Abstract
Despite accumulating evidence from animal models demonstrating that prenatal alcohol exposure (PAE) results in life-long neuroendocrine dysregulation, very little is known on this topic among humans with fetal alcohol spectrum disorders (FASD). We expected that alterations in gonadal hormones might interfere with the typical development of white matter (WM) myelination, and in a sex-dependent manner, in human adolescents with FASD. In order to investigate this hypothesis, we used diffusion tensor imaging (DTI) to assess: 1) whether or not sex moderates the impact of PAE on WM microstructure; and 2) how gonadal hormones relate to alterations in WM microstructure in children and adolescents affected by PAE.
Methods
61 youth (9 to 16 yrs.; 49% girls; 50% PAE) participated as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). DTI scans and passive drool samples were obtained to examine neurodevelopmental associations with testosterone (T) and dehydroepiandrosterone (DHEA) levels in boys and girls, and estradiol (E2) and progesterone (P) levels in girls. Tract-based spatial statistics were utilized to generate fractional anisotropy (FA) and mean diffusivity (MD) for 9 a priori WM regions of interest (ROIs).
Results
As predicted, alterations in FA were observed in adolescents with PAE relative to controls, and these differences varied by sex. Girls with PAE exhibited lower FA (Inferior fronto-occipital and Uncinate fasciculi) while boys with PAE exhibited higher FA (Callosal body, Cingulum, Corticospinal tract, Optic radiation, Superior longitudinal fasciculus) relative to age-matched controls. When gonadal hormone levels were examined in relation to DTI measures, additional group differences in FA were revealed, demonstrating that neuroendocrine factors are associated with PAE-related brain alterations.
Conclusions
These findings provide human evidence that PAE relates to sex-specific differences in WM microstructure, and underlying alterations in gonadal hormone function may, in part, contribute to these effects. Determining PAE-effects on neuroendocrine function among humans is an essential first step towards developing novel clinical (e.g., assessment or intervention) tools that target hormone systems to improve on-going brain development among children and adolescents with FASD.
1. Introduction
Following prenatal alcohol exposure (PAE), a highly complex pattern of impairments in cognition, self-regulation, and adaptive functioning can be observed; all of which are included under the term fetal alcohol spectrum disorder (FASD). Associated with these cognitive and behavioral problems among human adolescents with PAE are alterations in grey matter (Lebel et al., 2012). Similarly, smaller white matter (WM) volumes and altered shape of the corpus callosum are found in human adolescents with PAE (Sowell et al., 2001). WM is primarily made of organized myelinated axonal fiber bundles that connect functionally related brain regions. In combination, highly organized axonal fibers and myelin promote water diffusion in a parallel direction to axons while restricting perpendicular diffusion – resulting in a measureable MRI signal that reflects underlying tissue organization. Much of the increase in brain volume throughout development is resultant of increased myelination of fiber tracks, and the timing of this increase differs by region and sex in the developing human brain (Benes et al., 1994; Giedd et al., 1999).
Diffusion tensor imaging (DTI) can be used to measure directional restriction of water diffusion to assess white matter organization and coherency. A primary measure calculated from DTI data is FA (fractional anisotropy; i.e., magnitude of the directional diffusion coherence). Beyond FA, diffusivity averaged across three axes (i.e., MD; mean diffusivity), represents overall average water diffusion, with the highest MD values found in the ventricles and lowest values found in areas with organized parallel axonal tissue. As the human brain matures through adolescence with increasing myelin and axonal organization, directional water restriction in WM bundles increases with age (Beaulieu, 2002). In typically developing rodents, sex differences in myelination and synaptic pruning emerge in the context of rising levels of gonadal hormones (reviewed in (Brenhouse and Andersen, 2011)). Similarly, WM volumes and FA values increase steadily across adolescence, with boys exhibiting an overall steeper incline in volume compared to girls (Perrin et al., 2009), and these typical sex differences relating, in part, to gonadal hormone levels (Herting et al., 2012). Among typically developing individuals, positive associations are also observed between cognitive function and FA (Tamnes et al., 2010; Lee et al., 2017). Human DTI studies of adolescents with FASD have previously reported alterations in WM microstructure compared to controls, including regionally specific reduced FA and increased MD values (reviewed in (Wozniak and Muetzel, 2011)). Given the importance of WM to allow for efficient processing speeds, WM alterations may contribute, in part, to cognitive and behavioral problems associated with PAE 8,9. Reduced FA in youth with FASD may reflect less integrity or even delayed maturation of WM tracks via effects on signal transduction, processing speed and network efficiency. However, it remains to be determined how possible alterations in neuroendocrine function may contribute to observed WM alterations among youth with PAE.
Several animal models of PAE have demonstrated sex-specific effects of PAE on brain measures, including neurotransmitters, receptor expression, and neurogenesis (Schneider et al., 2002; Sliwowska et al., 2008; Weinberg et al., 2008; Sliwowska et al., 2010; Uban et al., 2010; Uban et al., 2013); thus, the presence of significant sex-specific and region-specific effects of PAE are a strong possibility among humans. Animal models suggest that the sexually dimorphic neurobiological impact of PAE is mediated in part through dysregulation of neuroendocrine systems (Weinberg et al., 2008). The hypothalamic-pituitary-gonadal (HPG) axis underlies reproduction, and is sensitive to the teratogenic effects of PAE (Esquifino et al., 1986; McGivern et al., 1998; Lan et al., 2006). In PAE male rodents, overall lower levels of testosterone have been observed, as well as a reduced impact of testosterone on other neuroendocrine and neurotransmitter systems (McGivern et al., 1988; Jungkuntz-Burgett et al., 1990). In PAE female rodents, HPG-dysregulation is evidenced by delays in puberty and sexual maturation (Esquifino et al., 1986; Lan et al., 2009b) and altered developmental patterns of hormone secretion including prolactin, follicle stimulating and luteinizing hormones (Esquifino et al., 1986; McGivern and Yellon, 1992; Weinberg et al., 2008). PAE also alters HPG interactions with the hypothalamic-pituitary-adrenal (HPA) axis, in a sex-specific manner in rodents (Lan et al., 2006; Lan et al., 2009a; Lan et al., 2009b). In general, female PAE rats tend to show greater HPA responsiveness to acute stressors, whereas male PAE rats tend to show greater responsiveness to chronic or prolonged stressors (Weinberg et al., 2008), and testosterone has a reduced capacity to inhibit HPA function in male PAE rats.
