Abstract
Many studies have assessed mercury (Hg) exposure in the Amazonian population. This article performs a literature search of the studies that used hair as a biomarker of Hg exposure in the Brazilian Amazonian population. The search covered the period from 1996 to 2016 and included articles which matched the following criteria: (1) articles related to Hg exposure into Brazilian Amazon; (2) articles that used hair as a biomarker of Hg exposure; (3) articles that used analytical tools to measure the Hg content on hair and (4) articles that presented arithmetic mean and/or minimum and maximum values of Hg. 36 studies were selected. The findings show that most of the studies were performed along margins of important rivers, such as Negro, Tapajós and Madeira. All the population presented mean levels of Hg on hair above 6 µg g−1 and general population, adults, not determined and men presented levels of Hg on hair above 10 µg g−1. The results show that most of the studies were performed by Brazilian institutions/researchers and the majority was performed in the State of Pará. The present study identified that Amazonian population has long-term been exposed to Hg. In terms of future perspectives, this study suggests the implementation of a strategic plan for environmental health surveillance in the region in order to promote health and benefit Amazonian population.
Keywords: mercury, methylmercury, Amazon, hair Amazonia
1. Background
The health effects of methylmercury (MeHg) exposure have been investigated since the accident that occurred in Minamata Bay, Japan. The clinical features of MeHg poisoning were classified as acute or chronic, based on the symptoms observed in patients living around the bay in the vicinity of the pollution source (a factory) and in patients living on the coast of the Shiranui Sea; both populations had consumed contaminated fish for almost 20 years [1].
Clinical studies of Japanese patients affected by dietary MeHg poisoning showed that mercury (Hg) had long-term effects on health. The patients from Goshoura Island, an area close to Minamata Bay, who had mean levels of total hair Hg of 37 µg g−1 in 1960 (n = 16) and 2.4 µg g−1 in 2002 (n = 23) showed persistent sensory disorders caused by their past history of MeHg exposure [2,3]. Evaluation of data from a population-based study performed in 1971 at Goshoura showed an increased incidence of neurological signs, such as ataxia (12%), dysarthria (5.9%), and paresthesia of extremities (5.7%) [4]. A study performed at Niigata, a town located along the Agano River that was affected by MeHg poisoning in 1965, showed that even people with chronic exposure to levels of Hg of less than 20 µg g−1 (n = 24) presented neurologic signs associated with MeHg poisoning [5]. Recently, a population-based study in Minamata and neighboring areas identified the association between neurological sign and the development of psychiatric symptoms.
In spite of the findings observed after the Minamata disaster, there is no clear consensus yet about a dose-response relationship between Hg exposure and health effects given that the genetic characteristics of the populations vary, the modes and times of exposure are diverse, and that life-styles and behavior can influence on the toxic effects of Hg exposure [6,7]. However, vulnerable populations (i.e., pregnant women, human fetuses, neonates, and children) are under the potential risks of Hg effects [8,9,10,11].
Analysis of scalp hair has been a valuable method used to assess the Hg exposure of different populations because hair is easy to collect, store, and manipulate [12]. Hair Hg levels strongly correlate with an individual’s dietary intake of MeHg. Moreover, its chemical stability facilitates retrospective studies [13]. However, Hg can be incorporated into hair in other ways, such as the sorption of volatile species (i.e., elemental Hg), and its level can be affected by hair color and growth rates, which are considered to be pre-analytical sources of variation that may cause bias and misleading interpretations [14,15]. In spite of these limitations, the versatility provided by scalp hair for assessing Hg exposure, especially in remote areas, has been valuable to access the Hg exposure in different populations.
In Brazil, many studies had been performed in the Amazonian region and hair has been selected as a biomarker of Hg exposure. The present article performs a systematic review of publications that analyzed Hg on hair of different populations into Brazilian Amazon. The objective of this article is to provide an overview of long-term exposure to Hg into Brazilian Amazon, identify populations under risk of Hg effects and give future perspectives for environmental health surveillance for the region.
2. Methods
This systematic review was registered in PROSPERO (registration number CRD42017056584). The search was performed using the following electronic databases: Pubmed, EBSCO, VHL (Virtual Health Library) and Scielo. Both authors performed independently the virtual search for articles titles and abstract using the search strategy showed in the Table 1. The search covered the studies that were published in the period from 1996 to 2016. The present study followed the following inclusion criteria for articles: (1) articles related to Hg exposure into Brazilian Amazon; (2) articles that used hair as a biomarker of Hg exposure; (3) articles that used analytical tools to measure the Hg content on hair and (4) articles that presented arithmetic mean and/or minimum and maximum values of Hg. As exclusion criteria, the present study followed: (1) review articles of Hg exposure and (2) articles which methodology was not clear, such as sample size, locality and analytical tools. The potential studies were screened and duplicates were removed. Articles not excluded were read by the two authors who extracted in detail the geographic location where the study was performed, sample size and type, the age range and/or mean age of the populations, the first author of the study, the year of the publication, and the arithmetic mean of Hg observed.
Table 1.
Search strategies in electronic database.
| Strategy | Keywords |
|---|---|
| #1 | Mercury and hair and Amazon |
| #2 | Methylmercury and hair and Amazon |
| #3 | Mercury and Brazil and Amazonia |
| #4 | Mercury and Amazon |
The present study classified the type of the studied populations as follows: general population (0 to >60 years old), adult population (>15 years old), children (<15 years old), women, men, indigenous and not determined (nd) when the study did not identify the type and/or the age range of the target population.
According to the type of each population, the data of the mean level of Hg was used in order to determine a weighted average in which the number of individuals studied contributed equally to a final average (Equation (1)).
| (1) |
where: WA = weighted average; X = arithmetic mean of Hg (µg g−1); I = Number of Individuals.
A cartographic quali-quantitative analysis of the data was performed. The classification of the populations determined for this study were considered for a qualitative evaluation and a quantitative analysis based on the degree of exposure followed the following criteria: lower (less than 2 µg g−1), medium (between 2 and 6 µg g−1) and higher (above 6 µg g−1).
The ArcMap 10.1 software (ESRI, Redlands, CA, USA) was used for georeferencing for studied where the geographic coordinates were not available. The geographic location was matched with the Brazilian Institute of Geography and Statistics (IBGE). The quantitative analysis was evaluated by a “choropleth map” where the differences of Hg exposure according to different populations and regions were shaded: yellow representing populations with low exposure to Hg and red high exposure.
Among the major source of risk of bias of the present study we can cite here:
(1) The period of the study.
The present study searched for studies that were published from 1996 to 2016 (20 years). Thus, the concentration of Hg on hair among the populations can vary, and, thus, the general visualization provided by the cartographic quali-quantitative analysis could not represent the current status.
(2) The methodologies for Hg measurement were not an exclusion or inclusion criteria.
Once the present study considered valid all the methodologies that measured Hg on hair, the results can not reflect a “gold standard” for Hg exposure in Amazon. Instead, all the selected articles were peer reviewed, thus the data could be considered to give an overview of long term Hg exposure in Amazonian population.
3. Results
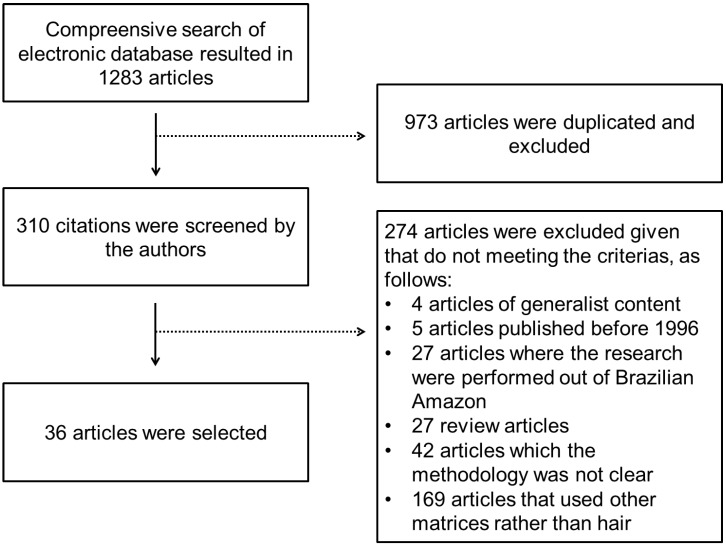
The database search identified 1283 articles, of which 973 were duplicated and, consequently, removed. The potential 310 articles were screened and 274 of them were removed given that did not attended to some inclusion/exclusion criteria: 169 used other matrices rather than hair, 42 which methodology was not clear, 27 were review articles, 27 were performed in other regions rather than Brazilian Amazon (7 in Peru, 6 in Bolivia, 4 in Ecuador, 3 in French Guiana, 1 in Venezuela, 1 in Suriname, 1 in Peru and Ecuador, 1 Faroe Island, 1 in Colombia, 1 in Africa and 1 in the Northwest of Brazil), 5 were published out of the period of investigation and 4 contained generalist subject about Hg exposure. The study selection is summarized in the flowchart described in Figure 1.
