Abstract
Salmonella and Campylobacter cause foodborne enteritis mainly via the consumption of raw/undercooked contaminated poultry meat and products. Broiler flocks are primarily colonized with these bacteria; however, the underlying etiology remains unclear. The present study was conducted in order to obtain further information on the prevalence and genotypic distribution of Salmonella and Campylobacter in free-living crows and broiler flocks in a region for 2 years, thereby facilitating estimations of the potential risk of transmission of C. jejuni from crows to broiler flocks. Salmonella serovars Bredeney and Derby were isolated from 8 and 3 out of 123 captured crows, respectively, both of which are not common in broiler chickens. Campylobacter were isolated from all 89 crows tested and C. jejuni was prevalent (85 crows). Pulsed field gel electrophoresis showed broad diversity in the crow isolates of C. jejuni. However, 3 crow isolates and 2 broiler isolates showing similar banding patterns were assigned to different sequence types in multi-locus sequence typing. These results indicate that crows do not share Salmonella serovars with broilers, and harbor various genotypes of C. jejuni that differ from those of broiler flocks. Thus, our results indicate that crows are not a potential vector of these bacteria to broiler flocks in this region.
Keywords: Salmonella, Campylobacter, crows, broilers
Salmonella and Campylobacter are the most common bacterial causes of human foodborne enteritis worldwide (21). These infections are mainly associated with the consumption of raw/undercooked contaminated poultry meat and products (33, 37, 41). In order to control human infection, countermeasures need to be simultaneously implemented at all stages of poultry production from farm to table (20, 34). Due to the high prevalence of Salmonella and Campylobacter in broiler flocks, the primary target to decrease the incidence of these infections is pre-harvest control at the farm level, which includes enhanced biosecurity to avoid the transmission of bacteria from the environment to bird flocks on the farm.
Potential reservoirs of Salmonella and Campylobacter have been identified or presumed, such as personnel, water supply, feed, transport crates, and proximal farms growing other livestock or poultry (1, 19, 24, 26, 43, 45). Wild animals have also been suggested as vectors of infections in poultry (14, 45). Recent studies provided the potential attribution of wild birds to Salmonella and Campylobacter infections in humans (8, 29). Wild birds living in close proximity to broiler farms were found to carry Salmonella spp. and Campylobacter spp. (10). Among wild birds, crows are generally known to breed in urban and agricultural settings and fly around their roosts and locations such as livestock farms at which they forage. Thus, if crows carry these bacteria, they may serve as a potential reservoir or carrier of Salmonella and Campylobacter to broilers.
The present study investigated the prevalence of Salmonella and Campylobacter in crows and their genetic relatedness with isolates from broilers by comparisons of pulsed field gel electrophoresis (PFGE) patterns and multi-locus sequence typing (MLST) in order to demonstrate the potential transmission of bacteria between crows and broiler flocks.
Materials and Methods
Sample collection
This study was conducted in Aomori prefecture, the most northeastern part of the main island of Japan. This region thrives with agriculture and animal husbandry. A total of 123 crows were captured on 19 occasions between October 2012 and April 2013 and 13 occasions between October 2013 and April 2014. Crows were captured using box traps placed at two sites 2.2 km from a city in Aomori prefecture. This area is semi-rural, and more than 20 broiler farms as well as a few scattered cattle and pig farms exist within a radius of 20 km from the trap sites. After being captured, crows were transferred to the Wildlife Quarantine Facility in the Kitasato University School of Veterinary Medicine. The species of crows were visually confirmed by the size and shape of the beaks. They consisted of 78 jungle crows (Corvus macrorhynchos), 28 carrion crows (C. corone), and 17 unidentified crows. The weights of the captive crows ranged between 340 and 770 g. All 123 crows were tested for Salmonella, of which 89 were also tested for Campylobacter (Table 1). The collection of wild animals for scientific investigation in this study was approved by the Prefectural governor of Aomori prefecture (Prefecture Directive No. 13) based on the Protection and Control of Wild Birds and Mammals and Hunting Management Law (Act No. 88 of 2002). Crows were euthanized by the inhalation of isoflurane followed by adjunctive exsanguination according to the AVMA Guidelines for the Euthanasia of Animals (2). The ceca were taken aseptically and divided into 2 parts of approximately 0.4 g each; one for the isolation of Salmonella and another for Campylobacter. Sex and age (adult or nestling) were presumed at necropsy by the presence of testes/ovaries and the bursa of Fabricius, respectively. The sex of seven crows was not established because of the immaturity of their genital organs.
Table 1.