In humans, sex differences in the effects of PAE on HPA, autonomic and behavioral reactivity to stressors have also been demonstrated in boys and girls (Haley et al., 2006), supporting the present hypothesis that, in humans, sex differences in the impact of PAE on brain development are associated with alterations in HPA-HPG interactions (Weinberg et al., 2008). 14 year old boys and girls with FASD exhibited dose-dependent alterations in testosterone – with greater doses of PAE related to increased testosterone levels (Carter et al., 2014). The presence of high testosterone levels in the absence of physical markers (Carter et al., 2014) may indicate decreased responsivity to hormones through altered number, binding and/or affinity of receptors in boys with PAE, similar to findings from PAE male rats. If PAE leads to changes in postnatal hormone functioning, this may have lasting effects on the developing brains of children and adolescents with FASD. The lack of human studies on HPG and brain development in FASD highlights a major gap in knowledge regarding whether or not neuroendocrine alterations contribute to PAE-related brain alterations in children and adolescents. The present study aimed to determine if there is evidence of HPG alterations among human adolescents affected by PAE. In order to do so, we examined group differences in DTI measures by sex; and whether or not inclusion of HPG (e.g., gonadal) hormone levels into the statistical models explained PAE- and sex- related WM alterations. We hypothesized that alterations in WM microstructure observed among adolescents with PAE would: 1) be sexually dimorphic; and 2) exhibit different associations with gonadal hormone levels compared to control adolescents.
2. Methods
2.1. Participants
Children and adolescents participated as part of the Collaborative Initiative on FASD (CIFASD) (Mattson et al., 2010) at two imaging sites between 2013 and 2016: Children’s Hospital Los Angeles (CHLA), CA USA and the University of Minnesota (UMN), Minneapolis, MN. Participants were referred through local FASD Clinics, responded to online postings of the study, or were obtained via word of mouth through local FASD-specific caregiver groups. Positive alcohol exposure histories were confirmed via review of records or maternal report and confirmed by a licensed medical doctor (Mattson et al., 2010). Participants in the PAE group were exposed to moderate to severe levels of alcohol (≥13 drinks/week or > 4 drinks/occasion) (Mattson et al., 2010). Children were excluded from CIFASD: 1) who were not fluent in English and/or did not learn English by age 5; 2) whose parents did not speak English; 3) who experienced claustrophobia; 4) who had metal on or in the body that could not be removed; 5) who had a vision or hearing problem that could not be corrected; or 6) who were or thought they may be pregnant (Mattson et al., 2010). In-person interviews were conducted with the primary caregiver to obtain detailed developmental histories of adolescents (including prenatal substance exposure). Control participants were excluded if they were exposed to > 1 drink per week on average or > 2 drinks on a single occasion (Mattson et al., 2010). For the present study, a total of 73 participants aged 9.3–16.0 years completed a DTI scan and salivary sample. Groups were carefully age-matched by frequency, mean, standard deviation and range (Table 1). After matching groups based on PAE status, sex, age and motion during DTI scan acquisition (see details below), 61 participants remained (49% girls; 50% PAE). All procedures were approved by CHLA and UMN’s IRBs and all subjects underwent a comprehensive informed consent/ assent procedure. Participants were compensated for their time with cash or gift cards.
Table 1.
Descriptives.
| Boys | Girls | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Control | PAE | Control | PAE | ||
| Sample Size | 15 | 16 | 15 | 15 | |
| Mean age at scan (years) | 12.9 ± 1.7 | 12.3 ± 1.6 | 13.3 ± 1.6 | 12.7 ± 1.6 | |
| Age range at scan (years) | (9.3–15.4) | (10.0–16.0) | (10.5–15.5) | (10.0–15.1) | |
| % from CHLA | 40% | 62% | 53% | 60% | |
| Gonadal Hormone Levels (pg/mL) | T | 66.3 ± 45 | 50.6 ± 39.7 | 50.4 ± 23 | 51.0 ± 23 |
| DHEA | 92.9 ± 62 | 56.9 ± 49 | 141.4 ± 80 | 114.1 ± 52 | |
| T:DHEA | 0.75 ± 0.2 | 1.11 ± 0.7 | 0.39 ± 0.1 | 0.49 ± 0.2 | |
| E2 | na | na | 1.7 ± 0.8 | 1.6 ± 0.5 | |
| P | na | na | 73.5 ± 76.8 | 73.1 ± 57.0 | |
| Motion (mm3) | FD | 0.51 ± 0.25 | 0.54 ± 0.18 | 0.52 ± 0.33 | 0.56 ± 0.17 |
Mean ± Standard Deviation, or range (low-high) when appropriate. Motion during acquisition of diffusion tensor images is represented by mean frame displacement (FD) across 30 diffusion tensor imaging (DTI) volumes. na = not applicable.
2.2. Image acquisition and preprocessing
2.2.1. Acquisition
DTI was utilized to examine FA and MD in 9 a priori WM regions of interest (ROIs). DTI was performed on a 3T Phillips Achieva MRI scanner at CHLA with 8 channel-head coil, and a 3T Siemens Tim Trio MRI scanner at UMN with 12-channel head coil. Acquisition protocols were coordinated between imaging sites, and based on the Pediatric Imaging Neurocognition and Genetics study (PING) (Brown et al., 2012), which harmonized parameters across imaging platforms. Prior to the study, a travelling human phantom was sent to each site in order to calibrate the acquisition sequences. Whole brain high-resolution structural anatomical images were acquired in the sagittal plane using a T1 wted MPRAGE scanning sequence (CHLA: flip angle = 8, TE = 3.185 ms, TR = 6.795, acquisition matrix = 256 × 256; UMN: flip angle = 7, TE = 4.33 ms, TR = 2170, acquisition matrix = 256 × 256). Whole-brain diffusion weighted volumes [gradient encoding pulses applied in 30 directions, b = 1000 s/mm2, axial slices with 2.5 mm thickness and 90° angle (CHLA: Acquisition matrix = 96 × 95, Echo Time = 86 ms, Repetition Time = 9000 ms; Voxel size = 2.5 × 2.5 × 2.5 mm3, 60 slices; UMN: Acquisition matrix = 96 × 96, Echo Time = 86 ms, Repetition Time = 17400 ms, Voxel size = 2.5 × 2.5 × 2.5 mm3)] and five non-diffusion weighted volume (b = 0s/mm2) were acquired from each participant.
2.2.2. Preprocessing
Using FMRIB Software Library v5.0 (FSL: http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLMotionOutliers), motion was assessed by examining frame-wise displacement (FD) with weighted scaling (Smith et al., 2004) to quantify the amount of movement between each gradient volume collected during the DTI sequence for each individual. Three individuals were excluded from further DTI analyses due to excessive motion that was defined in the present study as 2 or more volumes with FD ≥ 4 mm, or a single volume with FD ≥ 5.5 mm. This motion criterion was selected by confirming that the scans with visible artifacts were the same scans with the highest FD values, and therefore warrant exclusion. After exclusion, the final FD mean did not differ significantly between the four groups (i.e., Control boys vs boys with PAE vs Control girls vs girls with PAE).
