1 receptors could open KATP channels and hyperpolarize islet pericytes, thus reducing cytoplasmic Ca2+ levels. Pericytes would relax as a result, allowing for the capillary distension that increases blood flow locally.
Our findings further indicate that sympathetic nerves act on islet pericytes to reduce islet blood flow. Sympathetic nervous input increases cytosolic Ca2+ in islet pericytes and decreases capillary diameter. This mechanism helps explain the reduction in islet blood flow that is associated with increased sympathetic tonus (Atef et al., 1992; Jansson et al., 1989; Pettersson et al., 2009). Pericyte activation and relaxation thus provide mechanisms for localized, bidirectional regulation of blood flow in the islet. While we focused on the effects of beta cell activation and sympathetic input, pericytes may respond to additional local cues. Previous studies have shown that the endothelium-derived vasoactive factors nitric oxide and endothelin-1 are major regulators of islet blood flow by acting on the feeding arteriole (Jansson, 1994; Lai et al., 2007b; Moldovan et al., 1996; Olsson et al., 2000; Svensson et al., 1994). We show that these endothelium-derived vasoactive substances activate islet pericytes as well. We haven’t yet studied their effects on islet blood flow. Nevertheless, the emerging picture is that the responses of capillary pericytes are coordinated with those of feeding arterioles, providing an additional level of blood flow control.
Islet pericytes could function as an electrical syncytium, as proposed for mural cells in the retina (Borysova et al., 2013; Zhang et al., 2011). Indeed, islet vascular cells express the gap junction proteins connexin 43 (endothelial cells) and connexin 45 [smooth muscle cells; (Theis et al., 2004)]. Smooth muscle cells and pericytes could also be electrically coupled to endothelial cells (Figueroa and Duling, 2009). Gap junctions between these cells would spread the effects of local signaling to other islet regions, including the feeding arteriole, and ensure efficient regulation of blood flow, as shown in other tissues (Iadecola et al., 1997; Peppiatt et al., 2006; Segal and Duling, 1986). Interestingly, synchronized activity of beta cells also affects intraislet blood flow. Altering beta cell electrical coupling, by knocking out connexin 36, the gap junction protein that connects islet beta cells (Serre-Beinier et al., 2000), not only disrupts pulsatile insulin secretion (Head et al., 2012; Ravier et al., 2005), but also glucose-dependent increases in islet blood flow (Short et al., 2014). By producing a unified, temporally restricted secretory burst, coordinated beta cell activity may ensure robust and concerted vascular responses that efficiently increase blood flow throughout the islet.
Our findings showing that pericytes play an active role in regulating islet blood flow also have pathophysiological implications. Diabetes is a microvascular disease characterized by pericytic dropout and dysfunction and altered vascular responses (Dodge and D’Amore, 1992; Silva et al., 2017). Our study now shows that diabetic microvascular complications also affect the islet. The striking loss of pericytic coverage in capillaries in islets from obese mice and individuals with type 2 diabetes likely impairs the adaptation of islet blood flow to increased islet metabolism and sympathetic input. This may compromise hormone release into the circulation, thereby exacerbating glucose intolerance. Islet pericyte dysfunction could thus contribute to the natural history of type 2 diabetes.
Limitations of the study
We did not determine how blood flow control by pericytes affects the temporal dynamics of insulin and glucagon secretion into the circulation. To this end, we will have to manipulate selectively islet pericytes and measure simultaneously plasma hormone levels and glycemia. Studies are further required to understand how pericyte dysfunction disrupts islet hormonal output. This would allow revisiting the notion that alterations in islet vasculature contribute to the pathogenesis of diabetes (Gepts, 1981). Our study also raises the possibility that the sympathetic nervous system affects islet hormone secretion in part by controlling islet blood perfusion, which could be particularly relevant in human islets (Rodriguez-Diaz et al., 2011). Whether this mechanism affects islet hormone delivery into the circulation will be the focus of future work.
STAR METHODS
Contact for reagent and resource sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Joana Almaça (jalmaca@med.miami.edu).
