Abstract
Bacteria harbored in/on the hyphae of the dark septate endophyte, Veronaeopsis simplex Y34, were identified as a single Rhizobium species by molecular analyses of bacterial 16S rRNA genes, and were successfully isolated from the endophyte. The Rhizobium-cured fungus was prepared thoroughly by an antibiotic treatment, thereby allowing an examination of their effects on organic substrate utilization. Assays with Biolog® FF microplates revealed that the respiration potential for 52.6% of the tested compounds were significantly different between Rhizobium-harboring and -cured fungal hosts, indicating that organic substrate utilization by V. simplex Y34 was significantly influenced by the presence of the associated Rhizobium sp. VsBac-Y9.
Keywords: dark septate endophyte, Veronaeopsis simplex, endophyte-associated rhizobia, organic substrate utilization, tripartite symbiosis
Plant-microbe interactions drive plant health and the biogeochemical cycle in terrestrial ecosystems (1, 6, 7). Plants provide important habitats and deliver photosynthates to their associated microorganisms (15). In return, mutualistic microbes promote host growth via improved mineral and nutrient uptake, enhanced tolerance against environmental stress, and/or deterring encroachment by phytopathogens (19, 26, 28, 33). These symbiotic interactions have been adequately evaluated in the bipartite relationships between plants and mycorrhizal fungi or nodule bacteria (14, 32); however, increasing evidence has revealed more complex associations among plants, endophytic fungi, and rhizobacteria since the discovery of hyphae-epigenous (epihyphal) and endohyphal bacteria in various plant-associated fungi (13, 34). Phylogenetically diverse bacteria have been identified inside and outside (i.e., in/on) the hyphae of foliar Ascomycota, arbuscular mycorrhizal fungi (AMF), ectomycorrhizal basidiomycetes, and Sebacinalean endophytes. These bacteria have been shown to alter host morphology, sporulation, metabolite production, and other properties involved in symbiotic relationships with plants (2, 8, 27, 31). However, this tripartite symbiosis has rarely been reported for other widespread dark septate endophytes (DSE), which are defined as conidial or sterile ascomycetous fungi that live in symbiosis with certain plants and colonize plant root tissue intracellularly and intercellularly without any disease or typical mycorrhizal structures (17, 24). Due to the crucial importance of nutrient interchange between plants and endophytic fungi for maintaining their symbiotic relationship (15, 20), the mechanisms by which DSE-associated bacteria affect the metabolism of organic substrates by the host fungus need to be elucidated in order to assess the physiological and ecological roles of bacteria in tripartite symbioses. In our recent study, the DSE species, Veronaeopsis simplex, was found to be associated with bacterium-like cells (BLCs) on its hyphal surface in examinations of fluorescence microscopy images using a Live/Dead BacLight kit (Khastini, unpublished data). The aims of the present study were to (i) identify V. simplex-associated bacteria using a molecular analysis of 16S rRNA genes, (ii) isolate bacteria harbored in fungal hyphae, (iii) remove bacteria from living fungi by an antibiotic treatment, and (iv) further examine the effects of DSE-associated bacteria on organic substrate utilization by the host through comparisons of respiratory activity and mycelial growth between bacteria-harboring and -cured V. simplex isolates on 95 carbon sources using the Biolog FF MicroPlate (BiOLOG, Hayward, CA, USA).
