Abstract
The mosquito Aedes (Ae). aegypti transmits the viruses that cause dengue and chikungunya, two globally-important vector-borne diseases. We investigate how choosing alternate emissions and/or socioeconomic pathways may modulate future human exposure to Ae. aegypti. Occurrence patterns for Ae. aegypti for 2061-2080 are mapped globally using empirically downscaled air temperature and precipitation projections from the Community Earth System Model, for the Representative Concentration Pathway (RCP) 4.5 and 8.5 scenarios. Population growth is quantified using gridded global population projections consistent with two Shared Socioeconomic Pathways (SSPs), SSP3 and SSP5. Change scenarios are compared to a 1950-2000 reference period. A global land area of 56.9 M km2 is climatically suitable for Ae. aegypti during the reference period, and is projected to increase by 8% (RCP4.5) to 13% (RCP8.5) by 2061-2080. The annual average number of people exposed globally to Ae. aegypti for the reference period is 3794 M, a value projected to statistically significantly increase by 298-460 M (8-12%) by 2061-2080 if only climate change is considered, and by 4805-5084 M (127-134%) for SSP3 and 2232-2483 M (59-65%) for SSP5 considering both climate and population change (lower and upper values of each range represent RCP4.5 and RCP8.5 respectively). Thus, taking the lower-emissions RCP4.5 pathway instead of RCP8.5 may mitigate future human exposure to Ae. aegypti globally, but the effect of population growth on exposure will likely be larger. Regionally, Australia, Europe and North America are projected to have the largest percentage increases in human exposure to Ae. aegypti considering only climate change.
Keywords: Climate change, Aedes aegypti, dengue, chikungunya, BRACE
1. Introduction
The mosquito Aedes (Ae). aegypti serves as the primary vector for the viruses that cause dengue, the most prevalent vector-borne viral disease (WHO 2009). About 2.5 billion people in over 100 countries live in regions of high dengue risk (Kroeger and Nathan 2005), and a recent study estimated that up to 390 million dengue virus infections occur worldwide annually (Bhatt et al. 2013). When present, the febrile symptoms of dengue virus infections are generally mild to moderate, but about 1% of infections are manifested as the more severe dengue hemorrhagic fever (Gubler 1998). Aedes aegypti is also a vector of chikungunya virus, which can cause dengue-like symptoms in infected persons (Pialoux et al. 2007). The annual global occurrence of chikungunya is not well known; however, sporadic epidemics affecting >1M people have occurred in Asia and, more recently, the Americas (Pialoux et al. 2007, Nasci 2014, Halstead 2015). The ranges of dengue and chikungunya are expanding (Eisen and Moore 2013, Morrison 2014), making Ae. aegypti a vector of growing importance.
Climate variability largely determines the distribution and population dynamics of Ae. aegypti at the global scale (e.g., Capinha et al. 2014). Aedes aegypti is typically found in tropical and subtropical regions worldwide, within urban areas where it can exploit artificial water-filled containers for its immature (larval and pupal) stages (Tabachnick and Powell 1979). Its northern (southern) hemisphere range is generally equatorward of the January (July) average 10°C isotherm, but in some cases can extend as far poleward as the 1.8°C January average isotherm in North America and Europe (Christophers 1960). At local scales, temperature, humidity and precipitation influence Ae. aegypti development, reproduction and survival (Focks et al. 1993, Morin et al. 2013). Warmer temperatures are associated with higher adult mosquito abundance, because immature Ae. aegypti development rates increase as water temperatures rise concurrently with air temperatures (Eisen et al. 2014). However, extremely high temperatures (above ~ 32°C) can decrease development rates and increase adult mortality (Christophers 1960). Higher rainfall generally increases the number of suitable container habitats for Ae.aegypti, and higher humidity prevents desiccation of adults, facilitating greater mosquito abundance (Lucio et al. 2013).
Numerous studies employing varying methodologies have examined the potential for anthropogenic climate change to alter the global range of Ae. aegypti (Campbell et al. 2014, Capinha et al. 2014, Khormi and Kumar 2014, Rogers 2015). Collectively, the results suggest that climate change (in particular warmer temperatures) may cause the future range of Ae. aegypti to expand poleward from its present margins of survival. However, within some regions presently suitable for Ae. aegypti, the future results are not as consistent among studies. Less suitable habitats may result from climatic conditions that become too hot or dry (Rogers 2015). The effects of rising temperatures on Ae. aegypti populations are comparatively robust among the studies that consider temperature as a variable, but the effects of precipitation shifts are less certain. Manual filling of mosquito container habitats by humans may dampen the response of Ae. aegypti to rainfall fluctuations (Kearney et al. 2009), and in some instances, increased water storage practices in response to locally drier conditions may even enhance future habitat suitability for Ae. aegypti (Beebe et al. 2009). Some argue that such human factors, and others including intervention practices (e.g., pesticide use), urbanization, and transportation networks, may be stronger determinants of the range of Ae. aegypti than climate (e.g., Gubler 2002, Parham et al. 2015).
This paper is part of a project on the Benefits of Reduced Anthropogenic Climate changE (BRACE; O'Neill and Gettelman 2016.). BRACE aims to characterize the differential climate outcomes and subsequent impacts on a variety of sectors that result from differences in greenhouse gas (GHG) forcing from two distinct emissions pathways: one in which GHG emissions increase throughout the 21st century, and the other in which GHG emissions eventually decrease. Related BRACE papers on health impacts examine population exposure to extreme heat events (Xu et al. 2015, Jones et al. 2016) and future risks of heat-related mortality (Anderson et al. 2016a,b, Marsha et al. 2016). Here, we define four types of occurrence patterns for the virus vector mosquito Ae. aegypti on the basis of temperature and precipitation thresholds following Eisen et al. (2014). An ensemble of global climate model simulations is then employed to characterize how these patterns may shift in the late 21st century for two different climate change outcomes associated with the two distinct GHG emissions pathways. Finally, two plausible population pathways for humanity are combined with the projected mosquito occurrence patterns to estimate the number of humans exposed to Ae. aegypti in the late 21st century. The present study is unique in that 1) it uses straightforward climatic thresholds to define distinct Ae. aegypti occurrence patterns; 2) it explores the relative roles of climatic versus population changes on the future number of people exposed to Ae. aegypti; and 3) it quantifies the avoided climatic impacts of following a lower versus higher intensity GHG emissions pathway.