Figure 1.
Study selection flowchart.
In this context, the present study comprised 36 articles, comprising a total of 11,827 individuals (Table 2). According to the year of publication, the articles were published as follows: three in 1998 [16,17,18], two in 1999 [19,20], three in 2000 [21,22,23], two in 2001 [24,25], one in 2002 [26], two in 2003 [27,28], four in 2005 [29,30,31,32], three in 2006 [33,34,35], two in 2007 [36,37], one in 2008 [38], one in 2009 [39], two in 2010 [40,41], three in 2012 [42,43,44], one in 2013 [45], one in 2014 [46], three in 2015 [47,48,49] and two in 2016 [50,51]. Most of the studies (61%) were performed exclusively by Brazilians Institution [16,18,20,21,22,24,28,29,30,31,32,33,34,37,41,42,43,44,45,46,49,51] and others (36%) had been performed in collaboration with them [17,19,23,25,26,35,36,38,39,40,47,48,50]. Only one study (3%) was developed for a foreign country solely [27].
Table 2.
Characteristics of the included studies (n = 36).
| Year | Author | Locality | State | n | Type of Population According to the Author | Population Specificity | Type of the Population According to the Present Study | Range Hg (µg g−1) | Mean Hg (µg g−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1998 | Barbosa A.C. [16] | Fresco River | PA | 28 | Kayapo women | childbearing women | I | 0.8–13.7 | 8.11 |
| Madeira River | RO | 98 | non-indigenous women | childbearing women | W | 2.6–94.7 | 14.08 | ||
| Fresco River | PA | 54 | Kayapo children | nd | I | 2.0–20.4 | 7.30 | ||
| Madeira River | RO | 71 | non-indigenous children | nd | C | 0.8–44.4 | 10.82 | ||
| 1998 | Kehrig H.A. [17] | Balbina Village | AM | 53 | total of population studied | nd | G | nd | 6.54 |
| 16 | children | female | C | 1.3–22.0 | 7.7 | ||||
| 12 | children | male | C | 2.5–11.4 | 5.3 | ||||
| 12 | adults | female | A | 2.2–15.5 | 7.4 | ||||
| 13 | adults | male | A | 1.2–12.2 | 5.5 | ||||
| 1998 | Barbosa A.C. [18] | Madeira River | RO | 37 | women | nd | W | 2.0–37.2 | 14.3 |
| 37 | children | 0.5–15 months | C | 1.4–34.2 | 9.8 | ||||
| 1999 | Silva-Forsberg M.C. [19] | total (all the populations studied) | AM | 154 | total of population studied | 0.2–66 y.o | G | 5.76–171.24 | 75.46 |
| Acariquara, Rio Urubaxi | 15 | nd | 0.3–56 y.o | G | 14.37–146.25 | 69.18 | |||
| Tupuruquara, Rio Marie | 57 | nd | 0.2–66 y.o | G | 10.44–171.24 | 97.44 | |||
| Macuna, Rio Uneiuxi | 17 | nd | 0.7–52 y.o | G | 22.17–129.19 | 76.75 | |||
| Perseverança, Rio Negro | 23 | nd | 0.2–65 y.o | G | 15.77–122.32 | 65.72 | |||
| Ilha do Pinto. Rio Negro | 12 | nd | 1.6–37 y.o | G | 19.02–100.95 | 69.58 | |||
| Tapera. Rio Padauari | 11 | nd | 2–59 y.o | G | 19.20–55.59 | 37.48 | |||
| Tapereira. Rio Negro | 10 | nd | 2–47 y.o | G | 24.94–110.51 | 69.10 | |||
| Aldeia Maia. Rio Maia | 7 | nd | 13–40 y.o | G | 5.76–63.02 | 28.02 | |||
| Sitio Velho. Rio Marauia | 2 | nd | 42–62 y.o | A | 13.93–62.57 | 38.25 | |||
| 1999 | Guimaraes J.R.D. [20] | Pracuuba Lake | AP | 15 | fishermen and their family | nd | nd | nd | 16.7 |
| Duas Bocas Lake | 15 | fishermen and their family | nd | nd | nd | 28 | |||
| 2000 | Hacon S. [21] | Alta Floresta | MT | 75 | pregnant women | 14–45 y.o | W | 0.051–8.2 | 1.12 |
| 2000 | Santos E.C.O. [22] | Brasília Legal | PA | 220 | total of population studied | 0–>65 y.o | G | 0.53–49.99 | 11.75 |
| 30 | nd | 0–5 y.o | C | 1.09–20.46 | 5.84 | ||||
| 68 | nd | 6–10 y.o | C | 0.70–35.80 | 13.06 | ||||
| 33 | nd | 11–15 y.o | C | 1.22–47.00 | 14.2 | ||||
| 12 | nd | 16–20 y.o | A | 5.56–19.90 | 13.39 | ||||
| 10 | nd | 21–25 y.o | A | 1.40–29.50 | 15.25 | ||||
| 9 | nd | 26–30 y.o | A | 3.70–21.40 | 11.06 | ||||
| 16 | nd | 31–35 y.o | A | 2.84–37.20 | 12.57 | ||||
| 12 | nd | 36–40 y.o | A | 5.0–33.0 | 14.21 | ||||
| 1 | nd | 41–45 y.o | A | nd | 11.7 | ||||
| 7 | nd | 46–50 y.o | A | 1.02–14.24 | 7.06 | ||||
| 8 | nd | 51–55 y.o | A | 3.57–49.99 | 11.53 | ||||
| 5 | nd | 56–60 y.o | A | 0.53–7.07 | 4.93 | ||||
| 6 | nd | 61–65 y.o | A | 5.01–15.94 | 11.33 | ||||
| 3 | nd | >65 y.o | A | 2.78–16.46 | 7.45 | ||||
| São Luiz do Tapajós | 327 | total of population studied | 0–>65 y.o | G | 0.10–94.50 | 19.91 | |||
| 75 | nd | 0–5 y.o | C | 0.10–94.50 | 21.06 | ||||
| 74 | nd | 6–10 y.o | C | 2.40–52.50 | 22.1 | ||||
| 51 | nd | 11–15 y.o | C | 3.90–61.80 | 23.24 | ||||
| 21 | nd | 16–20 y.o | A | 2.10–33.60 | 19.11 | ||||
| 21 | nd | 21–25 y.o | A | 1.73–32.0 | 15.68 | ||||
| 15 | nd | 26–30 y.o | A | 3.90–34.90 | 15.34 | ||||
| 15 | nd | 31–35 y.o | A | 5.10–38.0 | 18.98 | ||||
| 15 | nd | 36–40 y.o | A | 2.60–27.8 | 14.31 | ||||
| 14 | nd | 41–45 y.o | A | 3.20–33.60 | 15.13 | ||||
| 8 | nd | 46–50 y.o | A | 4.0–47.0 | 21.71 | ||||
| 4 | nd | 51–55 y.o | A | 5.90–20.60 | 15.4 | ||||
| 6 | nd | 56–60 y.o | A | 7.90–27.60 | 17.13 | ||||
| 3 | nd | 61–65 y.o | A | 3.8–27.80 | 12.6 | ||||
| 5 | nd | >65 y.o | A | 3.20–20.80 | 12.6 | ||||