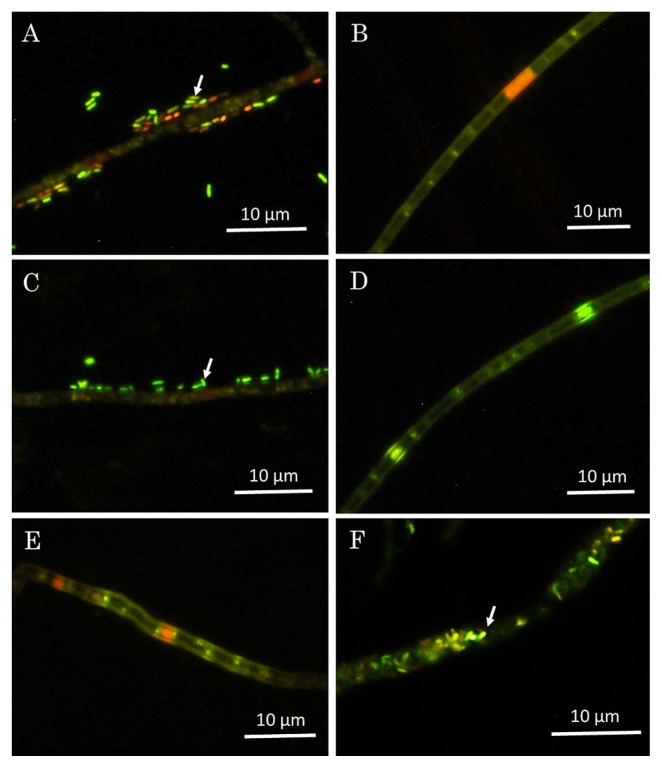
V. simplex was initially isolated from the litter below Acacia karroo in South Africa (isolate CBS 588.66) (3). In our previous study, two V. simplex isolates Y34 and IBAK45 (unpublished) were obtained in 2007 from the forest soil of Yaku Island and Ami, Japan, respectively, using the eggplant baiting method (18). These three isolates were used to identify DSE-associated bacteria. We removed loosely-associated bacteria (potentially contaminating bacteria) from the fungus using the modified van Tieghem method (29). The presence/absence of bacteria inside and outside of the hyphae of the original and van Tieghem-treated fungus was assessed microscopically by staining with a LIVE/DEAD BacLight™ Bacterial Viability Kit (Thermo Fisher Scientific, Waltham, MA, USA) and by fluorescence in situ hybridization (FISH) using the universal bacterial probe EUB338, as previously described (25). The endobacterium-harboring fungus, Mortierella elongata FMR23-6 I-B1, was simultaneously observed as a positive control for confirming the presence of endohyphal bacteria (25, 29). Fluorescent BLCs were only detected on the hyphal surface of the original V. simplex Y34 and IBAK45, and not on that of CBS 588.66 or any van Tieghem-treated isolates by the LIVE/DEAD BacLight Bacterial Viability Kit (Fig. 1) and 16S rRNA gene targeting FISH (Fig. S1).
Fig. 1.
Fluorescence microscopic observations of fungal mycelia using a Live/Dead Baclight kit. A and B, original isolate and van Tieghem-treated isolate of Veronaeopsis simplex Y34, respectively; C and D, original isolate and van Tieghem-treated isolate of V. simplex IBAK45, respectively; E, original isolate of V. simplex strain CBS 588.66; F, original isolate of Mortierella elongata FMR23-6 I-B1. Arrows in A, C, and F indicate bacterium-like cells (BLCs) epihyphally or endohyphally associated with hyphae.
In order to confirm BLC-free fungi, PCR amplification of the bacterial 16S rRNA gene was conducted using 0.1 μg of template DNA extracted from the hyphae of van Tieghem-treated isolates. Although BLC was not detected in microscopic observations, the targeted DNA band was obtained by PCR amplification for all van Tieghem treated-fungal isolates, suggesting that a very low population of bacteria harbored van Tieghem-treated fungi. In order to establish the number of bacterial species associated with the fungus, a terminal restriction fragment length polymorphism (T-RFLP) (29) analysis was performed using DNA extracted from the whole cell lysates of van Tieghem-treated fungi (Supplementary Methodology). T-RFLP profiles were composed of one major T-RF: a 189-base T-RF from the HaeIII digest, a 338-base T-RF from the HhaI digest, and a 126-base T-RF from the MspI digest (Fig. S2). The relative abundance of these major T-RFs ranged between 79.7% and 98.1% in each profile (Fig. S2). These results suggested that a single bacterial species was exclusively associated with the hyphae of V. simplex isolates.