Second, brain-extraction from DTI scans utilized BET, distortion correction utilized scans acquired in opposing directions (e.g., anterior-posterior (A-P) and P-A), and a 12-parameter affine registration was applied to correct for head movement and eddy currents (DTIFIT). FA images were created by fitting a tensor model to the raw diffusion data using FSL (Smith, 2002), and then, to give voxelwise maps of the 3 principle diffusion directions and magnitudes of diffusion along these three axes. Third, track based spatial statistics (TBSS) was used for nonlinear registration of all participants’ FA maps to every other participants’ FA map to identify “the most representative” participant to be utilized as the representative target (a control boy aged 13 years was automatically selected). For each DTI image, the nonlinear transformation to the target FA image was combined with the affine transformation from the target to MNI152 space. A mean skeleton comprising primarily the ‘core’ of the WM tracts was generated, and then projected back on each subject to generate subject-specific values with a FA threshold of 0.2–0.8 (for expansion on methods, see (Smith, 2002; Smith et al., 2004)). In FSL, a probabilistic mask was applied using the Jülich histological atlas (maxprop-Thr-50–1 mm) to identify 9 WM regions of interest (ROIs), with 7 of the 9 ROIs divided into the left and right hemispheres (cingulum (CGC), corticospinal tract (CST), inferior fronto-occipital fasciculus (IFO), optic radiation, superior fronto-occipital fasciculus (SFO), superior longitudinal fasciculus (SLF), uncinate fasciculus (UNC)) while the callosal body and fornix had one bilateral value (Eickhoff et al., 2007) (See Supplementary Table A.1). WM ROIs were initially selected based on previously established reproducibility (Hua et al., 2008), and then the final 9 ROIs were selected in the present study based on established neurocircuitries underlying documented cognitive deficits in individuals with FASD. Mean FA and MD values for each WM ROI were extracted for each subject from their skeletonized data for further statistical analyses in the statistical program R (v3.0.2) (Team, 2013).
2.3. Gonadal hormone assessment
To assess levels of gonadal hormones, 1.8 mL (mL) of passive drool was collected in the laboratory by a trained research assistant between the hours of 8:40am and 5:10pm, and on the same day as acquisition of DTI scan. Time of waking, saliva collection start, and saliva collection finish were recorded and utilized in statistical analyses. Briefly, participants were sampled > 30 min after eating, and instructed to rinse their mouth with water at least 10 min prior to saliva collection. Participants collected passive drool in their mouth, and pushed it through a short straw and into a 2 mL vial. Samples were frozen immediately and stored at −80° C. All samples at each site were shipped to Salimetrics (https://www.salimetrics.com), and assayed together to prevent batch-effects. Testosterone (T) and dehydroepiandrosterone (DHEA) levels were assessed in boys and girls. 17-β estradiol (E2) and progesterone (P; 4-pregenene-3, 20-dione) levels were assessed in girls only. Analyses were conducted in duplicates using ELISA/EIA and standard controls. Specificities for analysis of each hormone are reported in Supplementary Table A.2 including lower and higher limits of assays. All values obtained from participants were within lower/upper assay limits.
2.4. Statistical analyses
2.4.1. Descriptives
ANOVA was utilized to assess possible differences in: 1. age (Group (PAE vs Control) x Sex (Boys vs Girls) x Site (UMN vs CHLA)); and 2. Motion during the acquisition of the DTI scan (e.g., mean FD; Group (PAE vs Control) x Sex (Boys vs Girls) x age (continuous) x Site (UMN vs CHLA)).
2.4.2. Hormones
Linear regression was utilized to determine interactive effects of Group (PAE vs Control) x age (continuous) on gonadal hormone levels. Given robust sex differences in hormone levels, boys and girls were analyzed separately. To control for diurnal changes in hormone analyses, time of day and time since waking were coded and utilized as covariates.
2.4.3. Brain
In order to examine sex-specific patterns of PAE-related differences in FA or MD, Group (PAE vs Control) differences were examined separately in boys and girls. To examine group differences in FA and MD for the bilateral WM ROIs (n = 7), Group (PAE vs Control) × age × hemisphere (left, right) interactions were analyzed using linear mixed effects (lme) with hemisphere as the within-subject factor. For unilateral ROIs (n = 2; Callosal body, Fornix), Group (PAE vs Control) x age interactions were analyzed using linear regression. For both uni- and bi- lateral ROIs, if the interaction of all factors in the model was not significant, the model was reduced and re-analyzed. For bilateral tracts, non-significant 3-way interactions were followed by: 1) a lme examining two-way interactions (e.g., Group (PAE vs Control) × age + hemisphere (left, right) or Group (PAE vs Control) × hemisphere (left, right) + age), or 2) lme examining main effect of Group (PAE vs Control) with no interactions and age and hemisphere (left, right) as covariates. Final reduced models are reported for each ROI (Table 2).
Table 2.
Alterations in DTI measures between groups without hormones included into statistical model.
| FA | White Matter Tract | Group | Group × Age | Reduced Linear Model |
|---|---|---|---|---|
| Boys | Callosal Body | AE > C* | – | Group + Age |
| CGC | AE > C* | C: ↑**; AE: – | Group × Age + Hemisphere | |
| CST | AE > C* | – | Group + Age + Hemisphere | |
| Optic Radiation | AE > C* | C: ↑**; AE: – | Group × Age + Hemisphere | |
| SLF | AE > C** | – | Group + Age + Hemisphere | |
| Girls | IFO | C > AE# | – | Group + Age + Hemisphere |
| UNC | C > AE# | – | Group + Age + Hemisphere |
Fractional anisotropy (FA). Summary of results for mixed models without incorporating gonadal hormone levels. ↓ indicates significant negative association; ↑ indicates significant positive association; – indicates no significant association.
p < 0.10;
p < 0.05;
p < 0.01.
C:Control; AE:Alcohol-Exposed; DTI: diffusion tensor imaging; CGC: Cingulum; CST: Corticospinal Tract; IFO: Inferior fronto-occipital fasciculus; SLF: Superior longitudinal fasciculus; UNC: Uncinate fasciculus.
2.4.4. Brain × Hormones associations
To investigate potential associations between FA and gonadal hormone levels, lme (for bilateral tracts) or linear regression (for unilateral tracts) analyses described above (2.4.3) were replicated with the addition of hormone levels into each statistical model. All significant Group (PAE vs Control) x Hormone levels (continuous) effects were followed by post-hoc analyses utilizing linear regression within each subgroup separately (Control boys, PAE boys, Control girls, PAE girls) to assess associations between hormone levels and FA (summary of significant findings in Table 3). All post-hoc analyses controlled for Age and hemisphere (for bilateral tracts). Raw regression coefficients (beta; β) and their standard error (SE) are reported for each statistically significant model.
Table 3.
