Abstract
Objective
To determine the trajectory and magnitude of antidepressant response as well as the effect of antidepressant class and dose on symptomatic improvement in pediatric anxiety disorders.
Method
Weekly symptom severity data were extracted from randomized, parallel group, placebo-controlled trials of selective serotonin reuptake inhibitors (SSRIs) and selective serotonin-norepinephrine reuptake inhibitors (SSNRIs) in pediatric anxiety disorders. Treatment response was modeled for the standardized change in continuous measures of anxiety using Bayesian updating. Posterior distributions for each study served as informative conjugate priors to update subsequent study posteriors. Change in symptom severity was evaluated as a function of time, class and, for SSRIs, standardized dose.
Results
Data from 9 trials (SSRIs: n=5; SSNRIs, n=4) evaluating 7 medications in 1,673 youth were included. In the logarithmic model of treatment response, statistically—but not clinically—significant treatment effects emerged within 2 weeks of beginning treatment (standardized medication-placebo difference = −0.054, CI: −0.076 to −0.032, p=0.005, approximate Cohen’s d ≤ 0.2) and by week 6, clinically significant differences emerged (standardized medication-placebo difference = −0.120, CI: −0.142, −0.097, p=0.001, approximate Cohen’s d = 0.44). Compared to SSNRIs, SSRIs resulted in significantly greater improvement by the second week of treatment (p=0.0268) and this advantage remained statistically significant through week 12 (all ps<0.03). Improvement occurred earlier with high dose SSRI treatment (week 2, p=0.002) compared to low-dose treatment (week 10, p=0.025), but SSRI dose did not impact overall response trajectory (p>0.18 for weeks 1–12).
Conclusions
In pediatric patients with generalized, separation and/or social anxiety disorders, antidepressant-related improvement occurs early in the course of treatment and SSRIs are associated with more rapid and greater improvement compared to SSNRIs.
Keywords: selective serotonin reuptake inhibitor (SSRI, SRI); selective serotonin norepinephrine reuptake inhibitor (SSNRI, SNRI); separation anxiety disorder (SAD); social phobia (SoP); generalized anxiety disorder (GAD)
INTRODUCTION
Having an anxiety disorder during childhood or adolescence—a critical neurodevelopmental period—results in devastating psychosocial morbidity.1,2 Anxiety disorders during this period culminate in an increased risk for developing major depressive disorder,3–5 secondary anxiety disorders6 and suicidality.7 Importantly, pediatric anxiety disorders frequently respond to first-line psychopharmacologic treatments including selective serotonin reuptake inhibitors (SSRIs) and selective serotonin norepinephrine reuptake inhibitors (SSNRIs).8–10 To date, nearly all randomized controlled trials of these medications in pediatric patients with generalized, separation and/or social anxiety disorders support their efficacy;11–18 however, response varies among individual patients. Increased understanding of the time course of treatment response as well as the impact of specific antidepressant characteristics (including antidepressant class and dosing) on this response could substantially affect clinical practice. Further, understanding the variability in antidepressant treatment response could inform the duration of treatment trials and may decrease uncertainty related to the typical course of antidepressant-related improvement for patients and their families.
Recent meta-analyses that leverage longitudinal data reveal clinically relevant findings regarding the time course of antidepressant treatment response and medication dose in adults with MDD19 and OCD,20,21 as well as in pediatric patients with these disorders.22,23 These studies suggest that antidepressant-related improvement occurs early in the course of treatment in adolescents with MDD and OCD22,23 and, in adults with MDD, higher SSRI dose is associated with a greater response.19 However, in youth with anxiety disorders, the time course of antidepressant response and the effect of antidepressant dose or class on response trajectory and magnitude are unknown. Despite this, SSRIs (compared to SSNRIs) have been recommended as first-line psychopharmacologic interventions for pediatric anxiety disorders,24 and there is consensus that improvement may be dose-related.25 In fact, recommendations in the current AACAP Practice Parameters for the Treatment of Pediatric Anxiety Disorders are consistent with these beliefs: “clinicians should consider increasing SSRI doses for patients if significant improvement is not achieved by the fourth week of treatment.”25
While nearly all trials of SSRIs and SSNRIs in anxious youth demonstrate the superiority of individual antidepressant treatments compared to placebo,10 variability among individual clinical trials has precluded direct comparisons of SSRIs and SSNRIs. However, our prior meta-analysis of antidepressants in pediatric patients with anxiety disorders shed light on the degree to which the relative serotonergic selectivity of an antidepressant affects treatment response.10 Treatment effect size (weighted Cohen’s d) positively correlated with serotonergic selectivity (ratio of Ki,norepinephrine to Ki,5-HT), suggesting that antidepressants with greater serotonergic selectivity were associated with a larger effect size (R=0.79, p=0.021) in youth with generalized, separation and/or social anxiety disorders.10 There are no current recommendations regarding the dosing of SSRIs or the use of SSRIs over SSNRIs in pediatric anxiety disorders and it is unknown whether SSRIs are superior to SSNRIs for the treatment of anxious youth. Moreover, the only FDA-approved antidepressant for children and adolescents with anxiety (generalized anxiety disorder, ages 7–17) is the SSNRI duloxetine.16
Aggregating time course and symptom severity data from trials of SSRIs and SSNRIs in pediatric anxiety disorders allows the overall time course of treatment response and the impact of selected medication-specific and trial-specific variables to be evaluated with greater statistical power than can be accomplished in individual trials. With these considerations in mind, we conducted a Bayesian meta-analysis of antidepressant response in randomized, placebo-controlled trials of SSRIs and SSNRIs for the short-term treatment of generalized, social and/or separation anxiety disorders in children and adolescents. The objective of this meta-analysis was to examine weekly treatment data in pharmacotherapy trials of pediatric anxiety disorders. Specifically, we sought to: (1) examine the temporal course of antidepressant treatment response; (2) compare the trajectory and magnitude of SSRI and SSNRI treatment response; (3) determine if high doses of SSRIs are more effective than low doses in pediatric anxiety disorders. We hypothesized that (1) treatment response would be logarithmic as in youth with OCD22 and MDD;23 (2) SSRIs would be associated with a larger and more rapid improvement compared to SSNRIs; and (3) high dose treatment with SSRIs would be associated with greater treatment response compared to low-dose SSRI treatment.
METHOD
Search Strategy
All meta-analytic methods and sensitivity analyses were specified before conducting the meta-analysis proper. The studies included were obtained through an electronic search of English language articles in PubMed (1966 through July 2017) in addition to the Cochrane Database, Web of Science, Embase and PsychInfo as well as the government clinical trials registry, www.clinicaltrials.gov using the search strategy (adolescent* OR children OR pediatric OR youth) AND (anxiety OR social phobia OR social anxiety disorder OR SAD OR generalized anxiety disorder OR GAD OR separation anxiety disorder) AND (selective serotonin reuptake inhibitor OR SSRI OR selective serotonin norepinephrine reuptake inhibitor OR SNRI OR selective serotonin norepinephrine reuptake inhibitor OR fluoxetine OR fluvoxamine OR citalopram OR escitalopram OR fluoxetine OR paroxetine OR venlafaxine OR desvenlafaxine OR duloxetine OR vortioxetine OR vilazodone). The results of the search were then manually limited to randomized, placebo-controlled trials. The references of all eligible trials and review articles were searched for additional clinical trials.