In order to identify the fungus-associated bacterium exhibiting the major T-RF, clone libraries were constructed from the same DNA preparations mentioned above, as described in Supplementary Methodology. Since the detection of a single population was expected from T-RFLP profiling, only a few clone sequences were analyzed: 4 from V. simplex CBS 588.66, 7 from Y34, and 3 from IBAK45. Eleven of the 14 clone sequences showed 99.7–100% similarities to each other for the average length of 690 bp. BLAST searches with the NCBI database indicated that all bacterial sequences originating from the Y34 and IBAK45 isolates were closely related to Rhizobium sp. enrichment culture clone N312 with high similarity (99%). To the best of our knowledge, some intracellular bacteria in the genus Rhizobium were discovered in the hyphae of the endophytic Sebacinalean fungus Piriformospora indica (31) and diverse Ascomycota isolated as foliar endophytes of cupressaceous trees (4).
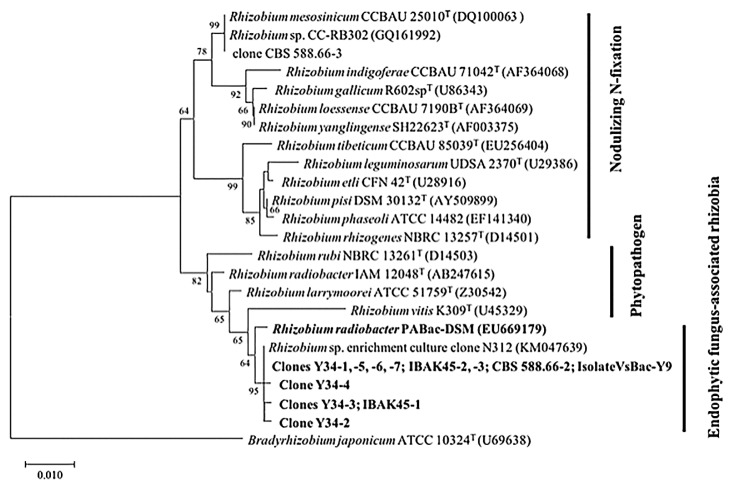
In an attempt to isolate the V. simplex-associated bacterium, aliquots of the filtered fungal homogenates of isolates Y34, IBAK45, and CBS 588.66 were separately spread on Difco™ nutrient broth (NB) agar plates and incubated at 30°C for 7 d. Bacterial colonies only appeared on the NB agar plate inoculated with the V. simplex Y34 homogenate. Since these colonies from the homogenate were very homogeneous, a single colony from the plate was subcultured repeatedly on an NB plate in order to assess its purity. The pure culture obtained, designated VsBac-Y9, was a Gram-negative rod-shaped bacterium. In order to establish whether this strain is a V. simplex-associated bacterium, a T-RFLP analysis of the 16S rRNA gene of strain VsBac-Y9 was performed, and showed combinations of a single T-RF (189-, 337-, and 126-base T-RFs from HaeIII, HhaI, and MspI digestion). In the phylogenetic tree (Fig. 2), strain VsBac-Y9 was affiliated with the cluster composed of the above-described clone sequences and Rhizobium sp, enrichment clone N312. A BLAST search of its almost-complete 16S rRNA gene sequence (1387 bp) with the NCBI database showed that isolate VsBac-Y9 was extremely close to the Rhizobium isolates P7 and YIC4260, which were cultured from the rhizospheres of gramineous Lolium multiflorum (37) and leguminous Sesbania cannabina (21), respectively.
Fig. 2.
Neighbor-joining phylogenetic tree based on partial 16S rRNA genes (829 positions), showing the relationship between Veronaeopsis simplex-associated bacteria and the endohyphal Rhizobium radiobacter PABac-DSM of Piriformospora indica, and other species of the genus Rhizobium. Numbers at nodes are posterior probability values (%); values lower than 60% are not shown. Horizontal lines show genetic distances, which are supported by values estimated with 1,000 bootstrap replicates. The scale bar indicates the number of substitutions per nucleotide position. Bradyrhizobium japonicum ATCC 10324T is used as the outgroup.