2. Methods
A brief overview of methods is provided here. A full description is in Online Resource 1.
Eisen et al. (2014) establish four types of occurrence patterns for Ae. aegypti, based on temperature and precipitation thresholds in representative cities in which Ae. aegypti is present:
Type 1. Year-around potential for high abundance of Ae. aegypti (e.g., Manila, Philippines);
Type 2. Year-around presence of Ae. aegypti but only seasonal potential for high abundance (e.g., Chiang Mai, Thailand);
Type 3. Only seasonal presence of Ae. aegypti active stages (larvae, pupae and adults), with overwintering eggs (e.g., Buenos Aires, Argentina);
Type 4. Only seasonal presence of Ae. aegypti active stages, with no overwintering eggs producing viable larvae (e.g., Puebla City, Mexico).
These distinctions are important, because they determine the duration and “intensity” to which humans in a given region may be exposed to Ae. aegypti. Table 1 shows the temperature and precipitation thresholds from Eisen et al. (2014) used here to define Types 1-4. Only one type is allowed to occur per grid box; where types overlap, the more suitable type for Ae. aegypti is selected (e.g., if Types 1 and 2 overlap, Type 1 is selected). Collectively, these thresholds indicate that warmer, wetter regions with small temperature variability are best suited for Ae. aegypti. The thresholds from Table 1 are applied to the gridded global temperature and precipitation fields described below to produce reference and future maps of the four Ae. aegypti occurrence patterns.
Table 1.
Thresholds used to define the occurrence patterns for Ae. aegypti.
| Type 1 | Type 2 | Type3 | Type 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Metric | ≥ | ≤ | ≥ | ≤ | ≥ | ≤ | ≥ | ≤ |
| Monthly Air Temperature (°C) | Annual Mean | 25.4 | - | 23.7 | - | 16.8 | - | 14.5 | - |
| Annual Min | 24.6 | - | 14.2 | - | 4.2 | - | 0.8 | - | |
| Annual Max | - | - | - | - | - | - | - | - | |
| Annual Range | - | 4.1 | - | 19.3 | - | 23.9 | 26.7 | ||
| Monthly Precipitation (mm) | Annual Mean | 1498 | - | 705 | - | 675 | - | 186 | - |
| Annual Min | - | - | - | - | - | - | - | - | |
| Annual Max | 304 | - | 148 | - | 134 | - | 27 | - | |
| Annual Range | 145 | - | 96 | - | 70 | - | 17 | - | |
A gridded 10 arc-minute monthly global climatology of near-surface temperature and precipitation for 1950-2000 was obtained from version 1.4 of WorldClim (Hijmans et al. 2005). WorldClim comprises the reference historical data used to produce maps of Ae. aegypti occurrence patterns, and provides the basis for a simple procedure to empirically downscale and bias correct projected climate model data described in Online Resource 1.
Maps of Ae. aegypti occurrence patterns for the late 21st century are produced with future climate projections from version 1 of the Community Earth System Model (CESM; Hurrell et al. 2013). CESM is a fully coupled atmosphere-ocean global climate model (AOGCM). The version used here is the same that supported the Coupled Model Intercomparison Experiment Phase 5 (CMIP5; Taylor et al. 2012). Simulations from the historical period (1950-2000) and two future projections for 2061-2080 are used. The future simulations are forced in accordance with the Representative Concentration Pathway (RCP; van Vuuren et al. 2011) 4.5 and 8.5 scenarios. RCP4.5 is a low-to-moderate emissions and concentration scenario with GHG radiative forcing reaching 4.5 W m−2 near 2100. RCP8.5 is a high-emissions and concentration scenario with GHG radiative forcing reaching 8.5 W m−2 near 2100. Monthly near-surface air temperature and precipitation fields are used from the first 15 members of a 30-member CESM initial condition ensemble based on the RCP8.5 scenario (Kay et al. 2014) and from a 15-member CESM ensemble based on the RCP 4.5 scenario (Sanderson et al. 2015), allowing internal model variability to be characterized. The differences in CESM temperature and precipitation between 1950-2000 and 2061-2080 for each ensemble member are then added to the corresponding WorldClim fields to generate bias-corrected, downscaled future projections, as described in Online Resource 1.
To assess the potential impacts of future population changes on exposure to Ae. aegypti, gridded global population projections (Jones, B., O'Neill, B.C., Spatially explicit global population scenarios consistent with the Shared Socioeconomic Pathways, submitted to Environmental Research Letters) from two of the five Shared Socioeconomic Pathways (SSPs) – SSP3 and SSP5 – are used. The SSP3, or “Regional Rivalry”, pathway describes a world in which there are high levels of inequality and little coordination across countries, and in which little progress is made toward meeting development goals, addressing environmental issues, or reducing fossil fuel consumption (O'Neill et al. 2015). Slow economic development and lower education trends stall the demographic transition in lower income countries, leading to rapid population growth relative to higher income nations, and a steadily increasing global population throughout the century. The SSP5, or “Fossil-fueled development”, pathway describes a world in which traditional economic growth is seen as the solution to social and economic issues, and is characterized by greater progress toward human development, but at the cost of intense usage of resources and high fossil fuel consumption (O'Neill et al. 2015). Economic growth is rapid and global population peaks by mid-century and then declines, driven by declining fertility in developing countries, while urbanization rates are very high worldwide. The gridded SSP3 and SSP5 population projections span each decade from 2010 to 2100, and are based on version 3 of the Gridded Population of the World (GPW) dataset for the year 2000 (CIESIN, 2005), and aggregate country-level population (KC and Lutz 2014) and urbanization (Jiang and O'Neill 2015) projections to 2100 consistent with each SSP. The SSP3 and SSP5 projections for 2070 are used to represent the 2061-2080 period, and the GPW 2000 dataset to represent the 1950-2000 reference period. The gridded population datasets are conservatively remapped from their native 7.5 arc-minute spatial resolution to the same 10 arc-minute resolution as the climate fields, and are used to estimate the recent and future number of persons exposed to Ae. aegypti for each occurrence pattern (Types 1-4), by summing the population for all grid cells corresponding to a given pattern and region of interest. Regions include the entire globe and the six permanently inhabited continents: Africa, Asia, Australia (including Oceania), Europe, North America and South America.