Criteria for Inclusion of Studies
Studies were included if they were prospective, randomized, parallel-group, placebo-controlled trials that evaluated the efficacy of SSRIs or SSNRIs in the treatment of social, generalized and/or separation anxiety disorder in children or adolescents, and used a validated rating scale to measure the severity of the anxiety symptoms. Exclusionary criteria were adapted from a recent meta-analysis of SSRIs in pediatric patients with major depressive disorder.23 As such, clinical trials were excluded if they met the following criteria: included adults (age >18 years); utilized a cross-over design; did not study an SSRI or SSNRI; were not randomized; were not placebo controlled; provided adjunctive psychotherapy to active or control group; or included <10 patients per treatment group.
Data Extraction
Data were extracted into an Excel (Microsoft, Redmond, WA) spreadsheet. Additional data related to the methods, demographics, SSRI/SSNRI dosing, duration of the trial, and other relevant aspects and results of the studies were collected (e.g., funding source, difference in SSRI/SSNRI and placebo dropout, etc.). Consistent with a prior meta-analysis of the efficacy and tolerability of these medications in anxious youth,26 the outcome measurement selected from each included clinical trial was the difference in mean improvement between the antidepressant-treated and placebo-treated groups. This difference in mean improvement was determined for the clinical rating scale measuring anxiety symptom severity at each reported time point. A hierarchy of symptom severity rating scales was developed based on (1) psychometric properties and comparability of the rating scales, (2) each scale’s appropriateness for use with children and adolescents, (3) consistency of use across trials and (4) inclusion of somatic symptoms that may be obscured by side effects of antidepressant treatment. The rating scale hierarchy decreases heterogeneity of measures as well as the likelihood of inflation and reporting bias and has been used in meta-analyses of antidepressants in pediatric anxiety disorders,26 antidepressants in pediatric patients with major depressive disorder (MDD),27 psychotherapy in youth with MDD28 and in comparative evaluations of antidepressant efficacy in anxiety and depressive disorders29 For the analyses described herein, the hierarchy of rating scales (in order of preference) consisted of: (1) the Pediatric Anxiety Rating Scale (PARS),30 (2) the Hamilton Anxiety Rating Scale (HAM-A),31 (3) Social Anxiety Scale for Children (SASC),32 (4) the Social Phobia and Anxiety Inventory (SPAI) and (5) the 9 delineated GAD items from the K-SADS.
Assessment of Bias
Two reviewers assessed risk of bias of each study with regard to sequence generation, allocation concealment, blinding of participant, blinding of researcher, blinding of assessor, selective reporting and attrition, as previously described.27,33 Consistent with prior risk of bias classification approaches to meta-analyses of antidepressants,27,33,34 each study was classified as having: (1) “low risk of bias” if no domain was rated as high risk of bias and ≤3 were classified as “unclear risk;” (2) “high risk of bias” if >1 domain was rated as high risk of bias or no domain was rated as high risk of bias but >3 domains were rated as unclear risk and (3) “uncertain risk of bias,” if other combinations of bias across domains were present.
Statistical Methods
The primary outcome for these analyses was the change in PARS total score (or other dimensional anxiety scale score) from baseline to endpoint. A set of treatment response models (linear, exponential, logarithmic and quadratic) was developed in which the relative treatment effects were modeled using a Bayesian inferential approach with parameters estimated using Markov chain Monte Carlo (MCMC) simulation. The best fitting model was selected by Akaike and Bayesian Information Criteria.35
For individual studies, the endpoint was typically week 8–12, except in two 16-week trials.13,18 Eight week data were available for the first of these trials. For the selected model (logarithmic-linear), the observed week 12 outcomes were assessed with regard to the credible interval of the predicted week 12 values based on a model that incorporated data from each study through week 8. For studies involving SSRIs, the endpoint dose, was converted (or imputed and converted) to fluoxetine equivalents based on the therapeutic dose range of each medication and as employed in similar meta-analyses of SSRI dose in depressive disorders.19 Based on fluoxetine equivalents (sertraline 120 mg/day; fluvoxamine 100 mg; paroxetine: 20 mg; fluoxetine 33 mg), dose was categorized as low (<1.5 fluoxetine equivalents/day) or high (>1.5 fluoxetine equivalents/day).19 The difference in change scores between each medication and its corresponding placebo arm was computed, and treatment response was modeled for the standardized change in continuous measures of anxiety. Specifically, the ratio of anxiety symptom severity score at each week to the initial anxiety symptom score was employed with the standard deviation in weekly anxiety score normalized using the initial anxiety score to allow for heterogeneity in variance across studies.
To preserve the heterogeneity in variance but allow comparison across studies, the mean and standard deviation for symptom severity in week t, x̄it and sit, , for each treatment group, i, were normalized using mean baseline symptom severity to obtain scaled mean and standard deviation at each week,
Given a distributional assumption of normality, we did not use Cohen’s d as it would scale by variance, imposing a homogeneity assumption that is invalid for this dataset (i.e., variance significantly differs across studies). Both the sample mean and variance of symptom severity measures are required for statistical sufficiency to allow recovery of the posterior density of the mean.36,37
To examine standardized mean change in continuous measures of anxiety, the Bayesian posterior from each study was used as an informative conjugate prior to update the posterior for subsequent studies. Change in anxiety symptom severity was evaluated as a function of time, class and, for SSRIs, standardized dose using posterior densities, and logarithmic trajectories of posterior means from Markov Chain Monte Carlo (MCMC) simulations as previously described.36
For comparison of antidepressant and placebo response, the posteriors for the mean symptom severity ratings for each group were obtained from posterior simulation. MCMC samples from each exact posterior distribution were then combined to numerically obtain the posterior distribution of the difference in means for inference and hypothesis testing. Specifically, given sample means, x̄1 and x̄2, sample standard deviations s1 and s2, and sample sizes n1 and n2, R values, , r = 1, 2, …, R, j = 1,2 … , N, were sampled from each of the marginal posterior distributions and , which, assuming normality of the population mean, are Student-t distributions. Then differences in means were computed and credible intervals, means, etc. were determined from the MCMC sample of R values from the posterior of differences in means. Additional details of this statistical approach have been previously described.36,37
For the trajectory analysis, a logarithmic trend was fit to the posterior mean differences for each week. The posterior MCMC sample of differences for each week for SSRIs and SSNRIs were then used to obtain the posterior density of the difference in efficacy of SSRIs vs. SSNRIs (or high vs. low dose SSRI treatment), both relative to placebo. These posterior mean differences were modeled with a logarithmic model as above. Predictive posterior simulation samples for week 12 projected outcomes were then obtained from MC simulation using the posterior estimates based on data up to week 8. These predictive samples were used to perform a statistical analysis of week 12 differences in SSRI vs. SSNRI (or high vs low dose SSRI treatment) outcomes, conditional on the log trajectory model. Finally, for the antidepressant class comparisons, because of the degree of norepinephrine reuptake blockade by atomoxetine, a sensitivity analysis was performed to determine the contribution of the atomoxetine study to the SSRI-SSNRI effect. In this regard, atomoxetine was among the most potent norepinephrine reuptake inhibitors evaluated in pediatric anxiety disorders, however, it was included in these analyses because of its ability to significantly inhibit the serotonin transporter38 and given preclinical data suggesting >85% of brain serotonin transporter antagonism at therapeutic doses.39
A suite of functions to perform the analysis was coded in Julia (versions 0.5 and 0.6),40 and will be included online upon publication of this article.