| Santana de Ituqui | 321 | total of population studied | 0–>65 y.o | G | 0.40–11.60 | 4.33 | |||
| 37 | nd | 0–5 y.o | C | 0.50–8.50 | 3.67 | ||||
| 81 | nd | 6–10 y.o | C | 0.40–10.9 | 4.44 | ||||
| 62 | nd | 11–15 y.o | C | 2.0–11.6 | 4.47 | ||||
| 25 | nd | 16–20 y.o | A | 2.5–9.60 | 5 | ||||
| 17 | nd | 21–25 y.o | A | 1.30–7.10 | 3.34 | ||||
| 16 | nd | 26–30 y.o | A | 1.70–9.20 | 4.69 | ||||
| 19 | nd | 31–35 y.o | A | 1.90–9.60 | 5.36 | ||||
| 18 | nd | 36–40 y.o | A | 1.20–6.0 | 3.44 | ||||
| 10 | nd | 41–45 y.o | A | 2.70–6.80 | 4.18 | ||||
| 10 | nd | 46–50 y.o | A | 1.90–6.40 | 4.02 | ||||
| 9 | nd | 51–55 y.o | A | 2.30–9.0 | 5.39 | ||||
| 6 | nd | 56–60 y.o | A | 3.10–6.90 | 4.37 | ||||
| 4 | nd | 61–65 y.o | A | 1.90–9.0 | 4.15 | ||||
| 7 | nd | >65 y.o | A | 0.70–5.70 | 3.61 | ||||
| 2000 | Dolbec J. [23] | Cametá | PA | 68 | total of population studied | 12–79 y.o | G | nd | 10.8 |
| 2001 | Barbosa A.C. [24] | Negro River | AM | 73 | children | <15 y.o | C | 0.51–45.89 | 18.52 |
| 76 | adults | >15 y.o | A | 1.66–59.01 | 21.4 | ||||
| 2001 | Harada M. [25] | Barreiras | PA | 76 | fisherman and family | 1–67, mean 28 y.o | G | 1.8–53.8 | 16.4 |
| Rainhas | 12 | fisherman and family | 7–53, mean 31 y.o | G | 3.1–34.5 | 14.1 | |||
| São Luiz do Tapajós | 44 | fisherman and family | 3–47, mean 21 y.o | G | 5.1–42.2 | 20.8 | |||
| Special group from Barreiras, Rainha and São Luiz do Tapajós | 50 | eligible subjects examined clinically that presented high level of Hg (>20 ppm) from March 1994 to February 1998 | 3–65, mean 25 y.o | G | 5.1–42.7 | 23.6 | |||
| 2002 | Crompton P. [26] | Jacareacanga | PA | 205 | total of population studied | general population, except children under 2 y.o | G | 0.3–83.2 | 8.6 |
| nd | men | nd | M | nd | 11.0 | ||||
| nd | women | nd | W | nd | 6.7 | ||||
| 2003 | Passos C.J. [27] | Brasília Legal | PA | 26 | adults women | 23–62, mean 41 y.o | W | 4.0–20.0 | 10.0 |
| 2003 | Santos E.C.O. [28] | Guajará Mirim e Nova Mamoré | RO | 910 | total of studied population (Pakaanova indigenous) | 0–>45 y.o | I | 0.52–83.89 | 8.37 |
| 57 | nd | 0–2 y.o | I | 1.48–83.89 | 10.54 | ||||
| 115 | nd | 3–5 y.o | I | 1.67–47.22 | 9.34 | ||||
| 152 | nd | 6–10 y.o | I | 0.52–63.81 | 8.16 | ||||
| 114 | nd | 11–15 y.o | I | 0.65–31.11 | 6.86 | ||||
| 177 | nd | 16–25 y.o | I | 0.65–39.42 | 8.45 | ||||
| 114 | nd | 26–35 y.o | I | 1.37–28.64 | 8.56 | ||||
| 50 | nd | 36–45 y.o | I | 1.49–21.25 | 8.39 | ||||
| 131 | nd | >45 y.o | I | 1.37–25.84 | 7.84 | ||||
| 2005 | Dorea J.G. [29] | Teles Pires (Tapajós Basin) | PA | 47 | Kayabi indigenous | Kayabi community from Teles Pires | I | nd | 12.8 |
| 249 | Munduruku indigenous | Munduruku community from Teles Pires | I | nd | 3.4 | ||||
| 2005 | Santos E.C.O. [30] | São Gabriel da Cachoeira | AM | 157 | total of population studied | 0–>40 y.o | G | 0.30–83.11 | 13.02 |
| 9 | nd | 0–5 y.o | C | 1.01–14.40 | 5.71 | ||||
| 8 | nd | 6–10 y.o | C | 2.05–15.00 | 7.35 | ||||
| 37 | nd | 11–20 y.o | NC | 0.94–22.81 | 7.346 | ||||
| 45 | nd | 21–30 y.o | A | 0.30–59.16 | 11.67 | ||||
| 33 | nd | 31–40 y.o | A | 1.03–60.00 | 16.56 | ||||
| 26 | nd | >40 y.o | A | 2.41–83.11 | 22.88 | ||||
| Barcelos | 242 | total of population studied | 0–>40 y.o | G | 0.07–52.04 | 9.671 | |||
| 17 | nd | 0–5 y.o | C | 0.83–25.89 | 7.46 | ||||
| 25 | nd | 6–10 y.o | C | 0.76–27.46 | 6.85 | ||||
| 44 | nd | 11–20 y.o | NC | 0.07–21.53 | 7.00 | ||||
| 62 | nd | 21–30 y.o | A | 0.25–42.64 | 9.05 | ||||
| 27 | nd | 31–40 y.o | A | 0.23–52.04 | 12.02 | ||||
| 67 | nd | >40 y.o | A | 2.60–32.86 | 12.67 | ||||
| 2005 | Tavares L.M.B. [31] | Riverine communities of Bocas de Conchas, Cuiabá Mirim, Estirão Cumprido and Porto Brandão located near of Barão de Melgaço | MT | 72 | riverine children | 3–7 y.o | C | 0.58–17.14 | 5.37 |
| Barão de Melgaço | 114 | urban children | 3–7 y.o | C | 0.38–7.57 | 2.08 | |||
| 2005 | Klautau-Guimaraes M.N. [32] | Teles Pires | PA | 65 | total of population studied (Kayabi indigenous) | 0–>61, mean 24.53 y.o | I | nd | 14.75 |
| 33 | Kayabi indigenous | 0–20 y.o | I | nd | 17.86 | ||||
| 25 | Kayabi indigenous | 21–40 y.o | I | nd | 11.97 | ||||
| 5 | Kayabi indigenous | 41–60 y.o | I | nd | 14.35 | ||||
| 2 | Kayabi indigenous | >61 y.o | I | nd | 15.17 | ||||
| 117 | total of population studied (Munduruku indigenous) | 0–>61, mean 30.90 y.o | I | nd | nd | ||||
| 34 | Munduruku indigenous | 0–20 y.o | I | nd | 4.26 | ||||
| 56 | Munduruku indigenous | 21–40 y.o | I | nd | 3.65 | ||||
| 20 | Munduruku indigenous | 41–60 y.o | I | nd | 3.75 | ||||
| 7 | Munduruku indigenous | >61 y.o | I | nd | 3.72 | ||||
| 2006 | Alves M.F.A. [33] | total (all the riverine populations studied) | AM | 105 | total of adult studied population | 18–50, mean 32 y.o | A | nd | 35.4 |
| Mariuá (Negro River) | 3 | adults | nd | A | nd | 24.9 | |||
| Marará (Negro River) | 17 | adults | nd | A | nd | 27.3 | |||