Summary of crows captured in the present study.
| Corvus macrorhynchos | Corvus corone | Unidentified | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Male | Female | N.D.* | Male | Female | N.D. | Male | Female | N.D. | |
| Juvenile | 26 (23)** | 27 (26) | 1 (1) | 1 (1) | 0 | 2 (1) | 8 (8) | 3 (3) | 4 (4) |
| Adult | 10 (7) | 14 (8) | 0 | 12 (2) | 13 (3) | 0 | 0 | 2 (2) | 0 |
|
| |||||||||
| Total | 36 (30) | 41 (34) | 1 (1) | 13 (3) | 13 (3) | 2 (1) | 8 (8) | 5 (5) | 4 (4) |
|
|
|
|
|||||||
| 78 (65) | 28 (7) | 17 (17) | |||||||
Not determined.
All 123 captive crows were tested for Salmonella, of which 89 were also tested for Campylobacter spp. in parentheses.
Isolation of Salmonella spp
Cecal samples were homogenized with a 9-fold volume of Hajna tetrathionate broth (Eiken, Tokyo, Japan) and incubated at 41.5°C for 24 h for enrichment. A loopful of each homogenate was then streaked on desoxycholate-hydrogen sulfate-lactose agar plates. After an incubation at 37°C for 24 h, black-colored colonies assumed to be Salmonella were then serotyped based on the Kauffmann-White scheme using a commercially available agglutination test (Denka Seiken, Tokyo, Japan) with O and H antigens.
Isolation of Campylobacter spp
Cecal samples were homogenized with Campylobacter enrichment medium (25) and incubated at 37°C for 48 h in a microaerobic environment for enrichment. A loopful of each homogenate was streaked on modified charcoal cefoperazone deoxycholate agar plates (Oxoid, Basingstoke, UK). After an incubation at 37°C for 48 h in a microaerobic environment, white-colored colonies assumed to be Campylobacter isolates were identified at the species level using multiplex PCR (53).
PFGE analysis
Isolates of Salmonella and Campylobacter were subtyped by PFGE following the PulseNet protocol (https://www.cdc.gov/pulsenet/pathogens/pfge.html). Genomic DNA was digested with XbaI (Takara, Shiga, Japan) for Salmonella and SmaI (Takara) for Campylobacter. PFGE banding patterns were analyzed using Fingerprint II software (Bio-Rad Laboratories, Hercules, CA, USA) with the Dice coefficient with 1.0% band tolerance and 1.0% optimization and the unweighted pair group method with arithmetic averages (UPGMA). The Salmonella serovar Braenderup H9812 genome digested with XbaI was used as the standard for both bacteria. Isolates collected from broilers in another study conducted in 2011–13 (Okamura et al., unpublished data) were also included in the analysis.
MLST analysis
MLST was performed as described (12). Genomic DNA was purified using a DNeasy Blood & Tissue kit (Qiagen, Düsseldorf, Germany). The amplification reactions for 7 housekeeping genes were performed with 7.5 pmol of each primer using KOD-Plus (Toyobo, Osaka, Japan). PCR products were gel-purified, mixed with the respective sequencing primers, and sequenced on a 3730XL DNA Analyzer using BigDye Terminator v3.1 Cycle sequence kit (Applied Biosystems, Foster city, CA, USA) following the manufacturer’s instructions. Sequence data were collated and alleles assigned using the Campylobacter MLST database (http://pubmlst.org/campylobacter/). ST9010 was newly submitted for ST designation following the procedure described on the Campylobacter MLST website. The assignment of STs to CCs was taken from the Campylobacter MLST database. The MLST profiles of these isolates are shown in the supplemental table.
Antimicrobial susceptibility test
The minimal inhibitory concentrations of ampicillin (ABPC), amoxicillin (AMPC), kanamycin (KM), nalidixic acid (NA), norfloxacin (NFLX), and enrofloxacin (ERFX) for C. jejuni isolates were obtained using an agar dilution method, in accordance with the Clinical Laboratory Standards Institute guidelines (7).
Results and Discussion
Prevalence of Salmonella
Salmonella spp. were isolated from the ceca of 11 out of 123 crows (8.9%). Eight and 3 isolates were identified as serovars Bredeney and Derby, respectively (Table 2). Serovar Bredeney rarely causes human infection with contaminated food such as poultry (32) and other products such as peanut butter (50). Serovar Derby is one of the prevalent serovars found in pigs in North America (36, 52). In Europe, S. Derby is one of the most prevalent serovars in slaughter pigs, and also ranks among the ten most frequently isolated serovars in humans (16). In Japan, this serovar has rarely been isolated from pigs (11, 47) and human cases (13), and to the best of our knowledge, these serovars have not been isolated from broiler flocks. One of the most frequently isolated serovars from broilers has generally been serovar Infantis (3). Therefore, Salmonella does not appear to be transmitted between crows and broiler flocks, but may provide a potential link between crows and pig farms in this area.
Table 2.
