Abstract
Microbial-based pest control is an attractive alternative to chemical insecticides. The present study sought to evaluate the toxicity of the entomopathogenic fungus Beauveria bassiana-28 ethyl acetate extracts on different larval stages and pupae of Culex quinquefasciatus mosquitoes. B. bassiana-28 ethyl acetate mycelial extracts produced mosquitocidal activity against larvae and pupae which was comparable to that of the commercial insecticide B. bassiana-22 extract. The LC50 (lethal concentration that kills 50% of the exposed larvae) values of B. bassiana-28 extracts for 1st to 4th instar larvae and pupae were 11.538, 6.953, 5.841, 3.581 and 9.041 mg/L respectively. Our results show that B. bassiana-28 ethyl acetate mycelial extract has strong insecticidal activity against larval and pupal stages of Cx. quinquefasciatus. Fourier transform infrared spectrum study of B. bassiana-28 extract shows peaks at 3226.91; 2927.94; 1593.13; 1404.18; 1224.18; 1247.94; 1078.21; 1018.41; 229.69; and 871.82 cm−1. Major spectral peaks were observed at 3226.91 cm−1, assigned to N–H stretching, 2927.94 cm−1 assigned to C–H bonding and 1595.13 cm−1 assigned to C–O stretching. Gas Chromatography-Mass Spectrometry studies of B. bassiana-28 ethyl acetate crude extract showed presence of six major compounds viz. N-hexadecanoic acids (13.6040%); Z,Z-9,12 octadecadienic acid (33.74%); 9-eicosyne (10.832%); heptacosane (5.148%); tetrateracontane (5.801%); and 7 hexyleicosane (5.723%). Histology of mosquito midgut tissue shows tissue lysis as a result of B.bassiana-28 extract exposure. The study shows that bioactive molecules obtained from B. bassiana-28 mycelial extract has insecticidal properties and can be used as alternative for mosquito control.
Keywords: Beauveria bassiana-28, Culex quinquefasciatus, FT-IR, GC-MS, ethyl acetate, midgut, biopesticide
1. Introduction
Mosquitoes are responsible for several vector-borne diseases [1]. Mosquitoes are classified into three subfamilies: Anophelinae, Culicinae and Toxorhynchitinae [2]. Female Culex quinquefasciatus mosquitoes are responsible for lymphatic filariasis in tropical and subtropical regions [3,4,5,6]. Human filariasis is a major public health hazard and remains a challenging socioeconomic problem in India and other tropical countries. Many researchers have reported that mosquitoes show resistance to synthetic chemical insecticides [7,8,9]. In this scenario, bioactive compounds of biological origin from bacteria, fungus, plants and entomopathogenic microbes are being bio-prospected as alternatives to chemical insecticides [10,11,12,13,14]. The advantages of these biopesticides are due to their biodegradability, target specificity, eco-friendly nature and their usefulness as tools to manage insecticide resistance in mosquitoes [15,16].
Several studies on entomopathogenic fungi (EPF) and their metabolites have shown their control potential in various stages of mosquitoes [17,18]. Secondary metabolites from Chrysosporium [19], Metarhizium and Beauveria [20,21]; Culicinomyces [22], Verticillium [19] and Piper [23] have been evaluated for their insecticidal potential on mosquitoes and houseflies [24]. Entomopathogenic fungus, particularly B. bassiana, are well known as insect pathogens against agricultural lepidopteran pests [25,26]. These EPF Beauveria spp., are highly specific to mosquitoes [27] and can be developed as potential insecticides for the control of mosquito larvae. B. bassiana is a cosmopolitan entomopathogenic fungus found in nature in soils. B. bassiana-derived insecticides have nowadays been registered and commercially developed worldwide as agricultural pest control methods [28]. Beauveria bassiana conidia, blastospores and their secondary metabolites are used for controlling the mosquito population at a laboratory level [29]. In the present study we investigated the toxicity of our isolate B. bassiana-28 extract in comparison with the commercially available microbial insecticide Beauveria bassiana-22 extract against different stages (Ist–IVth instar larvae) and pupae of Cx. quinquefasciatus mosquito and also performed larval histopathological studies at different time durations (6, 12 and 24 h post-treatment).
2. Materials and Methods
2.1. Source of Culture
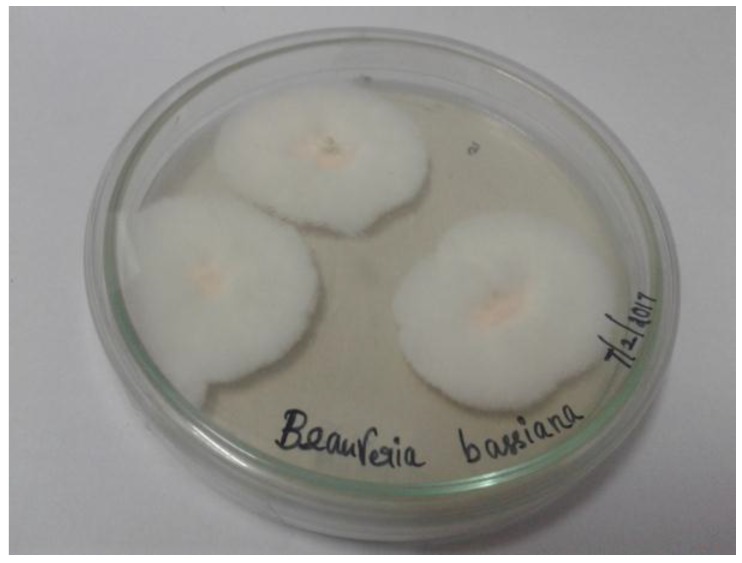
B. bassiana-28 fungal culture was isolated from dead cadavers of Spodoptera litura (tobacco cutworm or cotton leaf worm) insects in a cotton field in Dharmapuri, Tamil Nadu, India. B. bassiana-28 (Figure 1) was sub-cultured on potato dextrose agar (PDA) medium with added ampicillin (3 mg/100 mL) and incubated for 10 days at 25 °C ± 2 °C. B. bassiana-28 fungal culture was maintained and research work was carried out at the Molecular Entomology Laboratory (Salem, Tamil Nadu, India).