As shown in Fig. 2, the cluster composed of V. simplex-associated bacteria was close to R. radiobacter PABac-DSM identified as the endohyphal bacterium of P. indica (31). The most recent study of this endohyphal bacterium showed low numbers of R. radiobacter F4 (a subculture from R. radiobacter PABac-DSM) in axenically grown P. indica (long-term labcultured for 20 years), but high numbers in hyphae colonizing living plants or freshly re-isolated P. indica from plant roots (12), providing a potential explanation for why the population of associated bacteria was extremely small inside or outside the hyphae of V. simplex isolates (lab-cultured for 10 years). Furthermore, a phylogenetic analysis showed that these endophyte-associated bacteria were within the Agrobacterium/Rhizobium clade containing many phytopathogens and distant from the N-fixing symbiotic bacteria of leguminous plants, suggesting the necessity for further morphological and genomic characterizations to assign new positions for Rhizobium bacteria associated with endophytic fungi.
In order to assess the effects of V. simplex-associated rhizobia on organic substrate utilization by their host fungi, we eliminated bacteria present in the hyphae of van Tieghem-treated isolates using an antibiotic treatment (Supplementary Methodology). Antibiotic-treated hyphae were then grown on 1/2 CMMY agar to examine whether bacteria were present using PCR amplification of the partial 16S rRNA gene. The results obtained showed that only V. simplex Y34 was successfully cured of the associated Rhizobium spp. Therefore, only isolates of Rhizobium-harboring V. simplex Y34 and -cured Y34 were subsequently used to investigate the effects of rhizobia on organic substrate utilization by their fungal host V. simplex Y34 (Supplementary Methodology). The results demonstrated that the respiration potential for 50 out of 95 (52.6%) organic sources was significantly different between Rhizobium-associated and -cured isolates (Table 1, S1). Rhizobium-harboring V. simplex Y34 outperformed its cured isolate on 27 substrates including 9 saccharides, 7 saccharide derivatives, 3 amino acids and their derivatives, 3 carboxylic acids, 3 amides, 1 phosphorylated chemical, and 1 surfactant. In contrast, cured V. simplex Y34 was more active on 23 substrates than the Rhizobium-harboring isolate (i.e., 9 carboxylic acids, 8 saccharides and their derivatives, 3 amino acids, 1 ester, 1 amide, and 1 brominated chemical). On the other hand, the growth of Rhizobium-harboring V. simplex Y34 was greater than that of the cured isolate across most organic compounds (Table S1). In addition, Jaccard dissimilarity indices suggested that the global substrate utilization of V. simplex Y34 was significantly influenced by the presence of associated Rhizobium spp. (Table 2).
Table 1.
Comparison of significantly outperforming carbon sources by Rhizobium-harboring and -cured Veronaeopsis simplex Y34
| Category of organic substrate | Significantly outperformed organic substratea | |
|---|---|---|
|
| ||
| Rhizobium-harboring V. simplex Y34 | Rhizobium-cured V. simplex Y34 | |
| Monosaccharides (12)b | D-psicose; (1)c | D-arabinose, D-fructose, D-ribose, D-xylosed; (4)c |
| Disaccharides (10) | α-D-lactose, D-melibiose, D-trehalose, lactulose; (4) | — |
| Trisaccharides (3) | — | D-raffinose; (1) |
| Tetrasaccharide (1) | — | Stachyose; (1) |
| Oligosaccharide (1) | Dextrin; (1) | — |
| Cyclic oligosaccharides (2) | β-cyclodextrin; (1) | — |
| Nucleosides (2) | Adenosine; (1) | Uridine; (1) |