Alterations in DTI measures between groups with hormones included into statistical model.
| FA | White Matter Tract | T | DHEA | T:DHEA | P |
|---|---|---|---|---|---|
| Boys | CGC (AE > C*) | ||||
| Group | AE > C* | AE > C# | – | ||
| Hormone | ↑* | ↑# | – | ||
| Group × Hormone | C: ↑; AE: –* | – | – | ||
| IFO (–) | |||||
| Group | AE > C** | – | – | ||
| Group × Hormone | C: ↑*; AE: ↓# | – | – | ||
| Optic Radiation (AE > C*) | |||||
| Group | AE > C** | AE > C** | – | ||
| Hormone | – | ↑* | – | ||
| Group × Hormone | C: ↑*; AE: – | C: ↑*; AE: – | – | ||
| SFO (–) | |||||
| Group | AE > C** | AE > C** | – | ||
| Hormone | – | ↑* | – | ||
| Group × Hormone | C: ↑; AE: ↓* | C: ↑; AE: ↓** | – | ||
| Girls | CST (–) | ||||
| Group | – | C > AE# | – | – | |
| Group × Hormone | – | AE: ↑**; C: – | – | – | |
| Fornix (–) | |||||
| Group × Hormone | – | – | – | C: ↓ *; AE: – | |
| SLF (–) | |||||
| Group | – | – | AE > C* | – | |
| Hormone | – | – | ↑# | – | |
| Group × Hormone | AE: ↑*; C: – | AE: ↑*; C: ↓ | AE:↓*; C: ↑# | – |
Fractional anisotropy (FA). Summary of results for mixed models incorporating gonadal hormone levels. ↓ indicates significant negative association; ↑ indicates significant positive association; – indicates no significant association.
p < 0.10;
p < 0.05;
p < 0.01.
C:Control; AE:Alcohol-Exposed; T: testosterone; DHEA: dehydroepian-drosterone; P: progesterone. DTI: diffusion tensor imaging; CGC: Cingulum; CST: Corticospinal Tract; IFO: Inferior fronto-occipital fasciculus; SLF: Superior longitudinal fasciculus; UNC: Uncinate fasciculus.
3. Results
3.1. Descriptives
A summary of Descriptives can be found in Table 1. There were no significant differences in age between Groups (PAE vs Control), Sex (boys vs girls) or Site (CHLA vs UMN) (p values > 0.26). Time of saliva collection did not significantly differ between Groups, Sex or Site (p’s > 0.52). Mean FD did not differ between Groups or Sex (p’s > 0.26), but was significantly related to age overall (F(1.53) = 13.06, p < 0.001), with older participants moving less overall.
3.2. Gonadal hormones
3.2.1. Testosterone (T) levels
Boys
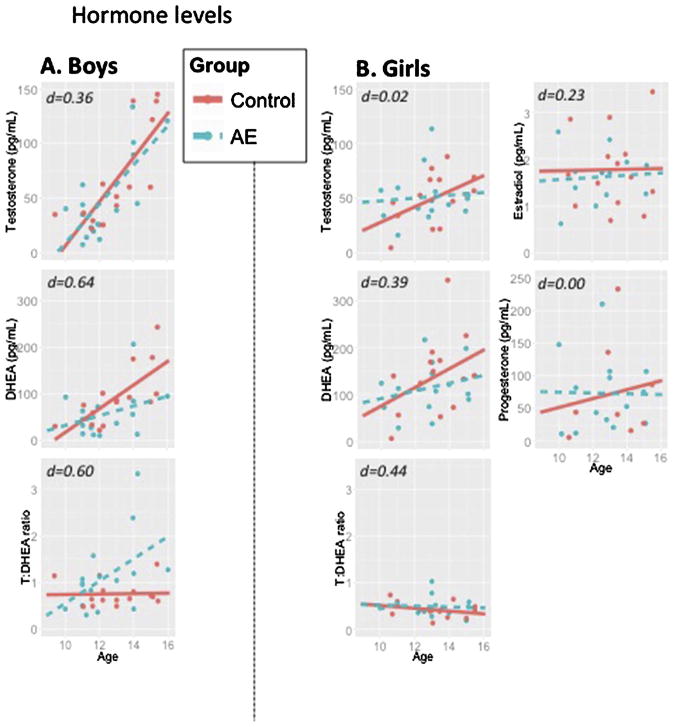
There was a significant effect of age [β = 20.36, SE = 22.55, p < 0.001] where T levels increased with age, but there was no significant effect of Group or Groupxage interaction (p’s > 0.86; Fig. 1A). Time of day was not a significant covariate (p = 0.14).
Fig. 1.
Hormone levels in: A) boys and B) girls. Salivary hormone levels by group and sex. Control in red; Alcohol-exposed in blue. Cohen’s d is represented to indicate expected size of a group (PAE vs Control) effect within same-sexed participants. No significant group differences were detected while controlling for age and time of saliva collection, with the exception of a statistical trend for testosterone:DHEA ratios among boys.
Abbreviations: AE: prenatal alcohol exposed; DHEA: dehydroepiandrosterone; pg/mL: Picogram/milliliter.
Girls
There was a statistical trend for age [β = 7.1, SE = 3.8, p = 0.07] where T levels increased with age, but there was no significant effect of Group or Groupxage interaction (p’s > 0.19; Fig. 1A). Time of day was not a significant covariate (p = 0.21).
3.2.2. Dehydroepiandrosterone (DHEA) levels
Boys
There was a significant effect of age [β = 23.8, SE = 7.2, p < 0.01], where DHEA levels increased with age, but no significant effect of Group or Groupxage interaction (p’s > 0.24; Fig. 1B). Time of day was not a significant covariate (p = 0.47).
Girls
There was a statistical trend for age [β = 19.5, SE = 10.9, p < 0.10] where DHEA levels increased with age, but no significant effect of Group or Groupxage interaction (p’s > 0.39; Fig. 1B). Time of day was not a significant covariate (p = 0.46).
3.2.3. T:DHEA ratios
Boys
There was a statistical trend for a Groupxage interaction [β = 0.3, SE = 0.6, p < 0.10, Cohen’s d = 0.60; Fig. 1C] where the T:DHEA ratio increased with age in PAE but not Control boys. Time of day was not a significant covariate (p = 0.17).
Girls
There were no significant effects of Group, age or Time of Day (p’s > 0.17; Fig. 1C).
3.2.4. Estradiol (E2) and progesterone (P) levels
In girls, there were no significant effects of Group, age or Time of day, or their interactions for either E2 or P (p’s > 0.14; Fig. 1D, E).
3.3. Brain measures
3.3.1. Fractional anisotropy (FA) group effects
Results: are summarized in Table 2 and Fig. 2.
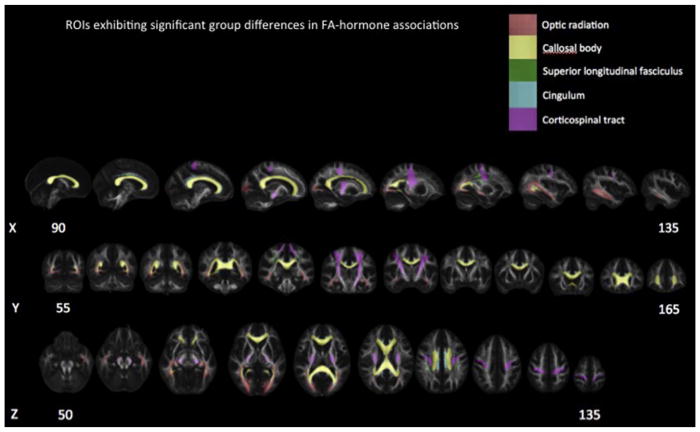
Fig. 2.