RESULTS
Selection of Studies and Study Characteristics
Nineteen articles were identified that were potentially eligible for inclusion in this meta-analysis The Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram41 illustrating the selection procedure—which yielded 9 studies—is shown in Figure S1 (available online). Overall, 1,243 citations were identified by the search and 340 potentially eligible articles were screened with 19 retrieved in full text (see Figure S1 and Table S1, available online). Overall, 9 double-blind, parallel RCTs (1,805 patients) conducted between 1997 and 2014, and comparing 8 antidepressants or placebo were included in the analysis. Table 1 tabulates the characteristics of included studies. The mean study sample size was 200 participants (range 22 to 320). Overall 923 participants were randomly assigned to an antidepressant and 882 to placebo. About half of the sample population was male (53%). The median duration of the acute treatment was 10 weeks (interquartile range [IQR] 9–12) and the majority of studies were multi-center (7 of 9; 78%) and all recruited outpatients (9 of 9; 100%).
TABLE 1.
Study, Patient and Treatment Characteristics of Included Randomized Controlled Trials of Selective Serotonin Norepinephrine Reuptake Inhibitors (SSNRIs) and Selective Serotonin Reuptake Inhibitors (SSRIs) in Children and Adolescents with Generalized, Social and/or Separation Anxiety Disorders.
| Author | Publicati on Year |
Recruitm ent Start Year |
Fundin g |
Grou p, N |
Durati on, wks |
Sex % male |
Age rang e, years |
Medicatio n |
Outco me measur e |
Endpoi nt dose, mg/day |
Maxim um dose, mg/day |
Hig h dose SSR I |
Medicati on- Placebo Attrition differen ce |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rynn et al.12 | 2001 | NR | Federal | 11 11 |
9 | 67 | 5–17 | Sertraline | HAMA | 50 | 50 | No | 9.1% |
| Birmaher et al.17 | 2003 | 1997 | Federal | 37 1137 |
12 | 46 | 7–17 | Fluoxetine | PARS | 20 | 20 | No | 8% |
| RUPP14 | 2001 | 1997 | Federal | 63 1165 |
8 | 51 | 6–17 | Fluvoxamine | PARS | 4.0±2.2b | 300 | Yes | 6% |
| March et al.13 | 2007 | 2003 | Industry | 137 11148 |
16a | 44 | 8–17 | Venlafaxine ER | SASCA | 142 | 225 | N/A | 8.0% |
| Rynn et al.11 | 2007 | 2000 | Industry | 157 11163 |
8 | 58 | 6–17 | Venlafaxine ER | PARS | NR | 225 | N/A | 1.6% |
| Walkup et al.15 | 2008 | 2003 | Federal | 133 1176 |
12 | 53 | 7–17 | Sertraline | PARS | 133 | 200 | Yes | 1.4% |
| Wagner et al.18 | 2004 | 1999 | Industry | 163 11156 |
16a | 50 | 8–17 | Paroxetine | PARS | 32.6 | 50 | Yes | 9.4% |
| Strawn et al.16 | 2015 | 2010 | Industry | 135 11137 |
10 | 47 | 7–17 | Duloxetine | PARS | 53.6 | 120 | N/A | <1% |
| Geller et al.71 | 2007 | 2003 | Industry | 87 1189 |
12 | 65 | 7–17 | Atomoxetine | PARS | 1.3b | 120 | N/A | 1.7% |
Note: DBPCT = double blind, placebo-controlled trial; HAM-A = Hamilton Anxiety Rating Scale; PARS = Pediatric Anxiety Rating Scale; SAS-CA = Social Anxiety Scale for Children and Adolescents; pbo = placebo; NR = not reported
This was a 16-week trial; however, 12-week data were used for the analyses described herein.
mg/kg/day, rather than mg/day.
Risk of bias was low across studies and the most common domain on which a possible source of bias emerged was the blinding of assessment (n=8, 88%) followed by sequence generation (n=5, 56%), although in the majority of those studies classified as “uncertain risk” the method of sequence generation was not reported. Differences in medication-placebo attrition rates were <10% in all studies (Table 1) and, as such, assessment of dropout bias based on imputation strategy was not conducted (see Table S2, available online), although the majority of studies employed last observation carried forward (LOCF) imputation of missing data. Overall, no trials were rated as having a high risk of bias. Finally, based on a priori defined outcome measures and analytic approaches (when available from the trial protocols, clinical study reports and trial registries such as clinical trials.gov), differences in planned and reported analytic approach were present in 3 studies (36%) and these were, as such, classified as “possible risk of bias” or “high risk of bias.”
Four different SSRIs were evaluated in these randomized controlled trials: fluoxetine (k=1, N=74), fluvoxamine (k=1, N=128), paroxetine (k=1, N=319), sertraline (k=2, N=231). Three SSNRIs were evaluated: atomoxetine (k=1, N=176), venlafaxine (k=2, N=605), duloxetine (k=1, N=272). Five of the studies (56%) were federally-funded, with the remaining 4 studies (44%) funded by industry and all studies were conducted in the outpatient setting.