| Polysaccharide (1) | Glycogen; (1) | — |
| Sugar alcohols (9) | — | Glycerol; (1) |
| Methyl sugars (5) | β-methyl-D-galactoside; (1) | — |
| Alcoholic β-glucoside (1) | Salicin; (1) | — |
| Glycoside (1) | Arbutind; (1) | — |
| Misc. carbohydrate (1) | Sedoheptulosan; (1) | — |
| Amino sugars (4) | D-glucosamine, N-acetyl-D-galactosamine, N-acetyl-D-glucosamine; (3) | — |
| Amino acids (14) | L-alanine, L-asparagine; (2) | L-threonine, N-acetyl-L-glutamic acid, L-pyroglutamic acid; (3) |
| Amino acid derivative (1) | Amygdalin; (1) | — |
| Carboxylic acids (16) | α-ketoglutaric acid, L-lactic acid, γ-hydroxy-butyric acid; (3) | 2-keto-D-gluconic Acid, β-hydroxy-butyric acid, D-glucuronic acid, D-galacturonic acid, D-gluconic acid, D-saccharic acid, D-malic acid, fumaric acid, sebacic acid; (9) |
| Esters (2) | — | Succinic acid monomethyl ester; (1) |
| Amides (5) | Alaninamide, Glucuronamide, Putrescine; (3) | Succinamic acid; (1) |
| Phosphorylated chemicals (2) | Glucose-1-Phosphate; (1) | — |
| Brominated chemical (1) | — | Bromosuccinic acid; (1) |
| Surfactant (1) | Tween® 80; (1) | — |
| Total (95) | 27e | 23e |
Significant differences in the respiration (OD 490 nm) of each organic substrate performed between Rhizobium-harboring and -cured Veronaeopsis simplex Y34 were examined by a one-way ANOVA with an FDR-adjusted P-value (P<0.05) (5)
Amount of organic substrates for each category
Amount of significantly outperformed organic substrates for each category by Rhizobium-harboring or -cured V. simplex Y34
Compounds printed in Bold indicate a significant difference in respiration with FDR-adjusted P<0.001
Total amount of significantly outperformed organic substrates within a BIOLOG FF Microplate by Rhizobium-harboring and -cured V. simplex Y34, respectively
Table 2.
Global dissimilarities in the profile of carbon source utilization between Rhizobium-harboring and -cured Veronaeopsis simplex Y34
| Sample | Rhizobium-harboring V. simplex Y34 | Rhizobium-cured V. simplex Y34 | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Replicate 1 | Replicate 2 | Replicate 3 | Replicate 1 | Replicate 2 | Replicate 3 | ||
| Rhizobium-harboring V. simplex Y34 | Replicate 1 | 0.0000a | |||||
| Replicate 2 | 0.0574 | 0.0000 | |||||
| Replicate 3 | 0.1377 | 0.1092 | 0.0000 | ||||
| Rhizobium-cured V. simplex Y34 | Replicate 1 | 0.2635 | 0.2593 | 0.2903 | 0.0000 | ||
| Replicate 2 | 0.2639 | 0.2610 | 0.2917 | 0.0595 | 0.0000 | ||
| Replicate 3 | 0.2732 | 0.2595 | 0.2830 | 0.0895 | 0.0764 | 0.0000 | |
This value was calculated by the Jaccard classic dissimilarity index (10)
An increasing number of studies have demonstrated that phylogenetically diverse bacteria associated with endophytic fungi critically influence host morphology, growth, sporulation, bioenergetic capacity, and metabolic products, which further affect the symbiotic capacity of the host with plants (23, 27, 35). The mechanisms by which endophyte-associated bacteria regulate the relationship between their fungal host and the specific symbiotic plant are of interest. Duponnois and Garbaye (1990) reported that ectomycorrhizal helper bacteria released citric and malic acids, which stimulated the growth of Paxillus involutus and metabolized self-toxins (i.e., polyphenolic substances) produced by P. involutus (9). In addition, a recent study showed that the composition of the bacterial community associated with the saprotrophic fungus Mucor hiemalis shifted after an antibiotic treatment, which led to significant reductions in fungal