Figure 1.
Seven days old strains of B. bassiana-28.
2.2. Commercial Microbial Insecticide B. bassiana-22
Commercially available powdered microbial insecticide B. bassiana-22 was procured from Manidharma Biotech, Pvt Ltd. (Chennai, Tamil Nadu, India). Commercial Beauveria bassiana-22 targets several insect pests such as root weevils, plant hoppers, Japanese beetle, black vine weevil, spittlebug and white grubs and lepidopteron pests. The procured B. bassiana-22 was cultured on PDA, supplemented with ampicillin (3 mg/100 mL) and incubated for 10 days at 25 °C ± 2 °C.
2.3. Morphological Identification of B. bassiana-28
Morphological identification was carried out for isolated or unknown B.bassiana-28 fungal strains based on morphological characteristics such as colony colour, aerial mycelial structures, pigment production and conidia stained with lacto-phenol cotton blue and viewed under light microscope (Olympus-CH20i, Mumbai, India) at 400× magnification.
2.4. Mass Culturing of B. bassiana-28
B. bassiana-28 broth was prepared for the mass culture of fungal mycelia as per the modified method of [30]. Four 1000 mL conical flasks, each containing 500 mL of potato dextrose broth (PDB), (dextrose 40 g, peptone 10 g, deionized water 1000 mL), were sterilized at 15 psi for 30 min. The broths were supplemented with 30 mg ampicillin, which acts as a bacterial control agent. B. bassiana-28 fungal conidia (1 × 107 per mL) were inoculated and grown in PDB. The flasks were incubated at 25 °C ± 2 °C for 25 days.
2.5. Crude Extraction from B. bassiana-28
Mass culturing of B. bassiana-28 and B. bassiana-22 was carried out in a 1000 mL Erlenmeyer flask containing 500 mL of PDB. The flasks were incubated under the optimized culture conditions (pH 7.0 at 27 °C) for 25 days. The fungal biomass was removed from the medium with help of Whatman No. 1 filter paper and washed more than five times with distilled water to remove the unwanted broth particles. Fungal biomass (100 g) was transferred to 500 mL glass beakers containing ethyl acetate (250 mL) which was mixed with the mycelium for cold extraction for 20 days at 25 °C ± 2 °C. After complete extraction the liquid portion was separated from the mycelium by filtering through Whatman No. 1 filter paper. Separated secondary metabolite ethyl acetate extracts were finally concentrated using a rotary vacuum evaporator (Superfit-R/150/11, Mumbai, India) at 45 °C.
2.6. Thin Layer Chromatography
Thin layer chromatography (TLC) was performed on commercial silica gel-H TLC plates (chloride-0.02%, sulphate-0.02%, iron-0.02%, heavy metals-0.02% and pH-7) for principal components separation. The developed TLC plates were dried at room temperature. After air drying B.bassiana-28 extract were spotted at center of the plate with the help of a capillary tube. Then we prepared different solvent systems as mobile phases for thin layer chromatography because the biological molecules can be separated by different solvent system. The mobile phase solvent systems were chloroform:methanol in several ratios (10; 9:1; 8:2; 7:3; 6:4; 5:5; 4:6; 3:7; 2:8; 1:9; 10). After the running process the plates were observed under UV light at 350 nm. The retention factor (Rf) values were calculated using Equation (1) and based on the movement of samples in TLC plate (Figure 2).
| (1) |
Figure 2.
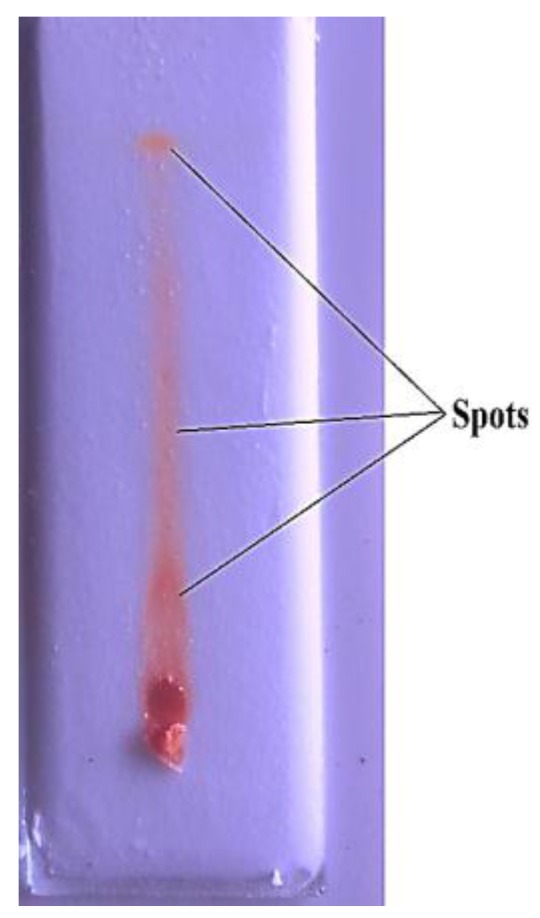
Thin layer chromatography of B. bassiana-28 extract. The mobile phases were chloroform: methanol in ratios of 10; 9:1; 8:2; 7:3; 6:4; 5:5; 4:6; 3:7; 2:8; 1:9; 10 and the retention factor (Rf) values of spots were 0.3333, 0.4444, 0.5555.
2.7. Mosquito Culture
Cx. quinquefasciatus egg rafts were obtained from the Institute of Vector Control Zoonoses, (IVCZ, Hosur, Tamil Nadu, India). The egg rafts were maintained in 2 L plastic jars containing tap water. The larvae were fed with dog biscuits and millet powder and yeast powder in 3:3:1 ratio. Larvae were kept at 27 °C ± 2 °C and 70–85% relative humidity with a 12:12 light and dark photoperiod.