Prevalence of Salmonella serovars in crows.
| Corvus macrorhynchos | Corvus corone | Unidentified | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Male | Female | N.D.* | Male | Female | N.D. | Male | Female | N.D. | |
| Juvenile | Derby 1** | Bredeney 1 Derby 1 |
— | — | — | — | Bredeney 1 | Derby 1 | Bredeney 2 |
|
| |||||||||
| Adult | Bredeney 1 | — | — | Bredeney 1 | Bredeney 1 | — | — | — | — |
not determined;
the number of Salmonella-positive crows.
Prevalence of Campylobacter
Campylobacter spp. were isolated from all 89 crows tested (100%). C. jejuni, C. lari and other unidentified Campylobacter were isolated from 85 (95.5%), 9 (10.1%), and 9 crows (10.1%), respectively, and the 2 species were simultaneously detected from 14 crows (Table 3). A previous study reported that wild birds harbored Campylobacter spp. in Denmark, wild birds on livestock farms carried C. jejuni at a high frequency, and the highest carriage rate of 61.8% was found in thrushes (23). In the Mid-Atlantic region of the US, the overall prevalence of C. jejuni was 7.2%, with the highest being crows (23%) and gulls (25%) (27). Weis et al. more recently reported that Campylobacter was isolated from 66.9% (85/127) of free-living American crows (C. brachyrhyncos) in California (51). The present study showed that the prevalence of Campylobacter was 100%, and C. jejuni (85/89) was predominant in crows. Since captured juvenile crows carried Campylobacter, even nestlings may be exposed to bacteria in roosts or their surroundings (46). C. jejuni is generally isolated more frequently than C. coli from broiler chickens (15). In the present study, C. coli was not isolated, while C. lari was detected albeit at a low prevalence. In Canada, C. lari was isolated from river water (66%) and waterfowl (32%), and one isolate from a seagull showed 100% homology to another from river water (49). Since there is a river within the 2-km radius of the box traps in the present study, free-living crows may also drink river water that may be contaminated with C. lari.
Table 3.
Prevalence of Campylobacter spp. in crows.
| Corvus macrorhynchos | Corvus corone | Unidentified | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Male | Female | N.D.* | Male | Female | N.D. | Male | Female | N.D. | |
| Juvenile | jejuni 21** | jejuni 25 | — | jejuni 1 | — | — | jejuni 8 | jejuni 3 | — |
| lari 4 | lari 1 | lari 1 | lari 1 | ||||||
| Others 3 | Others 2 | ||||||||
|
| |||||||||
| Adult | jejuni 6 | jejuni 8 | jejuni 1 | jejuni 2 | jejuni 3 | jejuni 1 | — | jejuni 2 | jejuni 4 |
| lari 1 | Others 2 | lari 1 | lari 1 | ||||||
| Others 1 | |||||||||
not determined;
the number of Campylobacter-positive crows.
Genetic subtypes of Salmonella and Campylobacter isolates
All 8 isolates of S. Bredeney were from crows captured at 5 different sampling occasions between October 2012 and March 2013; nevertheless, they showed an identical PFGE pattern (data not shown). This result indicates the clonal distribution of this serovar in the environment of this region, supported by Cormican et al. (9) who demonstrated high similarity in the PFGE patterns of 97 out of 112 isolates of serovar Bredeney from various sources such as humans, poultry, bovine, and deer. Three isolates of S. Derby were not subtyped due to a smeared band pattern (data not shown).
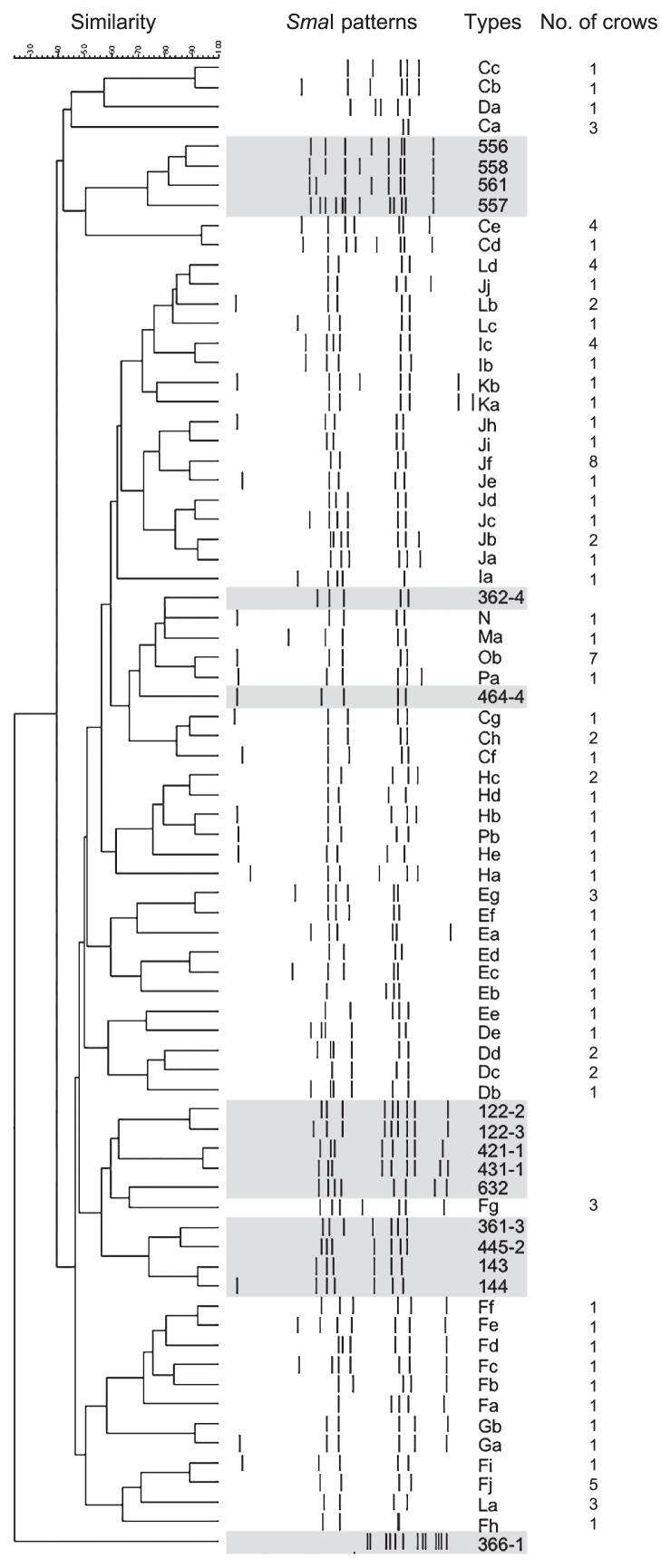