Experimental model and subject details
Mouse model
For in vivo and ex vivo measurements of cytosolic Ca2+ in pericytes, we generated mice that express GCaMP3 in pericytes using the Cre-Lox system. Briefly, we crossed NG2-CreER-tdTomato mice, kindly donated by Dr. Jae Lee, University of Miami (Soderblom et al., 2013), with mice that express GCaMP3 downstream of a loxP-flanked STOP cassette (The Jackson Laboratory, stock nr. 029043). Only F1 mice were used, from both sexes, 6-20 weeks old. To induce Cre recombinase expression, NG2-GCaMP3 received 0.124 mg/g body weight of tamoxifen injected i.p. for five consecutive days. Mice were used a week later. All experiments were conducted according to protocols and guidelines approved by the University of Miami Institutional Animal Care and Use Committee.
Human organ donors
We obtained human pancreatic tissue samples (from the head of the pancreas, n = 5 non-diabetic individuals, 4 type 2 diabetic individuals, male and female, ages = 15 – 52 years old) from the Human Islet Cell Processing Facility at the Diabetes Research Institute, University of Miami.
Method details
Preparation of living pancreatic slices
Tissue slices were prepared from NG2-GCaMP3 mice (6-20 weeks old) as previously described (Marciniak et al., 2014). Briefly, mice were anesthetized with isofluorane and euthanized by cervical dislocation. Low gelling temperature agarose (1.2%, Sigma Aldrich, cat. nr. 39346-81-1, dissolved in HEPES-buffered solution without BSA) was injected in the common bile duct using a 30-gauge needle and 5 mL syringe. After injection, small tissue pieces were cut and embedded further in agarose and placed at 4°C for 10 min. Pancreatic slices were then cut (150 μm thickness) on a vibrating blade microtome (VT1000S, Leica) and incubated in HEPES-buffered solution (125 mM NaCl, 5.9 mM KCl, 2.56 mM CaCl2, 1 mM MgCl2, 25 mM HEPES, 0.1% BSA, pH 7.4) containing 3 mM glucose. For blood vessel labeling, Lycopersicon esculentum lectin conjugated to DyLight 594 (Vector Labs, DL-1177) was injected (75μg) in the tail vein before sacrificing.
Confocal imaging of living pancreatic slices
Living pancreatic slices of NG2-GCaMP3 mice were placed on a coverslip in an imaging chamber (Warner instruments, Hamden, CT, USA) for imaging on a Leica TCS SP5 upright laser-scanning confocal microscope (Leica Microsystems, Wetzlar, Germany). Slices were continuously perfused with HEPES-buffered solution containing 3 mM glucose and confocal images were acquired with LAS AF software (Leica Microsystems) using a 40× water immersion objective (NA 0.8). We used a resonance scanner for fast image acquisition to produce time-lapse recordings spanning 50-100 μm of the slice (z-step: 5-10 μm, stack of ten confocal images with a size of 512 × 512 pixels) at 5 sec resolution (XYZT imaging). GCaMP3 fluorescence was excited at 488 nm and emission detected at 510–550 nm, DyLight 594 labeled tomato lectin was excited at 594 nm and emission detected at 610-650 nm.
We recorded changes in GCaMP3 fluorescence and blood vessel diameter induced by adrenaline, noradrenaline, endothelin-1, tyramine, l-nitro-arginine methyl ester (L-NAME), kainate, calcitonin gene-related peptide (CGRP), ATP, adenosine or 5′-(N-Ethylcarboxamido)adenosine powder (NECA) in basal glucose solution (3 mM), or by high glucose (16 mM) alone, with theophylline or A1 receptor antagonist PSB36. All chemicals were from Sigma, except kainate, CGRP and PSB36 that were from Tocris.
Transplantation into the anterior chamber of the eye and in vivo imaging
Mouse islet isolation was performed using collagenase digestion followed by purification with histopaque. Approximately 20 islets isolated from young NG2-GCaMP3 mice (6–8 weeks old) were aspirated into a 27G eye cannula connected to a 1 ml Hamilton syringe via a 0.4 mm polyethylene tubing. Young NG2-GCaMP3 mice (6–8 weeks old; n = 5 mice) were anesthetized with ~2% isoflurane and the eyes were kept humidified (ophthalmologic eye drops) to avoid drying of the cornea. Under a stereomicroscope, the cornea was punctured close to the sclera at the bottom part of the eye with a 31G insulin needle and a small radial incision of approximately the size of the eye cannula (~ 0.5 mm) was made. The blunt eye cannula was then gently inserted through this incision and the islets slowly injected into the anterior chamber, where they settle on the iris. After injection, the cannula was slowly withdrawn to avoid islets from flowing back through the incision. The mouse was left lying on the side before awakening. Mice were then put back in the cages and monitored until full recovery, and observed daily thereafter. Analgesia was achieved after surgical procedures with buprenorphine (0.05-0.1 mg/kg s.c.).