secondary metabolites such as trifluorbenzene and butanol (30). Since these volatiles may improve the growth of certain rhizobacteria (e.g., Burkholderia sp. AD024) (30), this finding suggests that fungal-associated bacteria affect the metabolic properties of a host, and these changes, in turn, influence the associated bacteria. Our comparative catabolism analysis revealed that Rhizobium-harboring V. simplex Y34 exhibited a preference for saccharides (e.g., dextrin and glycogen) and saccharide derivatives (e.g., arbutin, D-glucosamine, and N-acetyl-D-galactosamine), whereas cured isolate Y34 preferentially used carboxylic acids (e.g., β-hydroxy-butyric acid, D-glucuronic acid, D-saccharic acid, D-malic acid, and sebacic acid). Based on these differences in organic substrate utilization, we speculated that Rhizobium spp. present in V. simplex may regulate their host fungi to effectively and selectively catabolize certain compounds released by symbiotic plants or contained in the rhizosphere, potentially by secreting growth factors for the host fungus (11, 27), detoxifying harmful waste, or metabolizing byproducts such as reactive oxygen species, which are known to accumulate from the catabolism of certain compounds (22, 36). Approximately 20–40% of the photosynthetic products fixed by a plant are released into the rhizosphere as root exudates and further utilized by root-associated bacteria and fungi (16), whereas fungal-bacterial interactions in this “hot-spot” may affect plant-microbe symbiotic relationships. Therefore, our results provide novel insights into the metabolism of organic substrates, which may ultimately contribute to our understanding of the ecological role of each partner in tripartite symbiosis. Future studies that directly assess how Rhizobium species induce changes in organic compound catabolism and affect the symbiotic performance of host fungi with certain plants are needed.
In conclusion, this study identified DSE-associated bacteria with V. simplex using a 16S rRNA gene targeting clone library analysis despite negative detection by microscopic observations. We also successfully eliminated intimately associated Rhizobium species from V. simplex isolate Y34 using an antibiotic treatment. The results obtained demonstrated the effects of DSE-associated Rhizobium spp. on organic substrate utilization by their host endophytic fungus V. simplex Y34 across 95 compounds including most sugars, amino acids, and carboxylic acids, nearly all of which are involved in plant biology (e.g., global metabolism regulators: D-trehalose and m-inositol; metabolites involved in seed germination and root induction: dextrin, D-raffinose, L-asparagine, stachyose, and sucrose; structural components: D-mannose, L-alanine, L-arabinose and starch). To the best of our knowledge, this study is the first to have described the symbiotic bacteria associated with DSE and attempted to clarify the physiological and ecological roles of these bacteria in tripartite V. simplex symbiosis with plants by examining the effects of the associated Rhizobium bacteria on organic substrate utilization by their host fungus. In order to obtain a better understanding of plant-microbe symbiosis in natural and agroecological systems, future research is needed on the endophyte-associated bacteriome, bacterial localization, the mechanisms underlying interactions, and its importance for maintaining symbiotic relationships.
Supplementary Material
Acknowledgements
This study was supported by a grant from KAKENHI (17H03948), JSPS.