In all results presented below, uncertainty due to both the mapping methodology and internal climate model variability is considered. A validation of the global reference maps of Ae. aegypti occurrence patterns and the number of humans exposed is described in Online Resource 1, yielding an estimated methodological uncertainty of +/−10%. Statistical significance between means is assumed when a two-tailed Student's t-test is p<0.05.
3. Results
3.1 Future changes in climate fields
Widespread and statistically significant near-surface air temperature increases over land are projected for the RCP4.5 and RCP8.5 scenarios for 2061-2080, consistent with rising greenhouse gases in both emissions pathways (See Online Resource 2, Fig. S1). The global average near-surface temperature increase (over land) for 2061-2080 relative to 1950-2000 is 3.1 °C for RCP4.5 and 4.8 °C for RCP8.5. The largest warming is typically at middle to high latitudes. Statistically significant precipitation changes for 2061-2080 over land are also widespread for both RCP scenarios. Most regions exhibit increased precipitation, consistent with an overall increase in rainfall globally due to greater atmospheric water vapor resulting from warmer tropospheric temperatures (e.g., Held and Soden 2006). Notable exceptions where less rainfall is projected include Amazonian South America, Central America, western Africa, the Iberian Peninsula, southern Africa, northwestern India and Pakistan, and southern Australia. These patterns of climatic change play an important role in the projected 2061-2080 changes in the occurrence patterns of Ae. aegypti.
3.2 Future changes in the geographic distribution of Ae. aegypti
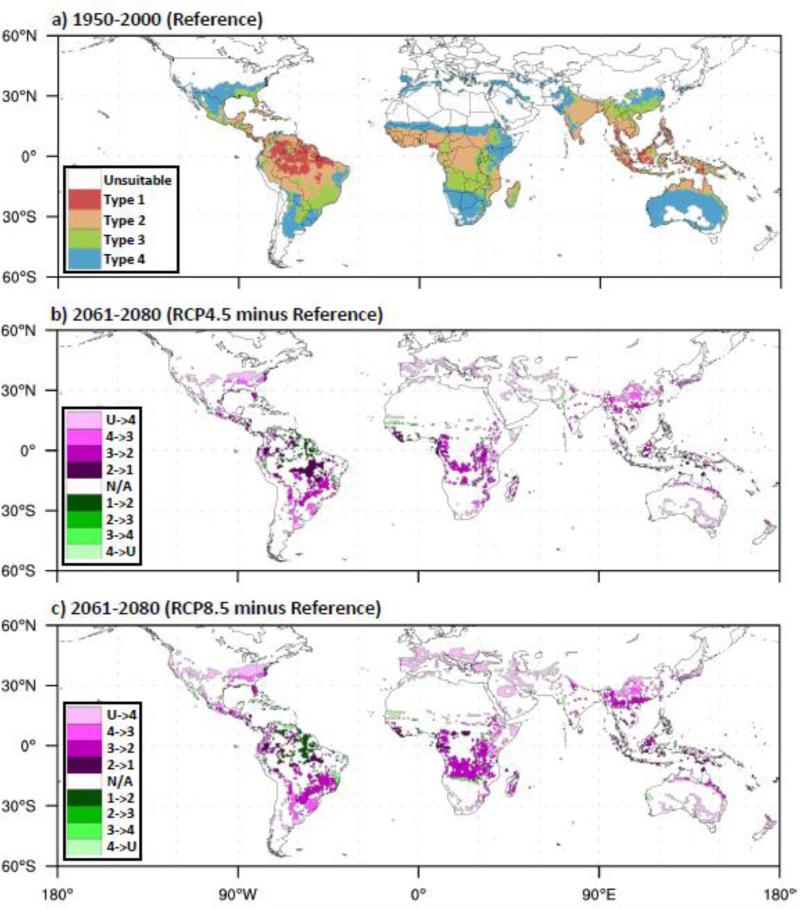
The global extents of the four Ae. aegypti occurrence patterns for the 1950-2000 reference period and their changes for the 2061-2080 RCP scenarios are shown in Fig. 1. In general, the most suitable occurrence patterns (Types 1 and 2) are near the equator and the less suitable patterns (Types 3 and 4) are poleward (Fig. 1a), following the climatic gradients from the relatively warm, wet tropics to the cooler, drier mid-latitudes. Table 2 accompanies Fig. 1 and presents the total surface area for the 1950-2000 reference period and the change in surface area for the two 2061-2080 RCP scenarios, for each continent and globally. Types 1 and 2 are projected to expand on all continents for both the RCP4.5 and RCP8.5 scenarios, with the exception of Europe where conditions remain too cool. Interestingly, strong drying in the Amazon rainforests for RCP8.5 leads to a contraction of Type 1 in that area (Fig. 1b), muting the overall expansion of Type 1 in South America. Type 3 exhibits areas of both expansion and contraction, depending on the continent. Statistically significant contraction of Type 3 occurs in Africa, Asia, Australia (RCP8.5 only), South America and globally, and is mainly due to the encroachment of the more suitable Type 2 pattern into areas previously categorized as Type 3. Significant northward expansion of Type 3 occurs in Europe and North America into areas previously categorized as Type 4. Type 4 significantly expands poleward into previously unsuitable areas in Asia, Europe and North America due to warmer temperatures, and expands in mainland Australia (where temperatures are already suitable) due to increases in rainfall. Type 4 significantly contracts in Africa (RCP4.5 only) and South America due to replacement by more suitable types. Globally there is a statistically significant expansion of Types 1, 2, 4, and “All Types” combined, and a contraction of Type 3. On a percentage basis, the “All Types” expansion is 8-13% globally depending on the RCP scenario, influenced by particularly large expansion of Type 1 (44-54%) and Type 2 (15-33%). In most cases where expansion occurs, it is larger under the RCP8.5 scenario compared to RCP4.5.
Fig. 1.
Global extent of Ae. aegypti occurrence patterns (Types 1-4) for (a) the 1950-2000 reference period, and changes in occurrence patterns for (b) 2061-2080 RCP4.5 minus the reference and (c) 2061-2080 RCP8.5 minus the reference. Magenta (green) shaded changes are toward more (less) suitable occurrence patterns; e.g., the magenta-shaded “U->4” category means an unsuitable area becomes Type 4. “N/A” means no change.
Table 2.