Time course of antidepressant response compared to placebo
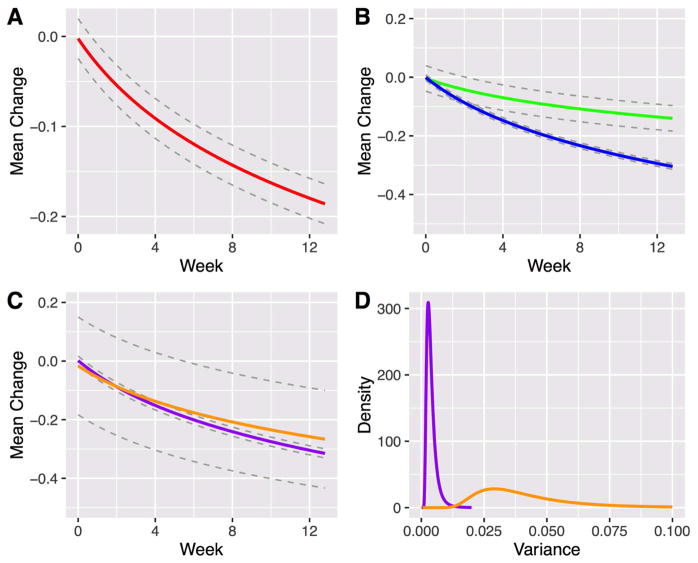
Based on AIC and BIC, the best fitting model for antidepressant treatment response was a linear-logarithmic model which suggested that antidepressant-related improvement in anxiety symptoms compared to placebo was greatest initially; this rate of improvement (vs. placebo) decreased over successive weeks. Statistically significant standardized medication-placebo differences (δ) emerged early in the course of treatment (δweek 2=−0.054, 95% confidence interval [CI]: −0.076 to −0.032, p=0.005, Figure 1A, Table 2) within 2 weeks of beginning treatment (approximate Cohen’s d ≤ 0.2), and by week 6, clinically significant differences emerged (standardized medication-placebo difference = −0.120, CI: −0.142, −0.097, p=0.001, approximate Cohen’s d = 0.44).
Figure 1.
Response trajectory in antidepressant-treated youth with generalized, separation and social anxiety disorders. Note: Standardized medication-placebo difference (“Mean Change”) was logarithmic in the best fitting model (A) and differed by antidepressant class (B) but not dose (C). Green and blue lines represent Selective Serotonin Norepinephrine Reuptake Inhibitors (SSNRIs) and Selective Serotonin Reuptake Inhibitors (SSRIs), respectively, while purple and orange lines denote high and low dose SSRI treatment, respectively. Dotted gray lines reflect the 95% confidence interval. Significant difference in variance posterior mean estimates (p<0.001) were observed between high (purple) and low (orange) dose SSRI treatment (D).
TABLE 2.
Antidepressant response in children and adolescents with generalized, separation and social anxiety disorders over time and across treatment models.
| Summary Response | SSRI Response | SSNRI Response | High Dose SSRI Response | Low Dose SSRI Response | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (week ) |
δ | 95% Credible Interval |
Significance (vs. baseline) |
δ | 95% Credible Interval |
Significance (vs. baseline) |
δ | 95% Credible Interval |
Significance (vs. baseline) |
δ | 95% Credible Interval |
Significance (vs. baseline) |
δ | 95% Credible Interval |
Significance (vs. baseline) |
| 0 | −0.002 | −0.024, −0.020 | p=0.698 | −0.001 | −0.011, 0.009 | p=0.718 | −0.004 | −0.047, 0.040 | p=0.714 | 0.02 | −0.014, 0.017 | p=0.729 | −0.016 | −0.183, 0.150 | p=0.711 |
| 2 | −0.054 | −0.076, −0.032 | p=0.005 | −0.087 | −0.096, −0.077 | p<0.001 | −0.042 | −0.086, 0.000 | p=0.021 | −0.088 | −0.103, −0.072 | p=0.002 | −0.087 | −0.254, 0.080 | p=0.149 |
| 4 | −0.091 | −0.113, −0.069 | p=0.002 | −0.148 | −0.157, −0.138 | p<0.001 | −0.069 | −0.113, −0.026 | p=0.006 | −0.151 | −0.167, −0.136 | p<0.001 | −0.137 | −0.304, 0.029 | p=0.065 |
| 6 | −0.120 | −0.142, −0.097 | p=0.001 | −0.195 | −0.204, −0.185 | p<0.001 | −0.091 | −0.134, −0.047 | p=0.003 | −0.201 | −0.216, −0.185 | p<0.001 | −0.176 | −0.343, −0.009 | p=0.051 |
| 8 | −0.143 | −0.165, −0.121 | p=0.001 | −0.234 | −0.243, −0.224 | p<0.001 | −0.108 | −0.152, −0.065 | p=0.002 | −0.241 | −0.256, −0.225 | p<0.001 | −0.208 | −0.374, −0.041 | p=0.025 |
| 10 | −0.163 | −0.185, −0.141 | p<0.001 | −0.266 | −0.276, −0.256 | p<0.001 | −0.122 | −0.166, −0.079 | p=0.002 | −0.275 | −0.290, −0.259 | p<0.001 | −0.235 | −0.401, −0.068 | p=0.023 |
| 12 | −0.180 | −0.202, −0.158 | p<0.001 | −0.294 | −0.304, 0.284 | p<0.001 | −0.135 | −0.179, −0.092 | p<0.001 | −0.304 | −0.320, −0.289 | p<0.001 | −0.258 | −0.425, −0.091 | p=0.018 |
Note: Negative values reflect improvement. SSRI = selective serotonin reuptake inhibitor; SSNRI = selective serotonin norepinephrine reuptake inhibitor; δ = standardized medication-placebo difference.
Effects of Antidepressant Class
For both SSRIs and SSNRIs, statistically significant improvement occurred at week 2 for both classes (δSSRI=−0.054, CI: −0.096 to −0.077, p<0.001; δSSNRI=−0.070, CI: −0.113 to 0, p=0.021). However, class-related differences in improvement between SSRIs and SSNRIs emerged at week 2 and remained statistically significant over the subsequent 10 weeks of treatment (Figure 1B, Table 2, Table 3). At week 12, the posterior density obtained from the difference in posterior predictive simulation samples for SSRIs vs. SSNRIs indicates that treatment response was greater for SSRIs compared to SSNRIs (p=0.003), although both treatments resulted in significant improvements (δSSRI −0.294, CI: −0.304 to −0.284, p<0.001; δSSNRI=−0.136, CI: −0.179 to −0.092, p<0.001).
TABLE 3.
Differences in Antidepressant Response in Children and Adolescents with Generalized, Separation and/or Social Anxiety Disorders for Antidepressant Class and Selective Serotonin Reuptake Inhibitor (SSRI) Dose.
| Difference in Response for SSRIs and SSNRI Treatment | Difference in Response High and Low Dose SSRI Treatment | |||||
|---|---|---|---|---|---|---|
| Time (week) | δ | 95% Credible Interval | Significance (vs. baseline) | δ | 95% Credible Interval | Significance (vs. baseline) |
| 0 | 0.003 | −0.031, 0.038 | p=0.681 | 0.018 | −0.017, 0.203 | p=0.648 |
| 4 | −0.078 | −0.112, −0.044 | p=0.009 | −0.014 | −0.199, 0.170 | p=0.381 |
| 8 | −0.125 | −0.160, −0.091 | p=0.004 | −0.033 | −0.218, 0.152 | p=0.249 |
| 12 | −0.159 | −0.194, −0.125 | p<0.0028 | −0.046 | −0.304, 0.139 | p=0.180 |
Note: Negative values reflect improvement. SSRI = selective serotonin reuptake inhibitor; SSNRI = selective serotonin norepinephrine reuptake inhibitor; δ = standardized medication-placebo difference.
