Abstract
Plant survival in many ecosystems requires tolerance of large radiation loads, unreliable water supply, and suboptimal soil fertility. We hypothesized that increased production of neutral lipids (triacylglycerols, TAGs) in plant leaves is a mechanism for dissipating excess radiation energy. In a greenhouse experiment, we combined drought and shade treatments and examined responses among four species differing in life form, habitat, and drought- and shade-tolerance. We also present a lipid extraction protocol suitable for sclerophyllous leaves of native Australian trees (e.g. Acacia, Eucalyptus). Fluorescence measurements indicated plants exposed to full sunlight experienced mild photoinhibition during our experiment. Accumulation of TAGs did not follow photosynthetic capacity, but instead, TAG concentration increased with non-photochemical quenching. This suggests plants under oxidative stress may increase biosynthesis of TAGs. Moderate drought stress resulted in a 60% reduction in TAG concentration in wheat (Triticum aestivum). Shading had no effect on TAGs, but increased concentrations of polar lipids in leaves; for example, acclimation to shade in Austrodanthonia spp., a native Australian grass, resulted in a 60% increase in associated polar lipids and higher foliar chlorophyll concentrations. Shading also reduced the digalactosyldiacylglycerol: monogalactosyldiacylglycerol (DGDG:MGDG) ratio in leaves, with a corresponding increase in the degree of unsaturation and thus fluidity of thylakoid membranes of chloroplasts. Our results suggest prevention of photodamage may be coordinated with accumulation of TAGs, although further research is required to determine if TAGs serve a photoprotective function in plant leaves.
Keywords: galactolipid, phospholipid, photoinhibition, shade tolerance, stress physiology
Introduction
Increased ability to quantify plant lipids, ranging up to full ‘lipidomic’ analyses, have revealed diverse roles for lipids in plant responses to stress, including heat tolerance (Grover et al. 2000, Higashi et al. 2015, Larkindale and Huang 2004), post-freezing recovery (Li et al. 2008, Welti et al. 2002), desiccation (Li et al. 2014), mechanical wounding responses (Buseman et al. 2006, Vu et al. 2014), and nutrient deficiencies (Hartel et al. 2000, Li et al. 2006). Development of mass spectrometry for high-throughput lipid analysis has been integral to these discoveries (Welti et al. 2007). Accordingly, novel lipid species are still being routinely discovered, often with unknown roles (Bogdanov et al. 2016, Garrett et al. 2009, Okazaki et al. 2013). Analytical techniques have advanced to the point where it is now possible to test hypotheses about how different classes of lipids respond to and regulate stress responses of plants. Here, we test the hypothesis that increased production of triacylglycerols (TAGs) in leaves of water-stressed plants is a mechanism for dissipating excess radiation energy.
Plant leaves only require a fraction of the energy in full sunlight (5–10%) to achieve light saturation of photosynthesis (Long et al. 1994, Ort 2001), and dissipation of excess light energy is necessary to prevent photoinhibition. Broadly defined, photoinhibition is the decrease in the intrinsic light-limited or light-saturated rate of photosynthesis as a result of exposure to excess light (Kok 1956). Many studies support the generalization that photoinhibition has flow-on negative effects on plant growth (Farage and Long 1991, Long et al. 1994, Losciale et al. 2010, Ögren and Sjöström 1990, Raven 2011, Reynolds et al. 2012). These effects stem from damage to the photosynthetic machinery, primarily the D1 protein of photosystem II (PSII), if excess absorbed energy is not continuously dissipated (Takahashi and Badger 2011). A number of known photoprotective mechanisms help plants avoid oxidative stress: (1) chloroplast or leaf movement (Brugnoli and Bjorkman 1992, Gamon and Pearcy 1989, Long et al. 1994), (2) increased reflectivity of leaves (e.g. trichomes; Camarero et al. 2012, Pierce 2007) or accumulation of cuticle waxes or UV-screening compounds (e.g. phenolics) in the epidermis (Balakumar et al. 1993, Robinson et al. 1993), (3) dissipation of energy as heat (i.e. non-photochemical quenching, NPQ; Bilger and Bjorkman 1990, Demmig et al. 1987, Demmig-Adams and Adams 1992), (4) maintenance of energy utilization via photorespiration (Takahashi and Badger 2011), and (5) formation of enzymes, antioxidants, and carotenoids that scavenge reactive oxygen species from cells (Asada 2006, Havaux and Niyogi 1999). Nonetheless, the relative importance of these different mechanisms for avoidance of oxidative stress is neither well defined nor understood, particularly for plants with a limited capacity (i.e. those exposed to nutrient deficiency, hot/cold temperatures, water limitations or drought) to utilize excess energy for the production of biomass.
Another possible outlet for excess energy is enhanced production of energy-rich, reduced-carbon compounds. Lipid biosynthesis – both of neutral lipids (e.g. diacylglycerols, DAGs and TAGs) and polar lipids (e.g. phospho- and galactolipids that comprise structural components of cell and organelle membranes) – requires about twice the energy of carbohydrate biosynthesis (Hu et al. 2008, Solovchenko 2012). Neutral storage lipids such as TAGs are accumulated as oil bodies in the cytoplasm of many algae species under nitrogen limitation (Shifrin and Chisholm 1981, Spoehr and Milner 1949, Thompson 1996) and excess light (Brown et al. 1996, Orcutt and Patterson 1974, Sukenik et al. 1989). In fact, this stress response underpins the utilization of microalgae for biofuel energy production (Hu et al. 2008).
Higher plants synthesize small amounts of TAGs in the endoplasmic reticulum, which then accumulate as lipid droplets in the cytosol (Chapman et al. 2013) or in chloroplasts where they accumulate as plastoglobules (Lippold et al. 2012). Increasing concentrations of TAGs during drought stress has been demonstrated for desert shrubs such as palo verde (Parkinsonia aculeata, Benadjaoud et al. 2013), forage grasses (Perlikowski et al. 2016), and a resurrection plant (Gasulla et al. 2013). Similarly, a number of studies have shown increasing concentrations of TAGs during drought stress of crop species such as corn (Douglas and Paleg 1981), soybean (Martin et al. 1986), and cotton (Pham Thi et al. 1989). In contrast to the membrane functions of polar lipids, the functional significance of TAGs in vegetative tissues is not well described, other than their role in energy storage. It has been suggested that lipid accumulation is associated with photosynthetic capacity (Lin and Oliver 2008, Perlikowski et al. 2016), but we lack even the most basic knowledge such as how light intensity affects lipids in higher plant leaves.
Here we report a greenhouse experiment combining drought and shade treatments to test responses of lipid biosynthesis across a diverse set of four plant species that differ in life form (trees vs. grasses) and habitat (cultivated vs. native Australian plants). Our objective was to determine if, across diverse plant species: (1) Lipid (including TAGs) concentration of leaves increases with drought, (2) TAG concentration decreases with shading, and (3) total polar lipid concentration (associated with chloroplast membranes) increases in leaves under shading.
Materials and Methods
Greenhouse experiment
Four study species were chosen, a native Australian species and a cultivated equivalent for each of two life forms: grass and tree (Table 1). Seeds for all species were sown into 4.5 L pots containing commercial potting mix and fertilized with Osmocote (Scotts Australia, Bella Vista, NSW, Australia). Seed for the native (Eucalyptus microcarpa (Maiden) Maiden, grey box) and cultivated (Chamaecytisus palmensis (Christ) F.A. Bisby & K.W. Nicholls, tagasaste) tree species were sown in August 2014 (five months prior to measurements), seed for the indigenous grass (Austrodanthonia spp., wallaby grass) was sown in October 2014 (four months prior to measurements), whilst seed for the cultivated grass (Triticum aestivum L., common wheat var. Gregory) was sown in December 2014 (one month prior to measurement). Individuals of C. palmensis were transferred into 14.5 L pots in December 2014.
Table 1.
Characteristics of the four plant species measured in this study. Tolerance/intolerance of the level of shading and drought in this experiment is indicated, as inferred from treatment impacts on growth (see Figure 1).
| Species | Habitat | Life Form | Shading | Drought |
|---|---|---|---|---|
| Austrodanthonia spp. | native to Australia | C3 grass | tolerant | intolerant |
| Eucalyptus microcarpa | native to Australia | tree | intolerant | intolerant |
| Chamaecytisus palmensis | cultivated (native to Canary Islands) | tree/shrub | tolerant | tolerant |
| Triticum aestivum | cultivated (native to southwest Asia) | C3 grass | intolerant | intolerant |
Plants were grown in one of two greenhouses (I.A. Watson Grains Research Centre, University of Sydney, Narrabri, NSW, Australia), a full-sunlight chamber or a shade chamber (50% shade cloth), each containing two watering treatments (well-watered, i.e. watered daily to saturation, vs. drought, i.e. daily provision of 100 mL water) which were randomly assigned to pots. Both shade and drought treatments were initiated mid-November 2014 (two months prior to measurements) for all species except T. aestivum, where drought was initiated in February 2015 (one month prior to measurements). Thus, our full-factorial experiment consisted of four treatments: full-sunlight/well-watered (sun), full-sunlight/drought (drought), shade/well-watered (shade), shade/drought (shade + drought). Shading reduced midday (12:00–14:00) photosynthetically active radiation (PAR) by an average of 25% on the sampling days (21–31 January, 1–4 March; Fig. S1). The maximum PAR was 1600 μmol m−2 s−1 inside the full-sunlight greenhouse and 960 μmol m−2 s−1 inside the shaded greenhouse. Total midday PAR was approximately 100 μmol m−2 s−1 dimmer in March than in January. Chamber temperature was maintained at 25:17 °C (day:night) with a daylength of 11 hr for the duration of the experiment (October 2014–March 2015). Chamber relative humidity was controlled at 70–100%, which led to variations in midday vapor pressure deficit of 1.5–3.5 kPa.