2.8. Larval Bioassay
Larval mortality bioassays were carried out according to the method suggested by the World Health Organization (WHO) [31], with slight modifications. Extracted B. bassiana-28 and B. bassiana-22 mycelia extract were transferred individually to 250 mL round bottom flasks, then the ethyl acetate solvent was removed by using a rotary evaporator, (Superfit- R/150/01, Mumbai, India). The bioassay has five testing concentrations and each concentration had three replicates of twenty larvae each. Test containers containing 20 mosquito larvae were stored in 150 mL plastic cups containing 99 mL of distilled water with the desired concentration (i.e., 25, 50,100,150, 200 and 250 μg/mL). In the control 20 individuals were exposed to the same dose of dimethyl sulfoxide (DMSO) as negative control. After 24 h exposure, mortality (%) was calculated and corrected with control mortality using the Abbott formula [32]. The larval mortality was calculated after 24 h post treatment. LC50 and LC90 values were calculated by probit analysis using the SPSS-16.0 software (IBM-Corporation, Bengaluru, Karnataka, India).
| (2) |
2.9. Pupal Toxicity Tests
Cx quinquefasciatus pupae from the laboratory maintained culture were used to examine the pupal toxicity of B. bassiana-28 and B. bassiana-22 extracts. Twenty pupae were transferred to 150 mL plastic containers containing 99 mL of distilled water. Five different concentrations of extracts (i.e., 25, 50, 100, 150, 200 and 250 μg/mL) were separately dissolved in DMSO (1 mL) and the dissolved fungal extracts were added to the water in the bioassay vessels. Each concentration had three replicates and each replicate had twenty pupae. Mortality was calculated 24 h post treatment, and mortality in the treatments and control was corrected using Abbott’s formula [32]. The LC50 and LC90 values were calculated from the toxicity data by probit analysis using SPSS-16.0 software.
3. Gas Chromatography-Mass Spectrophotometer (GC-MS) Analysis
A Clarus 680 30 m × 0.25 mm ID × 250 μm silica column was used for GC analysis of the chemical constituents. This column was packed with elite-5MS (5% biphenyl 95% dimethylpolysiloxane). The chemical constituents were separated by using He at a constant flow of 1 mL/min as a carrier gas. The crude extracts (1 μL) were injected to the GC-MS instrument at 260 °C during the column running time. The temperature ramp was as follows: 60 °C (2 min); followed by 300 °C at the rate of 10 °C min−1; and finally 300 °C, where it was held for 6 min. The mass detector conditions were 240 °C; ion source temperature at 240 °C; and ionization mode electron impact at 70 eV, a scan time 0.2 s and scan interval of 0.1 s. The fragments from 40 to 600 Da were collected.
Histological Studies
The 6, 12 and 24 h post-treated and control 4th instar Cx. quinquefasciatus larvae were fixed in 3% formaldehyde solution for 2 h at 4 °C. The blocks were cooled at 27 °C for 3 h and cut to 8 μm thickness 1.3 mm ribbons with a microtome (Berlin, Germany). Cross-sectioned larval gut was stained with haematoxylin and eosin stain. After air drying sections was viewed under a light microscope (Olympus-CH20i) at a magnification of 400×.
4. Results
4.1. Larval Bioassay
Based on LC50 and LC90 values it was found that B. bassiana-28 mycelium extract had insecticidal activity similar to that of the commercial microbial insecticide B. bassiana-22 on 1st to 4th instar of Cx. quinquefasciatus (Table 1 and Table 2).
Table 1.
Larvicidal and pupicidal activity of B. bassiana-28 fungal mycelium extract (ethyl acetate) against larvae and pupa of Cx. quinquefasciatus (after 24 h of exposure).
| Mosquito Species | Larval Stages | Concentration (mg/L) | Mortality (%) ± S.D. | LC50 (LCL-UCL) mg/L | LC90 (LCL-UCL) mg/L | χ2 (df) 3 |
|---|---|---|---|---|---|---|
| Cx. quinquefasciatus | 1st Instar | Control | 2.5 ± 0.12 | 11.538 (4.061–20.308) |
16.155 (6.575–26.375) |
4.276 |
| 25 | 15.24 ± 0.8 | |||||
| 50 | 43.33 ± 1.0 | |||||
| 100 | 53.33 ± 1.0 | |||||
| 150 | 71.66 ± 2.5 | |||||
| 200 | 83.33 ± 1.5 | |||||
| 250 | 91.66 ± 0.5 | |||||
| 2nd Instar | Control | 2.1 ± 0.11 | 6.953 (1.158–15.718) |
10.790 (2.345–21.689) |
3.089 | |
| 25 | 17.12 ± 1.0 | |||||
| 50 | 43.33 ± 2.5 | |||||
| 100 | 53.33 ± 1.0 | |||||
| 150 | 61.66 ± 1.0 | |||||
| 200 | 78.33 ± 2.0 | |||||
| 250 | 83.33 ± 1.0 | |||||
| 3rd Instar | Control | 1.8 ± 0.10 | 5.841 (1.151–12.787) |
8.337 (1.993–16.673) |
2.978 | |
| 25 | 21.45 ± 1.2 | |||||
| 50 | 63.33 ± 0.5 | |||||
| 100 | 73.33 ± 0.5 | |||||
| 150 | 83.33 ± 1.5 | |||||
| 200 | 93.33 ± 2.5 | |||||
| 250 | 96.66 ± 0.5 | |||||
| 4th Instar | Control | 2.1 ± 0.18 | 3.581 (2.254–18.730) |
5.265 (3.437–23.043) |
3.421 | |
| 25 | 37.23 ± 1.3 | |||||
| 50 | 71.66 ± 1.5 | |||||
| 100 | 78.33 ± 2.0 | |||||
| 150 | 86.66 ± 1.0 | |||||
| 200 | 93.33 ± 0.5 | |||||
| 250 | 100.00 ± 1.5 | |||||
| Pupa | Control | 2.7 ± 0.19 | 9.041 (2.975–16.369) |
12.104 (4.532–20.504) |
3.404 | |
| 25 | 25.42 ± 1.2 | |||||
| 50 | 61.66 ± 1.5 | |||||
| 100 | 78.33 ± 3.0 | |||||
| 150 | 86.66 ± 1.0 | |||||
| 200 | 93.33 ± 0.5 | |||||
| 250 | 100.00 ± 0.0 |
LC50: lethal concentration that kills 50% of the exposed larvae and pupa LC90: lethal concentration that kills 90% of the exposed larvae and pupa; UCL: upper confidence limit (95% fiducial limit); LCL: lower confidence limit (95% fiducial limit); χ2: chi-square; df: degrees of freedom; S.D.: standard deviation.