Sensitivity analysis of the trajectory and magnitude of antidepressant response revealed that removal of the atomoxetine study did not significantly change the trajectory or magnitude of modeled response (p>0.9 at all weeks, see Figure S2, available online). Additionally, given the possibility that variability in placebo response in industry-funded studies influences the relationship between funding source and the magnitude of the treatment effect, the density of placebo response was determined for SSNRI trials compared to SSRI trials and for industry-funded trials compared to Federally-funded trials. Differences in placebo response between industry- and Federally-funded studies did not significantly differ at baseline (0, 95%CI: −0.122 to 0.122), week 4 (−0.047, 95%CI: −0.226 to 0.131) or week 8 (−0.129, 95%CI: −0.276 to 0.238) (see Figure S3, available online). Similarly, differences in placebo response between SSRI and SSNRI studies did not significantly differ at baseline (0, 95%CI: −0.119 to 0.119), week 4 (−0.071, 95%CI: −0.255 to 0.111) or week 8 (−0.068, 95%CI: −0.33584 to 0.199826) (see Figure S4, available online).
Effect of Maximum SSRI Dose
Response, over time, did not differ between high dose SSRI treatment compared to low-dose treatment (δ=0.010; p=0.638, Figure 1C). However, statistically significant improvement occurred earlier (week 2) with high dose treatment (δhigh dose= −0.088, CI: −0.103 to −0.072, p=0.002), whereas treatment-related differences emerged just under the threshold for statistical significance at week 6 ((δlow dose=−0.176, CI: −0.343 to −0.009, p=0.051) and were statistically significant at week 8 ((δlow dose=−0.176, CI: −0.343 to −0.009, p=0.025). At week 12, both high-dose and low-dose were significantly improved compared to baseline (p<0.001 and p=0.018, respectively). Over the course of treatment, the variance was significantly (p<0.001) greater for low-dose SSRI studies compared to high-dose SSRI studies (Table 2; Figure 1D).
DISCUSSION
This meta-analysis of randomized, double-blind, placebo-controlled trials of SSRIs and SSNRIs in pediatric patients with anxiety disorders: (1) reveals a logarithmic response model; (2) highlights treatment-related early improvement in anxiety symptoms; (3) describes a greater trajectory and magnitude of response for SSRIs compared to SSNRIs and (4) suggests earlier improvement for trials involving high-dose SSRI compared to low-dose SSRIs.
Improvement occurred early in the course of antidepressant treatment (week 2 for both SSRIs and SSNRIs). In fact, approximately 50% of the treatment-related improvement, at week 12, occurred by the fourth week of treatment, consistent with meta-analyses of SSRIs in pediatric patients with MDD and OCD.22 SSRIs and SSNRIs differed in their response trajectories and magnitude. For SSNRIs, only 40% of the treatment response observed for SSRIs was observed at week 8, and the difference in trajectory was apparent by the second week of treatment. This is of interest in light of two recent meta-analyses of SSNRIs and SSRIs in pediatric anxiety.9,26 The most recent of these meta-analyses, by Wang and colleagues (2017) suggested a numeric, but not statistically significant advantage of SSRIs, compared to SSNRIs, for the endpoint response outcome9 and given that our prior meta-analysis revealed a relationship between serotonergic selectivity and the weighted effect size of the antidepressant (at endpoint).26 However, our prior analysis of serotonergic selectivity evaluated antidepressant class (i.e., SSNRI or SSRI) as a continuous variable (i.e., degree of serotonergic selectivity). In the present analyses, we dichotomized the mechanism of action, as is common clinically. Thus, these results are meaningful to clinicians as they choose which antidepressant class to use when treating anxious youth. Additionally, given the magnitude and trajectory of SSRI response, relative to SSNRI response observed herein, clinicians might preferentially use SSRIs as first-line psychopharmacologic interventions in pediatric patients with anxiety disorders.
The potential reasons for the difference in SSRI and SSNRI efficacy in pediatric anxiety disorders warrant further discussion. SSNRIs may be associated with class-specific tolerability concerns in youth. Consistent with this possibility, venlafaxine was associated with increased treatment-emergent suicidality in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) study42 and in a recent meta-analysis of antidepressants in pediatric patients with MDD.27 Additionally, the serotonin (5-hydroxytryptamine, 5-HT) system matures earlier than the noradrenergic system and this developmental lag in the noradrenergic system may underlie differences in the effectiveness of antidepressants that mechanistically target norepinephrine (e.g., SNRIs and tricyclic antidepressants) vs. 5-HT (i.e., SSRIs) between youth and adults.43 Finally, the pathophysiology of anxiety may involve more serotonergic dysfunction relative to noradrenergic dysfunction44 which could relate to the greater effectiveness of serotonergic agents relative to noradrenergic agents. Also, for SSNRIs, the degree of serotonergic blockade, at a given dose, may impact treatment response in pediatric patients with anxiety disorders; however, the degree to which an SSNRI blocks serotonin reuptake at a given dose in the pediatric population is unknown, and may relate to developmental differences in both target engagement and drug:metabolite ratios. While SSRIs are associated with more rapid and greater clinical improvement, side effects may impact the selection of medication class. For example, some side effects, including activation, may be more common with SSRIs compared to SSNRIs: a finding that may be of relevance in treating pediatric patients with specific co-morbidities (e.g., ADHD)45 or other factors (a family history of bipolar disorder)46 that may increase the risk of activation.
The relationship between the antidepressant class and the magnitude and trajectory of antidepressant response may be complicated by additional factors. Several specific limitations warrant further discussion. First, there is confounding between antidepressant class and funding source that may be relevant given that industry-funded trials in pediatric patients with MDD have lower effect sizes than Federally-funded studies. However, for pediatric anxiety disorders, effect sizes of Federally-funded and industry-funded studies of antidepressants do not statistically significantly differ (p=0.356).10 Second, given the possibility that variability in placebo response in industry-funded studies underlies a possible relationship between funding source and effect size,47 we examined the density of placebo response in SSNRI trials compared to SSRI trials and between industry-funded trials and Federally-funded trials and found no differences between the posterior densities of placebo response between SSRIs and SSNRIs or between Federally- and industry-funded trials (see Figure S2 and Figure S3, available online). For all comparisons (e.g., sponsorship or class), the 95% credible interval of placebo response includes 0 (at week 0, week 4 and week 8), indicating no statistically significant differences. Third, while the confounding of antidepressant class and funding represents a structural limitation of the data that evaluated in terms of the potential decreased effect size in industry-funded trials, this may represent a problematic assumption. Recently, Cipriani and colleagues,27 in a network meta-analysis of antidepressants in pediatric patients with MDD, raised the possibility that “trials without industry sponsors tend to have a smaller sample size, which might result in an exaggerated treatment effect.