Four uniform replicates per species per treatment were selected from the seven replicates that were initially sown; the same replicates were sampled for all analyses. Destructive harvests were performed on 11–13 February 2015 for all species except T. aestivum, which was harvested on 4 March 2015. For biomass measurements, each plant was separated into leaves, stems (for woody species), and roots and oven-dried at 60 °C to constant mass. Specific leaf area (m2 g−1) was determined for 1–5 leaves per species. Leaf area of C. palmensis, E. microcarpa, and T. aestivum was measured using a portable leaf area meter (LI-3050C, LI-COR, Lincoln, NE, USA); leaf length and width of the thin, rolled Austrodanthonia leaves were measured using calipers.
Gas exchange physiology and chlorophyll fluorescence
To understand how shading and drought affected the physiology of each plant species, we measured (1) instantaneous gas exchange rates, (2) the response of photosynthesis to irradiance, and (3) chlorophyll fluorescence. Maximum CO2 assimilation (Amax), photosynthetic rate under saturating light (Asat), and dark respiration (Rdark) was determined using a portable infrared gas analyzer (LI-6400XT, LI-COR, Lincoln, NE, USA) equipped with a fluorometer chamber (6400–40, LI-COR). Measurement of Amax was performed during on sunny days (9:00 to 15:00) on fully-developed leaves, which were enclosed in the cuvette at greenhouse temperature (Tleaf=26–30 °C) and vapor pressure deficit (VPDleaf=1.1–2.3 kPa), under saturating light (PAR=2000 μmol m−2 s−1) and CO2 concentration (2000 ppm). Measurement of Asat was performed from 9:00 to 15:00 on the same leaves as Amax, under the same temperature, vapor pressure deficit, and PAR (2000 μmol m−2 s−1) but at ambient CO2 concentration (420 ppm). Measurement of Rdark was performed at night (22:00 to 2:00) on fully-developed leaves, which were enclosed in the cuvette at greenhouse temperature (Tleaf=17–18 °C), vapor pressure deficit (VPDleaf=0.5–0.9 kPa), and CO2 concentration (420 ppm). After CO2 flux inside the cuvette stabilized, measurements of Amax, Asat, and Rdark were logged every five seconds for 1 min and averaged.
The response of net CO2 assimilation (An) to varying light intensities was measured on fully-developed leaves (n=4 per treatment) using a portable infrared gas analyzer (LI-6400XT, LI-COR, Lincoln, NE, USA) equipped with a fluorometer chamber (6400–40, LI-COR). These light curves were obtained at greenhouse temperature (Tleaf=22–30 °C), vapor pressure deficit (VPDleaf=0.7–2.2 kPa), and CO2 concentration (420 ppm). After photosynthesis stabilized under saturating light (2000 μmol m−2 s−1), the irradiance was decreased stepwise from 2000 to 2 μmol m−2 s−1 with a total of 13 points per light curve. Rates of photosynthesis were recorded after 1 min at each irradiance. The light compensation point (Icomp), light saturation point (Imax), and apparent quantum yield (Φ) for each curve were estimated using the Prioul and Chartier (1977) nonrectangular hyperbola-based model, as described by Lobo et al. (2013). All gas exchange measurements were made on 21–31 January for E. microcarpa, C. palmensis, and Austrodanthonia spp. or 1–4 March 2015 for T. aestivum. Because the small leaves of C. palmensis did not completely fill the cuvette, leaf area of each replicate was estimated from digital photos using ImageJ software (Schneider et al. 2012).
Maximum quantum yield of PSII (Fv:Fm), efficiency of heat dissipation (i.e. non-photochemical quenching, NPQ), and efficiency of PSII (ΦPSII) was measured using a portable infrared gas analyzer (LI-6400XT, LI-COR, Lincoln, NE, USA) equipped with a fluorometer chamber (6400–40, LI-COR). Measurement of Fv:Fm was performed at night (22:00 to 2:00) on fully-developed leaves of E. microcarpa, C. palmensis, and Austrodanthonia spp. on 24–25 January and on 3–4 March 2015 for T. aestivum to provide a measure of chronic photoinhibition. Measurement of NPQ and ΦPSII was performed on sunny days (9:00 to 15:00; 21–31 January for E. microcarpa, C. palmensis, Austrodanthonia spp., 1–3 March 2015 for T. aestivum). Non-photochemical quenching provides an index of photoprotection and of reversible downregulation of ΦPSII (Nichol et al. 2012). First, one fully-developed leaf was dark-adapted for at least 20 min before measurement of maximum fluorescence (Fm). A second, light-adapted leaf was inserted into the chamber under saturating PAR (2000 μmol m−2 s−1) for measurement of steady-state fluorescence (Fs) and maximum fluorescence in the light (F′m). A rectangular saturating flash of approximately 8000 μmol m−2 s−1 for 1 s duration was used.
Leaf reflectance and chlorophyll concentration index
Adaxial reflectance spectra of leaves were taken in the spectral range between 200 and 1000 nm with a spectral resolution of 0.5 nm with a spectrophotometer (Jaz Spectroclip, Ocean Optics, Dunedin, FL, USA) equipped with an integrating sphere. Leaf reflectance spectra were recorded against a WS-1 diffuse reflectance standard. Leaves of E. microcarpa, C. palmensis, and Austrodanthonia spp. were measured on 23 January, and T. aestivum was measured on 1 March 2015. Total chlorophyll content of leaves was estimated using the two-band model described by Gitelson et al. (2006):
where R540–560 is mean reflectance from a green band (540–560 nm) and R760–800 is mean reflectance from a near infrared band (760–800 nm). This chlorophyll index is positively and linearly related to total chlorophylls in leaves of many tree and crop species (Gitelson et al. 2003, Gitelson et al. 2006).
Lipid extraction from plant leaves
Leaves were collected at midday (11:00 to 14:00) on 25 January for E. microcarpa, C. palmensis, and Austrodanthonia spp. or 4 March 2015 for T. aestivum and immediately frozen in liquid nitrogen. Total lipids were extracted according to Welti et al. (2002), but several modifications were required for the sclerophyllous E. microcarpa leaves. To confirm that these modifications to the extraction protocol increased polar lipid yield from leaves of native Australian plants, we compared lipid concentration from a 2-day extraction to the 9-day extraction used in this study (n=10 trees, Table S1). This test revealed that a longer extraction time increased polar lipid yield by 27–77% for most Eucalyptus and Acacia trees (Table S1). Frozen leaf sections were ground into small leaf pieces in liquid nitrogen and transferred to 3 ml of isopropanol with 0.01% butylated hydroxytoluene (BHT) at 75 °C. After incubation for 15 min, 1.5 ml of chloroform and 0.6 ml of water were added to each sample tube. The tubes were shaken for 1 h and then rested overnight at −80 °C, followed by removal of the lipid extract. Over the next week, the leaves were re-extracted with 4 ml of chloroform/methanol (2:1) with 0.01% BHT three times and then with 4 ml of methanol/chloroform (2:1) with 0.01% BHT four times with overnight agitation each time. This 9-day extended extraction protocol was required to produce a white appearance in the thick Eucalyptus leaf pieces. The combined extracts (approximately 33 ml) were evaporated under nitrogen to 5 ml at 40 °C, then washed with 4 ml of chloroform and 1 ml of water and rested overnight. After discarding the upper-phase wash, the lipid extract was evaporated to 2 ml and stored at −80 °C. Leaf tissue was dried for 48 h at 60 °C to obtain dry weight, which ranged from 10–125 mg. For shipping, the solvent was evaporated completely under nitrogen and re-dissolved in 1 ml chloroform for analysis.