Table 2.
Larvicidal and pupicidal activity of commercial insecticide B. bassiana-22 fungal mycelium extract (ethyl acetate) against larvae and pupa of Cx. quinquefasciatus (after 24 h of exposure).
| Mosquito Species | Larval Stages | Concentration (mg/L) | Mortality (%) ± S.D. | LC50 (LCL-UCL) mg/L | LC90 (LCL-UCL) mg/L | χ2 (df) 3 |
|---|---|---|---|---|---|---|
| Cx. quinquefasciatus | 1st Instar | Control | 2.5 ± 0.10 | 10.523 (3.237–19.576) |
15.843 (5.871–26.807) |
0.774 |
| 25 | 18.02 ± 0.9 | |||||
| 50 | 41.66 ± 1.5 | |||||
| 100 | 60.00 ± 1.5 | |||||
| 150 | 78.33 ± 1.0 | |||||
| 200 | 88.33 ± 0.5 | |||||
| 250 | 98.33 ± 0.5 | |||||
| 2nd Instar | Control | 1.5 ± 0.00 | 6.840 (1.819–16.649) |
11.792 (2.069–24.581) |
0.721 | |
| 25 | 26.11 ± 0.8 | |||||
| 50 | 36.66 ± 2.0 | |||||
| 100 | 58.33 ± 1.5 | |||||
| 150 | 63.33 ± 1.0 | |||||
| 200 | 71.66 ± 1.0 | |||||
| 250 | 83.33 ± 2.5 | |||||
| 3rd Instar | Control | 2.0 ± 0.18 | 4.616 (1.010–11.781) |
7.631 (1.293–16.992) |
0.704 | |
| 25 | 28.32 ± 1.0 | |||||
| 50 | 55.00 ± 0.5 | |||||
| 100 | 73.33 ± 0.5 | |||||
| 150 | 81.66 ± 1.0 | |||||
| 200 | 91.66 ± 2.5 | |||||
| 250 | 98.33 ± 1.0 | |||||
| 4th Instar | Control | 2.3 ± 0.0 | 2.674 (1.343–8.466) |
4.605 (2.068–12.449) |
0.036 | |
| 25 | 34.12 ± 1.0 | |||||
| 50 | 61.66 ± 1.5 | |||||
| 100 | 81.66 ± 0.5 | |||||
| 150 | 91.66 ± 1.0 | |||||
| 200 | 96.66 ± 0.5 | |||||
| 250 | 100.00 ± 0. | |||||
| Pupa | Control | 0.00 | 8.364 (1.643–17.906) |
13.552 (3.516–25.583) |
0.465 | |
| 25 | 26.09 ± 0.7 | |||||
| 50 | 40.00 ± 0.5 | |||||
| 100 | 55.00 ± 0.5 | |||||
| 150 | 70.00 ± 2.5 | |||||
| 200 | 80.00 ± 1.0 | |||||
| 250 | 88.33 ± 0.5 |
4.2. Thin Layer Chromatography
Thin Layer Chromatography was analysed. B. bassiana-28 extract showed three spots with Rf values of 0.3333, 0.4444, and 0.5555, respectively (Figure 2).
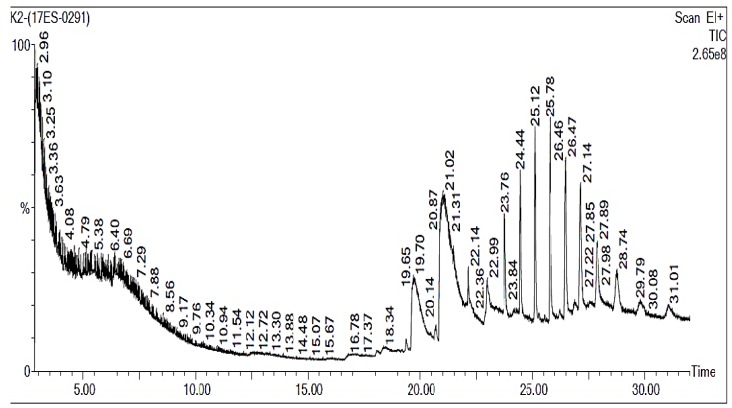
4.3. Gas Chromatography-Mass Spectrometry Analysis of B. bassiana-28 Ethyl Acetate Mycelial Extract
Gas Chromatography-Mass Spectrometry results obtained from the B. bassiana-28 indicated the presence of several major compounds viz. N-hexadecanoic acid (19.695%), eicosanoic acid (21.016%), octadecanoic acid (21.466%), tridecanoic acid (22.081%), pentadecanoic acid (22.136%), tetradecanoic acid (22.986%), octadecanoic acid (23.757%), eicosanoic acid (24.442%), heptadecanoic acid (25.117%), tridecanoic acid (25.778%), octadecanoic acid (26.468%), tridecanoic acid (27.143%), dodecanoic acid (27.888%), l-(+)-ascorbic acid 2,6-dihexadecanoate (28.749%) (Figure 3 and Table 3). Six major compounds—N-hexadecanoic acid (13.6040%), Z,Z-9,12 dectadecadienoic acid (33.74%), 9-eicosyne (10.832%), heptacosane (5.148%), tetrateracontane (5.801%); and 7 hexyleicosane (5.723%)—may be involved in the insecticidal activity.