C. jejuni isolates showed a broad diversity of PFGE patterns, which was consistent with previous findings (40). Fig. 1 includes all 59 patterns of crow isolates in the present study and 16 patterns of isolates from broiler flocks in our surveillance between 2011 and 2013 (Okamura et al., unpublished data). Each of the 43 different PFGE patterns originated from a single crow, while the other 16 patterns were isolated from 2–8 crows. This result suggests that crows obtain Campylobacter in diverse locations, and the roosts in which these crows congregate may be potential reservoirs retaining multiple strains of Campylobacter. Ten PFGE patterns (Ca, Dc, Dd, Fj, Ic, Jb, Jf, La, Ld, and Ob) were isolated at more than 2 different sampling occasions with an interval of 4 months for the pattern Ic and 11–15 months for the others. Campylobacter is known to survive against an atmospheric level of oxygen and/or poor nutrient supply (5), which may explain the persistence of the bacteria not only in poultry houses and farms (17, 44), but also in natural environments including crow roosts. This result implies that reservoirs are maintained for up to 1 year in crow roosts or their surroundings and may continuously expose juvenile and adult crows to different strains of C. jejuni.
Fig 1.
Dendrogram of Campylobacter jejuni isolates collected from crows in this study generated by pulsed-field gel electrophoresis (PFGE) patterns with SmaI. The isolates collected from broilers in the other study in 2011–13 were also included and shown in shaded area. The numbers of crows from which the respective PFGE patterns were isolated were also represented.
In the PFGE analysis, 80% similarity was found between patterns Ma, N, and Ob from 9 crows and 362-4 from broilers and between Cf from crows and 464-4 from broilers. These isolates appeared to be “closely related” epidemiologically according to the Tenover criteria (48). However, some difficulties are associated with accurately interpreting differences in or the similarity of banding patterns using a PFGE analysis due to the lack of reproducibility and its application to an unsuitable context (4, 48). In MLST for the further analysis of genetic relatedness, isolates with the similar PFGE patterns of Ma, N, and Ob from crows and 362-4 and 464-4 from broilers were assigned to different types: ST2761, ST9010, ST1540, ST22, and ST3727, respectively (Table 4). Crow isolates with the PFGE pattern of Cf were not recovered from frozen stock and not analyzed by MLST. ST2761 was included in the ST-952 complex. This complex was previously reported to be predominant among crow isolates (39) and has been detected in rabbits, environmental water and soil, and free-living birds (28). In the Campylobacter PubMLST database, ST1540 is associated with wild birds and environmental water. It belongs to the ST-1275 complex, which was also identified in environmental water samples in Canada (30) and New Zealand (6). These isolates possibly originated from or are related to environmental water, which is supported by the isolation of C. lari in the present study as described above. ST9010 was newly identified in the present study, submitted, and added to the PubMLST database. ST22 isolates from broilers belong to the ST-22 complex. The isolates in this clonal complex have predominantly been detected from human stools, and sometimes from chickens, cattle, cow milk, and other sources, but not from wild birds (PubMLST). The isolates with ST3727 (the ST-45 complex) have been found in chickens in Japan, except for two from human stools in New Zealand and Sweden (PubMLST), while the ST-45 complex is one of the most common clonal complexes with broad host ranges, a so-called “generalist” (22, 42).