Mice were imaged 3-4 months after transplantation after 5 consecutive i.p. injections of tamoxifen (see above). Imaging of islets in vivo in the anterior chamber of transplanted animals was performed as previously reported (Speier et al., 2008). Briefly, mice were anesthetized with ~2% isoflurane air mixture, placed on a heating pad and the head restrained with a headholder. The eyelid was carefully pulled back and the eye gently supported for fluorescence confocal imaging on an upright laser-scanning confocal microscope (Leica TCS SP5) using long distance water-dipping lenses (Leica HXC APO 20× 0.5 W), using PBS as immersion liquid. Blood vessels were labeled by tail vein injection of 150,000 Da Dextrans (FITC or TRITC conjugated (Sigma)). FITC and GCaMP3 were excited at 488 nm and emission light was collected between 500–550 nm; TRITC was excited at 561 nm and emission light collected at 570 nm. Reflected light was imaged by illumination at 633 nm and collection between 630–639 nm. We used a resonance scanner for fast image acquisition to produce time-lapse recordings of blood flowing through islet capillaries on confocal mode with a size of 512 × 512 pixels at 68 msec resolution (XYT imaging).
For functional hyperemia experiments, glucose was injected i.p. (2 g/kg body weight, stock 50% dextrose solution) in anesthetized mice and glycemia measured using a portable glucometer (Contour). The sympathomimetic phenylephrine was administered as eye drops (two drops; phenylephrine hydrochloride solution 2.5%; Alcon Laboratories, Forth Worth, TX) and imaging resumed 5 min later. The pulse and respiration of the animal was measured to ensure that the drug had not entered the systemic circulation. Data analysis was performed with ImageJ. For quantification of blood vessel diameter, we drew a line on the blood vessel and used the “plot profile” function to determine the vessel borders. Vessel diameter was calculated by subtracting these 2 position values. For quantification of blood flow, we manually counted the number of red blood cells (identified as black shadows crossing the vessel lumen) in different vessels and normalized it for an average vessel length of 50 μm.
Electron microscopy
Mouse and human pancreata were fixed in 2% glutaraldehyde in 0.1M PO4/100mM sucrose, overnight at 4°C. The next day, samples were rinsed with 0.15M PO4 three times, then post-fixed in 1% phosphate buffered osmium tetroxide overnight at 4°C. After fixation, samples were rinsed with 0.15M PO4 three times then dehydrated in an ascending series of cold ethanolic alcohols to 100% and infiltrated with a 1:1 mixture of propylene oxide and Epon-Araldite resin overnight at room temperature. The next day, the 1:1 mixture was replaced with fresh Epon-Araldite resin, left in the desiccator for 4 hours prior to being transferred to embedding molds and left to polymerize in a 64°C oven overnight. The resin blocks were removed from the molds, trimmed and cut using a Leica UC7 ultra-microtome. 100nm-sections were collected on Formvar coated copper grids and stained with uranyl acetate and lead citrate for contrast. Sections were scanned using a JEOL JEM-1400 transmission electron microscope equipped with a Gatan Orius digital camera.
Immunohistochemistry
Human pancreatic tissues from non-diabetic and type 2 diabetic donors (samples taken from the head of the pancreas) were fixed overnight in 4% PFA, cryoprotected in a sucrose gradient (10, 20 and 30% w/w sucrose), and frozen in Tissue-Tek Optimal Cutting Temperature (OCT) compound before cryosectioning (−20°C). Mouse pancreatic tissue was perfused with 4% PFA and processed similarly. Pancreatic slices used in physiological experiments were immersed in 4% PFA overnight and then placed in PBS. After a rinse with PBS-Triton X-100 (0.3%), pancreatic sections (40 μm) or slices (150 μm) were incubated in blocking solution (PBS-Triton X-100 0.3% and Universal Blocker Reagent; Biogenex, San Ramon, CA). Thereafter, sections were incubated for 48 h (20°C) with primary antibodies diluted in blocking solution. We immunostained GFP (Abcam, cat. nr. ab13970) pericytes using commonly used markers for pericytes (neuron-glial antigen 2, NG2, a chondroitin sulfate proteoglycan, 1:200; platelet-derived growth factor receptor-beta, PDGFRβ, 1:100; and alpha smooth muscle actin, αSMA,1:250), endothelial cells (CD31, 1:25), beta cells (insulin, 1:2000) and sympathetic fibers (tyrosine hydroxylase, 1:500). Immunostaining was visualized by using Alexa Fluor conjugated secondary antibodies (1:500 in PBS; 16 h at 20°C; Invitrogen, Carlsbad, CA). Cell nuclei were stained with DAPI. Slides were mounted with Vectashield mounting medium (Vector Laboratories). Confocal images of immunostained sections or slides were acquired on an inverted laser-scanning confocal microscope (Leica TCS SP5) with LAS AF software using a 63× oil immersion objective (NA 1.4) (Leica Microsystems).