Figure 3.
Compounds were identified from B.bassiana-28 extracts. Oven initial temp 60 °C for 2 min, ramp 10 °C min−1 to 300 °C, hold 6 min, Inject auto = 250 °C, volume = 1 μL, split = 10:1, carrier gas = He, solvent delay = 2.00 min, transfer temp = 240 °C, source temp = 240 °C, scan 50 to 600 Da, column 300 m~250 μm.
Table 3.
Major bioactive compounds identified in the ethyl acetate mycelium extracts of B. bassiana-28 using Gas Chromatography-Mass Spectrometry analysis.
| S. No. | Retention time (min) | Compound Name | Molecular Weight | Formula | Area (%) | Biological Activity |
|---|---|---|---|---|---|---|
| 1 | 16.695 | N-Hexadecanoic Acid | 256 | C16H32O2 | 13.640 | Pesticidal activity |
| 2 | 21.016 | (Z,Z)-9,12 Octadecadienoic Acid | 280 | C18H32O2 | 33.747 | Anti-inflammatory activity |
| 3 | 21.466 | 9-Eicosyne | 278 | C20H38 | 10.832 | No activity reported |
| 4 | 22.081 | cis-9,10-Epoxyoctadecan-1-ol | 280 | C18H32O2 | 1.352 | No activity reported |
| 5 | 22.136 | N-[Bromo-N-butyl]-2-piperidinone | 233 | C9H16ONBr | 2.612 | No activity reported |
| 6 | 22.986 | Hexatriacontane | 506 | C36H74 | 2.133 | No activity reported |
| 7 | 23.757 | 1-Bromo-2-methyldecane | 234 | C11H23Br | 3.121 | No activity reported |
| 8 | 24.442 | Tritetracontane | 604 | C43H88 | 3.647 | No activity reported |
| 9 | 25.117 | Heptacosane | 380 | C27H56 | 5.148 | Antibacterial |
| 10 | 25.778 | Tritetracontane | 618 | C44H90 | 5.801 | Anti-bacterial, Anti-fungal |
| 11 | 26.468 | 7-Hexyleicosane | 366 | C26H54 | 5.723 | No activity reported |
| 12 | 27.143 | Nonacosane | 408 | C29H60 | 5.408 | Anti-bacteria, |
| 13 | 27.888 | 1-Chloroheptacosane | 414 | C27H55CI | 3.866 | No activity reported |
| 14 | 28.749 | 9-Octyleicosane | 394 | C28H58 | 2.971 | No activity reported |
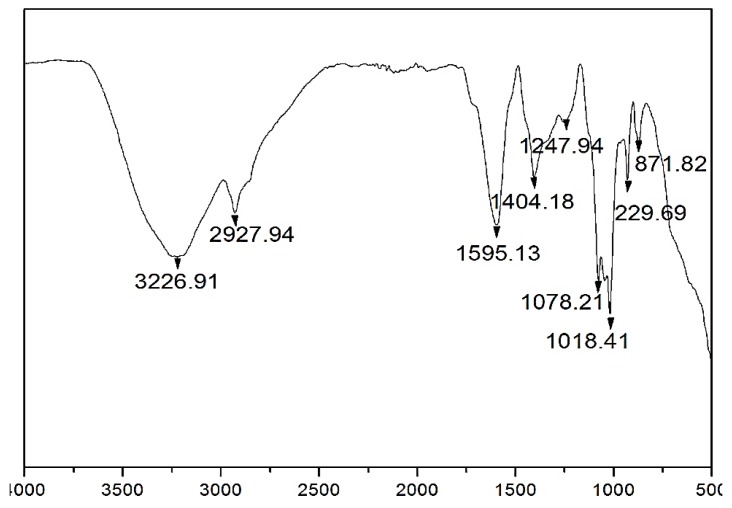
4.4. Fourier transform infrared spectrum Analysis of B. bassiana-28 Ethyl Acetate Mycelial Extract
The intracellular ethyl acetate metabolites were illustrated using Fourier transform infrared (FT-IR) analysis (Figure 4; Table 4). The results indicate the presence of bands with peak values at 3226.91; 2927.94; 2858.51; 1595.13; 1404.18; 1247.94; 1078.21; 1018.41; 929.69; 871.82 and 503.42 cm−1 (Table 4). B. bassiana -28 extracts have prominent peaks in the FT-IR spectra at 3226.91 cm−1 corresponding to N–H stretching vibrations. The strong band at 1595.13 cm−1 is assigned to C=O stretching. The two bands at 1078.21 cm−1, S=O stretching and 1018.41 cm−1, C–O stretching, are strong support to the presence of aliphatic and aromatic amines, respectively.
Figure 4.
Fourier transform infrared (FT-IR) spectrum analysis of B. bassiana-28 extract, scanning it in the range 500–4000 cm−1 at a resolution value of 4 cm−1.
Table 4.
Beauveria bassiana-28 mycelial crude metabolites of FT-IR Spectrum study.