Despite the long-held belief—ostensibly based on clinical experience—that antidepressants should be titrated, particularly in patients who have had partial responses at lower doses,48,49 we did not detect statistically significant SSRI dose-related effects. While in adults with OCD20,21 and MDD,19 higher SSRI dose is associated with greater—and in some cases—more rapid therapeutic response, the numerically larger treatment response of high dose SSRI treatment compared to low-dose SSRI treatment observed herein only trended towards statistical significance. Though prior meta-analyses of SSRI dose in youth with MDD23 and OCD22 failed to observe a dose-response relationship, the evaluation of dose-response in the pediatric population may be problematic. First, studies of titration strategy (e.g., slow vs. fast titration to target dose) for antidepressants as well as evaluations of antidepressant dose or plasma levels are remarkably rare in children and adolescents,50,51 yet serum drug levels may exhibit greater variability in pediatric populations.52,53 Second, evaluation of dose-response is difficult in individual trials and may be especially difficult in meta-analyses secondary to both the small number of trials and cross-trial variability that obscure the impact of dosing. In this regard, when a patient has “responded,” clinicians may not further titrate antidepressants, thus influencing the distribution of doses in the trial, resulting in “early” vs. “late” responders being dosed differently. Or, clinicians may not increase the antidepressant dose or may slow titration as a result of treatment-emergent side effects. These pediatric-specific adverse effects, including activation,26,54 are associated with higher serum concentrations of SSRIs55 and may result in more conservative dosing in clinical trials of pediatric patients. Further, despite the decades-long clinical practice of antidepressant titration in adults with MDD, only within the last year has a meta-analysis provided evidence of a dose-response relationship between SSRI response and dose.19 Potentially unknown or uncharacterized patient- and development-related factors likely influence the relationship between SSRI dose and treatment response in pediatric patients (e.g., cytochrome P450 enzyme activity shifts during development,56 variability in clearance (and half-life) of SSRIs).52,57,58 Finally, these dose-related findings should be considered in the context of the small number of included trials, although they highlight the need for more fixed-dose trials and greater access to patient level data.
The Bayesian updating approach37,59 described herein lends several important strengths to this meta-analysis. Findings from prior clinical trials, that represent probabilistic background knowledge, are leveraged.60 Bayesian updating utilizes the posterior distribution from each study as an informative conjugate prior to updating the posterior for subsequent studies—a process of “belief formation and change”60 that calibrates the meta-analytic results. Second, assumptions related to trial exchangeability, or in other words, assumptions related to individual trial homogeneity or heterogeneity, represent significant limitations in traditional meta-analyses. Importantly, trial-specific methodology (e.g., fixed-dose, forced-titration, randomization ratio), sample characteristics (e.g., age distribution, co-morbidity patterns) and reliability vary across trials47,61 and influence the results of individual studies. Thus, at one extreme, studies may be seen as “identical replications of each other”37 while, at the other extreme, studies may be seen as “so different that the results of any one study provide no information about the results of any of the others.”62 In reality, and particularly in the studies included in our report, most studies are comparable; however, there are studies with high placebo response and studies with high medication response. Bayesian updating preserves the relationship between treatment-specific (as opposed to pooled) variance and treatment effect for each comparison. As such, inter-trial differences are incorporated into the response model, thus attenuating the influence of assumptions related to exchangeability.
While this is the first meta-analytic evaluation of antidepressant class and dosing in children and adolescents with anxiety disorders, and one of three examinations of the time course of antidepressant response in pediatric patients, there are several important limitations. First, despite the general similarity of studies and our use of Bayesian updating to address the influence of exchangeability assumptions, unobserved factors may still affect the response and response trajectory described in this report. These factors have been increasingly recognized as determinants of placebo response and are often clinician-specific (e.g., experience with the disorder under study, expertise in the clinical trial population)63 or patient specific (e.g., treatment expectation)63 and are difficult, if not impossible to measure in meta-analyses. Second, some studies focused on specific disorders within the pediatric anxiety disorders triad (e.g., social anxiety disorder, GAD, separation anxiety disorder); however, there is a strong precedent for studying these disorders en block14,49,64,65 given their common comorbidity66 similar neurobiology67,68 and shared response to both psychopharmacologic and psychotherapeutic treatment.15 Third, unlike in pediatric studies of OCD22 and MDD,69 differences in the continuous outcome measures are common in studies of anxiety disorders and some measures may over-represent somatic symptoms (e.g., HAM-A) or differentially reflect impairment (e.g., PARS) or may reflect a narrower assessment of symptoms (e.g., SPAI); however, treatment-related improvement has been observed with all of the scales utilized in the component studies of this meta-analysis, and we selected the scale preference a priori to minimize outcome measure heterogeneity. Fourth, the studies differed in the severity of baseline anxiety symptoms, raising the possibility of a floor effect in studies that included children with less severity. Fifth, trials of low-dose SSRI had larger variance (Figure 1D) that increased the credible interval for the estimated magnitude of the treatment effect, resulting in a smaller difference between high and low-dose SSRI treatment. Sixth, imputation of missing outcome data (frequently by LOCF) could: (1) increase measured symptomatic improvement,33,70 for patients receiving placebo, given the waxing and waning nature of some anxiety disorders6 or (2) decrease symptomatic improvement observed in the treatment group if they do not improve. In this regard, diminished improvement on the continuous measure of anxiety in patients randomized to an antidepressant that is ultimately ineffective would be carried forward in the LOCF analysis thus reducing the apparent observed efficacy of that antidepressant. This reduction in apparent efficacy could further be perpetuated by the latency of antidepressant response in pediatric anxiety disorders. Finally, the risk of bias may vary among studies and influence these findings. Recent meta-analyses have observed moderate to high bias in some treatment studies9; however, this risk was ostensibly higher in studies wherein psychosocial interventions were provided.
In summary, our results suggest that antidepressant response in pediatric patients occurs early in the course of treatment and occurs with a greater magnitude and more rapid trajectory with SSRIs compared to SSNRIs. These data raise the possibility that SSRIs should be first-line antidepressants in youth with anxiety disorders and extend prior observations in pediatric patients with anxiety that more serotonergically selective agents may be more effective. Additionally, developments in Bayesian inference appear to allow a more precise and informative meta-analysis of available clinical trial data than was previously possible.
Supplementary Material
Acknowledgments
This work has been supported by the National Institute of Mental Health (MH106037) to J.R.S.
Drs. Mills and Welge served as the statistical experts for this research.
The authors thank the late Douglas Mossman, MD, of the University of Cincinnati, for his helpful comments and guidance.
Glossary
- RH
Antidepressant Response in Anxious Youth
Footnotes
Disclosure: Dr. Strawn has received research support from the National Institutes of Health (NIEHS) as well as Edgemont, Eli Lilly and Co., Forest, Shire, Lundbeck, and Neuronetics. He has received material support from Genesight/Assurex Health; has received royalties from the publication of two texts (Springer); and has served as an author for UpToDate and an Associate Editor for Current Psychiatry. Dr. Welge has received support from the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Mills and Mr. Sauley report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Jeffrey R. Strawn, University of Cincinnati College of Medicine., Cincinnati Children's Hospital Medical Center.