| Piloto (Negro River) | 25 | adults | nd | A | nd | 33.2 | |||
| Ponta da Terra (Cuiuni River) | 12 | adults | nd | A | nd | 38.2 | |||
| São Luiz (Negro River) | 21 | adults | nd | A | nd | 41.4 | |||
| Cumaru (Negro River) | 16 | adults | nd | A | nd | 43.7 | |||
| Baturité (Negro River) | 11 | adults | nd | A | nd | 33 | |||
| Manaus | 105 | total of studied population | 18–50, mean 28 y.o | A | nd | 1.0 | |||
| 2006 | Bastos W.R. [34] | total (all the populations studied) | RO | 713 | total of population studied | nd | nd | 5.99–150 | 15.22 |
| Calama | 34 | nd | nd | nd | 0.50–22.48 | 9.02 | |||
| Boa Vitoria | 3 | nd | nd | nd | 10.86–17.05 | 13.82 | |||
| Cujubim | 12 | nd | nd | nd | 1.55–14.67 | 6.30 | |||
| Firmesa | 4 | nd | nd | nd | 9.40–14.80 | 11.21 | |||
| Itacoa | 6 | nd | nd | nd | 5.28–16.00 | 11.97 | |||
| Nazaré | 64 | nd | nd | nd | 0.63–22.60 | 10.65 | |||
| Papagaios | 13 | nd | nd | nd | 4.76–27.22 | 13.72 | |||
| Santa Rosa | 19 | nd | nd | nd | 7.68–20.78 | 13.99 | |||
| São Carlos | 15 | nd | nd | nd | 1.84–22.83 | 9.51 | |||
| Terra Caida | 7 | nd | nd | nd | 5.01–14.61 | 9.61 | |||
| Sto Antônio do Pau Queimado | 14 | nd | nd | nd | 5.87–26.86 | 14.69 | |||
| Puruzinho | AM | 28 | nd | nd | nd | 4.57–28.27 | 14.83 | ||
| Livramento | 15 | nd | nd | nd | 18.96–63.54 | 36.89 | |||
| Valparaiso | 21 | nd | nd | nd | 2.98–82.38 | 18.93 | |||
| Auxiliadora | 34 | nd | nd | nd | 1.12–22.78 | 9.34 | |||
| Curralinho | 5 | nd | nd | nd | 10.70–34.49 | 19.69 | |||
| Nazaré do Retiro | 15 | nd | nd | nd | 9.69–24.77 | 17.90 | |||
| Novos Prazeres | 20 | nd | nd | nd | 2.77–24.28 | 11.90 | |||
| São Pedro | 14 | nd | nd | nd | 6.61–28.00 | 15.77 | |||
| Barreiras do Manicoré | 9 | nd | nd | nd | 1.45–23.04 | 10.82 | |||
| Cachoeirinha | 14 | nd | nd | nd | 1.54–37.22 | 14.74 | |||
| São Lazaro | 6 | nd | nd | nd | 2.50–23.37 | 9.48 | |||
| Maraca II | 6 | nd | nd | nd | 8.57–15.69 | 11.37 | |||
| Vista Nova | 4 | nd | nd | nd | 21.40–28.54 | 25.69 | |||
| Vista Alegre | 17 | nd | nd | nd | 7.28–26.28 | 16.02 | |||
| Bom Suspiro | 12 | nd | nd | nd | 6.43–30.06 | 16.29 | |||
| Carara | 39 | nd | nd | nd | 4.18–34.71 | 18.13 | |||
| Miriti | 16 | nd | nd | nd | 6.70–50.37 | 22.34 | |||
| São Sebastiao (Lago Lucio) | 17 | nd | nd | nd | 6.61–18.52 | 12.84 | |||
| Boca do Carapanatuba | 18 | nd | nd | nd | 3.43–19.22 | 10.45 | |||
| São Sebastiao do Tapuru | 18 | nd | nd | nd | 20.43–150.00 | 62.76 | |||
| Moanenses | 13 | nd | nd | nd | 3.26–20.49 | 12.73 | |||
| Três Casas | 9 | nd | nd | nd | 5.62–70.70 | 33.07 | |||
| Boa Ventura | 7 | nd | nd | nd | 4.73–35.79 | 16.55 | |||
| Auara Grande | 19 | nd | nd | nd | 6.21–24.98 | 15.97 | |||
| Fazenda Tabocal | 2 | nd | nd | nd | 0.50–1.50 | 1.00 | |||
| Remanso | 12 | nd | nd | nd | 8.36–29.02 | 18.16 | |||
| Arapapa | 7 | nd | nd | nd | 10.43–21.33 | 16.56 | |||
| Axinim | 13 | nd | nd | nd | 3.27–23.02 | 8.65 | |||
| Espirito Santo | 18 | nd | nd | nd | 3.51–21.28 | 12.47 | |||
| Santa Maria | 7 | nd | nd | nd | 6.70–16.84 | 9.28 | |||
| Caicara | 23 | nd | nd | nd | 1.94–17.98 | 10.04 | |||
| Paquique | 6 | nd | nd | nd | 7.49–11.57 | 9.23 | |||
| Uricurituba | 46 | nd | nd | nd | 0.36–19.12 | 9.09 | |||
| Santa Rosa II | 12 | nd | nd | nd | 5.81–16.89 | 11.65 | |||
| 2006 | Fillion M. [35] | Tapajós (São Luiz do Tapajós, Nova Canaã, Santo Antônio, Mussum, Vista Alegre, Açaituba) | PA | 251 | adults | 15–89, mean 35.2 y.o | A | 0.21–77.2 | 17.8 |
| 2007 | Passos C.J. [36] | Tapajós (São Luiz do Tapajós, Nova Canaã, Santo Antônio, Ipaupixuna, Novo Paraiso, Teca, Timbó, Açaituba, Campo Alegre, Sumauma, Vista Alegre, Mussum, Santa Cruz) | PA | 449 | adults | 15–89, mean 38.6 y.o | A | 0.2–58.3 | 16.8 |
| 2007 | Pinheiro M.C.N. [37] | Panacauera | PA | 8 | children | 0–1 y.o | C | 0.39–4.66 | 1.11 |
| 13 | children | 2–6 y.o | C | 0.65–5.16 | 2.27 | ||||
| 15 | children | 7–12 y.o | C | 0.86–9.46 | 2.99 | ||||
| Barreiras (Tapajós Basin) | 17 | children | 0–1 y.o | C | 1.80–15.70 | 5.35 | |||
| 45 | children | 2–6 y.o | C | 1.43–23.60 | 6.21 | ||||
| 22 | children | 7–12 y.o | C | 1.63–14.50 | 6.72 | ||||
| São Luiz do Tapajós | 11 | children | 0–1 y.o | C | 1.99–30.30 | 5.97 | |||
| 23 | children | 2–6 y.o | C | 2.76–53.80 | 13.22 | ||||
| 14 | children | 7–12 y.o | C | 1.34–38.80 | 10.83 | ||||
| 2008 | Passos C.J.S. [38] | Tapajós (São Luiz do Tapajós, Nova Canaã, Santo Antônio, Vista Alegre, Mussum, Açaituba) | PA | 256 | adults | 15–89, mean 35.3 y.o | A | 0.2–58.3 | 17.9 |
| 2009 | Fillion M. [39] | Tapajós (São Luiz do Tapajós, Nova Canaã, Santo Antônio, Mussum, Vista Alegre, Açaituba, Santa Cruz, Sumauma, Campo Alegre, Ipaupixuna, Novo Paraiso, Curi-Teca, Curi-Timbó) | PA | 456 | adults | 15–>65 y.o | A | 0.2–77.7 | 17.8 |
| 2010 | Grotto D. [40] | Tapajós | PA | 108 | total of population studied | mean 41.1 y.o | nd | 1–57.8 | 13.7 |
| 54 | men | nd | M | nd | 11.5 | ||||