Polar and neutral lipid analyses
Lipid samples (5–40 μl) were analyzed on a triple quadrupole/linear ion trap mass spectrometer (4000 QTRAP, Applied Biosystems, Foster City, CA, USA) at the Kansas Lipidomics Research Center Analytical Laboratory (Kansas State University, https://www.k-state.edu/lipid). The molecular species of polar lipids were defined by the presence of a head-group fragment and the mass of the ion from the intact lipid, which is determined by the total acyl carbons and acyl carbon-carbon double bonds (Welti et al. 2002, Xiao et al. 2010). To accurately quantify polar lipids at the level of class, galactolipids (monogalactosyldiacylglycerol, MGMG; digalactosyldiacylglycerol, DGDG) and phospholipids (phosphatidylglycerol, PG; phosphatidylcholine, PC; phosphatidylethanolamine, PE; phosphatidylinositol, PI; phosphatidylserine, PS; phosphatidic acid, PA) in each class were compared with two internal standards, as has been described previously (Welti et al. 2002, Xiao et al. 2010). Analysis of mass spectra involved background subtraction, smoothing, integration, and comparison of sample peaks with those of the internal standard using Analyst Software (v1.6, SCIEX). Limits of detection (0.002 nmol mg−1) and coefficients of variation (0.3) were used to remove poorly analyzed lipids from analyses. The data were normalized to the internal standard and to the dry weight of the sample, such that lipids can be expressed in units of nmol mg−1.
Neutral lipids (DAGs, TAGs) were quantified in a manner similar to the polar lipids. The TAG species were defined by the presence of one fatty acyl fragment and the mass of the ion formed from the intact lipid (Lee et al. 2011). This allows identification of one TAG acyl species, total acyl carbons, and total number of acyl double bonds in the other two chains, but does not allow identification of positions of acyl chains on the glycerol. Unlike classes of polar lipids, the mass spectral responses of individual TAG species are variable due to differential ionization and were repetitively scanned. Analysis of mass spectra involved background subtraction, smoothing, integration, and comparison of sample peaks with those of the internal standard using Analyst Software (v1.6, SCIEX). Limits of detection (0.002 nmol mg−1) and coefficients of variation (0.3) were used to remove poorly analyzed lipids from analyses. To allow direct comparison of TAG species with polar lipids, the data were normalized to the internal standard such that one unit equals the signal of 1 nmol of internal standard (tri17 : 1). Data were also normalized to the dry weight of the sample, and DAGs and TAGs were expressed in units of normalized signal mg−1.
Leaf energy content
For determination of leaf energy content, leaves of E. microcarpa, C. palmensis, and Austrodanthonia spp. were collected on 11 February and on 4 March 2015 for T. aestivum. Leaves were oven-dried at 60 °C for 48 h and ground to a fine powder in liquid nitrogen. The heat of combustion was measured on 0.7–0.8 g of ground leaf tissue using an oxygen bomb calorimeter (Parr 6400 Calorimeter, Parr Instrument Company, Moline, IL, USA) and was expressed relative to the heat obtained during initial standardization using approximately 1 g of benzoic acid.
Statistical Analyses
The effect of shading and drought was determined by using full-factorial, mixed-model analyses of variance (ANOVAs) with species and treatment as the main effects. Species was analyzed as a random effect, and treatment was analyzed as a fixed effect. When there was a significant species × treatment interaction (p≤0.05), individual species responses were analyzed using one-way, univariate ANOVAs. If differences among means were found (p≤0.05), we used the Tukey HSD to test for significant differences among treatments. The Q test for discordant data (Shoemaker et al. 1974) was performed on the replicates of the total amount of each lipid class, and outliers were removed. To test if leaf energy content, photosynthesis, and indicators of plant stress (i.e. Fv:Fm, NPQ) were correlated with lipid concentration, we used ordinary least squares regression. All data were tested for normality with the Shapiro and Wilk’s test; Rdark, Icomp, NPQ, lipid concentrations, and biomass measurements were ln-transformed to achieve normality. Analyses were performed using JMP 11.0 (SAS Institute, Cary, NC, USA). Means were considered significantly different at p≤0.05.
Results
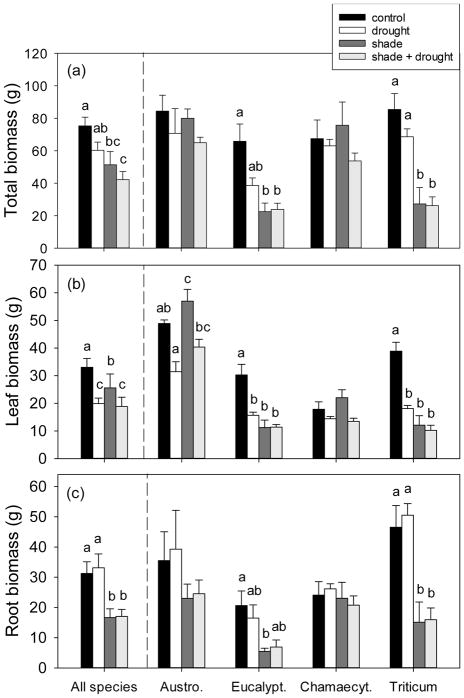
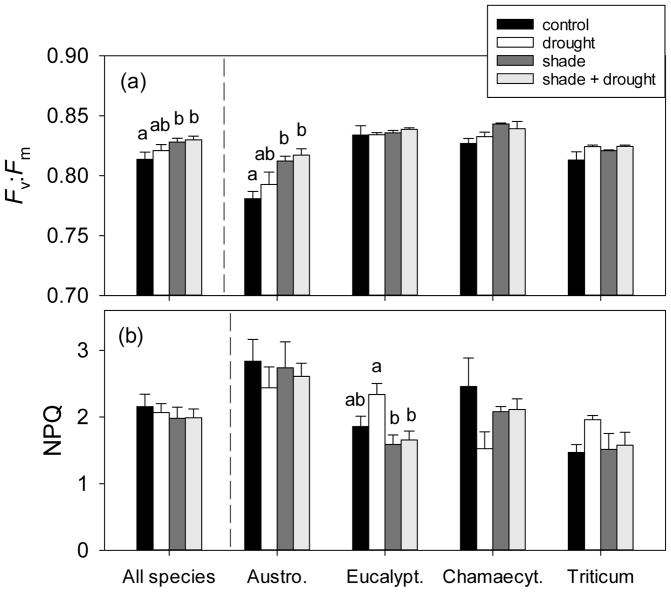
Both drought and shading treatments were effective in modifying plant biomass and physiology among our study species. Leaf biomass of droughted plants reached only about 60% that of control plants (Fig. 1), and drought reduced light-saturated photosynthetic rates by approximately 30% (Table 3). In addition, drought reduced the photosynthetic light saturation point by about 20% (Table 2). Shading decreased average plant biomass by over 20 g, whilst respiration, efficiency of PSII, and the photosynthetic light compensation point of shade-grown plants were all 30% less than that of control plants (Tables 2, 3, Fig. 1). Control plants had the highest biomass of all treatments (Fig. 1), but may have suffered slightly from overwatering, given their non-significant tendency for lower maximum quantum yield relative to droughted plants (Fig. 2a). Several lines of evidence indicate that un-shaded plants experienced mild photoinhibition; the ‘full-sunlight’ treatment produced a significant, albeit small decrease in maximum quantum yield of plants, relative to the ‘shade’ treatment (v:Fm=0.81 vs. 0.83, respectively; Fig. 2a). High rates of non-photochemical quenching (>2) were measured in nearly half of all study plants (i.e. 29 of 64 plants), although neither drought nor shading resulted in significantly increased NPQ when analyzed across all species (Fig. 2b).
Fig. 1.
The effect of shading and drought on (a) total, (b) leaf, and (c) root biomass of the four study species. The control is indicated by black bars, drought is indicated by white bars, shade is indicated by dark gray bars, and shade + drought is indicated by light gray bars. Means (±SE) of all species include 16 replicates per treatment, and individual species means (±SE) include 4 replicates per treatment. Means not connected by the same letter are significantly different (Tukey HSD, p<0.05).
Table 3.
The effect of shading and drought on dark respiration (Rdark), maximum CO2 assimilation (Amax), photosynthetic rate under saturating light (Asat), and efficiency of PSII (ΦPSII) of the four study species. Means (±SE) include 16 replicates per treatment, and means not connected by the same letter are significantly different (Tukey HSD, p<0.05).
| Treatment | Rdark (μmol CO2 m−2 s−1) | Amax (μmol CO2 m−2 s−1) | Asat (μmol CO2 m−2 s−1) | ΦPSII (μmol CO2 μmol−1 photons) |
|---|---|---|---|---|
|
| ||||
| Control | 1.3±0.1 a | 35.4±3.2 a,b | 19.3±2.7 a | 0.152±0.015 a |
| Drought | 1.1±0.1 a | 37.4±3.5 a | 13.1±1.7 b | 0.140±0.012 a |
| Shade | 0.9±0.1 b | 31.4±2.9 b | 16.5±2.2 a,b | 0.108±0.011 b |
| Shade + Drought | 0.8±0.1 b | 32.6±2.4 a,b | 15.3±2.0 a,b | 0.111±0.010 b |
Table 2.