References
- 1.Adesemoye A.O., Kloepper J.W. Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol. 2009;85:1–12. doi: 10.1007/s00253-009-2196-0. [DOI] [PubMed] [Google Scholar]
- 2.Arendt K.R., Hockett K.L., Araldi-Brondolo S.J., Baltrus D.A., Arnold A.E. Isolation of endohyphal bacteria from foliar Ascomycota and in vitro establishment of their symbiotic associations. Appl Environ Microbiol. 2016;82:2943–2949. doi: 10.1128/AEM.00452-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arzanlou M., Groenewald J.Z., Gams W., Braun U., Shin H.D., Crous P.W. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Stud Mycol. 2007;58:57–93. doi: 10.3114/sim.2007.58.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltrus D.A., Dougherty K., Arendt K.R., et al. Absence of genome reduction in diverse, facultative endohyphal bacteria. Microb Genom. 2017 Feb 28;3(2):e000101. doi: 10.1099/mgen.0.000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57:289–300. [Google Scholar]
- 6.Berendsen R.L., Pieterse C.M., Bakker P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Berg G., Grube M., Schloter M., Smalla K. Unraveling the plant microbiome: looking back and future perspectives. Front Microbiol. 2014;5:148. doi: 10.3389/fmicb.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertaux J., Schmid M., Hutzler P., Hartmann A., Garbaye J., Frey-Klett P. Occurrence and distribution of endobacteria in the plant-associated mycelium of the ectomycorrhizal fungus Laccaria bicolor S238N. Environ Microbiol. 2005;7:1786–1795. doi: 10.1111/j.1462-2920.2005.00867.x. [DOI] [PubMed] [Google Scholar]
- 9.Duponnois R., Garbaye J. Some mechanisms involved in growth stimulation of ectomycorrhizal fungi by bacteria. Can J Bot. 1990;68:2148–2152. [Google Scholar]
- 10.Faith D.P., Minchin P.R., Belbin L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio. 1987;69:57–68. [Google Scholar]
- 11.Ghignone S., Salvioli A., Anca I., et al. The genome of the obligate endobacterium of an AM fungus reveals an interphylum network of nutritional interactions. ISME J. 2012;6:136–145. doi: 10.1038/ismej.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo H., Glaeser S.P., Alabid I., Imani J., Haghighi H., Kämpfer P., Kogel K. The abundance of endofungal bacterium Rhizobium radiobacter (syn. Agrobacterium tumefaciens) increases in its fungal host Piriformospora indica during the tripartite sebacinalean symbiosis with higher plants. Front Microbiol. 2017;8:629. doi: 10.3389/fmicb.2017.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman M.T., Arnold A.E. Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Appl Environ Microbiol. 2010;76:4063–4075. doi: 10.1128/AEM.02928-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda S., Okubo T., Kaneko T., Inaba S., Maekawa T., Eda S., Sato S., Tabata S., Mitsui H., Minamisawa K. Community shifts of soybean stem-associated bacteria responding to different nodulation phenotypes and N levels. ISME J. 2010;4:315–326. doi: 10.1038/ismej.2009.119. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y., Wang W., Xie Q., et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 2017;356:1172–1175. doi: 10.1126/science.aam9970. [DOI] [PubMed] [Google Scholar]
- 16.Jones D.L., Nguyen C., Finlay R.D. Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil. 2009;321:5–33. [Google Scholar]
- 17.Jumpponen A., Trappe J.M. Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol. 1998;140:295–310. doi: 10.1046/j.1469-8137.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- 18.Khastini R.O., Ohta H., Narisawa K. The role of a dark septate endophytic fungus, Veronaeopsis simplex Y34, in fusarium disease suppression in Chinese cabbage. J Microbiol. 2012;50:618–624. doi: 10.1007/s12275-012-2105-6. [DOI] [PubMed] [Google Scholar]
- 19.Khastini R.O., Ogawara T., Sato Y., Narisawa K. Control of Fusarium wilt in melon by the fungal endophyte, Cadophora sp. Eur J Plant Pathol. 2014;139:333. [Google Scholar]
- 20.Lewis D. Interchange of metabolites in biotrophic symbioses between angiosperms and fungi. In: Sunderland N., Simon E.W., Heslop-Harrison J., editors. Perspectives in Experimental Biology. Pergamon; New York: 1976. pp. 207–219. [Google Scholar]