Table 4.
MLST types and antimicrobial susceptibility profiles of crow and broiler isolates showing similar PFGE patterns.
| ID of isolates | PFGE patterns | ST | Clonal complex | Minimal inhibitory concentration (μg mL−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ABPC | AMPC | KM | NA | NFLX | ERFX | ||||
| Crow | |||||||||
| 62217 | Ma | 2761 | ST-952 complex | 8 | 8 | 8 | 4 | 0.25 | ≦0.06 |
| 62221 | N | 9010 | Unassigned | 0.125 | ≦0.06 | 4 | 4 | ≦0.06 | ≦0.06 |
| 62220 | Ob | 1540 | ST-1275 complex | 0.125 | 0.25 | 4 | 4 | 0.25 | ≦0.06 |
|
| |||||||||
| Broiler | |||||||||
| 62218 | 362 | 22 | ST-22 complex | 2 | 4 | 8 | 4 | 0.25 | ≦0.06 |
| 62219 | 464 | 3727 | ST-45 complex | 2 | 2 | 4 | 8 | 1 | ≦0.06 |
The antibiotic resistance profiles of these 5 isolates were elucidated (Table 4). Crow isolates with ST1540 and ST9010 showed markedly greater susceptibility to ABPC and AMPC than the other isolates, suggesting that these 2 isolates are crow-associated C. jejuni that have not been exposed to ABPC or AMPC. However, ST2761, which was also isolated from crows, is resistant to ABPC and AMPC, compared to the broiler isolates. This may be due to its potential origin from different livestock such as pigs and cattle treated with these antibiotics. A possible explanation is that these antibiotics are extruded via multidrug efflux mechanisms. In the CmeABC system, for example, not only a broad range of antibiotics and bile salts, but also heavy metals, dyes, and detergents may serve as its substances (31, 35, 38), which are ubiquitous in the environment. Thus, the results of the PFGE and MLST analyses and antibiotic resistance profiles suggest that free-living crows harbored various types of C. jejuni and did not share common lineages with broiler flocks.
C. lari isolates represented 7 PFGE patterns, each of which was isolated from 1–2 crows (data not shown). The isolation of C. lari and C. jejuni from river water, although not attempted in the present study, may allow us to compare their PFGE patterns, providing epidemiological information on Campylobacter spp. in the environment and wildlife.
Free-living crows harbored Salmonella serovars Bredeney and Derby with low prevalence and C. jejuni with high prevalence in the region of the present study. A wide diversity of genotypes of C. jejuni appears to persist in various environmental reservoirs. Free-living crows and broilers do not share Salmonella serovars or the same lineages of C. jejuni. We conclude that free-living crows do not serve as a vector transmitting Salmonella serovars and C. jejuni between the natural environment and broiler farms in crow habitats. Salmonella serovars and Campylobacter spp. distribute in free-living crows and broilers in different manners.
Further investigations on the prevalence of these bacteria in pigs and cattle, which are also known to harbor S. Derby, as described above, and C. jejuni, respectively, and the characterization of isolates will provide a better understanding of the potential role(s) of crows in the distribution of Salmonella serovars and C. jejuni between poultry, livestock, and wild birds.
Supplementary Material
Acknowledgements
The authors thank K. Sakata, T. Okamoto, and Y. Aoyama for their technical assistance. This work was financially supported in part by the Ministry of Agriculture, Forestry and Fisheries of Japan.