| Observed Wave Numbers (cm−1) | Peak Assignment | Visible Intensity | Functional Group |
|---|---|---|---|
| 3226.91 | N–H stretching | Broad shape | Aliphatic |
| 2927.94 | C–H bending | Medium | Alkane |
| 2858.51 | C–H stretching | Medium | Alkane |
| 1595.13 | C=O Stretching | Medium | Alkane |
| 1404.18 | C–H bending | Medium | Alkane |
| 1247.94 | C–O Stretching | Medium | Alkane, Ether |
| 1078.21 | S=O Stretching | Sharp | Sulfone |
| 1018.41 | C–O Stretching | Sharp | Alkane |
| 929.69 | N–O Stretching | Sharp | Aliphatic |
| 871.82 | C=C bending | Sharp | Alkane |
| 503.42 | C–Br Stretching | Medium | Alkane |
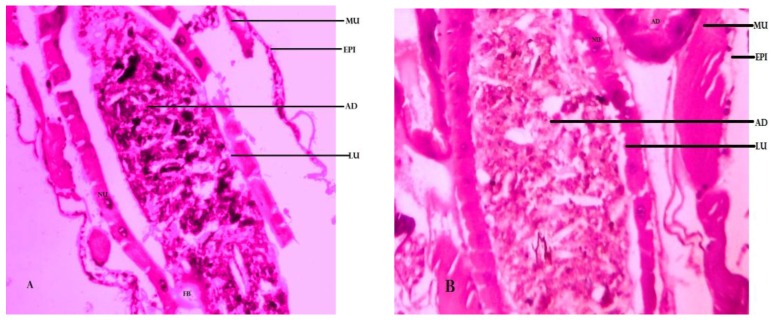
4.5. Histological Studies of Larvae of Cx. quinquefasciatus
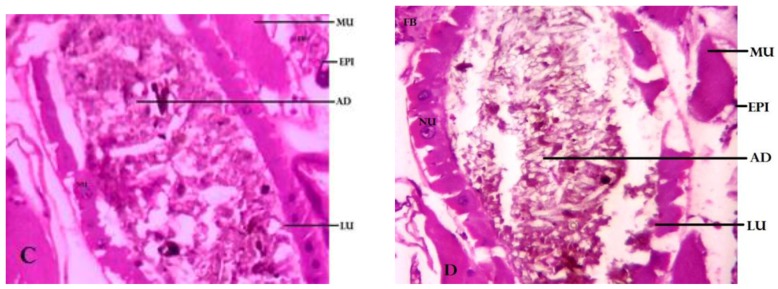
Histopathological results clearly show that midgut epithelial cells (epi) were severely damaged by B. bassiana-28 extract. Gut tissue of lumen was mutually by way of a thin peritrophic membrane (Pm) in 6 h treatment as compared to control (Figure 5B), whereas, at 12 h and 24 h treated midgut epithelium layer was damaged and cells were vacuolated but remained together with the nuclei and membrane was entirely bust during action with B. bassiana-28 (Figure 5B,C) also the lumen substance (lu) seeped out into muscles cells (mu). In muscles was appeared barely injured and fat bodies were confused.
Figure 5.
Cross section of Cx. quinquefasciatus larvae untreated and treated with B.bassiana-28 extract Control (A) (untreated) compared with (B) 6 h Treated (C) 12 h treated and (D) 24 h treated, Vacuolated gut epithelium (epi), gut lumen (lu), adipose tissue(ad), muscles (mu), nucleus (nu) and fat body (fb). Larval mid-gut section was stained with haematoxylin and eosin and stained mid-gut tissues viewed and photographed under light microscope at 400× magnification.
5. Discussion
In the present study, B. bassiana-28 and commercial B. bassiana-22 were found to produce toxicity in all stages like larval and pupal Cx. quinquefasciatus. Result clearly shows that B. bassiana-28 extract has strong mosquitocidal activity against 1st to 4th instar larvae of Cx. quinquefasciatus, (Table 1 and Table 2). Other fungi of the same species to which Beauveria belongs are sources of bioactive metabolites and conidia that have mosquitocidal properties under field and laboratory conditions [21,33,34]. Clark et al. [35] reported that Beauveria bassiana shows remarkable pathogenicity on three mosquito species such as Anopheles, Aedes and Culex. The entomopathogenic fungal pathogen Beauveria bassiana fungal conidia and blastospores reduced the malarial vector Anopheles stephensi survival rates [36].
B. bassiana-22 extracts toxicity has been proved with highest toxicity against the Cx. quinquefasciatus secondary metabolites from other reports on the same species. Beauveria bassiana show remarkable insecticidal properties against insecticide resistant and susceptible Anopheles arabiensis mosquitoes at different temperatures [37]. The FT-IR spectrum of the secondary metabolites of B. bassiana-28 contains important peaks indicating the presence of N–H stretching; C–H bending; C–H stretching; C=C bending; C=O stretching; C–H bending; S=O stretching; C–O stretching; N–O stretching; C=C bending; C–B stretching groups. Ethyl acetate mycelium extract showed the presence of prominent functional groups these are maybe involved in the mosquitocidal activity. GC-MS analysis marks exhibited important mosquitocidal compounds potentially responsible for insecticidal activity, namely, N-hexadecanoic acid (13.6040%) [38]; Z, Z-9,12 dectadecadienoic acid (33.74%); 9-eicosyne (10.832%); heptacosane (5.148%); tetrateracontane (5.801%); and 7-hexyleicosane (5.723%) are also thought to be involved in insecticidal activity. In our results show the major compound in assessment with the standard was N-hexadecanoic acid, and based on the above we infer that N-hexadecanoic acid from B. bassiana-28 mycelial extract may be the principal metabolite which confers insecticidal activity to the extract.
6. Conclusions
B. bassiana-28 mycelial extracts produced toxicity in larvae and pupae of Cx quinquefasciatus. In addition these extract produce considerable tissue damage to the midgut of mosquito larvae. Characterization of extracts showed that N-hexadecanoic acid is the main constituent of the extract which has insecticidal properties. In future work on control of mosquito larvae, N-hexadecanoic acid can be used as an alternative to chemical insecticides.
Acknowledgments
We would like to thank the sample analysis of GC-MS and FT-IR in Vellore Institute of Technology (Vellore, Tamil Nadu, India), Chennai also thanks to be Institute of Vector Control Zoonoses (IVCZ), Hosur, TN, India for providing Cx.quinquefasciatus mosquito larval for this study.