Quantification and statistical analyses
Quantification of pericyte coverage and αSMA expression
We used ImageJ software (http://imagej.nih.gov/ij/) to estimate in confocal planes the percentage of pericytes and endothelial cells versus other islet cells by manually counting the number of nuclei surrounded by cytoplasmic NG2 or CD31 immunostaining and the total number of endocrine cell nuclei. Ratio of pericytes over endothelial cells was calculated by dividing the number of pericytes by the number of endothelial cells in mouse and human islets. To determine NG2 and CD31 immunostained area, we used maximal projection of confocal images of mouse and human islets. After background subtraction, we manually adjusted threshold and used the “analyze particles” function in ImageJ to identify the different objects and calculate the total immunostained area. Pericyte coverage of islet capillaries is the ratio of NG2 immunostained area over CD31 immunostained area. To determine colocalization between NG2 (green channel) and αSMA (red channel) immunostaining, we used the ImageJ plugin “intensity correlation analysis” and calculated the Mander’s Colocalization coefficients (M1 and M2). These-coefficients avoid issues related to the absolute intensities of the signals, since they are normalized against total pixel intensity (Manders et al., 1993) and represent the percentage of NG2 that is present in αSMA-positive structures (M1) and vice –versa (M2). Quantifications were performed in a minimum of 3 islets per individual (4 lean mice and 3 ob/ob, ages 3-4 months; 5 non-diabetic and 4 type 2 diabetic individuals, ages 15 – 52 years).
Quantification of cytosolic Ca2+ levels
To quantify changes in intracellular Ca2+ levels, we drew regions of interest around individual islet pericytes, acinar pericytes and smooth muscle cells and measured changes in mean GCaMP3 fluorescence intensity using ImageJ. A detailed description of how Ca2+ imaging data were analyzed and quantified is provided as supplementary material (Figure S4). Briefly, changes in fluorescence intensity were expressed as percentage changes over baseline (ΔF/F). The baseline was defined as the mean of the three lowest intensity values during the control period of each recording [i.e. in non-stimulatory, basal glucose concentration conditions (3 mM)]. Ca2+ signals in pericytes showed transient, oscillating responses as well as slower changes in baseline. We therefore measured changes in total cytosolic Ca2+ levels by computing the area under the curve above baseline using Prism software (Prism 7, GraphPad software, La Jolla, CA). Areas under the curve were determined before, during, and after each stimulus for the same time period and compared with statistical tests.
Quantification of capillary diameter
A detailed description of the procedure to quantify blood vessel diameter is provided as supplementary material (Figure S5). Briefly, we drew a straight line transversal to the blood vessel borders and used the “reslice” z-function in ImageJ to generate a single image showing the changes in vessel diameter over time [xt scan, (Fischer et al., 2010)] (Figure S5). Noise from reslice images was removed using a median filter (radius = 1 pixel). We drew another line on the xt scan (resliced) image and, using the “plot profile” function, we determined the position of the pixels with the highest fluorescence intensity and considered these the vessel borders. Vessel diameter was calculated by subtracting these 2 position values. We measured vessel diameters at ≥ 5 different time points before and after the stimulus. Curves showing changes in diameter over time were smoothened by averaging 4–6 neighboring values. To establish that a capillary responded to a vasoactive stimulus, we pooled the diameter values before and after stimulation and determined if they were statistically different (unpaired t-test). If so, we considered that vessel to be responsive. To determine the extent of constriction/dilation, we pooled data on responsive capillaries from different islets from different slices/mice and calculated the average change in diameter (as percentage; Figure S5). We estimated the proportion of “responsive capillaries” by dividing the area of the islet vasculature that responded to a certain stimulus by the total area of islet microvasculature.