References
- 1.Allen V.M., Weaver H., Ridley A.M., Harris J.A., Sharma M., Emery J., Sparks N., Lewis M., Edge S. Sources and spread of thermophilic Campylobacter spp. during partial depopulation of broiler chicken flocks. J Food Prot. 2008;71:264–270. doi: 10.4315/0362-028x-71.2.264. [DOI] [PubMed] [Google Scholar]
- 2.American Veterinary Medical Association. Guidelines for the Euthanasia of Animals. American Veterinary Medical Association; 1931 N. Meacham Road Schaumburg, IL: 2013. [Google Scholar]
- 3.Asai T., Ishihara K., Harada K., Kojima A., Tamura Y., Sato S., Takahashi T. Long-term prevalence of antimicrobial-resistant Salmonella enterica subspecies enterica serovar infantis in the broiler chicken industry in Japan. Microbiol Immunol. 2007;51:111–115. doi: 10.1111/j.1348-0421.2007.tb03881.x. [DOI] [PubMed] [Google Scholar]
- 4.Barrett T.J., Gerner-Smidt P., Swaminathan B. Interpretation of pulsed-field gel electrophoresis patterns in foodborne disease investigations and surveillance. Foodborne Pathog Dis. 2006;3:20–31. doi: 10.1089/fpd.2006.3.20. [DOI] [PubMed] [Google Scholar]
- 5.Bronowski C., James C.E., Winstanley C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol Lett. 2004;356:8–19. doi: 10.1111/1574-6968.12488. [DOI] [PubMed] [Google Scholar]
- 6.Carter P.E., McTavish S.M., Brooks H.J., Campbell D., Collins-Emerson J.M., Midwinter A.C., French N.P. Novel clonal complexes with an unknown animal reservoir dominate Campylobacter jejuni isolates from river water in New Zealand. Appl Environ Microbiol. 2009;75:6038–6046. doi: 10.1128/AEM.01039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. CLSI supplement VET01S. 3rd ed. Wayne, PA: CLSI; 2015. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. [Google Scholar]
- 8.Cody A.J., McCarthy N.D., Bray J.E., Wimalarathna H.M., Colles F.M., Jansen van Rensburg M.J., Dingle K.E., Waldenstrom J., Maiden M.C. Wild bird-associated Campylobacter jejuni isolates are a consistent source of human disease, in Oxfordshire, United Kingdom. Environ Microbiol Rep. 2015;7:782–788. doi: 10.1111/1758-2229.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cormican M., DeLappe N., O’Hare C., Doran G., Morris D., Corbett-Feeney G., Fanning S., Daly M., Fitzgerald M., Moore J. Salmonella enterica serotype Bredeney: antimicrobial susceptibility and molecular diversity of isolates from Ireland and Northern Ireland. Appl Environ Microbiol. 2002;68:181–186. doi: 10.1128/AEM.68.1.181-186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craven S.E., Stern N.J., Line E., Bailey J.S., Cox N.A., Fedorka-Cray P. Determination of the incidence of Salmonella spp., Campylobacter jejuni, and Clostridium perfringens in wild birds near broiler chicken houses by sampling intestinal droppings. Avian Dis. 2000;44:715–720. [PubMed] [Google Scholar]
- 11.Dahshan H., Chuma T., Shahada F., Akiba M., Fujimoto H., Akasaka K., Kamimura Y., Okamoto K. Characterization of antibiotic resistance and the emergence of AmpC-producing Salmonella Infantis from pigs. J Vet Med Sci. 2010;72:1437–1442. doi: 10.1292/jvms.10-0186. [DOI] [PubMed] [Google Scholar]
- 12.Dingle K.E., Colles F.M., Wareing D.R., Ure R., Fox A.J., Bolton F.E., Bootsma H.J., Wilems R.J., Urwin R., Maiden M.C. Multilocus sequence typing system for Campylobacter jejuni. J Clin Microbiol. 2001;39:14–23. doi: 10.1128/JCM.39.1.14-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebuchi S., Baba A., Uryu K., Hiwaki H. Two outbreaks caused by Salmonella Derby and S. Anatum at grilled-meat restaurants in Fukuoka city. Jpn J Infect Dis. 2006;59:405–406. [PubMed] [Google Scholar]
- 14.Ellis-Iversen J., Ridley A., Morris V., Sowa A., Harris J., Atterbury R., Sparks N., Allen V. Persistent environmental reservoirs on farms as risk factors for Campylobacter in commercial poultry. Epidemiol Infect. 2012;140:916–924. doi: 10.1017/S095026881100118X. [DOI] [PubMed] [Google Scholar]
- 15.Esteban J.I., Oporto B., Aduriz G., Juste R.A., Hurtado A. A survey of food-borne pathogens in free-range poultry farms. Int J Food Microbiol. 2008;123:177–182. doi: 10.1016/j.ijfoodmicro.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 16.European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. EFSA J. 2013;11:3129, 250. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans S.J., Sayers A.R. A longitudinal study of Campylobacter infection of broiler flocks in Great Britain. Prev Vet Med. 2000;46:209–223. doi: 10.1016/s0167-5877(00)00143-4. [DOI] [PubMed] [Google Scholar]