The effect of shading and drought on the light compensation point (Icomp), light saturation point (Imax), and apparent quantum yield (Φ) of the four study species. These parameters were obtained by measuring the response of net CO2 assimilation (An) to varying light intensities. Means (±SE) include 16 replicates per treatment, and means not connected by the same letter are significantly different (Tukey HSD, p<0.05).
| Treatment | Icomp (μmol photons m−2 s−1) | Imax (μmol photons m−2 s−1) | Φ (μmol CO2 μmol−1 photons) |
|---|---|---|---|
|
| |||
| Control | 42.3±3.2 a | 856±114 a | 0.041±0.004 a |
| Drought | 38.4±1.6 a | 669±82 b | 0.038±0.004 a |
| Shade | 29.1±1.6 b | 768±112 a,b | 0.042±0.004 a |
| Shade + Drought | 29.2±1.1 b | 691±88 a,b | 0.042±0.003 a |
Fig. 2.
The effect of shading and drought on (a) maximum quantum yield of PSII (Fv:Fm) and (b) efficiency of heat dissipation (i.e. non-photochemical quenching, NPQ) of the four study species. The control is indicated by black bars, drought is indicated by white bars, shade is indicated by dark gray bars, and shade + drought is indicated by light gray bars. Means (±SE) of all species include 16 replicates per treatment, and individual species means (±SE) include 4 replicates per treatment. Means not connected by the same letter are significantly different (Tukey HSD, p<0.05).
Despite these general responses across species, there was considerable variation in tolerance of shading and drought. There were significant species × treatment interactions for nearly every gas exchange (Imax, Rdark, ΦPSII, Amax, NPQ, Fv:Fm, but not Asat, Icomp) and growth parameter (total, leaf, and stem biomass, but not root biomass; Table 4). Two of the four study species exhibited shade-tolerant responses; shading did not affect total biomass or photosynthetic rate of Austrodanthonia or Chamaecytisus (Fig. 1, S2). Chamaecytisus was more tolerant of drought than other species, showing no decrease in growth (see responses of leaf, stem, and root biomass; Fig. 1). The grass Austrodanthonia was less drought-tolerant (31–40 g leaf biomass under drought vs. 49–57 g when well-watered; Fig. 1) and showed clear signs of photoinhibition (Fv:Fm=0.78 under shade vs. 0.81 under full-sun; Fig. 2a). On the other hand, shading reduced growth of Eucalyptus and Triticum by over 60% (Fig. 1), and both Eucalyptus and Triticum were susceptible to drought (see Fig. 1, S2). Full-sun, droughted Eucalyptus plants exhibited high non-photochemical quenching (2.34 vs. 1.66 for shaded, droughted plants; Fig. 2b).
Table 4.
Results of full-factorial, mixed-model ANOVAs including species as a random effect, treatment as a fixed effect, and measures of gas exchange physiology, chlorophyll fluorescence, and growth as dependent variables. Rdark, Icomp, NPQ, leaf, root, and stem biomass were ln-transformed to achieve normality. Degrees of freedom (df), F-statistics, and p-values are given. Asterisks denote significance (* p≤0.05, ** p≤0.01, *** p≤0.001).
| Response | Effect | df | F | p | |
|---|---|---|---|---|---|
| Icomp | Treatment | 3 | 13.07 | <0.001 | *** |
| (μmol photons m−2 s−1) | Species × Treatment | 9 | 1.01 | 0.447 | |
|
| |||||
| Imax | Treatment | 3 | 3.15 | 0.034 | * |
| (μmol photons m−2 s−1) | Species × Treatment | 9 | 2.10 | 0.048 | * |
|
| |||||
| Rdark | Treatment | 3 | 11.53 | <0.001 | *** |
| (μmol CO2 m−2 s−1) | Species × Treatment | 9 | 2.22 | 0.037 | * |
|
| |||||
| ΦPSII | Treatment | 3 | 10.14 | <0.001 | *** |
| (μmol CO2 μmol−1 photons) | Species × Treatment | 9 | 2.51 | 0.019 | * |
|
| |||||
| Amax | Treatment | 3 | 3.17 | 0.033 | * |
| (μmol CO2 m−2 s−1) | Species × Treatment | 9 | 2.35 | 0.028 | * |
|
| |||||
| Asat | Treatment | 3 | 5.43 | 0.003 | ** |
| (μmol CO2 m−2 s−1) | Species × Treatment | 9 | 1.89 | 0.076 | |
|
| |||||
| NPQ | Treatment | 3 | 0.42 | 0.742 | |
| Species × Treatment | 9 | 2.32 | 0.029 | * | |
|
| |||||
| Fv:Fm | Treatment | 3 | 9.26 | <0.001 | *** |
| Species × Treatment | 9 | 2.20 | 0.039 | * | |
|
| |||||
| Total biomass | Treatment | 3 | 10.93 | <0.001 | *** |
| (g) | Species × Treatment | 9 | 2.83 | 0.009 | ** |
|
| |||||
| Leaf biomass | Treatment | 3 | 28.32 | <0.001 | *** |
| (g) | Species × Treatment | 9 | 9.70 | <0.001 | *** |
|
| |||||
| Stem biomass | Treatment | 3 | 3.27 | 0.038 | * |
| (g) | Species × Treatment | 3 | 3.38 | 0.034 | * |
|
| |||||
| Root biomass | Treatment | 3 | 11.92 | <0.001 | *** |
| (g) | Species × Treatment | 9 | 1.85 | 0.082 | |
Drought effects on leaf lipids
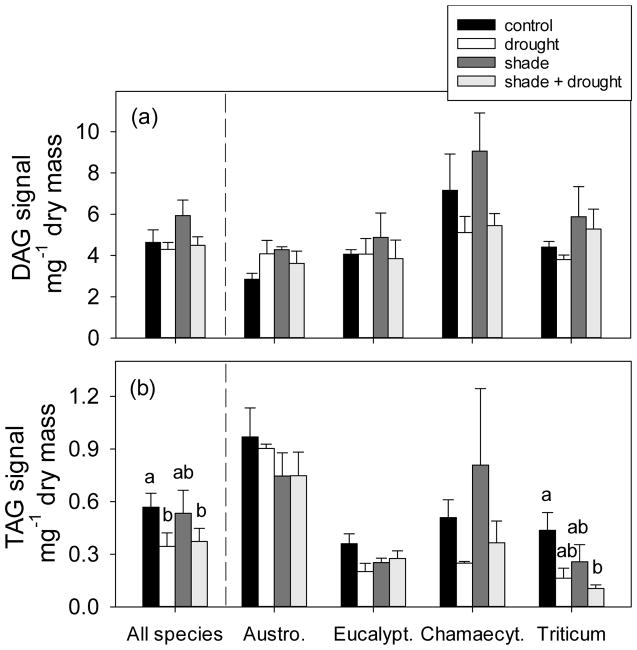
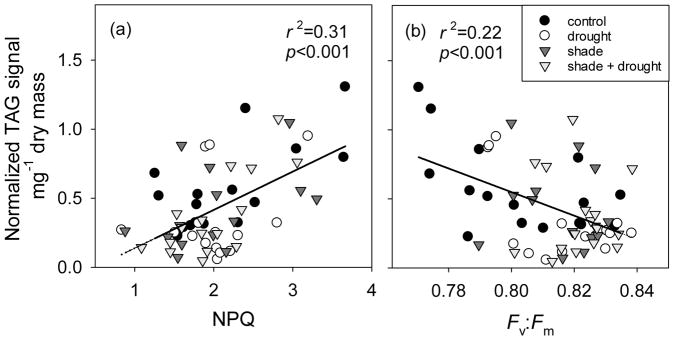
Across all species, drought decreased concentrations of TAGs in leaves by nearly 40% (Fig. 3b), but had no effect on DAGs (Fig. 3a) or total polar lipids (Fig. 5). Concentrations of neutral storage lipids varied among plant species; leaves of Austrodanthonia and Chamaecytisus were richer in DAGs and TAGs than those of Eucalyptus and Triticum (Fig. 3). Only leaves of Triticum showed reduced concentrations of TAGs during drought (Fig. 3b). Non-photochemical quenching, an indicator of photoinhibition, was positively correlated to TAG concentrations in leaves (Fig. 4a). Similarly, TAG concentrations were negatively correlated to Fv:Fm (Fig. 4b) and to photosynthetic capacity (Fig. S4). Responses of specific TAGs (based on their fatty acyl group) were consistent with responses observed for total TAGs; drought reduced concentrations of TAG species defined as 16:0, 18:0, 18:2, and 18:3 (Fig. S3). There was no effect of drought on 18:1 TAGs (Fig. S3).
Fig. 3.
The effect of shading and drought on (a) diacylglycerols (DAGs) and (b) triacylglycerols (TAGs) in leaves of four plant species. The control is indicated by black bars, drought is indicated by white bars, shade is indicated by dark gray bars, and shade + drought is indicated by light gray bars. Means (±SE) of all species include 14–16 replicates per treatment, and individual species means (±SE) include 3–4 replicates per treatment; means are normalized to internal standards. Means not connected by the same letter are significantly different (Tukey HSD, p<0.05).