Change in land surface area (millions km2) for each type of Ae. aegypti occurrence pattern, for 2061-2080 versus 1950-2000, for each region and RCP scenario. Pixels were weighted by the sin of the latitude when calculating surface area.*
| Type1 | Type 2 | Type 3 | Type 4 | All Types | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1950-2000 | Δ 2061-2080 | 1950-2000 | Δ 2061-2080 | 1950-2000 | Δ 2061-2080 | 1950-2000 | Δ 2061-2080 | 1950-2000 | Δ 2061-2080 | ||||||
| Region | Reference | RCP4.5 | RCP8.5 | Baseline | RCP4.5 | RCP8.5 | Baseline | RCP4.5 | RCP8.5 | Reference | RCP4.5 | RCP8.5 | Baseline | RCP4.5 | RCP8.5 |
| Africa | 0.2 | 0.5 | 0.9 | 7.3 | 1.9 | 3.2 | 5.1 | −1.9 | −3.4 | 7.0 | −0.1 | −0.2 | 19.5 | 0.3 | 0.4 |
| Asia | 1.1 | 0.7 | 0.9 | 4.4 | 0.3 | 0.6 | 2.9 | −0.2 | −0.5 | 3.2 | 0.4 | 1.5 | 11.6 | 1.3 | 2.6 |
| Australia | 0.0 | 0.1 | 0.1 | 0.9 | 0.2 | 0.3 | 0.3 | 0.0 | −0.1 | 4.9 | 0.7 | 0.7 | 6.1 | 1.0 | 1.0 |
| Europe | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.4 | 0.5 | 1.1 | 0.4 | 0.6 | 1.2 |
| N. America | 0.1 | 0.1 | 0.1 | 0.8 | 0.2 | 0.3 | 1.1 | 0.3 | 0.3 | 2.5 | 0.4 | 0.8 | 4.5 | 1.0 | 1.5 |
| S. America | 3.5 | 1.4 | 0.2 | 5.6 | 0.2 | 1.9 | 3.2 | −0.9 | −1.3 | 2.6 | −0.4 | −0.3 | 15.0 | 0.4 | 0.5 |
| Global | 4.9 | 2.7 | 2.2 | 19.0 | 2.9 | 6.3 | 12.5 | −2.7 | −4.8 | 20.5 | 1.6 | 3.6 | 56.9 | 4.5 | 7.3 |
underlined italicized values indicate statistically significant (p<0.05) changes for 2061-2080 compared to 1950-2000.
3.3 Future changes in human exposure to Ae. aegypti
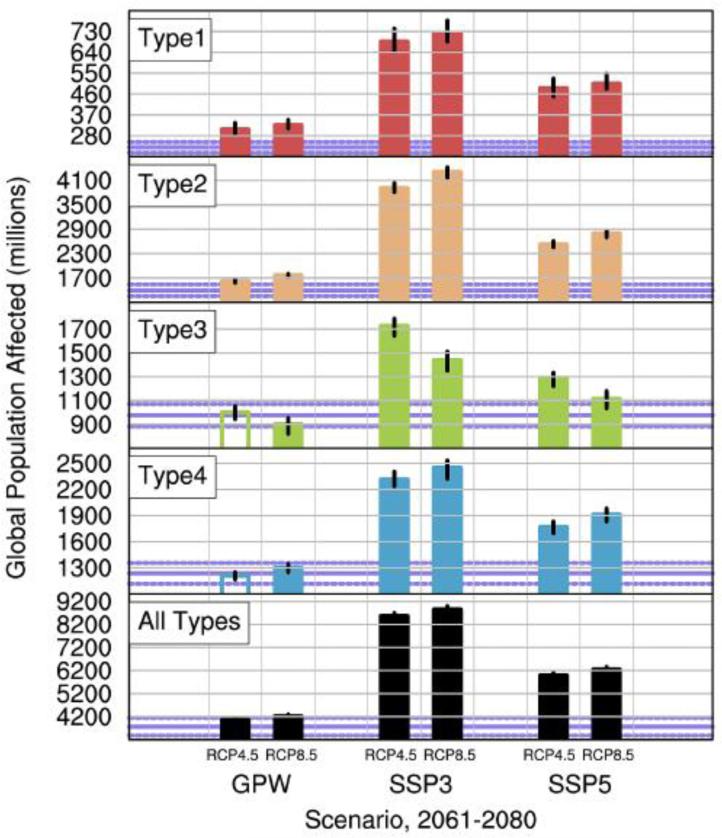
Figure 2 shows the projected number of persons exposed to Ae. aegypti globally for 2061-2080 as a result of changes to the areal extents of the four occurrence patterns. Similar figures for each of the 6 continents are in Online Resource 2. Table 3 accompanies Figure 2 and presents the changes numerically for “All Types” combined as well as for the difference between RCP8.5 and RCP4.5, globally and for each continent. The GPW pathway assumes that population does not change from year 2000 levels, and therefore isolates the specific impact of climate change on persons exposed to Ae. aegypti. The GPW results for 2061-2080 are largely consistent with the changes in areal extent discussed in the previous section; globally the number of persons exposed increases for Types 1, 2, 4 and “All Types” and decreases for Type 3. When population growth is accounted for in addition to climate change (the SSP3 and SSP5 pathways), in nearly every instance changes in the number of persons exposed to Ae. aegypti far exceed those due to climate change alone (GPW). Globally, the number of persons exposed for the 1950-2000 reference period for “All Types” is 3794 million; this value expands by 298 million (8%) for the GPW case (climate change only), but by 4805 million (127%) and 2232 million (59%) for the SSP3 and SSP5 pathways.
Fig. 2.
Global population affected (millions of persons) for each Ae. aegypti occurrence pattern (Types 1-4) and all patterns combined (“All Types”). Colored bars indicate the ensemble mean value, and the black lines at the top of each bar indicate the range of values among ensemble members. To aid comparison, the bar colors for each Type are identical to the colors used in the global extent map (Fig. 1a). Results are shown for the 2061-2080 RCP4.5 and RCP8.5 emissions and concentration scenarios for each of the three population pathways: GPW 2000, SSP3 2070 and SSP5 2070. Violet-colored solid horizontal lines on each panel indicate the population affected for the 1950-2000 reference period, assuming the GPW 2000 population; the dashed violet lines are +/− 2 standard deviations from the mean. Filled (unfilled) bars indicate populations statistically significantly (insignificantly) different from the 1950-2000 reference period (p<0.05)
Table 3.