- 18.Flint J.A., Van Duynhoven Y.T., Angulo F.J., et al. Estimating the burden of acute gastroenteritis, foodborne disease, and pathogens commonly transmitted by food: an international review. Clin Infect Dis. 2005;41:698–704. doi: 10.1086/432064. [DOI] [PubMed] [Google Scholar]
- 19.Franz E., van der Fels-Klerx H.J., Thissen J., van Asselt E.D. Farm and slaughterhouse characteristics affecting the occurrence of Salmonella and Campylobacter in the broiler supply chain. Poult Sci. 2012;91:2376–2381. doi: 10.3382/ps.2009-00367. [DOI] [PubMed] [Google Scholar]
- 20.Gast R.K. Serotype-specific and serotype-independent strategies for preharvest control of food-borne Salmonella in poultry. Avian Dis. 2007;51:817–828. doi: 10.1637/8090-081807.1. [DOI] [PubMed] [Google Scholar]
- 21.Greig J.D., Ravel A. Analysis of foodborne outbreak data reported internationally for source attribution. Int J Food Microbiol. 2009;130:77–87. doi: 10.1016/j.ijfoodmicro.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Gripp E., Hlahla D., Didelot X., et al. Closely related Campylobacter jejuni strains from different sources reveal a generalist rather than a specialist lifestyle. BMC Genomics. 2011;12:584. doi: 10.1186/1471-2164-12-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hald B., Skov M.N., Nielsen E.M., Rahbek C., Madsen J.J., Wainø M., Chriél M., Nordentoft S., Baggesen D.L., Madsen M. Campylobacter jejuni and Campylobacter coli in wild birds on Danish livestock farms. Acta Vet Scand. 2016;58:11. doi: 10.1186/s13028-016-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson I., Ederoth M., Andersson L., Vagsholm I., Olsson Engvall E. Transmission of Campylobacter spp. to chickens during transport to slaughter. J Appl Microbiol. 2005;99:1149–1157. doi: 10.1111/j.1365-2672.2005.02689.x. [DOI] [PubMed] [Google Scholar]
- 25.Itoh T., Sakai S. Distribution and pathogenicity of Campylobacter in poultry. J Jpn Soc Poult Dis. 1984;20:2–12. [Google Scholar]
- 26.Jonsson M.E., Chriél M., Norström M., Hofshagen M. Effect of climate and farm environment on Campylobacter spp. colonisation in Norwegian broiler flocks. Prev Vet Med. 2012;107:95–104. doi: 10.1016/j.prevetmed.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Keller J.I., Shriver W.G., Waldenström J., Griekspoor P., Olsen B. Prevalence of Campylobacter in wild birds of the mid-Atlantic region, USA. J Wildl Dis. 2011;47:750–754. doi: 10.7589/0090-3558-47.3.750. [DOI] [PubMed] [Google Scholar]
- 28.Kwan P.S., Barrigas M., Bolton F.J., French N.P., Gowland P., Kemp R., Leatherbarrow H., Upton M., Fox A.J. Molecular epidemiology of Campylobacter jejuni populations in dairy cattle, wildlife, and the environment in a farmland area. Appl Environ Microbiol. 2008;74:5130–5138. doi: 10.1128/AEM.02198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson B., de Pinna E., Horton R.A., et al. Epidemiological evidence that garden birds are a source of human salmonellosis in England and Wales. PLoS One. 2014;9:e88968. doi: 10.1371/journal.pone.0088968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lévesque S., Frost E., Arbeit R.D., Michaud S. Multilocus sequence typing of Campylobacter jejuni isolates from humans, chickens, raw milk, and environmental water in Quebec, Canada. J Clin Microbiol. 2008;46:3404–3411. doi: 10.1128/JCM.00042-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J., Michel L.O., Zhang Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob Agents Chemother. 2002;46:2124–2131. doi: 10.1128/AAC.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore J.E., Murray L., Fanning S., Cormican M., Daly M., Delappe N., Morgan B., Murphy P.G. Comparison of phenotypic and genotypic characteristics of Salmonella bredeney associated with a poultry-related outbreak of gastroenteritis in Northern Ireland. J Infect. 2003;47:33–39. doi: 10.1016/s0163-4453(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 33.Moore J.E., Corcoran D., Dooley J.S., et al. Campylobacter. Vet Res. 2005;36:351–382. doi: 10.1051/vetres:2005012. [DOI] [PubMed] [Google Scholar]
- 34.Newell D.G., Elvers K.T., Dopfer D., et al. Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl Environ Microbiol. 2011;77:8605–8614. doi: 10.1128/AEM.01090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payot S., Cloeckaert A., Chaslus-Dancla E. Selection and characterization of fluoroquinolone-resistant mutants of Campylobacter jejuni using enrofloxacin. Microb Drug Resist. 2002;8:335–343. doi: 10.1089/10766290260469606. [DOI] [PubMed] [Google Scholar]