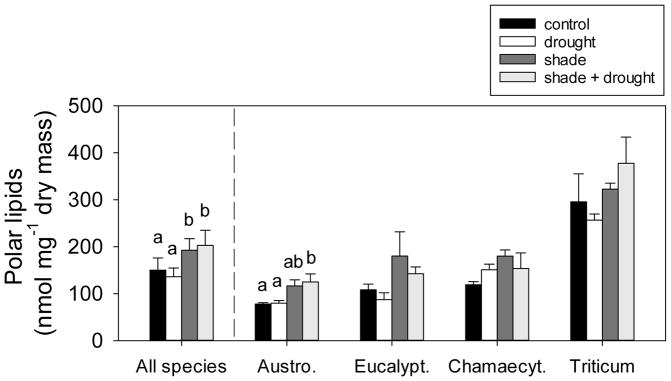
Fig. 5.
The effect of shading and drought on total polar lipids of the four study species. The control is indicated by black bars, drought is indicated by white bars, shade is indicated by dark gray bars, and shade + drought is indicated by light gray bars. Means (±SE) of all species include 14–16 replicates per treatment, and individual species means (±SE) include 3–4 replicates per treatment. Means not connected by the same letter are significantly different (Tukey HSD, p<0.05).
Fig. 4.
Relationship between (a) non-photochemical quenching (NPQ) and (b) maximum quantum yield of PSII (Fv:Fm) and triacylglycerol (TAG) concentration in leaves of four plant species under shading and drought. Plants in full-sunlight treatments are represented by circles, whereas plants in shaded treatments are represented by inverted triangles. Fluorescence was not measured on the same leaves as lipid concentration, but leaves for lipid determination were collected in the same week as fluorescence measurements.
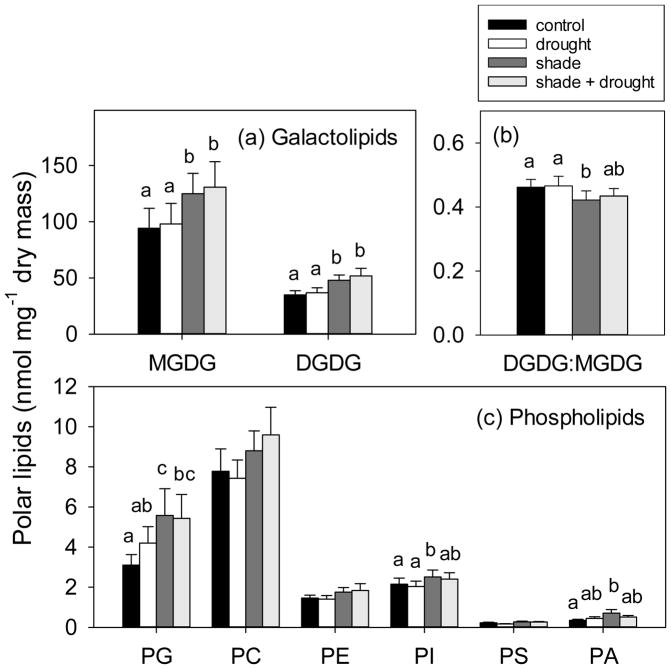
Shading effects on leaf lipids
Shading increased total concentrations of polar lipids in leaves by nearly 30% (Fig. 5), but had no effect on concentrations of neutral lipids (Fig. 3). Total concentrations of polar lipids were greatest in Triticum, compared to the other three species (Fig. 5). In considering individual responses of our study species, shading only significantly increased total polar lipids of Austrodanthonia (Fig. 5). Total concentrations of polar lipids in leaves were positively related to chlorophyll index (Fig. 6) and thus chlorophyll concentration. Shading increased galactolipids by 33–37% (Fig. 7a) and decreased the ratio of DGDG:MGDG (Fig. 7b). Shading also had dramatic effects on some phospholipid classes. For example, shading increased PG by 80%, PI by 17%, and PA by 102% (Fig. 7c), but did not affect concentrations of PC, PE, PS (Fig. 7c), or the ratio of PC:PE (F3,44=2.18, p=0.104; data not shown). Increases in phospholipids with shading were found in both the grass species, but not in the tree species (Table S2). The energy content of plant leaves was positively correlated with total polar lipid concentration (Fig. S5), but this trait was a poor indicator for leaf lipid concentration, as it only explained 14% of variation in polar lipids.
Fig. 6.
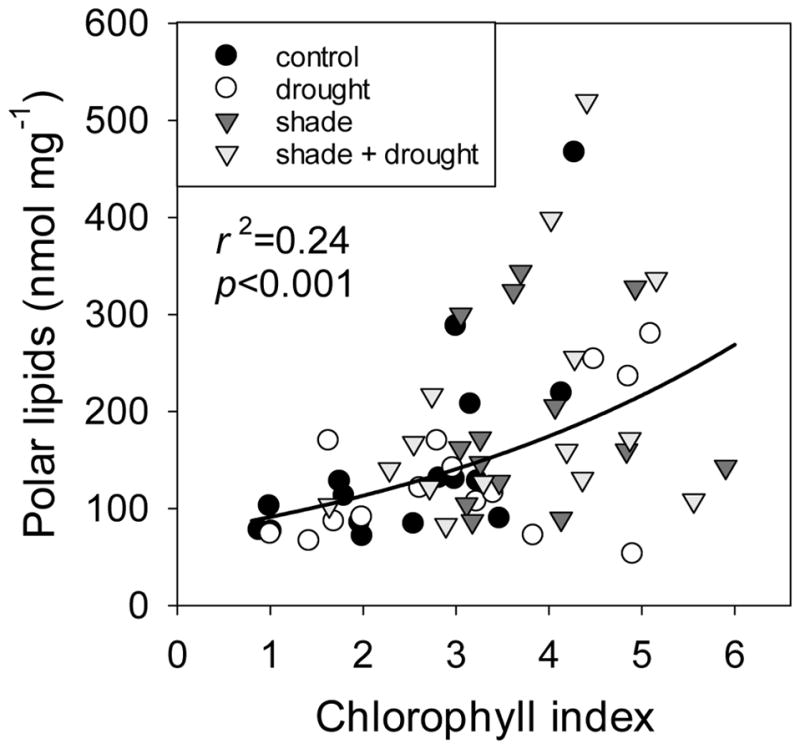
Relationship between chlorophyll index (R760–800/R540–560 − 1) and ln-transformed total polar lipid concentration for leaves of four plant species under shading and drought. The chlorophyll index is positively linearly related to chlorophyll concentration, i.e. larger numbers indicate higher chlorophyll concentrations. Plants in full-sunlight treatments are represented by circles, whereas plants in shaded treatments are represented by inverted triangles. Reflectance was not measured on the same leaves as lipid concentrations, but leaves were collected within three days of reflectance measurements.
Fig. 7.
The effect of shading and drought on polar lipid classes of (a) galactolipids, represented by monogalactosyldiacylglycerol (MGMG) and digalactosyldiacylglycerol (DGDG), (b) the ratio of DGDG:MGDG, and (c) phospholipids, represented by phosphatidylglycerol (PG), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), and phosphatidic acid (PA), in leaves of four study species. The control is indicated by black bars, drought is indicated by white bars, shade is indicated by dark gray bars, and shade + drought is indicated by light gray bars. Means (±SE) include 13–16 replicates per treatment, and means not connected by the same letter are significantly different (Tukey HSD, p<0.05).
Discussion
Four diverse plant species spanning a range of drought- and shade-tolerance (Table 1) were exposed to moderate levels of drought and shading in this experiment. To test our hypothesis that production of TAGs in leaves of water-stressed plants is a mechanism for dissipating excess radiation energy, we expected that TAG concentration of leaves would (1) increase with drought and (2) decrease with shading. Instead, we observed a 40% decrease in TAGs of leaves under drought and no effect of shading on TAG concentration (Fig. 3b). While these results do not match our expectation, we observed that high TAG accumulation in leaves corresponded to higher rates of non-photochemical quenching and reduced maximum quantum yield of PSII (Fv:Fm; Fig. 4). This suggests that plants under oxidative stress may increase biosynthesis of TAGs, in support of our main hypothesis. Accumulation of TAGs did not follow photosynthetic capacity (Fig. S4), as has been previously suggested (Lin and Oliver 2008, Perlikowski et al. 2016). Concentration of TAGs changes diurnally (greatest during the day, least at night) and seasonally (greatest in the second half of the growing season) for crabapple (Malus sp.; Lin and Oliver 2008); both patterns support our hypothesis that accumulation of TAGs is a mechanism for dissipation of excess radiation energy in leaves. Synthesis of a typical TAG requires 993 kJ energy per unit carbon and thus uses 53% more energy than for synthesis of storage carbohydrate (650 kJ/C, Subramanian et al. 2013). The weak overall response of TAGs in our experiment (Fig. 3b) seems most likely due to reduction in light intensity within the greenhouse (i.e. maximum PAR=1600 μmol m−2 s−1) compared to full sunlight. Despite high rates of energy dissipation as heat, all plants in this experiment had predawn Fv:Fm greater than 0.77, indicating only mild photoinhibition (Lawlor and Tezara 2009, Nichol et al. 2012, Valladares and Pearcy 2002). This light level was insufficient to induce strong photoinhibition and thus substantial increases in TAG biosynthesis. Further research is required to determine if TAGs serve a photoprotective function in plant leaves.