| 54 | women | nd | W | nd | 8.8 | ||||
| 2010 | Bortoli M.C. [41] | Novo Airão | AM | 55 | women | mean 32.3 y.o | W | 0.04–18.67 | 5.67 |
| 2012 | Barcelos G.R.M. [42] | Tapajós | PA | 144 | adults | 15–83, mean 43 y.o | A | 1–43.3 | 10.4 |
| 2012 | Dutra M.D.S. [43] | Itaituba | PA | 90 | children | population from urban area, samples collected in 2004 | C | nd | 1.01 |
| 47 | children | population from urban area, samples collected in 2006 | C | nd | 1.18 | ||||
| 90 | children | population from urban area, samples collected in 2010 | C | nd | 1.18 | ||||
| 2012 | Farias L.A. [44] | Manaus | AM | 201 | children | 2–7 y.o | C | 0.02–34.4 | 1.93 |
| 2013 | Khoury E.D.T. [45] | Barreiras | PA | 78 | general population | 13–53 y.o | G | nd | 8.66 |
| São Luiz do Tapajós | 30 | general population | 13–53 y.o | G | nd | 9.19 | |||
| Furo do Maracujá | 49 | general population | 13–53 y.o | G | nd | 0.73 | |||
| 2014 | Rocha A.V. [46] | Demarcação—Machado River | RO | 10 | children | 3–9 y.o | C | nd | 3.57 |
| Gleba do Rio Preto | 10 | children | 3–9 y.o | C | nd | 6.24 | |||
| 2015 | Faial K. [47] | Itaituba | PA | 6 | male | 0–2 y.o | C | 4.14–9.79 | 6.85 |
| 6 | male | 3–5 y.o | C | 16.01–23.80 | 19.57 | ||||
| 10 | male | 6–10 y.o | C | 12.59–24.93 | 18.58 | ||||
| 6 | male | 11–15 y.o | C | 5.83–15.57 | 13.08 | ||||
| 7 | male | 16–20 y.o | A | 17.82–24.16 | 20.87 | ||||
| 4 | male | 21–25 y.o | A | 9.42–20.09 | 15.52 | ||||
| nd | male | 26–30 y.o | A | nd | nd | ||||
| 3 | male | 31–35 y.o | A | 8.55–10.83 | 9.69 | ||||
| 5 | male | 36–40 y.o | A | 10.92–20.03 | 15.29 | ||||
| 1 | male | 41–45 y.o | A | 14.81–14.81 | 14.81 | ||||
| 1 | male | 46–50 y.o | A | 2.07–2.07 | 2.07 | ||||
| 1 | male | 51–55 y.o | A | 13.89–13.89 | 13.89 | ||||
| 3 | male | 56–60 y.o | A | 14.00–14.16 | 14.08 | ||||
| 5 | male | >60 y.o | A | 12.04–21.47 | 14.57 | ||||
| 4 | female | 0–2 y.o | C | 8.25–12.89 | 10.38 | ||||
| 7 | female | 3–5 y.o | C | 12.20–19.29 | 15.83 | ||||
| 7 | female | 6–10 y.o | C | 11.46–23.21 | 15.84 | ||||
| 8 | female | 11–15 y.o | C | 8.41–21.71 | 13.12 | ||||
| 5 | female | 16–20 y.o | A | 13.52–22.01 | 16.74 | ||||
| 14 | female | 21–25 y.o | A | 6.33–17.66 | 11.22 | ||||
| 7 | female | 26–30 y.o | A | 4.84–10.6 | 7.16 | ||||
| 8 | female | 31–35 y.o | A | 4.95–20.78 | 13.52 | ||||
| 3 | female | 36–40 y.o | A | 7.30–15.73 | 11.09 | ||||
| 3 | female | 41–45 y.o | A | 14.69–23.02 | 18.62 | ||||
| 3 | female | 46–50 y.o | A | 7.31–14.98 | 10.27 | ||||
| 4 | female | 51–55 y.o | A | 15.52–18.52 | 17.02 | ||||
| 1 | female | 56–60 y.o | A | 14.29–14.29 | 14.29 | ||||
| 9 | female | >60 y.o | A | 14.60–27.02 | 20.39 | ||||
| 2015 | Castilhos Z. [48] | São Chico | PA | 172 | nd | nd | nd | 0.14–35.90 | 3.44 |
| Creporizinho | 146 | nd | nd | nd | 0.23–10.49 | 2.25 | |||
| 2015 | Hoshino A. [49] | Lago do Puruzinho | AM | 58 | general population | 1–47, mean 17.3 y.o | G | nd | 12.78 |
| 2016 | Rocha A.V. [50] | Porto Velho | RO | 200 | women | 18–48, mean 26.60 y.o | W | nd | 0.60 |
| 2016 | Carvalho L.V.B. [51] | Belmont | RO | 42 | nd | mean 11.3 y.o | nd | nd | 2.71 |
| Cunia | 52 | nd | mean 11.3 y.o | nd | nd | 7.18 | |||
| 11.827 |
Legend. NC: not included for map classification, nd: not determined; G: general population; C: children; A: adults; W: women; M: men; I: Indigenous, >older than. y.o: year(s) old.
There was a predominance of studies that were performed in the State of Pará (47%) [22,23,25,26,27,29,35,36,37,38,39,40,42,43,45,47,48]. Some studies had been performed in the State of Amazonas (22%) [17,19,24,30,33,41,44,49], Rondônia (14%) [18,28,46,50,51], Mato Grosso (8%) [21,31,32] and Amapá (3%) [20]. Two studies were performed using samples from populations from two different States: Pará and Rondônia (3%) [16] and Rondônia and Amazonas (3%) [34].
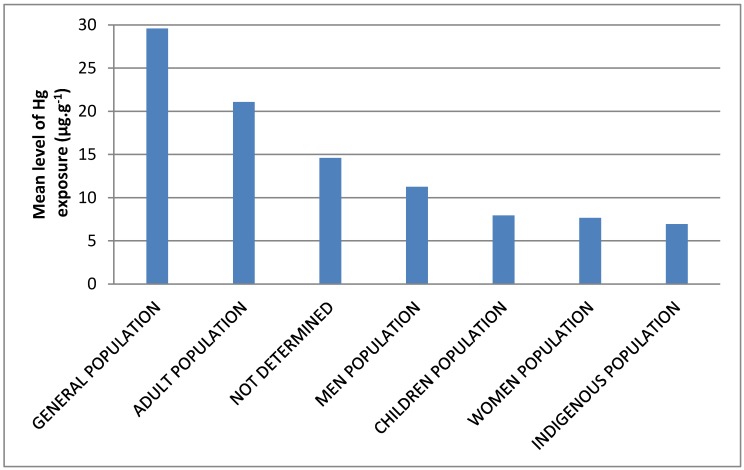
The results shows that general population presented the highest mean level of Hg exposure (29.59 µg g−1, ranging from 0.73 to 97.44 µg g−1), followed by adult population (21.08 µg g−1, ranging from 1.00 to 43.70 µg g−1), not determined (14.60 µg g−1, ranging from 1.00 to 62.76 µg g−1), men population (11.25 µg g−1, ranging from 11.00 to 11.50 µg g−1), children population (7.95 µg g−1, ranging from 1.11 to 22.00 µg g−1), women population (7.66 µg g−1, ranging from 0.60 to 14.30 µg g−1) and Indigenous population (6.95 µg g−1, ranging from 4.90 to 8.37 µg g−1) (Figure 2).
Figure 2.
Mean level (µg g−1) of Hg exposure in populations of Brazilian Amazon according to the present study.
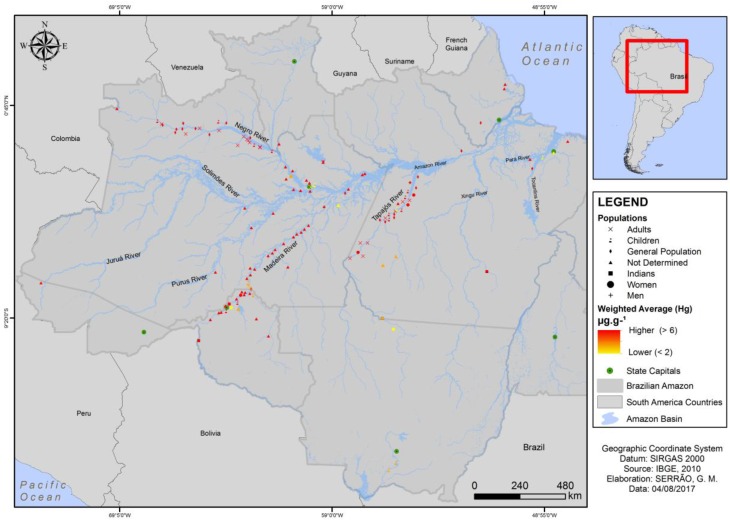
The analysis of the georeferencing data is presented as a map which shows a geospatial distribution of the populations and their respective degree of Hg exposure. The results show that most of the studies were performed along the margins of the Amazonian rivers and that most of these populations are highly exposed (Figure 3).