- 36.Pires A.F., Funk J.A., Bolin C. Risk factors associated with persistence of Salmonella shedding in finishing pigs. Prev Vet Med. 2014;116:120–128. doi: 10.1016/j.prevetmed.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Pires S.M., Vieira A.R., Hald T., Cole D. Source attribution of human salmonellosis: an overview of methods and estimates. Foodborne Pathog Dis. 2014;11:667–676. doi: 10.1089/fpd.2014.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pumbwe L., Piddock L.J. Identification and molecular characterisation of CmeB, a Campylobacter jejuni multidrug efflux pump. FEMS Microbiol Lett. 2002;206:185–189. doi: 10.1111/j.1574-6968.2002.tb11007.x. [DOI] [PubMed] [Google Scholar]
- 39.Ramonaitė S., Kudirkiene E., Tamuleviciene E., Leviniene G., Malakauskas A., Gölz G., Alter T., Malakauskas M. Prevalence and genotypes of Campylobacter jejuni from urban environmental sources in comparison with clinical isolates from children. J Med Microbiol. 2014;63:1205–1213. doi: 10.1099/jmm.0.072892-0. [DOI] [PubMed] [Google Scholar]
- 40.Ramonaitė S., Novoslavskij A., Zakarienė G., Aksomaitienė J., Malakauskas M. High prevalence and genetic diversity of Campylobacter jejuni in wild crows and pigeons. Curr Microbiol. 2015;71:559–565. doi: 10.1007/s00284-015-0881-z. [DOI] [PubMed] [Google Scholar]
- 41.Schlundt J., Toyofuku H., Jansen J., Herbst S.A. Emerging food-borne zoonoses. Rev Sci Technol. 2004;23:513–533. doi: 10.20506/rst.23.2.1506. [DOI] [PubMed] [Google Scholar]
- 42.Sheppard S.K., Cheng L., Méric G., et al. Cryptic ecology among host generalist Campylobacter jejuni in domestic animals. Mol Ecol. 2014;23:2442–2451. doi: 10.1111/mec.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirota K., Katoh H., Ito T., Otsuki K. Salmonella contamination in commercial layer feed in Japan. J Vet Med Sci. 2000;62:789–791. doi: 10.1292/jvms.62.789. [DOI] [PubMed] [Google Scholar]
- 44.Shreeve J.E., Toszeghy M., Ridley A., Newell D.G. The carry-over of Campylobacter isolates between sequential poultry flocks. Avian Dis. 2011;46:378–385. doi: 10.1637/0005-2086(2002)046[0378:TCOOCI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 45.Sommer H.M., Heuer O.E., Sørensen A.I., Madsen M. Analysis of factors important for the occurrence of Campylobacter in Danish broiler flocks. Prev Vet Med. 2013;111:100–111. doi: 10.1016/j.prevetmed.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Taff C.C., Weis A.M., Wheeler S., et al. Influence of host ecology and behavior on Campylobacter jejuni prevalence and environmental contamination risk in a synanthropic wild bird species. Appl Environ Microbiol. 2016;82:4811–4820. doi: 10.1128/AEM.01456-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takada H., Inoue N. Serotypes, antimicrobial resistance, and genomic analysis of Salmonella isolates from slaughtered swine in Gunma Prefecture, Japan. J Jpn Vet Med Assoc. 2008;61:65–69. [Google Scholar]
- 48.Tenover F.C., Arbeit R.D., Goering R.V., Mickelsen P.A., Murray B.E., Persing D.H., Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Dyke M.I., Morton V.K., McLellan N.L., Huck P.M. The occurrence of Campylobacter in river water and waterfowl within a watershed in southern Ontario, Canada. J Appl Microbiol. 2010;109:1053–1066. doi: 10.1111/j.1365-2672.2010.04730.x. [DOI] [PubMed] [Google Scholar]
- 50.Viazis S., Beal J.K., Monahan C., et al. Laboratory, environmental, and epidemiologic investigation and regulatory enforcement actions in response to an outbreak of Salmonella Bredeney infections linked to peanut butter. Open Forum Infect Dis. 2015;2:ofv114. doi: 10.1093/ofid/ofv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weis A.M., Miller W.A., Byrne B.A., Chouicha N., Boyce W.M., Townsend A.K. Prevalence and pathogenic potential of Campylobacter isolates from free-living, human-commensal american crows. Appl Environ Microbiol. 2014;80:1639–1644. doi: 10.1128/AEM.03393-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkins W., Rajić A., Waldner C., McFall M., Chow E., Muckle A., Rosengren L. Distribution of Salmonella serovars in breeding, nursery, and grow-to-finish pigs, and risk factors for shedding in ten farrow-to-finish swine farms in Alberta and Saskatchewan. Can J Vet Res. 2010;74:81–90. [PMC free article] [PubMed] [Google Scholar]
- 53.Yamazaki-Matsune W., Taguchi M., Seto K., Kawahara R., Kawatsu K., Kumeda Y., Kitazato M., Nukina M., Misawa N., Tsukamoto T. Development of a multiplex PCR assay for identification of Campylobacter coli, Campylobacter fetus, Campylobacter hyointestinalis subsp. hyointestinalis, Campylobacter jejuni, Campylobacter lari and Campylobacter upsaliensis. J Med Microbiol. 2007;56:1467–1473. doi: 10.1099/jmm.0.47363-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.