Regions of Interest exhibiting significant group differences. ROIs demonstrated along the X, Y and Z planes.
Boys
Main effects of Group revealed that FA was significantly higher in boys with PAE compared to Controls in the callosal body, cingulum (CGC), corticospinal tract (CST), optic radiation and superior longitudinal fasciculus (SLF) (p’s < 0.05). Significant Groupxage interactions were observed for the CGC and optic radiation (p’s < 0.01), where a positive association between FA and age was observed in Controls (p’s < 0.05) but not boys with PAE (p’s > 0.28).
Girls
There was a trend toward lower FA in girls with PAE compared to Controls, as revealed by statistical trends for main effects of Group for the IFO and UNC (p’s < 0.10).
3.3.2. FA with t levels
Results: are summarized in Table 3.
Boys
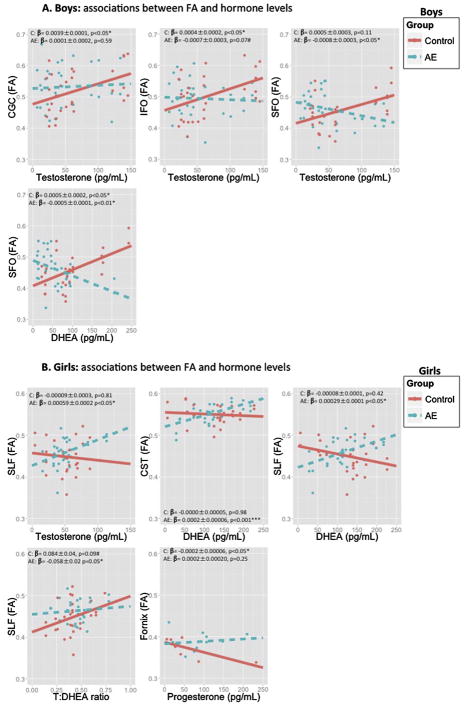
There was a statistical trend for a GroupxT interaction predicting mean FA values in the Cingulum (β = −0.0005, SE = 0.0002, p < 0.10), where a significant positive association between FA and T levels was observed in Control boys (β = 0.0039, SE = 0.0001, p < 0.05), but no significant association in boys with PAE (β = 0.0001, SE = 0.0002, p = 0.59). There was a significant GroupxT interaction predicting mean FA values in the IFO (β=−0.0009, SE = 0.0003, p < 0.05; Fig. 3A), where a significant positive association between FA and T levels was observed in Control boys (β = 0.0004, SE = 0.0002, p < 0.05), and a statistical trend for a negative association was observed in boys with PAE (β = −0.0007, SE = 0.0003, p < 0.10). There was a significant GroupxT interaction predicting mean FA values in the optic radiation (β = −0.0004, SE = 0.0002, p < 0.05), but post-hoc analyses revealed that this association was no longer significant when explored among Control boys only (β = 0.0004, SE = 0.0001, p = 0.79), or boys with PAE only (β = −0.0002, SE = 0.0001, p = 0.15). There was a significant GroupxT interaction predicting mean FA values in the SFO (β = −0.0010, SE = 0.0003, p < 0.05), where a significant negative association was observed between FA and T levels in boys with PAE (β = −0.0008, SE = 0.0003, p < 0.05), but no significant association observed in Control boys (β = 0.0005, SE = 0.0003, p = 0.11).
Fig. 3.
Associations between FA and hormone levels in: A) boys and B) girls. Control in red; Alcohol-exposed in blue. Raw regression coefficients (Beta) ± standard error and p values are presented from post-hoc analyses for each group to represent effect size.
Abbreviations: AE: prenatal alcohol exposed; CST: corticospinal tract; DHEA: dehydroepiandrosterone; FA: fractional anisotropy; IFO; inferior fronto-occipital tract; SFO: superior fronto-occipital tract; SLF; superior longitudinal fasciculus; pg/mL: Picogram/milliliter.
Girls
There was a statistical trend for a GroupxT interaction predicting mean FA values in the SLF (β = 0.0009, SE = 0.0004, p < 0.10; 3B), where a significant positive association between FA and T levels was observed in girls with PAE (β = 0.00059, SE = 0.0002, p < 0.05) but not in Control girls (β = −0.00009, SE = 0.0003, p = 0.81).
3.3.3. FA with DHEA levels
Boys
There was a significant GroupxDHEA interaction predicting mean FA values in the optic radiation (β = −0.0003, SE = 0.0001, p < 0.05), but post-hoc analyses revealed that this association was no longer significant when explored among Control boys only (β = 0.0001, SE = 0.0001, p = 0.14), or boys with PAE only (β = −0.0004, SE = 0.0005, p = 0.58). There was a significant GroupxDHEA interaction predicting mean FA values in the SFO (β = −0.0009, SE = 0.0002, p < 0.01; Fig. 3A), where a positive association between FA and DHEA levels was observed in Control boys (β = 0.0005, SE = 0.0002, p < 0.05), but a negative association observed in boys with PAE (β = −0.0005, SE = 0.0001, p < 0.01).
Girls
There was a significant GroupxDHEA interaction predicting mean FA values in the CST (β=0.0002, SE = 0.0001, p < 0.05; Fig. 3B), where a significant positive association between FA and DHEA levels was observed in girls with PAE (β = 0.00027, SE = 0.00006, p < 0.001), but not in Control girls (β = −0.00000, SE = 0.00005, p = 0.98). There was a significant GroupxDHEA interaction predicting mean FA values in the SLF (β = 0.0003, SE = 0.0001, p < 0.05) where a significant positive association between FA and DHEA levels was observed in girls with PAE (β = 0.00029, SE = 0.0001, p < 0.05), but not in Control girls (β = −0.00008, SE = 0.0001, p = 0.42).
3.3.4. FA with T:DHEA ratio
Boys
No significant associations were observed between T:DHEA ratios and FA values (p’s > 0.13).
Girls
There was a significant GroupxT:DHEA ratio interaction predicting mean FA values in the SLF (β = −0.15, SE = 0.06, p < 0.05; Fig. 3B) where a statistical trend for a positive association between FA and T:DHEA ratios was observed in Control girls (β = 0.08, SE = 0.04, p < 0.10), but a significant negative association in girls with PAE (β = −0.05, SE = 0.02, p < 0.05).
3.3.5. FA with E2 levels
Girls
No associations between E2 and FA were observed among girls (p’s > 0.35).
3.3.6. FA with P levels
Girls
There was a statistical trend for a GroupxP interaction predicting mean FA values in the Fornix (β=0.0005, SE = 0.0002, p < 0.10; Fig. 3B), where a negative association between FA and P levels was observed in Control girls (β= −0.0002, SE = 0.00006, p < 0.05), but not in girls with PAE (β = 0.0002, SE = 0.00021, p = 0.25).
3.3.7. Mean diffusivity (MD)
Boys & Girls
For MD, there were no significant Group effects or Groupxage interactions among boys (p’s > 0.19) or girls (p’s > 0.39).