Statistical analyses
For statistical comparisons we used Prism 7 (GraphPad software, La Jolla, CA) and performed Student’s t tests (paired or unpaired) or one-way analysis of variance (ANOVA) corrected for multiple comparisons (using Tukey’s Multiple Comparison Test; each row represented matched data). P-values are shown in the figures. Throughout the manuscript we present data as mean ± SEM.
Supplementary Material
HIGHLIGHTS.
Contractile pericytes covering capillaries regulate pancreatic islet blood flow
Beta cell activation relaxes pericytes and dilates islet capillaries through adenosine signaling
Sympathetic input activates islet pericytes to locally constrict capillaries and decrease islet blood flow
Pericyte coverage of islet capillaries sharply drops during type 2 diabetes progression
Acknowledgments
We thank Drs. Jae Lee and Amber Hackett (University of Miami) for donating the NG2-CreER-tdTomato mice, Kevin Johnson for histological work, and Melissa Canales for data quantification. This work was funded by NIH grants K01DK111757 (J.A.), R56DK084321 (A.C.), R01DK084321 (A.C.), R01DK111538 (A.C.), R01DK113093 (A.C.), and R21ES025673 (A.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.A. performed immunohistochemistry, physiological experiments with slices and in vivo imaging; J.W. prepared mouse pancreatic slices; R.R.D performed electron microscopy; E.P. helped with tissue processing and immunohistochemistry; J.A. and A.C. designed the study, analyzed data, and wrote the paper. All authors discussed the results and commented on the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Atef N, Ktorza A, Picon L, Penicaud L. Increased islet blood flow in obese rats: role of the autonomic nervous system. Am J Physiol. 1992;262:E736–740. doi: 10.1152/ajpendo.1992.262.5.E736. [DOI] [PubMed] [Google Scholar]
- Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36:451–455. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31:705–714. doi: 10.1007/s00268-006-0719-8. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne RM, Knabb RM, Ely SW, Rubio R. Adenosine in the local regulation of blood flow: a brief overview. Fed Proc. 1983;42:3136–3142. [PubMed] [Google Scholar]
- Borysova L, Burdyga T. Evidence that NO/cGMP/PKG signalling cascade mediates endothelium dependent inhibition of IP(3)R mediated Ca(2)(+) oscillations in myocytes and pericytes of ureteric microvascular network in situ. Cell Calcium. 2015;58:535–540. doi: 10.1016/j.ceca.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysova L, Wray S, Eisner DA, Burdyga T. How calcium signals in myocytes and pericytes are integrated across in situ microvascular networks and control microvascular tone. Cell Calcium. 2013;54:163–174. doi: 10.1016/j.ceca.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunicardi FC, Stagner J, Bonner-Weir S, Wayland H, Kleinman R, Livingston E, Guth P, Menger M, McCuskey R, Intaglietta M, et al. Microcirculation of the islets of Langerhans. Long Beach Veterans Administration Regional Medical Education Center Symposium. Diabetes. 1996;45:385–392. doi: 10.2337/diab.45.4.385. [DOI] [PubMed] [Google Scholar]
- Burdyga T, Borysova L. Calcium signalling in pericytes. J Vasc Res. 2014;51:190–199. doi: 10.1159/000362687. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- Carlsson PO, Jansson L, Ostenson CG, Kallskog O. Islet capillary blood pressure increase mediated by hyperglycemia in NIDDM GK rats. Diabetes. 1997;46:947–952. doi: 10.2337/diab.46.6.947. [DOI] [PubMed] [Google Scholar]
- Carlsson PO, Olsson R, Kallskog O, Bodin B, Andersson A, Jansson L. Glucose-induced islet blood flow increase in rats: interaction between nervous and metabolic mediators. Am J Physiol Endocrinol Metab. 2002;283:E457–464. doi: 10.1152/ajpendo.00044.2002. [DOI] [PubMed] [Google Scholar]
- Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK. PDGF signalling controls age-dependent proliferation in pancreatic beta-cells. Nature. 2011;478:349–355. doi: 10.1038/nature10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X, Gao X, Jansson L, Quach M, Skogseid B, Barbu A. Multiple microvascular alterations in pancreatic islets and neuroendocrine tumors of a Men1 mouse model. Am J Pathol. 2013;182:2355–2367. doi: 10.1016/j.ajpath.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Collis MG. The vasodilator role of adenosine. Pharmacol Ther. 1989;41:143–162. doi: 10.1016/0163-7258(89)90104-6. [DOI] [PubMed] [Google Scholar]