Figure 3.
The georeferencing results showing a geospatial distribution of the Brazilian Amazonian populations and their respective degree of Hg exposure on hair.
4. Discussion
The theme of Hg exposure in Amazonian population is an intriguing form of environmental contamination for some reasons: (1) the mean level of Hg exposure in Amazonian population usually exceed the normal limit preconized by WHO [24,30,33,52,53,54]; (2) there is a dichotomy in the clinical findings in different Amazonian populations, where some studies associate Hg exposure with the development of clinical symptoms [35,55,56,57] while others do not [45,58,59,60,61] and (3) even “non-exposed” populations are at risk of Hg effects [58].
The present study performed a systematic review of Hg exposure in Brazilian Amazon population. The results show that the majority of the studies were performed by Brazilian Institutions and researchers, reflecting the low international insertion despite that Hg is a global problem [62]. The high number of studies that were performed in the State of Pará is in agreement with the high prevalence of gold miners in the region (legal and illegal miners), especially in the Tapajós Basin [26,63].
The analysis shows that most of the studies were performed along the margins of important rivers, such as Negro, Tapajós and Madeira Rivers. The results show that all the populations presented mean levels above 6 µg g−1 of Hg on hair and that general population, adults, not determined and men presented mean levels above 10 µg g−1 of Hg on hair. Thus, the findings support the idea that Amazonian population present (along the period of time covered by this study) mean levels of Hg above the normal limit preconized by WHO (1–2 µg g−1 and levels above 10 µg g−1 for daily fish consumers) [64]. Although, affirm that this population is under risk of Hg effect is premature. Discussions about this issue should be evaluated based on other studies.
As future perspectives, the present study suggests an implementation of a strategic plan for the region in order to promote health and benefit the population. As strategies, we propose:
-
(1)
Increase the technical capacity for Hg determination in the region;
-
(2)
Implement an environmental health surveillance program that considerers the Amazonian life style, behavior and ecosystem dynamics;
-
(3)
A follow up program to monitor the Hg content and health of individuals that presents high levels of Hg on biological matrices.
5. Conclusions
The Hg exposure in the Amazonian population is a fact. The high level of Hg on hair revealed by this study shows that this population has been long-term exposed to this metal.
The data reveals that the studies focused on population that lives along the margins of the rivers and, of utmost importance, populations under risk of Hg exposure from gold mining activities, especially from Tapajós Basin located in the State of Pará.
Author Contributions
Nathália Santos Serrão de Castro and Marcelo de Oliveira Lima were responsible for: (1) Design, analysis and interpretation of data; (2) Article writing and critical review of the intellectual content; (3) Final approval of the version to be published; (4) All aspects of the work, ensuring the accuracy and integrity of any part of the work.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer
The opinions or assertions contained herein are the private views of the author(s) and are not to be construed as official or to reflect the views of the Evandro Chagas Institute or the Metropolitan College of Amazon.
References
- 1.Ekino S., Susa M., Ninomiya T., Imamura K., Kitamura T. Minamata disease revisited: An update on the acute and chronic manifestations of methyl mercury poisoning. J. Neurol. Sci. 2007;262:131–144. doi: 10.1016/j.jns.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 2.Ninomiya T., Ohmori H., Hashimoto K., Tsuruta K., Ekino S. Expansion of methylmercury poisoning outside of Minamata: An epidemiological study on chronic methylmercury poisoning outside of Minamata. Environ. Res. 1995;70:47–50. doi: 10.1006/enrs.1995.1045. [DOI] [PubMed] [Google Scholar]
- 3.Ninomiya T., Imamuraa K., Kuwahatab M., Kindaichid M., Susac M., Ekino S. Reappraisal of somatosensory disorders in methylmercury poisoning. Neurotoxicol. Teratol. 2005;27:643–653. doi: 10.1016/j.ntt.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Yorifuji T., Tsuda T., Takao S., Harada M. Long-term exposure to methylmercury and neurologic signs in Minamata and neighboring communities. Epidemiology. 2008;19:3–9. doi: 10.1097/EDE.0b013e31815c09d2. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama K., Yorifuji T., Tsuda T., Sekikawa T., Nakadaira H., Saito H. Methyl mercury exposure at Niigata, Japan: Results of neurological examinations of 103 adults. J. Biomed. Biotechnol. 2012;2012:635075. doi: 10.1155/2012/635075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karagas M.R., Choi A.L., Oken E., Horvat M., Schoeny R., Kamai E., Cowell W., Grandjean P., Korrick S. Evidence on the human health effects of low-level methylmercury exposure. Environ. Health Perspect. 2012;120:799–807. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes P., James K.A.F., Levy L.S. Is low-level environmental mercury exposure of concern to human health? Sci. Total Environ. 2009;408:171–182. doi: 10.1016/j.scitotenv.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Grandjean P., White R.F., Weihe P., Jørgensen P.J. Neurotoxic risk caused by stable and variable exposure to methylmercury from seafood. Ambul. Pediatr. 2003;3:18–23. doi: 10.1367/1539-4409(2003)003<0018:NRCBSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Grandjean P., Weihe P., White R.F., Debes F. Cognitive performance of children prenatally exposed to ‘safe’ levels of methylmercury. Environ. Res. 1998;77:165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- 10.Amin-Zaki L., Elhassani S., Majeed M.A., Clarkson T.W., Doherty R.A., Greenwood M. Intra-uterine Methylmercury Poisoning in Iraq. Pediatrics. 1974;54:587–595. [PubMed] [Google Scholar]
- 11.Yorifuji T., Kashima S., Tsuda T., Harada M. What has methylmercury in umbilical cords told us?—Minamata disease. Sci. Total Environ. 2009;408:272–276. doi: 10.1016/j.scitotenv.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Airey D. Mercury in human hair due to environment and diet: A review. Environ. Health Perspect. 1983;52:303–316. doi: 10.1289/ehp.8352303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosselin N.H., Brunet R.C., Carrier G., Bouchard M., Feeley M. Reconstruction of methylmercury intakes in indigenous populations from biomarker data. J. Expo. Sci. Environ. Epidemiol. 2006;16:19–29. doi: 10.1038/sj.jea.7500433. [DOI] [PubMed] [Google Scholar]
- 14.Kempson I.M., Lombi E. Hair analysis as a biomonitor for toxicology, disease and health status. Chem. Soc. Rev. 2011;40:3915–3940. doi: 10.1039/c1cs15021a. [DOI] [PubMed] [Google Scholar]
- 15.Grandjean P., Jørgensen P.J., Weihe P. Validity of mercury exposure biomarkers. In: Samuel H., Wilson W.A.S., editors. Biomarkers of Environmentally Associated Disease. CRC Press; Boca Raton, FL, USA: 2002. pp. 235–247. [Google Scholar]