Change in population for “All Types” of Ae. aegypti occurrence patterns for 2061-2080 versus 1950-2000, for each region, SSP pathway, and RCP scenario, as well as the difference of the RCP8.5 and RCP4.5 scenarios.*
| Reference | RCP4.5 | RCP8.5 | RCP8.5-minus-RCP4.5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1950-2000 | Δ 2061-2080 | Δ 2061-2080 | Δ 2061-2080 | |||||||
| Region | GPW | GPW | SSP3 | SSP5 | GPW | SSP3 | SSP5 | GPW | SSP3 | SSP5 |
| Africa | 664 | 16 | 2093 | 1023 | 19 | 2104 | 1029 | 3 | 10 | 6 |
| Asia | 2538 | 145 | 2172 | 747 | 202 | 2334 | 846 | 58 | 162 | 99 |
| Australia | 17 | 4 | 17 | 43 | 5 | 18 | 45 | 1 | 1 | 2 |
| Europe | 44 | 64 | 48 | 113 | 129 | 106 | 194 | 65 | 58 | 81 |
| N. America | 253 | 56 | 245 | 236 | 84 | 280 | 290 | 28 | 35 | 54 |
| S. America | 277 | 13 | 230 | 70 | 20 | 243 | 78 | 7 | 13 | 9 |
| Global | 3794 | 298 | 4805 | 2232 | 460 | 5084 | 2483 | 162 | 279 | 251 |
underlined italicized values indicate statistically significant (p<0.05) changes for 2061-2080 compared to 1950-2000 for the RCP4.5 and RCP8.5 scenarios. For the RCP8.5-minus-RCP4.5 scenario, italicized underlined values indicate statistically significant differences (p<0.05) for the RCP8.5 versus RCP4.5 scenario
Changes in the number of persons exposed under SSP3 are larger than SSP5 for continents primarily comprised of developing and middle-income countries (Africa, Asia and South America) because population grows most rapidly in developing countries for the SSP3 pathway. By contrast, for continents with predominantly industrialized countries (Australia, Europe and North America), changes are largest for SSP5 because population grows most rapidly in industrialized countries for this pathway. On a percentage basis, growth of persons exposed for the climate change only pathway (GPW) is greatest for the continents with a large fraction of land in the mid-latitudes: Australia, Europe and North America. For example, Europe exhibits particularly large percentage growth in persons exposed to Ae. aegypti for the GPW pathway of 144% for RCP4.5 and 292% for RCP8.5, and trails only much-larger Asia in terms of absolute growth.
Compared to RCP4.5, there is larger growth in the number of persons exposed under the RCP8.5 scenario on all continents and for all 3 population pathways for “All Types” (Table 3). However, only for North America and Europe are the differences significant for all three population pathways. The differences are insignificant for all three of the population pathways for Africa, Asia and South America, where strong population growth far outweighs the impact of climate change. For RCP8.5 an additional 162 (GPW), 279 (SSP3) and 251 (SSP5) million persons globally would be exposed to Ae. aegypti compared to RCP4.5, equivalent to about 4-7% of the 3974 million people exposed for the 1950-2000 reference period, though only the SSP5 changes are significant.
4. Discussion and Conclusions
By 2061-2080, for both RCP scenarios, many regions already suitable for Ae. aegypti may maintain or shift toward Type 1 and Type 2 occurrence patterns associated with greater mosquito abundance for a longer annual duration. The areal extent of the Type 1 pattern is projected to increase by 44-54% globally, and Type 2 by 15-33%, with the majority of expansion in Africa, southern Asia and South America. Accordingly, the number of persons exposed to these more suitable Ae. aegypti occurrence patterns may increase substantially. For example, the number of humans exposed to Ae. aegypti in 2061-2080 for the Type 1 pattern is projected to increase by about 35-45% (GPW), 200-215% (SSP3) and 115-120% (SSP5) from a present-day exposed population of about 230 million. Percentage increases are similar for the Type 2 pattern. Comparing these results to the global “All Types” increases in humans exposed by 2061-2080 of 8-12% (GPW), 127-134% (SSP3), and 59-64% (SSP5), it is evident that even climate change alone (the GPW case), in the absence of population growth, leads to relatively large increases of these more suitable occurrence patterns.
In regions that have marginal or no suitability in the present-day, by 2061-2080 a shift toward enhanced seasonal suitability for Ae. aegypti is projected, particularly due to the expansion of the Type 4 occurrence pattern into areas previously unsuitable for the establishment of Ae. aegypti. The areal extent of the Type 4 pattern is projected to grow by 8-18% globally, but will likely grow at a more rapid rate at the cool mid-latitude margins of present-day suitability, particularly in Europe (138-308%). Consequently, the number of Europeans exposed to Ae. aegypti in 2061-2080 for the Type 4 pattern is projected to increase by about 140-265% (GPW), 105-220% (SSP3) and 240-400% (SSP5) from a present-day exposed population of 44 million. Therefore, in addition to population change, by 2061-2080 climate change alone (the GPW case) may drive large increases in the number of humans exposed seasonally to Ae. aegypti in areas that have marginal present-day suitability.
We therefore find that within regions, for certain Ae. aegypti occurrence patterns, climate change plays an important role in driving increases in human exposure to Ae. aegypti. However, when considering human exposure to Ae. aegypti for all occurrence patterns collectively, we find that climate change is a comparatively modest driver of increased exposure relative to population growth globally and particularly for continents comprised mainly of developing and middle income countries. For example, the average growth in the population exposed to all Ae. aegypti occurrence patterns by 2061-2080 for Africa, Asia and South America combined is 4-6% for GPW (climate change only), compared to 161-165% for SSP3 and 70-72% for SSP5 (pathways for which both climate change and population growth are accounted for). As a result, the differences in human exposure to Ae. aegypti between the RCP4.5 and RCP8.5 climate change scenarios are statistically insignificant for all three continents for all three population pathways, meaning there is no apparent advantage to taking the lower-emissions RCP4.5 pathway in terms of reducing human exposure to Ae. aegypti in these regions. There are however, statistically significant differences between RCP4.5 and RCP8.5 for North America and Europe for all three population pathways, and globally for the SSP5 storyline, and thus in these regions there is an advantage to taking the lower emissions RCP4.5 pathway, though it is still modest compared to overall changes in exposure due to population growth.