- Crawford C, Wildman SS, Kelly MC, Kennedy-Lydon TM, Peppiatt-Wildman CM. Sympathetic nerve-derived ATP regulates renal medullary vasa recta diameter via pericyte cells: a role for regulating medullary blood flow? Front Physiol. 2013;4:307. doi: 10.3389/fphys.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Corselli M, Chen WC, Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16:2851–2860. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Dai C, Brissova M, Reinert RB, Nyman L, Liu EH, Thompson C, Shostak A, Shiota M, Takahashi T, Powers AC. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes. 2013;62:4144–4153. doi: 10.2337/db12-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detimary P, Jonas JC, Henquin JC. Stable and diffusible pools of nucleotides in pancreatic islet cells. Endocrinology. 1996;137:4671–4676. doi: 10.1210/endo.137.11.8895332. [DOI] [PubMed] [Google Scholar]
- Dodge AB, D’Amore PA. Cell-cell interactions in diabetic angiopathy. Diabetes Care. 1992;15:1168–1180. doi: 10.2337/diacare.15.9.1168. [DOI] [PubMed] [Google Scholar]
- Feil R, Gappa N, Rutz M, Schlossmann J, Rose CR, Konnerth A, Brummer S, Kuhbandner S, Hofmann F. Functional reconstitution of vascular smooth muscle cells with cGMP-dependent protein kinase I isoforms. Circ Res. 2002;90:1080–1086. doi: 10.1161/01.res.0000019586.95768.40. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MJ, Uchida S, Messlinger K. Measurement of meningeal blood vessel diameter in vivo with a plug-in for ImageJ. Microvasc Res. 2010;80:258–266. doi: 10.1016/j.mvr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Geisler JC, Corbin KL, Li Q, Feranchak AP, Nunemaker CS, Li C. Vesicular nucleotide transporter-mediated ATP release regulates insulin secretion. Endocrinology. 2013;154:675–684. doi: 10.1210/en.2012-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepts W. The islet of Langerhans: Biochemistry, Physiology and Pathology. 1981 Chapter 13. [Google Scholar]
- Gilliam LK, Palmer JP, Taborsky GJ., Jr Tyramine-mediated activation of sympathetic nerves inhibits insulin secretion in humans. J Clin Endocrinol Metab. 2007;92:4035–4038. doi: 10.1210/jc.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graefe KH, Bossle F, Wolfel R, Burger A, Souladaki M, Bier D, Dutschka K, Farahati J, Bonisch H. Sympathomimetic effects of MIBG: comparison with tyramine. J Nucl Med. 1999;40:1342–1351. [PubMed] [Google Scholar]
- Guyton AC, Hall JE. Text book of medical physiology. Elsevier Saunders; 2006. [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2 doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MR, Karuparthi PR, Habibi J, Lastra G, Patel K, Wasekar C, Manrique CM, Ozerdem U, Stas S, Sowers JR. Ultrastructure of islet microcirculation, pericytes and the islet exocrine interface in the HIP rat model of diabetes. Exp Biol Med (Maywood) 2008;233:1109–1123. doi: 10.3181/0709-RM-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazama A, Hayashi S, Okada Y. Cell surface measurements of ATP release from single pancreatic beta cells using a novel biosensor technique. Pflugers Arch. 1998;437:31–35. doi: 10.1007/s004240050742. [DOI] [PubMed] [Google Scholar]
- Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RK. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes. 2012;61:1700–1707. doi: 10.2337/db11-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstrom C, Westman S, Zachrisson U, Hellman B. The number of red blood cells in the islets of Langerhans as an index of the B cell activity. Acta Endocrinol (Copenh) 1960;34:611–618. doi: 10.1530/acta.0.xxxiv0611. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtz J, Borden P, Ceasrine A, Minichiello L, Kuruvilla R. Neurotrophin Signaling Is Required for Glucose-Induced Insulin Secretion. Dev Cell. 2016;39:329–345. doi: 10.1016/j.devcel.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–659. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- Jansson L. The regulation of pancreatic islet blood flow. Diabetes Metab Rev. 1994;10:407–416. doi: 10.1002/dmr.5610100405. [DOI] [PubMed] [Google Scholar]
- Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, Grapensparr L, Kallskog O, Lau J, Liljeback H, et al. Pancreatic islet blood flow and its measurement. Ups J Med Sci. 2016;121:81–95. doi: 10.3109/03009734.2016.1164769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson L, Eizirik DL, Sandler S. Terbutaline decreases the blood flow of the pancreatic islets but does not reduce the diabetogenic action of streptozotocin in the rat. Eur J Pharmacol. 1989;161:79–83. doi: 10.1016/0014-2999(89)90182-9. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Haire MF, Palade GE. Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J Cell Biol. 1985;100:1379–1386. doi: 10.1083/jcb.100.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang JH, Kuo CH, Peng SJ, Tang SC. 3-D Imaging Reveals Participation of Donor Islet Schwann Cells and Pericytes in Islet Transplantation and Graft Neurovascular Regeneration. EBioMedicine. 2015;2:109–119. doi: 10.1016/j.ebiom.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EY, Jansson L, Patzak A, Persson AE. Vascular reactivity in arterioles from normal and alloxan-diabetic mice: studies on single perfused islets. Diabetes. 2007a;56:107–112. doi: 10.2337/db06-0623. [DOI] [PubMed] [Google Scholar]
- Lai EY, Persson AE, Bodin B, Kallskog O, Andersson A, Pettersson U, Hansell P, Jansson L. Endothelin-1 and pancreatic islet vasculature: studies in vivo and on isolated, vascularly perfused pancreatic islets. Am J Physiol Endocrinol Metab. 2007b;292:E1616–1623. doi: 10.1152/ajpendo.00640.2006. [DOI] [PubMed] [Google Scholar]
- Lavoie EG, Fausther M, Kauffenstein G, Kukulski F, Kunzli BM, Friess H, Sevigny J. Identification of the ectonucleotidases expressed in mouse, rat, and human Langerhans islets: potential role of NTPDase3 in insulin secretion. Am J Physiol Endocrinol Metab. 2010;299:E647–656. doi: 10.1152/ajpendo.00126.2010. [DOI] [PubMed] [Google Scholar]
- Li Q, Puro DG. Adenosine activates ATP-sensitive K(+) currents in pericytes of rat retinal microvessels: role of A1 and A2a receptors. Brain Res. 2001;907:93–99. doi: 10.1016/s0006-8993(01)02607-5. [DOI] [PubMed] [Google Scholar]
- Liu YM, Guth PH, Kaneko K, Livingston EH, Brunicardi FC. Dynamic in vivo observation of rat islet microcirculation. Pancreas. 1993;8:15–21. doi: 10.1097/00006676-199301000-00005. [DOI] [PubMed] [Google Scholar]
- Lukinius A, Jansson L, Korsgren O. Ultrastructural evidence for blood microvessels devoid of an endothelial cell lining in transplanted pancreatic islets. Am J Pathol. 1995;146:429–435. [PMC free article] [PubMed] [Google Scholar]
- Manders EMM, Verbeek FJ, Aten JA. Measurement of co-localization of objects in dual-colour confocal images. Journal of Microscopy. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- Marciniak A, Cohrs CM, Tsata V, Chouinard JA, Selck C, Stertmann J, Reichelt S, Rose T, Ehehalt F, Weitz J, et al. Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nat Protoc. 2014;9:2809–2822. doi: 10.1038/nprot.2014.195. [DOI] [PubMed] [Google Scholar]
- McCuskey RS, Chapman TM. Microscopy of the living pancreas in situ. Am J Anat. 1969;126:395–407. doi: 10.1002/aja.1001260402. [DOI] [PubMed] [Google Scholar]
- Moldovan S, Livingston E, Zhang RS, Kleinman R, Guth P, Brunicardi FC. Glucose-induced islet hyperemia is mediated by nitric oxide. Am J Surg. 1996;171:16–20. doi: 10.1016/s0002-9610(99)80066-x. [DOI] [PubMed] [Google Scholar]
- Nyman LR, Ford E, Powers AC, Piston DW. Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo. Am J Physiol Endocrinol Metab. 2010;298:E807–814. doi: 10.1152/ajpendo.00715.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell FM, Mastitskaya S, Hammond-Haley M, Freitas F, Wah WR, Attwell D. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. Elife. 2017;6 doi: 10.7554/eLife.29280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson R, Jansson L, Andersson A, Carlsson PO. Local blood flow regulation in transplanted rat pancreatic islets: influence of adenosine, angiotensin II, and nitric oxide inhibition. Transplantation. 2000;70:280–287. doi: 10.1097/00007890-200007270-00007. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson US, Henriksnas J, Jansson L. Reversal of high pancreatic islet and white adipose tissue blood flow in type 2 diabetic GK rats by administration of the beta3-adrenoceptor inhibitor SR-59230A. Am J Physiol Endocrinol Metab. 2009;297:E490–494. doi: 10.1152/ajpendo.00140.2009. [DOI] [PubMed] [Google Scholar]