- 16.Barbosa A.C., Silva S.R.L., Dórea J.G. Concentration of mercury in hair of indigenous mothers and infants from the Amazon Basin. Arch. Environ. Contam. Toxicol. 1998;34:100–105. doi: 10.1007/s002449900291. [DOI] [PubMed] [Google Scholar]
- 17.Kehrig H.A., Malm O., Akagi H., Guimarães J.R., Torres J.P. Methylmercury in fish and hair samples from the Balbina Feservoir, Brazilian Amazon. Environ. Res. 1998;77:84–90. doi: 10.1006/enrs.1998.3836. [DOI] [PubMed] [Google Scholar]
- 18.Barbosa A.C., Dórea J.G. Indices of mercury contamination during breast feeding in the Amazon Basin. Environ. Toxicol. Pharmacol. 1998;6:71–79. doi: 10.1016/S1382-6689(98)00031-3. [DOI] [PubMed] [Google Scholar]
- 19.Silva-Forsberg M.C., Forsberg B.R., Zeldemann V.K. Mercury contamination in humans linked to river chemistry in the Amazon Basin. Ambio. 1999;28:519–521. [Google Scholar]
- 20.Guimaraes J.R.D., Forti M.C. Mercury in human and environmental samples from two lakes in Amapa, Brazilian Amazon. Ambio. 1999;28:296–301. [Google Scholar]
- 21.Hacon S., Yokoo E., Valente J., Campos R.C., Da Silva V.A., de Menezes A.C.C., De Moraes L.P., Ignotti E. Exposure to mercury in pregnant women from Alta Floresta-Amazon Basin, Brazil. Environ. Res. 2000;84:204–210. doi: 10.1006/enrs.2000.4115. [DOI] [PubMed] [Google Scholar]
- 22.De Oliveira Santos E.C., de Jesus I.M., da Silva Brabo E., Loureiro E.C.B., da Silva Mascarenhas A.F., Weirich J., Câmara V.D.M., Cleary D. Mercury exposures in riverside Amazon communities in Pará, Brazil. Environ. Res. 2000;84:100–107. doi: 10.1006/enrs.2000.4088. [DOI] [PubMed] [Google Scholar]
- 23.Dolbec J., Mergler D., Sousa Passos C.J., Sousa de Morais S., Lebel J. Methylmercury exposure affects motor performance of a riverine population of the Tapajós river, Brazilian Amazon. Int. Arch. Occup. Environ. Health. 2000;73:195–203. doi: 10.1007/s004200050027. [DOI] [PubMed] [Google Scholar]
- 24.Barbosa A.C., Jardim W., Dórea J.G., Fosberg B., Souza J. Hair mercury speciation as a function of gender, age, and body mass index in inhabitants of the Negro River Basin, Amazon, Brazil. Arch. Environ. Contam. Toxicol. 2001;40:439–444. doi: 10.1007/s002440010195. [DOI] [PubMed] [Google Scholar]
- 25.Harada M., Nakanishi J., Yasoda E., Maria da Conceicâo N.P., Oikawa T., de Assis Guimarâes G., da silva Cardoso B., Kizaki T., Ohno H. Mercury pollution in the Tapajos River Basin, Amazon mercury level of head hair and health effects. Environ. Int. 2001;27:285–290. doi: 10.1016/S0160-4120(01)00059-9. [DOI] [PubMed] [Google Scholar]
- 26.Crompton P., Ventura A.M., de Souza J.M., Santos E., Strickland G.T., Silbergeld E. Assessment of mercury exposure and malaria in a Brazilian Amazon riverine community. Environ. Res. 2002;90:69–75. doi: 10.1006/enrs.2002.4358. [DOI] [PubMed] [Google Scholar]
- 27.Passos C.J., Mergler D., Gaspar E., Morais S., Lucotte M., Larribe F., Davidson R., de Grosbois S. Eating tropical fruit reduces mercury exposure from fish consumption in the Brazilian Amazon. Environ. Res. 2003;93:123–130. doi: 10.1016/S0013-9351(03)00019-7. [DOI] [PubMed] [Google Scholar]
- 28.Santos E.C.O., Câmara V.M., Brabo E.S., Loureiro E.C.B., Jesus I.M., Fayal K., Sagica F. Mercury exposure evaluation among Pakaanóva Indians, Amazon Region, Brazil. Cad. Saude Publica. 2003;19:199–206. doi: 10.1590/S0102-311X2003000100022. [DOI] [PubMed] [Google Scholar]
- 29.Dórea J.G., de Souza J.R., Rodrigues P., Ferrari I., Barbosa A.C. Hair mercury (signature of fish consumption) and cardiovascular risk in Munduruku and Kayabi Indians of Amazonia. Environ. Res. 2005;97:209–219. doi: 10.1016/j.envres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Santos E.C.O., Sá G.C., Jesus I.M., Brabo E.S., Câmara V.M., Lima M.O., Faial K.F., Mendes R.A., Mascarenhas A.F.S. Mercury in the Negro river, Brazilian Amazon—Preliminary study of exposure indicators in fish and human populations. Cad. Saúde Coletiva. 2005;13:225–235. [Google Scholar]
- 31.Tavares L.M.B., Câmara V.M., Malm O., Santos E.C.O. Performance on neurological development tests by riverine children with moderate mercury exposure in Amazonia, Brazil. Cad. Saude Publica. 2005;21:1160–1167. doi: 10.1590/S0102-311X2005000400018. [DOI] [PubMed] [Google Scholar]
- 32.Klautau-Guimarães M.N., D´Ascenção R., Caldart F.A., Grisolia C.K., Souza J.R., Barbosa A.C., Cordeiro C.M.T., Ferrari I. Analysis of genetic susceptibility to mercury contamination evaluated through molecular biomarkers in at-risk Amazon Amerindian populations. Genet. Mol. Biol. 2005;832:827–832. doi: 10.1590/S1415-47572005000500027. [DOI] [Google Scholar]
- 33.Alves M.F.A., Fraijo N.A., Barbosa A.C., De Lima D.S., Souza J.R., Dórea J.G., Cordeiro G.W.O. Fish consumption, mercury exposure and serum antinuclear antibody in Amazonians. Int. J. Environ. Health Res. 2006;16:255–262. doi: 10.1080/09603120600734147. [DOI] [PubMed] [Google Scholar]
- 34.Bastos W.R., Gomes J.P.O., Oliveira R.C., Almeida R., Nascimento E.L., Bernadi J.V.E., Lacerda L.D., Silveira E.G., Pfeiffer W.C. Mercury in the environment and riverside population in the Madeira River Basin, Amazon, Brazil. Sci. Total Environ. 2006;368:344–351. doi: 10.1016/j.scitotenv.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 35.Fillion M., Mergler D., Passos C.J.S., Larribe F., Lemire M., Guimarães J.R.D. A preliminary study of mercury exposure and blood pressure in the Brazilian Amazon. Environ. Health. 2006;5:29. doi: 10.1186/1476-069X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passos C.J.S., Mergler D., Fillion M., Lemire M., Mertens F., Guimarães J.R.D., Philibert A. Epidemiologic confirmation that fruit consumption influences mercury exposure in riparian communities in the Brazilian Amazon. Environ. Res. 2007;105:183–193. doi: 10.1016/j.envres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Pinheiro M.C.N., Cresco-López M.E., Vieira J.L.F., Oikawa T., Guimarães G.A., Araújo C.C., Amoras W.W., Ribeiro D.R., Herculano A.M., do Nascimento J.L.M., et al. Mercury pollution and childhood in Amazon riverside villages. Environ. Int. 2007;33:56–61. doi: 10.1016/j.envint.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 38.Passos C.J.S., Silva D.S., Lemire M., Fillion M., Guimarães J.R.D., Lucotte M., Mergler D. Daily mercury intake in fish-eating populations in the Brazilian Amazon. J. Expo. Sci. Environ. Epidemiol. 2008;18:76–87. doi: 10.1038/sj.jes.7500599. [DOI] [PubMed] [Google Scholar]
- 39.Fillion M., Passos C.J.S., Lemire M., Fournier B., Mertens F., Guimarães J.R.D., Mergler D. Quality of life and health perceptions among fish-eating communities of the Brazilian amazon: An ecosystem approach to well-being. Ecohealth. 2009;6:121–134. doi: 10.1007/s10393-009-0235-z. [DOI] [PubMed] [Google Scholar]