- 21.Li Y., Li X., Liu Y., et al. Genetic diversity and community structure of rhizobia nodulating Sesbania cannabina in saline-alkaline soils. Syst Appl Microbiol. 2016;39:195–202. doi: 10.1016/j.syapm.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Li Z., Yao Q., Dearth S.P., et al. Integrated proteomics and metabolomics suggests symbiotic metabolism and multimodal regulation in a fungal—endobacterial system. Environ Microbiol. 2017;19:1041–1053. doi: 10.1111/1462-2920.13605. [DOI] [PubMed] [Google Scholar]
- 23.Lumini E., Bianciotto V., Jargeat P., Novero M., Salvioli A., Faccio A., Bécard G., Bonfante P. Presymbiotic growth and sporal morphology are affected in the arbuscular mycorrhizal fungus Gigaspora margarita cured of its endobacteria. Cell Microbiol. 2007;9:1716–1729. doi: 10.1111/j.1462-5822.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 24.Newsham K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011;190:783–793. doi: 10.1111/j.1469-8137.2010.03611.x. [DOI] [PubMed] [Google Scholar]
- 25.Ohshima S., Sato Y., Fujimura R., Takashima Y., Hamada M., Nishizawa T., Narisawa K., Ohta H. Mycoavidus cysteinexigens gen. nov., sp. nov., an endohyphal bacterium isolated from a soil isolate of the fungus Mortierella elongata. Int J Syst Evol Microbiol. 2016;66:2052–2057. doi: 10.1099/ijsem.0.000990. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez H., Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999;17:319–339. doi: 10.1016/s0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 27.Salvioli A., Ghignone S., Novero M., Navazio L., Venice F., Bagnaresi P., Bonfante P. Symbiosis with an endobacterium increases the fitness of a mycorrhizal fungus, raising its bioenergetic potential. ISME J. 2016;10:130–144. doi: 10.1038/ismej.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato I., Yoshida S., Iwamoto Y., Aino M., Hyakumachi M., Shimizu M., Takahashi H., Ando S., Tsushima S. Suppressive potential of Paenibacillus strains isolated from the tomato phyllosphere against Fusarium crown and root rot of tomato. Microbes Environ. 2014;29:168–177. doi: 10.1264/jsme2.ME13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato Y., Narisawa K., Tsuruta K., Umezu M., Nishizawa T., Tanaka K., Yamaguchi K., Komatsuzaki M., Ohta H. Detection of Betaproteobacteria inside the mycelium of the fungus Mortierella elongata. Microbes Environ. 2010;25:321–324. doi: 10.1264/jsme2.me10134. [DOI] [PubMed] [Google Scholar]
- 30.Schulz-Bohm K., Tyc O., de Boer W., Peereboom N., Debets F., Zaagman N., Janssens T.K.S., Garbeva P. Fungus-associated bacteriome in charge of their host behavior. Fungal Genet Biol. 2017;102:38–48. doi: 10.1016/j.fgb.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M., Schmid M., Rothballer M., et al. Detection and identification of bacteria intimately associated with fungi of the order Sebacinales. Cell Microbiol. 2008;10:2235–2246. doi: 10.1111/j.1462-5822.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith S.E., Smith F.A., Jakobsen I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003;133:16–20. doi: 10.1104/pp.103.024380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surono, Narisawa K. The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol. 2017;28:1–10. [Google Scholar]
- 34.Toljander J.F., Artursson V., Paul L.R., Jansson J.K., Finlay R.D. Attachment of different soil bacteria to arbuscular mycorrhizal fungal extraradical hyphae is determined by hyphal vitality and fungal species. FEMS Microbiol Lett. 2006;254:34–40. doi: 10.1111/j.1574-6968.2005.00003.x. [DOI] [PubMed] [Google Scholar]
- 35.Uehling J., Gryganskyi A., Hameed K., et al. Comparative genomics of Mortierella elongata and its bacterial endosymbiont Mycoavidus cysteinexigens. Environ microbiol. 2017;19:2964–2983. doi: 10.1111/1462-2920.13669. [DOI] [PubMed] [Google Scholar]
- 36.Vannini C., Carpentieri A., Salvioli A., et al. An interdomain network: the endobacterium of a mycorrhizal fungus promotes antioxidative responses in both fungal and plant hosts. New Phytol. 2016;211:265–75. doi: 10.1111/nph.13895. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X., Jin L., Sun K., Li S., Li X., Ling W. Phenanthrene and Pyrene Modify the Composition and Structure of the Cultivable Endophytic Bacterial Community in Ryegrass (Lolium multiflorum Lam) Int J Environ Res Public Health. 2016;13:1081. doi: 10.3390/ijerph13111081. < http://dx.doi.org/10.3390/ijerph13111081>. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.