4. Discussion
Novel findings support the hypothesis that PAE is associated with a different pattern of WM microstructure in boys compared to girls, and that HPG alterations may, in part, relate to PAE-effects on white matter (WM) microstructure. Consistent with previous studies demonstrating a pattern of reduced FA following PAE in humans (Wozniak and Muetzel, 2011), girls with PAE exhibited lower FA values in the inferior fronto-occipital (IFO) and uncinate (UNC) WM tracts. Contrasting with previous literature, boys with PAE exhibited higher FA values in the callosal body, cingulum (CGC), corticospinal tract (CST), optic radiation and superior longitudinal fasciculus (SLF). This pattern of sex differences in PAE-related alterations in FA was observed throughout the brain among projection fibers (CST), association tracts (CGC, optic radiation, SLF) and commissural tracts (callosal body). Second, the expected positive association between age and FA was absent among boys with PAE in two association fibers (CGC and optic radiation), suggesting possible alterations in developmental trajectories of WM tracts that cross through multiple cortical areas. Third, adolescents with PAE exhibit a different pattern of associations between FA and gonadal hormones compared to Control adolescents, and with prominent sex differences. Control boys tended to exhibit positive associations between hormones and FA, whereas Control girls exhibited negative associations; and this sex-difference was reversed among adolescents with PAE. Together, these results suggest that alterations in WM observed among adolescents with PAE are sex-specific and implicate underlying HPG dysregulation, as commonly observed in animal models of PAE (Weinberg et al., 2008). Major implications of sex differences in the effects of PAE on WM development include enhanced understanding of mental health disorders that are more likely to occur in one sex, and informed treatment plans that take sex into consideration. Further understanding of sex-specific neuroendocrine dysregulation following PAE in human adolescents may inform future clinical tools allowing for individualized assessment and intervention measures for FASD by using measures of neuroendocrine function as indices of wellbeing.
4.1. Sex-specific alterations in FA among adolescents with PAE
As reviewed in (Wozniak and Muetzel, 2011), previous DTI studies spanning across ages 5–25 years old collectively demonstrate a pattern of lower FA, and higher MD among humans affected by PAE, and this pattern of effects is typically thought to be indicative of underlying WM pathology. Girls with PAE in our study were consistent with this profile (e.g., lower FA) while boys with PAE exhibited the opposite (e.g., higher FA) when compared to same-sex Controls. It is possible that restricting the range of participants’ ages to the dynamic developmental window of pubertal maturation, when gonadal hormones are significantly increasing, we were able to capture marked sex differences in the effects of PAE on WM maturation, which have not been detected in previous studies with wider age ranges. Specifically, lower FA in girls with PAE is consistent with a developmental profile of delayed WM maturation, whereas higher FA in boys with PAE may indicate altered maturation of WM tracts. In boys with PAE, the absence of positive associations between age and FA may further reflect disruption of the expected continual maturation of WM between childhood and early adulthood. Delayed, or accelerated, maturation of underlying WM may have long-term implications for the ongoing integration of cortical-subcortical networks: a process that continues well into early adulthood (Simmonds et al., 2014). Early or rapid myelination of WM may result in reduced plasticity of structural networks underlying integration of cognition, emotion and behavior in adulthood, although further research in humans is needed to examine this as a possible mechanism. It is important to note that although boys with PAE may exhibit elevated FA levels during pubertal maturation, it is possible that the continued maturation of WM post-puberty may be slowed or absent in boys with PAE, resulting in reduced FA at an older age. Thus, future research is needed to examine the hypothesis that both girls and boys with PAE eventually exhibit delayed maturation of WM (as evidenced by lower FA). In girls, this effect of lower FA may be observed throughout adolescence, but in boys, perhaps not until late adolescence or early adulthood.
Typical sex differences in WM maturation include peak growth into early adulthood in boys, but primarily during mid-adolescence in girls (Simmonds et al., 2014). According to the mean age range for peak growth of WM tracts (see (Simmonds et al., 2014)), the WM tracts with lower FA in girls with PAE are tracts that mature post-puberty [e.g., IFO (~age 15.8–17.7 years); UNC (~ 19.7–28.2 years)], whereas WM tracts with higher FA in boys with PAE included tracts that likely mature during early puberty [e.g., Callosal Body (~age 11.9–15.4 years); CST (~age 10.1–15.8 years)], mid/late-puberty [e.g., SLF (~age 14.9–16.6 years)], and post-puberty [e.g., CGC (~age 19.7–20.8 years)]. Cross-species studies demonstrate that sex differences in myelination are observed around the onset of puberty (reviewed in (Brenhouse and Andersen, 2011)); therefore, implicating a potential role for gonadal hormones in explaining, in part, sex differences in myelination of WM. PAE-related alterations in HPG-function may interfere with the typical developmental timeline of myelination in a sex-dependent manner. Across the life span of typically developing humans, males have higher levels of FA, as well as higher rates of decline later in life, compared to females (Kochunov et al., 2012). It is possible that this typical sex difference may be augmented by PAE in human adolescents. However, future longitudinal DTI studies with hormone and puberty measures across adolescence and early adulthood are needed to explore this hypothesis among individuals affected by PAE.
4.2. Evidence for neuroendocrine dysregulation relating to FA alterations in girls and boys with PAE
The presence of sex differences in brain structure implicates a role for gonadal hormones (Galea et al., 2008). Among animal models of PAE, alterations in neuroendocrine function (including HPG-dysregulation) are a key underlying mechanism for PAE-related brain alterations: dysregulated HPG-function continually impacts the developing PAE brain, and does so in a sex-specific manner (Weinberg et al., 2008). Similar to other studies in typically developing human adolescents (Herting et al., 2012), the present results demonstrate a positive association between FA and testosterone levels among control boys for 4 of the 9 WM ROIs examined. However, boys with PAE did not exhibit this typical positive relationship between testosterone and FA in the CGC, optic radiation, IFO, and SFO. All four of these tracts are association fibers (Oishi et al., 2011), further suggesting that the relationship between HPG-function and fibers connecting different areas of the cortex are significantly altered among boys with PAE. The CGC is a major bundle of association fibers carrying afferent connections from the cingulate gyrus to the entorhinal cortex, and receives input from the thalamus, which is highly sensitive to gonadal hormones (Oishi et al., 2011). Given its integral role in limbic function, the CGC may be particularly relevant to mental health (depression, schizophrenia) (Drevets et al., 2008), executive functioning (Bettcher et al., 2016), and learning and memory (Shapira-Lichter et al., 2016): all behaviors known to be affected by hormones (Galea et al., 2008) and PAE (Mattson et al., 2011). Projecting from the geniculate body to the primary visual cortex, a large degree of inter-individual variability in size and course has been observed in the optic radiation (Burgel et al., 1999). The IFO connects the frontal and occipital lobes, while the anatomy of the SFO is less clear and appears to connect the caudate and merge with the corona radiate (Oishi et al., 2011). A similar pattern of PAE-related alterations was observed between FA and DHEA in the optic radiation and SFO, suggesting that these WM tracts may be highly sensitive to PAE-related alterations in HPG-function in boys.