Role of neutral lipids in plant leaves under drought
In plant leaves, DAGs are mostly transient building blocks for synthesis of other lipids, including TAGs, phospho-, and galactolipids (Cagliari et al. 2011). Severe drought has been reported to enhance synthesis of TAGs from DAGs (Gasulla et al. 2013, Perlikowski et al. 2016), which has been associated with stabilization of membranes during dehydration (Gasulla et al. 2013). Increased membrane stability could be facilitated by (1) the conical shape of DAG molecules rendering membranes less stable (Goni and Alonso 1999), and/or (2) TAG oil bodies absorbing excess membrane lipids that form as cells shrink during dehydration (Gasulla et al. 2013). Not all species may be capable of this conversion process. For example, neither desiccation-sensitive Arabidopsis and Lindernia subracemosa, nor desiccation-tolerant Lindernia brevidens and Paraisometrum mileense, showed any change in DAG/TAG concentrations during dehydration (Gasulla et al. 2013, Li et al. 2014). While we observed no effect of moderate drought on concentrations of DAGs (Fig. 3a), further research is needed to determine if our study species are capable of converting DAGs to TAGs under more severe drought.
The literature contains numerous examples of increasing TAG concentrations under severe drought conditions (Benadjaoud et al. 2013, Douglas and Paleg 1981, Gasulla et al. 2013, Martin et al. 1986, Perlikowski et al. 2016, Pham Thi et al. 1989). For example, large increases (125%) in TAGs were observed in desiccated leaves of palo verde (Parkinsonia aculeate; Benadjaoud et al. 2013). Here, we found that moderate drought decreased concentrations of all TAG molecular species (Fig. S3), as well as overall TAG concentrations of leaves by nearly 40% (Fig. 3b). Nearly all previous studies have examined plant responses to the onset of dehydration (i.e. no watering for days or weeks), rather than during sustained moderate drought stress. In the study by Perlikowski et al. (2016), a gradual drought stimulated TAG accumulation in only one of two genotypes of forage grass (Lolium–Festuca hybrids) after 11 days, corresponding to a soil water concentration of only 3% of field capacity. Our finding that TAG concentrations decline with drought (Fig. 3b), at least initially, argue in support of inhibition of TAG biosynthesis and/or degradation of neutral storage lipids. Degradation of foliar TAGs may represent an early adaptive response to water deficit in drought-sensitive plants, that is then followed by accumulation of TAGs as water deficit becomes more severe (e.g. Perlikowski et al. 2016).
Despite decades of research, the functional role of neutral lipids remains unclear. A difficulty in comparing TAG concentrations across studies is the lack of standard protocols for lipid extractions from plant leaves. Methods originally described by Folch (1957) and Bligh and Dyer (1959) are widely used, but are often modified (Booij and Vandenberg 1994, De Boer 1988, Smedes and Askland 1999). The lipid extraction protocol developed for this study requires a longer extraction time than previously described methods (e.g. Welti et al. 2002), but is suitable for thick, long-lived leaves of native Australian trees (e.g. Eucalyptus, Acacia species, Table S1). The method-dependence of lipid data for plant leaves has been well recognized (Roose and Smedes 1996), and comparisons across studies are thus necessarily relative and not absolute (Furse et al. 2015). A standardization of extraction method would facilitate comparisons across studies.
Effect of shading on polar lipids in plant leaves
Shading increased overall polar lipid concentrations (Fig. 5), especially concentrations of the two galactolipids, MGDG and DGDG (and their precursor PA), and the phospholipid PG (Fig. 7). All are essential components of chloroplast membranes (Dubots et al. 2012, Fuller and Nas 1987, Sato et al. 2000). These changes confirmed our hypothesis that leaves contain more polar lipids when formed in the shade (Fig. 5). Structural and chemical alterations that accompany shade-acclimation of chloroplasts include development of larger granal stacks, proliferation of thylakoid membranes and PSII complexes, and increased concentration of chlorophyll b (Lambers et al. 2008). We estimated chlorophyll concentrations of leaves to confirm that shaded leaves with high polar lipid concentrations contain more chlorophyll (Fig. 6). The capacity to modify chloroplast physiology, and accompanying polar lipid profiles, facilitated a photosynthetic response to shade in our study species – the light compensation point, photosynthetic rate, and respiration rate were all diminished when plants were grown under shade (Tables 2, 3).
A novel result reported here is the increase in polar lipid concentrations (MGDG, PG, PC, PI, PA) in response to shade by the native Australian grass, Austrodanthonia (Fig. 5, Table S2). We could find only a single previous study that examined the response of leaf lipids to shading. This previous study reported that polar lipid concentration (per unit leaf mass) of upper canopy leaves was not sensitive to shading in soybean (Burkey et al. 1997). The substantial investment in total polar lipids under shaded conditions (57% greater than the control) by the shade-tolerant Austrodanthonia was accompanied by greater leaf biomass (Fig. 1b) and reduced photoinhibition (Fig. 2a).
Polar lipid profiles in leaves produced under shade differed from those produced in the sun, with resources preferentially allocated to MGDG over DGDG (i.e. reduced DGDG:MGDG ratio; Fig. 7b). Production of the unsaturated polar lipid MGDG (Gounaris and Barber 1983) appears more aligned with stress than DGDG (Gigon et al. 2004, Pham Thi et al. 1989), allowing for increased fluidity of the thylakoid membrane bilayer (Dörmann and Benning 2002, Gounaris and Barber 1983). Maintaining fluidity may be more important for shade leaves due to the greater number of granal stacks and PSII that are physically separated from PSI. Interaction between PSI, located in the non-appressed regions of the thylakoid membrane, and PSII, located in the appressed regions, is required for the photochemical reactions of photosynthesis (Lambers et al. 2008).
The phospholipid PG increased with shading in both grass species (Table S2). This minor lipid component is important in stabilizing the trimeric form of light-harvesting complex II (LHCII, Standfuss et al. 2005) and the dimeric form of PSII (Kruse et al. 2000) in thylakoid membranes. In Arabidopsis thaliana mutants deficient in PG, thylakoid membranes do not develop into a granum structure (Hagio et al. 2002). High concentrations of PG may allow for increased rates of electron transfer (Kim et al. 2007) in shade-adapted leaves and/or disaggregation of the LHCII (Schaller et al. 2011), leading to increased energy transfer from PSII to PSI (Apostolova et al. 2006). We have shown that shading increases foliar polar lipid concentration and the degree of unsaturation of thylakoid membranes, which appears to be a common response in shade-tolerant plant species.
Supplementary Material
Acknowledgments
We thank Antony Vuragu and Tom Buckley for help with planting, watering, and climate control of the greenhouses at the I.A. Watson Grains Research Centre. The lipid analyses described in this work were performed by M. Roth and R. Welti at the Kansas Lipidomics Research Center Analytical Laboratory; instrument acquisition and lipidomics method development was supported by National Science Foundation (EPS 0236913, MCB 1413036, MCB 0920663, DBI 0521587, DBI 1228622), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20GM103418), and Kansas State University.
Abbreviations
- DAGs
diacylglycerols
- DGDG
digalactosyldiacylglycerol
- MGMG
monogalactosyldiacylglycerol
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PS
phosphatidylserine
- TAGs
triacylglycerols
Footnotes
Author contributions
M.A.A. and T.L.T. designed research; R.M.M. and A.I.D. performed research; R.M.M. analyzed data; R.M.M., T.L.T., and M.A.A. wrote the paper.