- Ravier MA, Guldenagel M, Charollais A, Gjinovci A, Caille D, Sohl G, Wollheim CB, Willecke K, Henquin JC, Meda P. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 2005;54:1798–1807. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev. 2010;31:343–363. doi: 10.1210/er.2009-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res Bull. 2000;51:363–369. doi: 10.1016/s0361-9230(99)00260-9. [DOI] [PubMed] [Google Scholar]
- Ruth P, Wang GX, Boekhoff I, May B, Pfeifer A, Penner R, Korth M, Breer H, Hofmann F. Transfected cGMP-dependent protein kinase suppresses calcium transients by inhibition of inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci U S A. 1993;90:2623–2627. doi: 10.1073/pnas.90.7.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson A, Rachi E, Sakhneny L, Baer D, Lisnyansky M, Epshtein A, Landsman L. Islet Pericytes Are Required for beta-Cell Maturity. Diabetes. 2016;65:3008–3014. doi: 10.2337/db16-0365. [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- Serre-Beinier V, Le Gurun S, Belluardo N, Trovato-Salinaro A, Charollais A, Haefliger JA, Condorelli DF, Meda P. Cx36 preferentially connects beta-cells within pancreatic islets. Diabetes. 2000;49:727–734. doi: 10.2337/diabetes.49.5.727. [DOI] [PubMed] [Google Scholar]
- Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Short KW, Head WS, Piston DW. Connexin 36 mediates blood cell flow in mouse pancreatic islets. Am J Physiol Endocrinol Metab. 2014;306:E324–331. doi: 10.1152/ajpendo.00523.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L, Subiabre M, Araos J, Saez T, Salsoso R, Pardo F, Leiva A, San Martin R, Toledo F, Sobrevia L. Insulin/adenosine axis linked signalling. Mol Aspects Med. 2017;55:45–61. doi: 10.1016/j.mam.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci. 2013;33:13882–13887. doi: 10.1523/JNEUROSCI.2524-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, Pileggi A, Moede T, Kohler M, Wilbertz J, Leibiger B, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson AM, Ostenson CG, Sandler S, Efendic S, Jansson L. Inhibition of nitric oxide synthase by NG-nitro-L-arginine causes a preferential decrease in pancreatic islet blood flow in normal rats and spontaneously diabetic GK rats. Endocrinology. 1994;135:849–853. doi: 10.1210/endo.135.3.7520863. [DOI] [PubMed] [Google Scholar]
- Tabrizchi R, Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharmacol Ther. 2001;91:133–147. doi: 10.1016/s0163-7258(01)00152-8. [DOI] [PubMed] [Google Scholar]
- Tang SC, Peng SJ, Chien HJ. Imaging of the islet neural network. Diabetes Obes Metab. 2014;16(Suppl 1):77–86. doi: 10.1111/dom.12342. [DOI] [PubMed] [Google Scholar]
- Terzic A, Tung RT, Inanobe A, Katada T, Kurachi Y. G proteins activate ATP-sensitive K+ channels by antagonizing ATP-dependent gating. Neuron. 1994;12:885–893. doi: 10.1016/0896-6273(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Theis M, Mas C, Doring B, Degen J, Brink C, Caille D, Charollais A, Kruger O, Plum A, Nepote V, et al. Replacement by a lacZ reporter gene assigns mouse connexin36, 45 and 43 to distinct cell types in pancreatic islets. Exp Cell Res. 2004;294:18–29. doi: 10.1016/j.yexcr.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Tian F, Fessenden JD, Schacht J. Cyclic GMP-dependent protein kinase-I in the guinea pig cochlea. Hear Res. 1999;131:63–70. doi: 10.1016/s0378-5955(99)00015-5. [DOI] [PubMed] [Google Scholar]
- Xian X, Hakansson J, Stahlberg A, Lindblom P, Betsholtz C, Gerhardt H, Semb H. Pericytes limit tumor cell metastasis. J Clin Invest. 2006;116:642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wu DM, Xu GZ, Puro DG. The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J Physiol. 2011;589:2383–2399. doi: 10.1113/jphysiol.2010.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