Similar to previous human studies (Herting et al., 2012), there were no significant positive associations between FA and testosterone, or FA and DHEA, levels in control girls. However, positive associations between FA and testosterone or DHEA were observed in girls with PAE. In light of findings in males, our present results suggest that the typical sex difference in hormone-FA associations may be reversed among adolescents with PAE, albeit region specific. In girls with PAE, this effect was observed in the SLF: a tract passing laterally through the frontal, occipital, parietal and temporal lobes. Higher ratios of testosterone to DHEA were negatively associated with FA levels in girls with PAE, but positively associated in control girls, indicating that T:DHEA ratios may be sensitive to revealing PAE-related WM alterations in girls. A negative association between progesterone and FA was observed in the fornix among control girls, and this effect was absent among girls with PAE. The fornix connects the hippocampus to mammillary bodies and the thalamus: all regions highly sensitive to HPG-hormones and known to be impacted by PAE in human adolescents (reviewed in (Lebel et al., 2011)). Overall, the current findings suggest that PAE may alter typical associations between hormone levels and WM patterns in girls and boys. Moving forward, it will be important to determine if alterations in hormone-brain associations in adolescents with PAE are age-dependent. Moreover, given that PAE has been shown to reduce cellular sensitivity to circulating hormones and disrupt regulation of neurosteroid levels in animal models (Caldeira et al., 2004), future studies should determine if sex-specific brain alterations reflect a compensatory mechanism as a consequence of reduced cellular sensitivity to hormones in youth with PAE.
4.3. PAE-effects on basal HPG-hormone levels
Despite robust PAE- and sex- related differences in hormone-FA associations, there were very few statistically significant group differences in basal hormone levels on their own. The T:DHEA ratio increased with age in boys with PAE, whereas the ratio remains relatively constant across age in all other groups. The present findings suggest that examination of T:DHEA ratios may be a more sensitive measure for detecting PAE-related alterations in boys. It is also possible that measuring hormones at a single time point in the present study resulted in limited power to detect group differences given significant fluctuations across the day as part of the natural circadian pattern in HPG-function. Although time of day when the hormone sample was collected was utilized as a covariate, the variable collection times may have reduced our ability to detect group differences in basal hormone levels. Animal models of PAE are readily able to experimentally test HPG-systems (via gonadectomy, hormone injections, stressor/drug challenge), and it is following an experimental manipulation that PAE-effects on HPG-function are often revealed (Lan et al., 2006; Lan et al., 2009a; Lan et al., 2009b; Uban et al., 2013). Future research could capitalize on naturally occurring challenges to HPG and HPA systems (e.g., hormone-based birth control, stressful first day of school, or other naturally occurring stressors) among adolescents with PAE to more comprehensively assess neuroendocrine dysregulation. Alterations in hormone receptor densities or affinities, binding of circulating hormones, or downstream effects of hormone receptor activation may contribute to PAE-related brain-hormone alterations observed in the present study. Indeed, PAE has been shown to reduce cellular sensitivity to circulating hormones and disrupt regulation of neurosteroid levels (Caldeira et al., 2004); therefore indicating that hormone-brain alterations may be observed in the absence of basal hormone alterations. It is important to note that detecting neuroendocrine effects in girls is more challenging, particularly at this age, when both onset and stage of menses varies across female participants. Further research is needed to better elucidate neuroendocrine dysregulation as a function of pubertal status, onset and stage of menstruation, while controlling for contraception use, in girls with PAE.
4.4. Limitations, methodological considerations and future directions
The present findings of elevated FA in boys with PAE oppose previous literature (Wozniak et al., 2011) and should therefore be considered tentative until future replication. However, a lack of sex differences in previous literature may be primarily due to the failure to statistically investigate sex differences in the effects of PAE on WM; overall smaller PAE sample sizes relative to the present study may have played a role. Additionally, motion affects the reconstruction of WM tracts and alters DTI measures with higher motion relating to lower FA (Aksoy et al., 2010; Roalf et al., 2016); thus, we implemented more stringent criterion for acceptable motion relative to previous DTI studies with PAE participants, possibly increasing power to detect sex-specific effects of PAE on WM.
Several diagnoses fall under the umbrella term of fetal alcohol spectrum disorder (FASD), and have been shown to relate to severity of outcomes, with the diagnosis of fetal alcohol syndrome (FAS) typically identifying individuals with more severe deficits compared to other FASD diagnoses. In the present study, individuals with varying levels of PAE were all classified into the same PAE group; thus, not having the ability to statistically address differences as a function of PAE-related diagnosis is a major limitation, and should be considered in future studies. Another significant limitation of the present study is the use of retrospective reports of maternal alcohol consumption. Future research utilizing prospective reports of maternal alcohol consumption are needed to address the effects of dose and timing of PAE on neuroendocrine function. Additionally, prospective data collection better allows for the ability to assess the impact of co-substance exposure, as mothers consuming moderate to high levels of alcohol during pregnancy may also engage in use of other harmful substances, which could explain the present findings. Accurate co-substance use was often unknown for most participants with PAE in the present sample, making this a major limitation.
5. Conclusions
The present findings suggest that PAE-related alterations in HPG-function are associated with alterations in WM microstructure. Neuroendocrine dysregulation has not been fully characterized among humans with FASD, and is therefore an overlooked, yet promising, avenue of research with potential for informing future medical interventions and revealing underlying mechanisms among human FASD research. Given the significant impact of hormones on brain maturation (and related cognitive, behavioral and mental health outcomes), further investigation of how neuroendocrine systems are impacted by PAE in human adolescents is needed.
Supplementary Material
Acknowledgments
Role of the funding sources
The neuroimaging research was funded by grants from National Institute on Alcohol Abuse and Alcoholism (NIAAA) through the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) U01 AA017122 to Elizabeth Sowell, PhD and Jeff Wozniak, PhD, LP. Megan Herting, PhD was funded by F32HD078084. The gonadal hormone collection and analyses were funded by NIAAA through a F32AA022561 to Kristina Uban, PhD.
We would like to thank the families for their participation, and the research assistants for executing data collection at CHLA (Max Orozco, Alexy Andrade, Lawrence Wong, Trinh Luu, Jordan Barlam) and UMN (Julia Tang, Timothy Hendrickson). We would also like to thank Dr. Bryon Mueller and Dr. Christopher Boys for their assistance with MRI data and participant acquisition, respectively. This work was performed in collaboration with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD; E. Riley, San Diego State University, Principal Investigator) that includes 16 different centers where data collection and analysis take place. The data collection sites and associated investigators described in this paper are: Children’s Hospital Los Angeles, Los Angeles (E.R. Sowell) and University of Minnesota, Minneapolis (J.R. Wozniak). Additional information about CIFASD can be found at www.cifasd.org.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2017.05.019.
Footnotes
Conflict of interest
No financial support from any individual or corporate body has been received for compensation of work over the past three years; therefore the authors declare no potential conflict of interest.