Author Contributions
Perumal Vivekanandhan, Thangaraj Kavitha, Sengodan Karthi and Muthugoundar Subramanian Shivakumar designed the experiments; Perumal Vivekanandhan cultured the fungi; Perumal Vivekanandhan and Thangaraj Kavitha conducted the experiments including inoculation of EPF; Perumal Vivekanandhan carried out experiments and Muthugoundar Subramanian Shivakumar and Sengottayan Senthil-Nathan analysed experimental results. Perumal Vivekanandhan and Sengodan Karthi interpreted the data and wrote the manuscript; Muthugoundar Subramanian Shivakumar and Sengottayan Senthil-Nathan revised and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization . Global Programme to Eliminate Lymphatic Filariasis-Progress Report on Mass Drug Administration in 2016. Volume 85. Weekly Epidemiological Record; World Health Organization; Geneva, Switzerland: 2016. pp. 365–372. [Google Scholar]
- 2.Knight K.L., Stone A. A Catalogue of the Mosquitoes of the World (Diptera: Culicidae) 2nd ed. Entomological Society of America; College Park, MD, USA: 1997. p. 611. Thomas Say Foundation. [Google Scholar]
- 3.Solomon T. Flavivirus encephalitis. N. Engl. J. Med. 2004;351:370–378. doi: 10.1056/NEJMra030476. [DOI] [PubMed] [Google Scholar]
- 4.Amerasan D., Murugan K., Kovendan K., Mahesh Kumar P., Panneerselvam C., Subramaniam J., William S.J., Hwang J.S. Adulticidal and repellent properties of Cassia tora Linn. (Family: Caesalpinaceae) against Culex quinquefasciatus, Aedes aegypti, and Anopheles stephensi. Parasitol. Res. 2012;111:1953–1964. doi: 10.1007/s00436-012-3042-3. [DOI] [PubMed] [Google Scholar]
- 5.Wada Y. Vector Mosquitoes of Filariasis in Japan. Trop. Med. Health. 2011;39:39–45. doi: 10.2149/tmh.39-1-suppl_2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura M., Darbro J.M., Harrington L.C. Avian malaria parasites share congeneric mosquito vectors. J. Parasitol. 2010;96:144–151. doi: 10.1645/GE-2060.1. [DOI] [PubMed] [Google Scholar]
- 7.Ramkumar G., Shivakumar M.S. Laboratory development of Permethrin resistance and cross-resistance pattern of Culex quinquefasciatus to other insecticides. Parasitol. Res. 2015;114:2553–2560. doi: 10.1007/s00436-015-4459-2. [DOI] [PubMed] [Google Scholar]
- 8.Muthusamy R., Shivakumar M.S. Susceptibility status of Aedes aegypti (L.) (Diptera: Culicidae) to temephos from three districts of Tamil Nadu, India. J. Vector Borne Dis. 2015;52:159–165. [PubMed] [Google Scholar]
- 9.Hemingway J., Ranson H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 10.Benelli G., Flamini G., Canale A., Cioni P.L., Conti B. Toxicity evaluation of different essential oil formulations against the Mediterranean Fruit Fly Ceratitis capitata (Wiedemann) (Diptera Tephritidae) Crop Prot. 2012;42:223–229. doi: 10.1016/j.cropro.2012.05.024. [DOI] [Google Scholar]
- 11.Benelli G., Flamini G., Canale A., Molfetta I., Cioni P.L., Conti B. Repellence of Hyptis suaveolens L. (Lamiaceae) whole essential oil and major constituents against adults of the granary weevil Sitophilus granarius (L.) (Coleoptera: Dryophthoridae) Bull. Insectol. 2012;65:177–183. [Google Scholar]
- 12.Ramkumar G., Karthi S., Muthusamy R., Suganya P., Natarajan D., Kweka E.J., Shivakumar M.S. Mosquitocidal Effect of Glycosmis pentaphylla Leaf Extracts against Three Mosquito Species (Diptera: Culicidae) PLoS ONE. 2016;11:e0158088. doi: 10.1371/journal.pone.0158088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivekanandhan P., Senthil-Nathan S., Shivakumar M.S. Larvicidal, pupicidal and adult smoke toxic effects of Acanthospermum hispidum (DC) leaf crude extracts against mosquito vectors. Physiol. Mol. Plant Pathol. 2017;101:156–162. doi: 10.1016/j.pmpp.2017.05.005. [DOI] [Google Scholar]
- 14.Senthil-Nathan S., Chung P.G., Murugan K. Effect of botanicals and bacterial toxin on the gut enzyme of Cnaphalocrocis medinalis. Phytoparasitica. 2004;32:433–443. doi: 10.1007/BF02980437. [DOI] [Google Scholar]
- 15.Malik A., Singh N., Satya S. House Fly (Musca domestica): A review of control strategies for a challenging pest. J. Environ. Sci. Health Part B. 2007;42:453–469. doi: 10.1080/03601230701316481. [DOI] [PubMed] [Google Scholar]
- 16.Ponsankar A., Vasantha-Srinivasan P., Senthil-Nathan S., Thanigaivel A., Edwin E., Selin-Rani S., Kalaivani K., Hunter W.B., Alessandro R.T., Abel-Megeed A., et al. Target and non-target toxicity of botanical insecticide derived from Couroptia guianensis L. flower against generalist herbivore, Spodoptera litura Fab, and an earthworm, Eisenia foetida Savigny. Ecotoxicol. Environ. Saf. 2016;133:260–270. doi: 10.1016/j.ecoenv.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Govindrajan M., Jebamesan A., Reetha D. Larvicidal effect of extracellular secondary metabolites of different fungi against the mosquito, Cx quinquefasciatus Say. Trop. Biomed. 2005;22:1–3. [PubMed] [Google Scholar]
- 18.Selin-Rani S., Senthil-Nathan S., Thanigaivel A., Vasantha-Srinivasan P., Edwin E., Ponsankar A., Lija-Escaline J., Kalaivani K., Abdel-Megeed A., Hunter W.B., et al. Toxicity and physiological effect of quercetin on generalist herbivore, Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Chemosphere. 2016;165:257–267. doi: 10.1016/j.chemosphere.2016.08.136. [DOI] [PubMed] [Google Scholar]