References
- Apostolova EL, Dobrikova AG, Ivanova PI, Petkanchin IB, Taneva SG. Relationship between the organization of the PSII supercomplex and the functions of the photosynthetic apparatus. J Photochem Photobiol B:Biol. 2006;83:114–122. doi: 10.1016/j.jphotobiol.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakumar T, Vincent VHB, Paliwal K. On the interaction of UV-B radiation (280–315 nm) with water stress in crop plants. Physiol Plant. 1993;87:217–222. [Google Scholar]
- Benadjaoud A, Benhassaine-Kesri G, Zachowski A, Aid F. Effects of dehydration and rehydration on the leaf lipids and lipid metabolism in Parkinsonia aculeata (Caesalpiniaceae) Botany. 2013;91:505–513. [Google Scholar]
- Bilger W, Bjorkman O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbency changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res. 1990;25:173–185. doi: 10.1007/BF00033159. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bogdanov IV, Shenkarev ZO, Finkina EI, Melnikova DN, Rumynskiy EI, Arseniev AS, Ovchinnikova TV. A novel lipid transfer protein from the pea Pisum sativum: isolation, recombinant expression, solution structure, antifungal activity, lipid binding, and allergenic properties. BMC Plant Biol. 2016;16:107. doi: 10.1186/s12870-016-0792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij K, Vandenberg C. Comparison of techniques for the extraction of lipids and PCBs from benthic invertebrates. B Environ Contam Tox. 1994;53:71–76. doi: 10.1007/BF00205141. [DOI] [PubMed] [Google Scholar]
- Brown MR, Dunstan GA, Norwood SJ, Miller KA. Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. J Phycology. 1996;32:64–73. [Google Scholar]
- Brugnoli E, Bjorkman O. Chloroplast movements in leaves - Influence on chlorophyll fluorescence and measurements of light-induced absorbency changes related to ΔpH and zeaxanthin formation. Photosynth Res. 1992;32:23–35. doi: 10.1007/BF00028795. [DOI] [PubMed] [Google Scholar]
- Burkey KO, Wilson RF, Wells R. Effects of canopy shade on the lipid composition of soybean leaves. Physiol Plant. 1997;101:591–598. [Google Scholar]
- Buseman CM, Tamura P, Sparks AA, Baughman EJ, Maatta S, Zhao J, Roth MR, Esch SW, Shah J, Williams TD, Welti R. Wounding stimulates the accumulation of glycerolipids containing oxophytodienoic acid and dinor-oxophytodienoic acid in Arabidopsis leaves. Plant Physiol. 2006;142:28–39. doi: 10.1104/pp.106.082115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliari A, Margis R, dos Santos Maraschin F, Turchetto-Zolet AC, Loss G, Margis-Pinheiro M. Biosynthesis of triacylglycerols (TAGs) in plants and algae. Int J Plant Biol. 2011;2:40–52. [Google Scholar]
- Camarero JJ, Olano JM, Alfaro SJA, Fernandez-Marin B, Becerril JM, Garcia-Plazaola JI. Photoprotection mechanisms in Quercus ilex under contrasting climatic conditions. Flora. 2012;207:557–564. [Google Scholar]
- Chapman KD, Dyer JM, Mullen RT. Commentary: Why don’t plant leaves get fat? Plant Sci. 2013;207:128–134. doi: 10.1016/j.plantsci.2013.03.003. [DOI] [PubMed] [Google Scholar]
- De Boer J. Chlorobiphenyls in bound and non-bound lipids of fishes - Comparison of different extraction methods. Chemosphere. 1988;17:1803–1810. [Google Scholar]
- Demmig-Adams B, Adams WW. Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Molec Biol. 1992;43:599–626. [Google Scholar]
- Demmig B, Winter K, Kruger A, Czygan FC. Photoinhibition and zeaxanthin formation in intact leaves - A possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987;84:218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörmann P, Benning C. Galactolipids rule in seed plants. Trends in Plant Science. 2002;7:112–118. doi: 10.1016/s1360-1385(01)02216-6. [DOI] [PubMed] [Google Scholar]
- Douglas TJ, Paleg LG. Lipid composition of Zea mays seedlings and water stress-induced changes. J Exp Bot. 1981;32:499–508. [Google Scholar]
- Dubots E, Botte C, Boudiere L, Yamaryo-Botte Y, Jouhet J, Marechal E, Block MA. Role of phosphatidic acid in plant galactolipid synthesis. Biochimie. 2012;94:86–93. doi: 10.1016/j.biochi.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Farage PK, Long SP. The occurrence of photoinhibition in an over-wintering crop of oil-seed rape (Brassica napus L.) and its correlation with changes in crop growth. Planta. 1991;185:279–286. doi: 10.1007/BF00194071. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fuller G, Nas WD. Plant lipids and their interactions. In: Fuller G, editor. Ecology and Metabolism of Plant Lipids. American Chemical Society; Washington D.C: 1987. pp. 2–8. [Google Scholar]
- Furse S, Egmond MR, Killian JA. Isolation of lipids from biological samples. Mol Membr Biol. 2015;32:55–64. doi: 10.3109/09687688.2015.1050468. [DOI] [PubMed] [Google Scholar]
- Gamon JA, Pearcy RW. Leaf movement, stress avoidance and photosynthesis in Vitis californica. Oecologia. 1989;79:475–481. doi: 10.1007/BF00378664. [DOI] [PubMed] [Google Scholar]
- Garrett TA, Raetz CRH, Richardson T, Kordestani R, Son JD, Rose RL. Identification of phosphatidylserylglutamate: a novel minor lipid in Escherichia coli. J Lipid Res. 2009;50:1589–1599. doi: 10.1194/jlr.M800549-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasulla F, vom Dorp K, Dombrink I, Zahringer U, Gisch N, Dormann P, Bartels D. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: a comparative approach. Plant J. 2013;75:726–741. doi: 10.1111/tpj.12241. [DOI] [PubMed] [Google Scholar]
- Gigon A, Matos AR, Laffray D, Zuily-Fodil Y, Pham-Thi AT. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (ecotype Columbia) Ann Bot. 2004;94:345–351. doi: 10.1093/aob/mch150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelson AA, Gritz Y, Merzlyak MN. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J Plant Physiol. 2003;160:271–282. doi: 10.1078/0176-1617-00887. [DOI] [PubMed] [Google Scholar]
- Gitelson AA, Keydan GP, Merzlyak MN. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys Res Lett. 2006;33:114–120. [Google Scholar]
- Goni FM, Alonso A. Structure and functional properties of diacylglycerols in membranes. Progress Lipid Res. 1999;38:1–48. doi: 10.1016/s0163-7827(98)00021-6. [DOI] [PubMed] [Google Scholar]
- Gounaris K, Barber J. Monogalactosyldiacylglycerol: the most abundant polar lipid in nature. Trends Biochem Sci. 1983;8:378–381. [Google Scholar]
- Grover A, Agarwal M, Katiyar-Agarwal S, Sahi C, Agarwal S. Production of high temperature tolerant transgenic plants through manipulation of membrane lipids. Current Sci. 2000;79:557–559. [Google Scholar]
- Hagio M, Sakurai I, Sato S, Kato T, Tabata S, Wada H. Phosphatidylglycerol is essential for the development of thylakoid membranes in Arabidopsis thaliana. Plant Cell Physiol. 2002;43:1456–1464. doi: 10.1093/pcp/pcf185. [DOI] [PubMed] [Google Scholar]
- Hartel H, Dormann P, Benning C. DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc Natl Acad Sci USA. 2000;97:10649–10654. doi: 10.1073/pnas.180320497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Okazaki Y, Myouga F, Shinozaki K, Saito K. Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci Reports. 2015;5:10533. doi: 10.1038/srep10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 2008;54:621–639. doi: 10.1111/j.1365-313X.2008.03492.x. [DOI] [PubMed] [Google Scholar]
- Kim EH, Razeghifard R, Anderson JM, Chow WS. Multiple sites of retardation of electron transfer in Photosystem II after hydrolysis of phosphatidylglycerol. Photosynth Res. 2007;93:149–158. doi: 10.1007/s11120-006-9126-0. [DOI] [PubMed] [Google Scholar]
- Kok B. On the inhibition of photosynthesis by intense light. Biochim Biophys Acta. 1956;21:234–244. doi: 10.1016/0006-3002(56)90003-8. [DOI] [PubMed] [Google Scholar]
- Kruse O, Hankamer B, Konczak C, Gerle C, Morris E, Radunz A, Schmid GH, Barber J. Phosphatidylglycerol is involved in the dimerization of photosystem II. J Biol Chem. 2000;275:6509–6514. doi: 10.1074/jbc.275.9.6509. [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapin FS, Pons TL. Plant Physiological Ecology. Springer; New York: 2008. [Google Scholar]
- Larkindale J, Huang BR. Changes of lipid composition and saturation level in leaves and roots for heat-stressed and heat-acclimated creeping bentgrass (Agrostis stolonifera) Environ Exp Bot. 2004;51:57–67. [Google Scholar]