References
- Aksoy M, Skare S, Holdsworth S, Bammer R. Effects of motion and b-matrix correction for high resolution DTI with short-axis PROPELLER-EPI. NMR Biomed. 2010;23:794–802. doi: 10.1002/nbm.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Mungas D, Patel N, Elofson J, Dutt S, Wynn M, Watson CL, Stephens M, Walsh CM, Kramer JH. Neuroanatomical substrates of executive functions:Beyond prefrontal structures. Neuropsychologia. 2016;85:100–109. doi: 10.1016/j.neuropsychologia.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, Jr, Venkatraman VK, Akshoomoff N, Amaral DG, Bloss CS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kaufmann WE, Kenet T, Kennedy DN, Murray SS, Sowell ER, Jernigan TL, Dale AM. Neuroanatomical assessment of biological maturity. Curr Biol. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgel U, Schormann T, Schleicher A, Zilles K. Mapping of histologically identified long fiber tracts in human cerebral hemispheres to the MRI volume of a reference brain: position and spatial variability of the optic radiation. Neuroimage. 1999;10:489–499. doi: 10.1006/nimg.1999.0497. [DOI] [PubMed] [Google Scholar]
- Caldeira JC, Wu Y, Mameli M, Purdy RH, Li PK, Akwa Y, Savage DD, Engen JR, Valenzuela CF. Fetal alcohol exposure alters neurosteroid levels in the developing rat brain. J Neurochem. 2004;90:1530–1539. doi: 10.1111/j.1471-4159.2004.02686.x. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Dodge NC, Granger DA, Jacobson SW. Effects of prenatal alcohol exposure on testosterone and pubertal development. Alcohol Clin Exp Res. 2014;38:1671–1679. doi: 10.1111/acer.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Esquifino AI, Sanchis R, Guerri C. Effect of prenatal alcohol exposure on sexual maturation of female rat offspring. Neuroendocrinology. 1986;44:483–487. doi: 10.1159/000124690. [DOI] [PubMed] [Google Scholar]
- Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormonesthe brain, and behaviour. Can J Exp Psychol. 2008;62:247–260. doi: 10.1037/a0014501. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2055–2064. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungkuntz-Burgett L, Paredez S, Rudeen PK. Reduced sensitivity of hypothalamic-preoptic area norepinephrine and dopamine to testosterone feedback in adult fetal ethanol-exposed male rats. Alcohol. 1990;7:513–516. doi: 10.1016/0741-8329(90)90041-a. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2012;33:9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic-pituitary-adrenal activity in male rats. J Neuroendocrinol. 2006;18:672–684. doi: 10.1111/j.1365-2826.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Lan N, Hellemans KG, Ellis L, Viau V, Weinberg J. Role of testosterone in mediating prenatal ethanol effects on hypothalamic-pituitary-adrenal activity in male rats. Psychoneuroendocrinology. 2009a;34:1314–1328. doi: 10.1016/j.psyneuen.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Sliwowska JH, Viau V, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal function across the estrous cycle. Alcohol Clin Exp Res. 2009b;33:1075–1088. doi: 10.1111/j.1530-0277.2009.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev. 2011;21:102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, Bookheimer SY, O’Connor MJ, Narr KL, Kan E, Abaryan Z, Sowell ER. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci. 2012;32:15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Steiner RJ, Yu Y, Short SJ, Neale MC, Styner MA, Zhu H, Gilmore JH. Common and heritable components of white matter microstructure predict cognitive function at 1 and 2 y. Proc Natl Acad Sci U S A. 2017;114:148–153. doi: 10.1073/pnas.1604658114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund A, Autti-Ramo I, May PA, Adnams CM, Konovalova V, Wetherill L, Arenson AD, Barnett WK, Riley EP. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44:635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivern RF, Yellon SM. Delayed onset of puberty and subtle alterations in GnRH neuronal morphology in female rats exposed prenatally to ethanol. Alcohol. 1992;9:335–340. doi: 10.1016/0741-8329(92)90077-n. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Raum WJ, Salido E, Redei E. Lack of prenatal testosterone surge in fetal rats exposed to alcohol: alterations in testicular morphology and physiology. Alcohol Clin Exp Res. 1988;12:243–247. doi: 10.1111/j.1530-0277.1988.tb00188.x. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Handa RJ, Raum WJ. Ethanol exposure during the last week of gestation in the rat: inhibition of the prenatal testosterone surge in males without long-term alterations in sex behavior. Neurotoxicol Teratol. 1998;20:483–490. doi: 10.1016/s0892-0362(98)00009-9. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, van Zijl P, Mori S. MRI Atlas of Human White Matter. Elsevier; 2011. pp. 1–255. [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45:1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Quarmley M, Elliott MA, Satterthwaite TD, Vandekar SN, Ruparel K, Gennatas ED, Calkins ME, Moore TM, Hopson R, Prabhakaran K, Jackson CT, Verma R, Hakonarson H, Gur RC, Gur RE. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903–919. doi: 10.1016/j.neuroimage.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- Shapira-Lichter I, Weinstein M, Lustgarten N, Ash E, Litinsky I, Aloush V, Anouk M, Caspi D, Hendler T, Paran D. Impaired diffusion tensor imaging findings in the corpus callosum and cingulum may underlie impaired learning and memory abilities in systemic lupus erythematosus. Lupus. 2016;25:1200–1208. doi: 10.1177/0961203316636471. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–368. doi: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Effects of prenatal ethanol exposure on regulation of basal hypothalamic-pituitary-adrenal activity and hippocampal 5-HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology. 2008;33:1111–1123. doi: 10.1016/j.psyneuen.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Barker JM, Barha CK, Lan N, Weinberg J, Galea LA. Stress-induced suppression of hippocampal neurogenesis in adult male rats is altered by prenatal ethanol exposure. Stress. 2010;13:301–313. doi: 10.3109/10253890903531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, Fjell AM. Intellectual abilities and white matter microstructure in development: a diffusion tensor imaging study. Hum Brain Mapp. 2010;31:1609–1625. doi: 10.1002/hbm.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org/ [Google Scholar]
- Uban KA, Sliwowska JH, Lieblich S, Ellis LA, Yu WK, Weinberg J, Galea LA. Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Horm Behav. 2010;58:835–843. doi: 10.1016/j.yhbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Comeau WL, Ellis LA, Galea LA, Weinberg J. Basal regulation of HPA and dopamine systems is altered differentially in males and females by prenatal alcohol exposure and chronic variable stress. Psychoneuroendocrinology. 2013;38:1953–1966. doi: 10.1016/j.psyneuen.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol Rev. 2011;21:133–147. doi: 10.1007/s11065-011-9162-1. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Muetzel RL, Bell CJ, Hoecker HL, Nelson ML, Chang PN, Lim KO. Inter-hemispheric functional connectivity disruption in children with prenatal alcohol exposure. Alcohol Clin Exp Res. 2011;35:849–861. doi: 10.1111/j.1530-0277.2010.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.