- 19.Soni N., Prakash S. Entomopathogenic fungus generated nanoparticles for enhancement of efficacy in Culex quinquefasciatus and Anopheles stephensi. Asian Pac. J. Trop. Dis. 2012;2:356–361. doi: 10.1016/S2222-1808(12)60181-9. [DOI] [Google Scholar]
- 20.Mnyone L.L., Kirby M.J., Mpingwa M.W., Lwetoilera D.W., Knols B.G.J., Takken W., Koenraadt C.J.M., Russel T.L. Infection of Anopheles gambiae mosquitoes with entomopathogenic fungi: Effect of host age and blood feeding stage. Parasitol. Res. 2011;108:317–322. doi: 10.1007/s00436-010-2064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bukhari T., Takken W., Koenraadt C.J.M. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasites Vectors. 2011;4:23. doi: 10.1186/1756-3305-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh G., Prakash S. Evaluation of culture filtrates of Culicinomyces clavisporus: Myco adulticides for Culex quinquefasciatus, Aedes aegypti and Anopheles stephensi. Parasitol. Res. 2010;110:267–272. doi: 10.1007/s00436-011-2482-5. [DOI] [PubMed] [Google Scholar]
- 23.Prakash B., Shukla R., Singh P., Kumar A., Mishra P.K., Dubey N.K. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Int. J. Food Microbiol. 2010;142:114–119. doi: 10.1016/j.ijfoodmicro.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Pimmental D., Perkins J.H. Pest Control: Cultural and Environmental Aspects. Westview Press; Boulder, CO, USA: 1980. p. 243. AAAS Symposium. [Google Scholar]
- 25.Nguyen N.T.H., Borgemeister C., Poehling H.M., Zimmermann G. Laboratory investigations on the potential of entomopathogenic fungi for biocontrol of Helicoverpa armigera (Lepidoptera: Noctuidae) larvae and pupae. Biocontrol. Sci. Technol. 2007;17:853–864. doi: 10.1080/09583150701546375. [DOI] [Google Scholar]
- 26.Godonou I., James B., Atcha-Ahowe C., Vodouhe S., Kooyman C., Ahanchede A., Korie S. Potential of Beauveria bassiana and Metarhizium anisopliae isolates from Benin to control Plutella xylostella L. (Lepidoptera: Plutellidae) Crop Prot. 2009;28:220–224. doi: 10.1016/j.cropro.2008.10.009. [DOI] [Google Scholar]
- 27.Mnyone L.L., Kirby M.J., Lwetoijera D.W., Mpingwa M.W., Simfukwe E.T., Knols B.G.J., Takken W., Russell T.L. Anopheline and culicine mosquitoes are not repelled by surfaces treated with the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Parasites Vectors. 2010;3:80. doi: 10.1186/1756-3305-3-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajek A.E., Wraight S.P., Vandenberg J.D. Control of arthropods using pathogenic fungi. In: Pointing S.B., Hyde K.D., editors. Bio-Exploitation of Filamentous Fungi. Volume 6. Fungal Diversity Press; Hong Kong, China: 2001. pp. 309–347. (Fungal Diversity Research Series). [Google Scholar]
- 29.Howard A.F.V., Guessan R.N., Koenraadt C.J.M., Asidi A., Farenhorst M., Akogbéto M., Thomas M.B., Knols B.G.J., Takken W. The entomopathogenic fungus Beauveria bassiana reduces instantaneous blood feeding in wild multi-insecticide-resistant Culex quinquefasciatus mosquitoes in Benin, West Africa. Parasites Vectors. 2010;3:87. doi: 10.1186/1756-3305-3-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragavendran C., Natarajan D. Insecticidal potency of Aspergillus terreus against larvae and pupae of three mosquito species Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti. Environ. Sci. Pollut. Res. 2015;22:17224–17237. doi: 10.1007/s11356-015-4961-1. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. Communicable Disease Control, Prevention and Eradication. WHO Pesticide Evaluation Scheme . Guidelines for Laboratory and Field Testing of Mosquito Larvicides. WHO; Geneva, Switzerland: 2005. WHO/CDS/WHOPES/GCDPP/1.3. [Google Scholar]
- 32.Abbott W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 1987;3:302–303. doi: 10.1093/jee/18.2.265a. [DOI] [PubMed] [Google Scholar]
- 33.Achonduh O.A., Tondje P.R. First report of pathogenicity of Beauveria bassiana RBL 1034 to the malaria vector, Anopheles gambiae s.l. (Diptera; Culicidae) in Cameroon. Afr. J. Biotechnol. 2008;7:931–935. [Google Scholar]
- 34.Inglis D.G., Johnson D.L., Goettel M.S. Effects of temperature and thermoregulation on mycosis by Beauveria bassiana in grasshoppers. Biol. Control. 1996;7:131–139. doi: 10.1006/bcon.1996.0076. [DOI] [Google Scholar]
- 35.Clark T.B., Kellen W., Fukuda T., Lindegren J.E. Field and laboratory studies on the pathogenicity of the fungus Beauveria bassiana to three genera of mosquitoes. J. Invertebr. Pathol. 1968;11:1–7. doi: 10.1016/0022-2011(68)90047-5. [DOI] [PubMed] [Google Scholar]
- 36.Blanford S., Chan B.H.K., Jenkins N., Sim D., Turner R.J., Read A.F., Thomas M.B. Fungal pathogen reduces potential for malaria transmission. Science. 2005;308:1638–1641. doi: 10.1126/science.1108423. [DOI] [PubMed] [Google Scholar]
- 37.Kikankie C.K., Brooke B.D., Knols B.G.J., Koekemoer L.L., Farenhorst M., Hunt R.H., Thomas M.B., Coetzee M. The infectivity of the entomopathogenic fungus Beauveria bassiana to insecticide-resistant and susceptible Anopheles arabiensis mosquitoes at two different temperatures. Malar. J. 2010;9:71. doi: 10.1186/1475-2875-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senthilkumar G., Madhanraj P., Panneerselvam S. A studies on the compounds and its antifungal potentiality of fungi isolated from paddy field soils of Jenbagapuram Village, Thanjavur District, and South India. Asian J. Pharm. Res. 2011;1:19–21. [Google Scholar]