- Lawlor DW, Tezara W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot. 2009;103:561–579. doi: 10.1093/aob/mcn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Welti R, Schapaugh WT, Trick HN. Phospholipid and triacylglycerol profiles modified by PLD suppression in soybean seed. Plant Biotechnol J. 2011;9:359–372. doi: 10.1111/j.1467-7652.2010.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AH, Wang DD, Yu BZ, Yu XM, Li WQ. Maintenance or Collapse: Responses of extraplastidic membrane lipid composition to desiccation in the resurrection plant Paraisometrum mileense. PLoS ONE. 2014;9:e103430. doi: 10.1371/journal.pone.0103430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MY, Welti R, Wang XM. Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of PLDzeta1 and PLDzeta2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol. 2006;142:750–761. doi: 10.1104/pp.106.085647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang R, Li M, Li L, Wang C, Welti R, Wang X. Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in Arabidopsis thaliana. J Biol Chem. 2008;283:461–468. doi: 10.1074/jbc.M706692200. [DOI] [PubMed] [Google Scholar]
- Lin WL, Oliver DJ. Role of triacylglycerols in leaves. Plant Sci. 2008;175:233–237. [Google Scholar]
- Lippold F, vom Dorp K, Abraham M, Holzl G, Wewer V, Yilmaz JL, Lager I, Montandon C, Besagni C, Kessler F, Stymne S, Dormann P. Fatty acid phytyl ester synthesis in chloroplasts of Arabidopsis. Plant Cell. 2012;24:2001–2014. doi: 10.1105/tpc.112.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo FD, de Barros MP, Dalmagro HJ, Dalmolin AC, Pereira WE, de Souza EC, Vourlitis GL, Ortiz CER. Fitting net photosynthetic light-response curves with Microsoft Excel - a critical look at the models. Photosynthetica. 2013;51:445–456. [Google Scholar]
- Long SP, Humphries S, Falkowski PG. Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Molec Biol. 1994;45:633–662. [Google Scholar]
- Losciale P, Chow WS, Grappadelli LC. Modulating the light environment with the peach ‘asymmetric orchard’: effects on gas exchange performances, photoprotection, and photoinhibition. J Exp Bot. 2010;61:1177–1192. doi: 10.1093/jxb/erp387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BA, Schoper JB, Rinne RW. Changes in soybean (Glycine max [L.] Merr.) glycerolipids in response to water stress. Plant Physiol. 1986;81:798–801. doi: 10.1104/pp.81.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol CJ, Pieruschka R, Takayama K, Forster B, Kolber Z, Rascher U, Grace J, Robinson SA, Pogson B, Osmond B. Canopy conundrums: building on the Biosphere 2 experience to scale measurements of inner and outer canopy photoprotection from the leaf to the landscape. Funct Plant Biol. 2012;39:1–24. doi: 10.1071/FP11255. [DOI] [PubMed] [Google Scholar]
- Ögren E, Sjöström M. Estimation of the effect of photoinhibition on the carbon gain in leaves of a willow canopy. Planta. 1990;181:560–567. doi: 10.1007/BF00193011. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Otsuki H, Narisawa T, Kobayashi M, Sawai S, Kamide Y, Kusano M, Aoki T, Hirai MY, Saito K. A new class of plant lipid is essential for protection against phosphorus depletion. Nat Commun. 2013;4:1510. doi: 10.1038/ncomms2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcutt DM, Patterson GW. Effect of light intensity upon lipid composition of Nitzschia closterium (Cylindrotheca fusiformis) Lipids. 1974;9:1000–1003. doi: 10.1007/BF02533825. [DOI] [PubMed] [Google Scholar]
- Ort DR. When there is too much light. Plant Physiol. 2001;125:29–32. doi: 10.1104/pp.125.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlikowski D, Kierszniowska S, Sawikowska A, Krajewski P, Rapacz M, Eckhardt A, Kosmala A. Remodeling of leaf cellular glycerolipid composition under drought and re-hydration conditions in grasses from the Lolium-Festuca complex. Front Plant Sci. 2016;7:1027. doi: 10.3389/fpls.2016.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham Thi AT, El-Hafid L, Zuily-fodil Y, da Silva JV. Enzymatic breakdown of polar lipids in cotton leaves under water stress. In: Biacs PA, Gruiz K, Kremmer T, editors. Biological Role of Plant Lipids. Springer; US: 1989. pp. 527–529. [Google Scholar]
- Pierce S. The jeweled armor of Tillandsia—Multifaceted or elongated trichomes provide photoprotection. Aliso: J Syst Evol Bot. 2007;23:44–52. [Google Scholar]
- Prioul JL, Chartier P. Partitioning of transfer and carboxylation components of intracellular resistance to photosynthetic CO2 fixation: A critical analysis of the methods used. Ann Bot. 1977;41:789–800. [Google Scholar]
- Raven JA. The cost of photoinhibition. Physiol Plant. 2011;142:87–104. doi: 10.1111/j.1399-3054.2011.01465.x. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G. Achieving yield gains in wheat. Plant Cell Environ. 2012;35:1799–1823. doi: 10.1111/j.1365-3040.2012.02588.x. [DOI] [PubMed] [Google Scholar]
- Robinson SA, Lovelock CE, Osmond CB. Wax as a mechanism for protection against photoinhibition - A study of Cotyledon orbiculata. Botanica Acta. 1993;106:307–312. [Google Scholar]
- Roose P, Smedes F. Evaluation of the results of the QUASIMEME lipid intercomparison: The Bligh & Dyer total lipid extraction method. Mar Pollut Bull. 1996;32:674–680. [Google Scholar]
- Sato N, Hagio M, Wada H, Tsuzuki M. Requirement of phosphatidylglycerol for photosynthetic function in thylakoid membranes. Proc Natl Acad Sci USA. 2000;97:10655–10660. doi: 10.1073/pnas.97.19.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller S, Latowski D, Jemiola-Rzeminska M, Dawood A, Wilhelm C, Strzalka K, Goss R. Regulation of LHCII aggregation by different thylakoid membrane lipids. Biochimica Et Biophysica Acta. 2011;1807:326–335. doi: 10.1016/j.bbabio.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin NS, Chisholm SW. Phytoplankton lipids - Interspecific differences and effects of nitrate, silicate and light-dark cycles. J Phycol. 1981;17:374–384. [Google Scholar]
- Shoemaker DP, Garland CW, Steinfeld DP. Experiments in Physical Chemistry. McGraw-Hill Inc; New York: 1974. [Google Scholar]
- Smedes F, Askland TK. Revisiting the development of the Bligh and Dyer total lipid determination method. Mar Pollut Bull. 1999;38:193–201. [Google Scholar]
- Solovchenko AE. Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses. Russ J Plant Physiol. 2012;59:167–176. [Google Scholar]
- Spoehr HA, Milner HW. The chemical composition of Chlorella - Effect of environmental conditions. Plant Physiol. 1949;24:120–149. doi: 10.1104/pp.24.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standfuss J, van Scheltinga ACT, Lamborghini M, Kuehlbrandt W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. Eur Mol Biol Organization J. 2005;24:919–928. doi: 10.1038/sj.emboj.7600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Barry AN, Pieris S, Sayre RT. Comparative energetics and kinetics of autotrophic lipid and starch metabolism in chlorophytic microalgae: implications for biomass and biofuel production. Biotechnol Biofuels. 2013;6:150. doi: 10.1186/1754-6834-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukenik A, Carmeli Y, Berner T. Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J Phycol. 1989;25:686–692. [Google Scholar]
- Takahashi S, Badger MR. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 2011;16:53–60. doi: 10.1016/j.tplants.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Thompson GA. Lipids and membrane function in green algae. Biochim Biophys Acta-Lipids and Lipid Metabolism. 1996;1302:17–45. doi: 10.1016/0005-2760(96)00045-8. [DOI] [PubMed] [Google Scholar]
- Valladares F, Pearcy RW. Drought can be more critical in the shade than in the sun: a field study of carbon gain and photo-inhibition in a Californian shrub during a dry El Nino year. Plant Cell Environ. 2002;25:749–759. [Google Scholar]
- Vu HS, Shiva S, Roth MR, Tamura P, Zheng LQ, Li MY, Sarowar S, Honey S, McEllhiney D, Hinkes P, Seib L, Williams TD, Gadbury G, Wang XM, Shah J, Welti R. Lipid changes after leaf wounding in Arabidopsis thaliana: expanded lipidomic data form the basis for lipid co-occurrence analysis. Plant J. 2014;80:728–743. doi: 10.1111/tpj.12659. [DOI] [PubMed] [Google Scholar]
- Welti R, Li WQ, Li MY, Sang YM, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang XM. Profiling membrane lipids in plant stress responses - Role of phospholipase Da in freezing-induced lipid changes in Arabidopsis. J Biol Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- Welti R, Shah J, Li WQ, Li MY, Chen JP, Burke JJ, Fauconnier ML, Chapman K, Chye ML, Wang XM. Plant lipidomics: Discerning biological function by profiling plant complex lipids using mass spectrometry. Front Biosci. 2007;12:2494–2506. doi: 10.2741/2250. [DOI] [PubMed] [Google Scholar]
- Xiao S, Gao W, Chen QF, Chan SW, Zheng SX, Ma JY, Wang MF, Welti R, Chye ML. Overexpression of Arabidopsis acyl-CoA binding protein ACBP3 promotes starvation-induced and age-dependent leaf senescence. Plant Cell. 2010;22:1463–1482. doi: 10.1105/tpc.110.075333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.