Total population growth in developing and middle income countries in Africa, Asia and South America is greater for the SSP3 (“Regional Rivalry”) pathway compared to the SSP5 (“Fossil-fueled Development”) pathway, due to more rapidly declining birthrates in developing and middle income countries for SSP5 (O'Neill et al. 2015). Conversely, population growth is larger in developed countries in Australia, Europe and North America for SSP5, driven by urbanization. As a result, human exposure to Ae. aegypti increases most for the SSP3 pathway in Africa, Asia and South America, whereas it increases most for the SSP5 pathway in Australia and Europe (and is about the same for either pathway in North America due to developing and middle income countries in Central America versus the developed United States). These are important distinctions because SSP3 and SSP5 also differ in dimensions not directly included in our modeling results, but that would be expected to affect the extent of future outbreaks of dengue and their control. SSP3 is a world with weak investments in health, institutions, and research and development with a declining ability to identify, communicate, prevent and manage dengue outbreaks (Ebi 2013). Most urban growth in low-income countries is in unplanned settlements, with limited attention to increasing access to safe water and improved sanitation, implying increases in the number of possible breeding sites for Ae. aegypti. On the other hand, SSP5 is a world with strong investments in health, education, institutions, and research and development with the capacity and political will to prepare for, prevent and manage dengue outbreaks (Ebi 2013), even though enhanced global trade networks (and presumably transportation infrastructure) may facilitate the expansion and/or reintroduction of Ae. aegypti into marginally suitable areas. Significant progress is achieved in improving access to safe water and improved sanitation, thus reducing breeding grounds for Ae. aegypti. These distinctions imply that the more vulnerable developing and middle income countries in Africa, Asia and South America may be disproportionately impacted under the SSP3 pathway, not only because more humans in these countries would be exposed to Ae. aegypti under SSP3, but also because of comparatively diminished capacity to mitigate dengue risk.
The analysis is subject to a number of model and data limitations. We employ a simple modeling approach for Ae. aegypti occurrence based on long-term monthly and annual average temperature and precipitation thresholds. This approach does not directly address important complex system interactions related to Ae. aegypti ecology, development, behavior and survival that occur on shorter timescales, and that more sophisticated process-based modeling approaches may resolve (e.g., Focks et al. 1993, Morin et al. 2015). For example, several climatic processes known to impact Ae. aegypti life-history traits are not represented, such as rainfall intensity (Koenraadt et al. 2008), diurnal temperature variability (Carrington et al. 2013) and upper temperature limits (Williams et al. 2014). Our approach also neglects microclimatic impacts that may buffer mosquitoes from temperature and precipitation extremes (Kearney et al. 2009). It is noteworthy that while the projections of populations exposed to Ae. aegypti (Fig. 2 and Table 3) apply only to areas that are inhabited by humans, our calculations of the areal extent of occurrence patterns (Fig. 1 and Table 2) are independent of population and thus imply suitability in some uninhabited regions. Only results from the CESM AOGCM are used, an approach that does not allow inter-model uncertainty to be estimated (CESM is one of about 20 CMIP5 AOGCMs). The SSP population projections are subject to numerous sources of uncertainty, not the least the unknown geopolitical landscape of the future (O'Neill et al. 2015).
Other study limitations are related to human and mosquito behavior. Aedes aegypti is highly dependent on human behavior for finding suitable container habitats, shade, and shelter (e.g., Eisen et al. 2014). For example, a study in San Juan, Puerto Rico found that most Ae. aegypti pupae were produced in human-managed containers (Barrera et al. 2011). How such behavioral factors may change in the future is unaccounted for. Interspecies competition among Ae. aegypti and other mosquitoes may affect the rate and extent of range expansion; for example, it has been suggested that Ae. albopictus may reduce or displace Ae. aegypti populations in regions of the United States, Brazil and Thailand where both species co-occur (Moji et al. 1988, Lounibos et al. 2002, Braks et al. 2004). How human interventions aimed at reducing Ae. aegypti populations may change in the future is unknown. For example, controversial releases of genetically-modified “sterile” male mosquitoes (Ernst et al. 2015) may become more common in the future, and, if they do, would differ between the SSPs. Additionally, how other human factors such as cultural practices, water access, urbanization, transportation networks and global trade may evolve and impact the spread of Ae. aegypti is unclear (Mackenzie et al. 2004). Gubler (2002) notes that a resurgence of dengue (likely linked to the proliferation of Ae. aegypti) over the past half century is associated with the establishment of peri-urban shantytowns that lack reliable water and sewer services, as well as a large increase in international transportation.
Finally, it is noteworthy that increases in population exposure to Ae. aegypti do not necessarily translate into increased risk for dengue or chikungunya virus transmission, due to other factors such as the potential for a vaccine to be developed (Thomas 2015), and other climatic impacts on mosquito ecology (Patz et al. 1998, Liu-Helmersson et al. 2014). Despite these caveats, numerous studies project that 21st century climate change may, overall, facilitate enhanced dengue transmission (Hopp and Foley 2001, Hales et al. 2002, Colon-Gonzalez 2013, Bouzid et al. 2014).
We conclude that both climate change and population growth may increase human exposure to the virus vector mosquito Ae. aegypti by the late 21st century. The global population is projected to grow from year 2000 levels of 6053 million (GPW) to 11143 million for SSP3 or 8468 million for SSP5 by 2070. The percentage of this total global population exposed to Ae. aegypti for the reference 1950-2000 case is 63%, a value projected to increase to 68-70% by 2061-2080 if population is held constant (GPW; i.e., climate change only), to 77-80% for SSP3 and to 71-74% for SSP5. Developing economies in tropical and subtropical Africa, southern Asia and South America may be particularly impacted by the expansion of year-round Ae. aegypti occurrence patterns, whereas industrialized mid-latitude economies may be impacted by the expansion of seasonal occurrence patterns. The benefits of reduced anthropogenic climate change on human exposure to Ae. aegypti due to taking the RCP4.5 emissions scenario instead of the RCP8.5 scenario may be more pronounced in industrialized countries than in developing and middle income countries.