- 40.Grotto D., Valentini J., Fillion M., Passos C.J., Garcia S.C., Mergler D., Barbosa F., Jr. Mercury exposure and oxidative stress in communities of the Brazilian Amazon. Sci. Total Environ. 2010;408:806–811. doi: 10.1016/j.scitotenv.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 41.De Bortoli M.C. Assessment of Thyroid Hormone (T3) Levels and Selenium Status of Women Living in Mercury Exposure Area. Universidade de São Paulo; de São Paulo, Brazil: 2009. [Google Scholar]
- 42.Barcelos G.R.M., Marco K.C., Grotto D., Valentino J., Garcia S.C., Braga G.U.L., Barbosa F., Jr. Evaluation of Glutathione S-transferase GSTM1 and GSTT1 Polymorphisms and Methylmercury Metabolism in an Exposed Amazon Population. J. Toxicol. Environ. Health Part A. 2012;75:960–970. doi: 10.1080/15287394.2012.695232. [DOI] [PubMed] [Google Scholar]
- 43.Dutra M.D.S., Jesus I.M., Santos E.C.O., Lima M.O., Medeiros R.L.F., Cavadas M., Luiz R.R., Câmara V.M. Longitudinal assessment of mercury exposure in schoolchildren in an urban area of the Brazilian Amazon. Cad. Saude Publica. 2012;28:1539–1545. doi: 10.1590/S0102-311X2012000800012. [DOI] [PubMed] [Google Scholar]
- 44.Farias L.A., Fávaro D.I.T., Pessoa A., Aguiar J.P.L., Yuyama L.K.O. Mercury and methylmercury concentration assessment in children’s hair from Manaus, Amazonas State, Brazil. Acta Amaz. 2012;42:279–286. doi: 10.1590/S0044-59672012000200015. [DOI] [Google Scholar]
- 45.Khoury E.D.T., Souza G.S., Silveira L.C.L., Araújo A.A., Pinheiro M.C.N. Neurological manifestations in riverine populations from areas exposed to mercury in the Brazilian Amazon. Cad. Saude Publica. 2013;29:2307–2318. doi: 10.1590/0102-311x00158012. [DOI] [PubMed] [Google Scholar]
- 46.Rocha A.V., Cardoso B.R., Cominetti C., Bueno R.B., Bortoli M.C., Farias L.A., Favaro D.I.T., Camargo L.M.A., Cozzolino S.M.F. Selenium status and hair mercury levels in riverine children from Rondônia, Amazonia. Nutrition. 2014;30:1318–1323. doi: 10.1016/j.nut.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Faial K., Deus R., Deus S., Neves R., Jesus I., Santos E., Alvez C.N., Brasil D. Mercury levels assessment in hair of riverside inhabitants of the Tapajós River, Pará State, Amazon, Brazil: Fish consumption as a possible route of exposure. J. Trace Elem. Med. Biol. 2015;30:66–76. doi: 10.1016/j.jtemb.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Castilhos Z., Rodrigues-Filho S., Cesar R., Rodrigues A.P., Villas-Boas R., Jesus I., Lima M., Faial K., Miranda A., Brabo E., et al. Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon. Environ. Sci. Pollut. Res. 2015;22:11255–11264. doi: 10.1007/s11356-015-4340-y. [DOI] [PubMed] [Google Scholar]
- 49.Hoshino A., Pacheco-Ferreira H., Sanches S.G.G., Carvallo R., Cardoso N., Perez M., Câmara V.M. Mercury exposure in a riverside Amazon population, Brazil: A study of the ototoxicity of methylmercury. Int. Arch. Otorhinolaryngol. 2015;19:135–140. doi: 10.1055/s-0034-1389030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rocha A.V., Cardoso B.R., Zavarize B., Almondes K., Bordon I., Hare D.J., Favaro D.I.T., Cozzolino S.M.F. GPX1 Pro198Leu polymorphism and GSTM1 deletion do not affect selenium and mercury status in mildly exposed Amazonian women in an urban population. Sci. Total Environ. 2016;571:801–808. doi: 10.1016/j.scitotenv.2016.07.054. [DOI] [PubMed] [Google Scholar]
- 51.De Carvalho L.V.B. Avaliação dos Níveis de Estresse Oxidativo Induzido por Exposição ao Mercúrio em População Ribeirinha Infantojuvenil do Rio Madeira (RO) Escola Nacional de Saúde Pública Sergio Arouca; Rio de Janeiro, Brazil: 2016. [Google Scholar]
- 52.Barbosa A.C., Garcia A.M., Souza J.R. Mercury contamination in hair of riverine populations of Apiacas reserve in the Brazilian Amazon. Water Air Soil Pollut. 1997;97:1–8. doi: 10.1007/BF02409639. [DOI] [Google Scholar]
- 53.Soares de Campos M., Sarkis J.E.S., Müller R.C.S., Brabo E.S., Santos E.C.O. Correlation between mercury and selenium concentrations in Indian hair from Rondônia State, Amazon region, Brazil. Sci. Total Environ. 2002;287:155–161. doi: 10.1016/S0048-9697(01)01002-6. [DOI] [PubMed] [Google Scholar]
- 54.Pinheiro M.C.N., Muller R.C.S., Sarkis J.E., Vieira J.L.F., Oikawa T., Gomes M.S.V., Guimarães G.A., do Nascimento J.L.M., Silveira L.C.L. Mercury and selenium concentrations in hair samples of women in fertile age from Amazon riverside communities. Sci. Total Environ. 2005;349:284–288. doi: 10.1016/j.scitotenv.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 55.Lebel J., Mergler D., Lucotte M., Amorin M., Dolbec J., Miranda D., Aranttes G., Rheaut R., Pichet P. Evidence of early nervous system dysfunction in Amazonian populations exposed to low-levels of methylmercury. Neurotoxicology. 1996;17:157–167. [PubMed] [Google Scholar]
- 56.Lebel J., Mergler D., Branches F., Lucotte M., Amorim M., Larribe F., Dolbec J. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ. Res. 1998;79:20–32. doi: 10.1006/enrs.1998.3846. [DOI] [PubMed] [Google Scholar]
- 57.Rodrigues A.R., Souza C.R.B., Braga A.M., Rodrigues P.S.S., Silveira A.T., Damim E.T.B., Côrtes M.I.T., Castro A.J.O., Mello G.A., Vieira J.L.F. Mercury toxicity in the Amazon: Contrast sensitivity and color discrimination of subjects exposed to mercury. Braz. J. Med. Biol. Res. 2007;40:415–424. doi: 10.1590/S0100-879X2007000300018. [DOI] [PubMed] [Google Scholar]
- 58.Santos E.C.O., Câmara V.M., Jesus I.M., Brabo E.S., Loureiro E.C.B., Mascarenhas A.F.S., Fayal K.F., Sá Filho G.C., Sagica F.E.S., Lima M.O., et al. A contribution to the establishment of reference values for total mercury levels in hair and fish in Amazonia. Environ. Res. 2002;90:6–11. doi: 10.1006/enrs.2002.4366. [DOI] [PubMed] [Google Scholar]
- 59.Santos E.C.O., Jesus I.M., Brabo E.S., Fayal K.F., Sá Filho G.C., Lima M.O., Miranda A.M.M., Mascarenhas A.S., de Sá L.L.C., Silva A.P., et al. Exposure to mercury and arsenic in Amazon States: A summary of studies by the Evandro Chagas Institute/FUNASA. Rev. Bras. Epidemiol. 2003;6:171–185. doi: 10.1590/S1415-790X2003000200010. [DOI] [Google Scholar]
- 60.Marques R.C., Dórea J.G., Bastos W.R., Rebelo M.F., Fonseca M.F., Malm O. Maternal mercury exposure and neuro-motor development in breastfed infants from Porto Velho (Amazon), Brazil. Int. J. Hyg. Environ. Health. 2007;210:51–60. doi: 10.1016/j.ijheh.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Dorea J., Barbosa A.C., Ferrari I., de Souza J.R. Mercury in hair and in fish consumed by Riparian women of the Rio Negro, Amazon, Brazil. Int. J. Environ. Health Res. 2003;13:239–248. doi: 10.1080/0960312031000122398. [DOI] [PubMed] [Google Scholar]
- 62.Larson H.J. The Minamata convention on mercury: Risk in perspective. Lancet. 2014;383:198–199. doi: 10.1016/S0140-6736(13)62000-0. [DOI] [PubMed] [Google Scholar]
- 63.Telmer K., Costa M., Simões Angélica R., Araujo E.S., Maurice Y. The source and fate of sediment and mercury in the Tapajós River, Pará, Brazilian Amazon: Ground- and space-based evidence. J. Environ. Manag. 2006;81:101–113. doi: 10.1016/j.jenvman.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 64.WHO/UNEP DTIE Chemicals Branch . Guidance for Identifying Populations at Risk from Mercury Exposure. World Health Organization; Geneva, Switzerland: 2008. p. 167. [Google Scholar]