Dr. Jeffrey A. Mills, Carl H. Lindner College of Business, University of Cincinnati.
Mr. Beau A. Sauley, Carl H. Lindner College of Business, University of Cincinnati.
Dr. Jeffrey A. Welge, University of Cincinnati College of Medicine.
References
- 1.Ramsawh HJ, Chavira DA. Association of Childhood Anxiety Disorders and Quality of Life in a Primary Care Sample. J Dev Behav Pediatr. 2016;37:269–276. doi: 10.1097/DBP.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 2.Asselmann E, Wittchen H-U, Lieb R, Beesdo-Baum K. Sociodemographic, clinical, and functional long-term outcomes in adolescents and young adults with mental disorders. Acta Psychiatr Scand. 2018;137(1):6–17. doi: 10.1111/acps.12792. [DOI] [PubMed] [Google Scholar]
- 3.Beesdo K, Bittner A, Pine DS, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry. 2007;64(8):903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- 4.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 5.Meier SM, Petersen L, Mattheisen M, Mors O, Mortensen PB, Laursen TM. Secondary depression in severe anxiety disorders: A population-based cohort study in Denmark. The Lancet Psychiatry. 2015;2(6):515–523. doi: 10.1016/S2215-0366(15)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beesdo-Baum K, Knappe S. Developmental Epidemiology of Anxiety Disorders. Child Adolesc Psychiatr Clin N Am. 2012;21:457–478. doi: 10.1016/j.chc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Husky MM, Olfson M, He J, Nock MK, Swanson SA, Merikangas KR. Twelvemonth suicidal symptoms and use of services among adolescents: results from the National Comorbidity Survey. Psychiatr Serv. 2012;63(10):989–996. doi: 10.1176/appi.ps.201200058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locher C, Koechlin H, Zion SR, et al. Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo for Common Psychiatric Disorders Among Children and Adolescents: A Systematic Review and Meta-analysis. JAMA psychiatry. 2017;74(10):1011–1020. doi: 10.1001/jamapsychiatry.2017.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, SHW, Sim L, et al. Comparative effectiveness and safety of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders: A systematic review and meta-analysis. JAMA Pediatr. 2017 doi: 10.1001/jamapediatrics.2017.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149–157. doi: 10.1002/da.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rynn MA, Riddle MA, Yeung PP, Kunz NR. Efficacy and Safety of Extended-Release Venlafaxine in the Treatment of Generalized Anxiety Disorder in Children and Adolescents: Two Placebo-Controlled Trials. The American journal of psychiatry. 2007;164:290–300. doi: 10.1176/ajp.2007.164.2.290. [DOI] [PubMed] [Google Scholar]
- 12.Rynn MA, Siqueland L, Rickels K. Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. Am J Psychiatry. 2001;158(12):2008–2014. doi: 10.1176/appi.ajp.158.12.2008. [DOI] [PubMed] [Google Scholar]
- 13.March JS, Entusah AR, Rynn M, Albano AM, Tourian KA. A Randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry. 2007;62(10):1149–1154. doi: 10.1016/j.biopsych.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Pine DS, Walkup JT, Labellarte MJ, et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344:1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- 15.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283–293. doi: 10.1016/j.jaac.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Birmaher B, Axelson Da, Monk K, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- 18.Wagner KD, Berard R, Stein MB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry. 2004;61(11):1153–1162. doi: 10.1001/archpsyc.61.11.1153. [DOI] [PubMed] [Google Scholar]
- 19.Jakubovski E, Varigonda AL, Freemantle N, Taylor MJ, Bloch MH. Systematic review and meta-analysis: Dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174–183. doi: 10.1176/appi.ajp.2015.15030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Issaria Y, Jakubovski E, Bartley CA, Pittenger C, Bloch MH. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016;77(5):e605–e611. doi: 10.4088/JCP.14r09758. [DOI] [PubMed] [Google Scholar]
- 21.Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850–855. doi: 10.1038/mp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: Early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851–859. e2. doi: 10.1016/j.jaac.2016.07.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varigonda AL, Jakubovski E, Taylor MJ, Freemantle N, Coughlin C, Bloch MH. Systematic Review and Meta-Analysis: Early Treatment Responses of Selective Serotonin Reuptake Inhibitors in Pediatric Major Depressive Disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557–564. doi: 10.1016/j.jaac.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Mohatt J, Bennett SM, Walkup JT. Treatment of Separation, Generalized, and Social Anxiety Disorders in Youths. Am J Psychiatry. 2014;171(7):741–8. doi: 10.1176/appi.ajp.2014.13101337. [DOI] [PubMed] [Google Scholar]
- 25.Connolly SD, Bernstein GA. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267–283. doi: 10.1097/01.chi.0000246070.23695.06. [DOI] [PubMed] [Google Scholar]
- 26.Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and Tolerability of Antidepressants in Pediatric Anxiety Disorders: a Systematic Review and Meta-Analysis. Depress Anxiety. 2015;32(3):149–157. doi: 10.1002/da.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016:1263–1271. doi: 10.1016/S0140-6736(16)30385-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Cipriani A, Zhang Y, et al. Comparative efficacy and acceptability of antidepressants, psychological interventions, and their combination for depressive disorder in children and adolescents: protocol for a network meta-analysis. BMJ Open. 2017;7(8):e016608. doi: 10.1136/bmjopen-2017-016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuijpers P, Sijbrandij M, Koole SL, Andersson G, Beekman AT, Reynolds CF. The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: A meta-analysis of direct comparisons. World Psychiatry. 2013;12(2):137–148. doi: 10.1002/wps.20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.RUPP The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2002;41(9):1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 32.Spence SH. A measure of anxiety symptoms among children. Behav Res Ther. 1998;36(5):545–566. doi: 10.1016/s0005-7967(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa TA, Salanti G, Atkinson LZ, et al. Comparative efficacy and acceptability of first-generation and second-generation antidepressants in the acute treatment of major depression: protocol for a network meta-analysis. BMJ Open. 2016;6(7):e010919. doi: 10.1136/bmjopen-2015-010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins P, Green TJ, Alderson S, Clarke P, Mulrow M, Oxman DC, DA Cochrane Handbook: Cochrane Reviews: Ch 8: Assessing risk of bias in included studies. Cochrane Handbook for: Systematic Reviews of Interventions. 2011;6:3–10. [Google Scholar]
- 35.Mills JA, Prasad K. A comparison of model selection criteria. Econom Rev. 1992;11(2):201–233. [Google Scholar]
- 36.Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in Children and Adolescents with Anxiety: A Review and Bayesian Analysis of Abandoned Randomized Controlled Trials. J Child Adolesc Psychopharmacol. 2018;28(1):2–9. doi: 10.1089/cap.2017.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis. 2004 [Google Scholar]