Supplementary Material
Acknowledgements
This research was supported by the National Science Foundation (GEO-1010204) and the National Institutes of Health (IR01AI091843). The National Center for Atmospheric Research is sponsored by the National Science Foundation. The SSP data is available on request from Bryan Jones; other data sources are cited within. Lars Eisen of the Centers for Disease Control and Prevention provided invaluable guidance.
Contributor Information
A.J. Monaghan, National Center for Atmospheric Research, P.O. Box 3000, Boulder, CO 80307
K.M. Sampson, National Center for Atmospheric Research, P.O. Box 3000, Boulder, CO 80307
D.F. Steinhoff, National Center for Atmospheric Research, P.O. Box 3000, Boulder, CO 80307
K.C. Ernst, University of Arizona, College of Public Health, P.O. Box 245163, Tucson, AZ 85724
K.L. Ebi, University of Washington, School of Public Health, 1705 NE Pacific St, Box 357965, Seattle, WA 98195-7965
B. Jones, City University of New York, CUNY Institute for Demographic Research, 135 East 22nd St, New York, NY 10010
M.H. Hayden, National Center for Atmospheric Research, P.O. Box 3000, Boulder, CO 80307
References
- Anderson GB, Oleson KW, Jones B, Peng RD. Predicting high-mortality heatwaves: Developing health-based models to predict whether a heatwave is high-mortality or less dangerous based on heatwave and community characteristics. 2016a Submitted to Clim Chang. [Google Scholar]
- Anderson GB, Oleson KW, Jones B, Peng RD. High-mortality heatwaves and health in a changing future: Projecting frequency of high-mortality heatwaves in 82 U.S. communities in 2061-2080 under different climate, population, and adaptation scenarios. 2016b doi: 10.1007/s10584-016-1779-x. Submitted to Clim Chang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Amador M, MacKay AJ. Populaton dynamics of Aedes aegypti and dengue as influence by weather and human behavior in San Juan, Puerto Rico. PLoS Neg Trop Dis. 2011;5:e1378. doi: 10.1371/journal.pntd.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe NW, Cooper RD, Mottram P, Sweeney AW. Australia's dengue risk driven by human adaptation to climate change. PLoS Neg Trop Dis. 2009;3:e429. doi: 10.1371/journal.pntd.0000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. doi:10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzid M, Colón-González FJ, Lung T, Lake IR, Hunter PR. Climate change and the emergence of vector-borne diseases in Europe: case study of dengue fever. BMC Pub Health. 2014;14:781. doi: 10.1186/1471-2458-14-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourenço-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann Entomol Soc Am. 2004;97:130–139. [Google Scholar]
- Campbell LP, Luther C, Peterson AT. Climate change influences on global distributions of dengue and chikungunya virus vectors. Phil Trans R Soc B. 2014;370:20140135. doi: 10.1098/rstb.2014.0135. doi:10.1098/rstb.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capinha C, Rocha J, Sousa CA. Macroclimate determines the global range limit of Aedes aegypti. EcoHealth. 2014;11:420–428. doi: 10.1007/s10393-014-0918-y. [DOI] [PubMed] [Google Scholar]
- Carrington LB, Seifert SN, Willits NH, Lambrechts L, Scott TW. Large diurnal temperature fluctuations negatively influence Aedes aegypti (Diptera: Culicidae) life-history traits. J Med Entomol. 2013;50:43–51. doi: 10.1603/me11242. [DOI] [PubMed] [Google Scholar]
- Christophers SR. Aedes aegypti (L.), the yellow fever mosquito; its life history, bionomics, and structure. University Press; Cambridge: 1960. [Google Scholar]
- CIESIN (Center for International Earth Science Information Network) Gridded Population of the World, Version 3 (GPWv3) Data Collection. Columbia University; 2005. [1 December 2014]. http://sedac.ciesin.columbia.edu/gpw/index.jsp. [Google Scholar]
- Colón-González FJ, Fezzi C, Lake IR, Hunter PR. The effects of weather and climate change on dengue. PLoS Neg Trop Dis. 2013;7:e2503. doi: 10.1371/journal.pntd.0002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebi KL. Health in the new scenarios for climate change research. Int J Environ Res Pub Health. 2013;11:30–46. doi: 10.3390/ijerph110100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Monaghan AJ, Lozano-Fuentes S, et al. The impact of temperature on the bionomics of Aedes (Stegomyia) aegypti, with special reference to the cool geographic range margins. J Med Entomol. 2014;51:496–516. doi: 10.1603/me13214. [DOI] [PubMed] [Google Scholar]
- Eisen L, Moore CG. Aedes (Stegomyia) aegypti in the continental United States: A vector at the cool margin of its geographic range. J Med Entomol. 2013;50:467–478. doi: 10.1603/me12245. [DOI] [PubMed] [Google Scholar]
- Ernst KC, Haenchen S, Dickinson K, et al. Awareness and support of release of genetically modified “sterile” mosquitoes, Key West, Florida, USA. Emerg Infect Dis. 2015 doi: 10.3201/eid2102.141035. in press. doi:10.3201/eid2102.141035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focks DA, Haile DG, Daniels E, Mount GA. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): analysis of the literature and model development. J Med Entomol. 1993;30:1003–1017. doi: 10.1093/jmedent/30.6.1003. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- Hales S, De Wet N, Maindonald J, Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- Halstead SB. Reappearance of chikungunya, formerly called dengue, in the Americas. Emerg Infect Dis. 2015;21:557–561. doi: 10.3201/eid2104.141723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held IM, Soden BJ. Robust responses of the hydrological cycle to global warming. J Clim. 2006;19:5686–5699. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- Hopp MJ, Foley JA. Global-scale relationships between climate and the dengue fever vector, Aedes aegypti. Clim Chang. 2001;48:441–463. [Google Scholar]
- Hurrell JW, Holland MM, Gent PR, et al. The Community Earth System Model: a framework for collaborative research. Bull Am Meteorol Soc. 2013;94:1339–1360. [Google Scholar]
- Jiang L, O'Neill BC. Global urbanization projections for the Shared Socioeconomic Pathways. Glob Env Chang. 2015 doi:10.1016/j.gloenvcha.2015.03.008. [Google Scholar]
- Jones B, O'Neill BC, Oleson K. High-mortality heatwaves and health in a changing future: Projecting frequency of high-mortality heatwaves in 82 U.S. communities in 2061-2080 under different climate, population, and adaptation scenarios. 2016 Submitted to Clim Chang. [Google Scholar]
- Kay JE, Deser C, Phillips A, et al. The Community Earth System Model (CESM) Large Ensemble Project: A community resource for studying climate change in the presence of internal climate variability. Bull Am Meteorol Soc. 2014 doi://10.1175/BAMS-D-13-00255.1. [Google Scholar]
- KC S, Lutz W. The human core of the shared socioeconomic pathways: Population scenarios by age, sex and level of education for all countries to 2100. Glob Env Chang. 2014 doi: 10.1016/j.gloenvcha.2014.06.004. doi:10.1016/j.gloenvcha.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M, Porter WP, Williams C, Ritchie S, Hoffmann AA. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: the dengue mosquito Aedes aegypti in Australia. Funct Ecol. 2009;23:528–538. [Google Scholar]
- Khormi HM, Kumar L. Climate change and the potential global distribution of Aedes aegypti: spatial modelling using geographical information system and CLIMEX. Geosp Health. 2014;8:405–415. doi: 10.4081/gh.2014.29. [DOI] [PubMed] [Google Scholar]
- Koenraadt CJM, Harrington LC. Flushing effect of rain on container-inhabiting mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae). J Med Entomol. 2008;45:28–35. doi: 10.1603/0022-2585(2008)45[28:feoroc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kroeger A, Nathan MB. Dengue: setting the global research agenda. Lancet. 2006;368:2193–2195. doi: 10.1016/S0140-6736(06)69873-5. [DOI] [PubMed] [Google Scholar]
- Liu-Helmersson J, Stenlund H, Wilder-Smith A, Rocklöv J. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PloS ONE. 2014;9:e89783. doi: 10.1371/journal.pone.0089783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP, Suarez S, Menendez Z, et al. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus. J Vector Ecol. 2002;27:86–95. [PubMed] [Google Scholar]
- Lucio PS, Degallier N, Servain J, et al. A case study of the influence of local weather on Aedes aegypti (L.) aging and mortality. J Vector Ecol. 2013;38:20–37. doi: 10.1111/j.1948-7134.2013.12005.x. [DOI] [PubMed] [Google Scholar]
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature medicine. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- Marsha A, Sain SR, Heaton MJ, Monaghan AJ, Wilhelmi OV. Climate change influences on extreme heat mortality in Houston, Texas. 2016 Submitted to Clim Chang. [Google Scholar]
- Moji M, Khamboonruang C, Choochote W, Suwanpanit P. Ovitrap surveys of dengue vector mosquitoes in Chiang Mai, northern Thailand: seasonal shifts in relative abundance of Aedes albopictus and Ae. aegypti. Med Vet Entomol. 1988;2:319–324. doi: 10.1111/j.1365-2915.1988.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Morin CW, Comrie AC, Ernst KC. Climate and dengue transmission: evidence and implications. Env Health Perspect. 2013;121:1264–1272. doi: 10.1289/ehp.1306556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CW, Monaghan AJ, Hayden MH, Barrera R, Ernst K. Meteorologically driven simulations of dengue epidemics in San Juan, PR. PLoS Neg Trop Dis. 2015;9:e0004002. doi: 10.1371/journal.pntd.0004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TE. Reemergence of chikungunya virus. J Virol. 2014;88:11644–11647. doi: 10.1128/JVI.01432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasci RS. Movement of chikungunya virus into the Western Hemisphere. Emerg Infect Dis. 2014;20:1394. doi: 10.3201/eid2008.140333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill BC, Gettleman A, editors. The Benefits of Reduced Anthropogenic Climate changE (BRACE): Introduction to the special issue on the BRACE Project. 2016 Submitted to Clim Chang. [Google Scholar]
- O'Neill BC, Ebi KL, Kemp-Benedict E, et al. The roads ahead: narratives for the Shared Socioeconomic Pathways. Glob Environ Chang. 2015 in press. doi:10.1016/j.gloenvcha.2015.01.004. [Google Scholar]
- Parham PE, Waldock J, Christophides GK, et al. Climate, environmental and socioeconomic change: weighing up the balance in vector-borne disease transmission. Phil Trans R Soc B. 2015;370:20130551. doi: 10.1098/rstb.2013.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Martens WJ, Focks DA, Jetten TH. Dengue fever epidemic potential as projected by general circulation models of global climate change. Env Health Perspect. 1998;106:147. doi: 10.1289/ehp.98106147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pialoux G, Gauzere B-A, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- Rogers DJ. Dengue: recent past and future threats. Phil Trans R Soc B. 2015;370:20130562. doi: 10.1098/rstb.2013.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson B, Tebaldi C, Knutti R. A new ensemble of GCM simulations to assess avoided impacts in a climate mitigation scenario. Clim Chang. 2015 doi:10. 1007/ s10584-015-1567-z. [Google Scholar]
- Tabachnick WJ, Powell JR. A world-wide survey of genetic variation in the yellow fever mosquito, Aedes aegypti. Genet Res. 1979;34:215–229. doi: 10.1017/s0016672300019467. [DOI] [PubMed] [Google Scholar]
- Taylor KE, Stouffer RJ, Meehl GA. An Overview of CMIP5 and the Experiment Design. Bull Am Meteorol Soc. 2012;93:485–498. [Google Scholar]
- Thomas SJ. Preventing Dengue—Is the Possibility Now a Reality? New Engl J Med. 2015 doi: 10.1056/NEJMe1413146. Doi:10.1056/NEJMe1413146. [DOI] [PubMed] [Google Scholar]
- van Vuuren DP, Edmonds J, Kainuma M, et al. The representative concentration pathways: an overview. Clim Chang. 2011;109:5–31. [Google Scholar]
- Williams CR, Mincham G, Ritchie SA, Viennet E, Harley D. Bionomic response of Aedes aegypti to two future climate change scenarios in far north Queensland, Australia: implications for dengue outbreaks. Parasites and Vectors. 2014;7:447. doi: 10.1186/1756-3305-7-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization) Dengue: guidelines for diagnosis, treatment, prevention and control – New edition. WHO Press; Geneva: 2009. [PubMed] [Google Scholar]
- Xu Y, Lamarque J-F, Sanderson B. The importance of aerosol scenarios in projections of future heat extremes. Clim Chang. 2015 doi:10.1007/s10584-015-1565-1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.