- 38.Ding YS, Naganawa M, Gallezot JD, et al. Clinical doses of atomoxetine significantly occupy both norepinephrine and serotonin transports: Implications on treatment of depression and ADHD. Neuroimage. 2014;86:164–171. doi: 10.1016/j.neuroimage.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani M, Dooley DJ, Weyerbrock A, Jackisch R, Feuerstein TJ. Differential inhibitory effects of drugs acting at the noradrenaline and 5-hydroxytryptamine transporters in rat and human neocortical synaptosomes. Br J Pharmacol. 2009;158(7):1848–1856. doi: 10.1111/j.1476-5381.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bezanson J, Edelman A, Karpinski S, Shah VB. Julia: A fresh approach to numerical computing. SIAM Rev. 2017;59(1):65–98. [Google Scholar]
- 41.Moher D, Liberati ATJ, AD The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 42.Brent DA, Emslie GJ, Clarke GN, et al. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am J Psychiatry. 2009;166(4):418–426. doi: 10.1176/appi.ajp.2008.08070976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: Implications for differential drug effects on juveniles and adults. Biochem Pharmacol. 2007;73(8):1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(SUPPL 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 45.Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: Is there an association with mania? Pediatr Drugs. 2011;13:225–243. doi: 10.2165/11591660-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strawn JR, Adler CM, McNamara RK, et al. Antidepressant tolerability in anxious and depressed youth at high risk for bipolar disorder: A prospective naturalistic treatment study. Bipolar Disord. 2013;16(5):523–530. doi: 10.1111/bdi.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walkup JT. Antidepressant Efficacy for Depression in Children and Adolescents: Industry- and NIMH-Funded Studies. Am J Psychiatry. 2017:1–8. doi: 10.1176/appi.ajp.2017.16091059. [DOI] [PubMed] [Google Scholar]
- 48.Strawn JR, Sakolsky DJ, Rynn Ma. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527–539. doi: 10.1016/j.chc.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Wehry AM, Beesdo-Baum K, Hennelly MM, Connolly SD, Strawn JR. Assessment and Treatment of Anxiety Disorders in Children and Adolescents. Curr Psychiatry Rep. 2015;17(52):99–110. doi: 10.1007/s11920-015-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid AM, McNamara JPH, Murphy TK, et al. Side-effects of SSRIs disrupt multimodal treatment for pediatric OCD in a randomized-controlled trial. J Psychiatr Res. 2015;71:140–147. doi: 10.1016/j.jpsychires.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakolsky DJ, JS, JMP, Emslie GJ, et al. Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. J Clin Psychopharmacol. 2011;31(1):92–97. doi: 10.1097/JCP.0b013e318204b117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Findling RL, Nucci G, Piergies AA, et al. Multiple dose pharmacokinetics of paroxetine in children and adolescents with major depressive disorder or obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(6):1274–1285. doi: 10.1038/sj.npp.1300960. [DOI] [PubMed] [Google Scholar]
- 53.Findling RL, McNamara NK, Stansbrey RJ, et al. The relevance of pharmacokinetic studies in designing efficacy trials in juvenile major depression. J Child Adolesc Psychopharmacol. 2006;16(1–2):131–145. doi: 10.1089/cap.2006.16.131. [DOI] [PubMed] [Google Scholar]
- 54.Rynn MA, Walkup JT, Compton SN, et al. Child/Adolescent Anxiety Multimodal Study: Evaluating Safety. J Am Acad Child Adolesc Psychiatry. 2015;54(3):180–190. doi: 10.1016/j.jaac.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reinblatt SP, DosReis S, Walkup JT, Riddle MA. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119–126. doi: 10.1089/cap.2008.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 57.Findling RL, Robb AS, DelBello M, et al. Pharmacokinetics and Safety of Vortioxetine in Pediatric Patients. J Child Adolesc Psychopharmacol. 2017;27(6):526–534. doi: 10.1089/cap.2016.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emslie GJ, Prakash A, Zhang Q, Pangallo Ba, Bangs ME, March JS. A double-blind efficacy and safety study of duloxetine fixed doses in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol. 2014;24:170–179. doi: 10.1089/cap.2013.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kruschke JK. Posterior predictive checks can and should be Bayesian: Comment on Gelman and Shalizi, “Philosophy and the practice of Bayesian statistics”. Br J Math Stat Psychol. 2013;66(1):45–56. doi: 10.1111/j.2044-8317.2012.02063.x. [DOI] [PubMed] [Google Scholar]
- 60.Williamson J. Bayesian Nets and Causality3: Philosophical and Computational Foundations. Oxford University Press; 2005. [Google Scholar]
- 61.Dobson ET, Strawn JR. Placebo response in pediatric anxiety disorders: Implications for clinical trial Design and interpretation. J Child Adolesc Psychopharmocology. 2016;26(8):686–693. doi: 10.1089/cap.2015.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gelman A, Hwang J, Vehtari A. Understanding predictive information criteria for Bayesian models. Stat Comput. 2014;24:997–1016. [Google Scholar]
- 63.Strawn JR, Dobson ET, Mills JA, et al. Placebo response in pediatric anxiety disorders: results from the child/adolescent anxiety multimodal study. J Child Adolesc Psychopharmacol. 2017;27(6):501–508. doi: 10.1089/cap.2016.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Compton SN, Walkup JT, Albano AM, et al. Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child Adolesc Psychiatry Ment Heal. 2010;4:1. doi: 10.1186/1753-2000-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strawn JR, Compton SN, Robertson B, Albano AM, Hamdani M, Rynn MA. Extended release guanfacine in pediatric anxiety disorders: a pilot, randomized, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2017;27(1):29–37. doi: 10.1089/cap.2016.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kendall PC, Compton SN, Walkup JT, et al. Clinical characteristics of anxiety disordered youth. J Anxiety Disord. 2010;24(3):360–365. doi: 10.1016/j.janxdis.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blackford JU, Pine DS. Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child Adolesc Psychiatr Clin N Am. 2012;21(3):501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strawn JR, Dominick KC, Patino LR, Doyle CD, Picard LS, Phan KL. Neurobiology of Pediatric Anxiety Disorders. Curr Behav Neurosci Reports. 2014;1(3):154–160. doi: 10.1007/s40473-014-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Varigonda AL, Jakubovski E, Taylor MJ, Freemantle N, Coughlin C, Bloch MH. Systematic Review and Meta-Analysis: Early Treatment Responses of Selective Serotonin Reuptake Inhibitors in Pediatric Major Depressive Disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557–564. doi: 10.1016/j.jaac.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Little RJ, D’Agostino R, Cohen ML, et al. The Prevention and Treatment of Missing Data in Clinical Trials. N Engl J Med. 2012;367(14):1355–1360. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(9):1119–1127. doi: 10.1097/chi.0b013e3180ca8385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.