Abstract
Background
Voriconazole has previously been associated with increased risk for cutaneous squamous cell carcinoma (SCC) in solid organ transplant recipients. Less is known about the risk in patients after hematopoietic cell transplantation (HCT).
Objective
We evaluated the effect of voriconazole on the risk for nonmelanoma skin cancer (NMSC), including SCC and basal cell carcionoma, among those who have undergone allogeneic and autologous HCT.
Methods
In all, 1220 individuals who had undergone allogeneic HCT and 1418 who had undergone autologous HCT were included in a retrospective cohort study. Multivariate analysis included voriconazole exposure and other known risk factors for NMSC.
Results
In multivariate analysis, voriconazole use increased the risk for NMSC (hazard ratio, 1.82; 95% confidence interval, 1.13–2.91) among those who had undergone allogeneic HCT, particularly for SCC (hazard ratio, 2.25; 95% confidence interval, 1.30–3.89). Voriconazole use did not appear to confer increased risk for NMSC among those who had undergone autologous HCT.
Limitations
This is a retrospective study.
Conclusion
Voriconazole use represents an independent factor that may contribute to increased risk specifically for SCC in the allogeneic HCT population.
Keywords: basal cell carcinoma, bone marrow transplantation, hematopoietic cell transplantation, nonmelanoma skin cancer, squamous cell carcinoma, voriconazole
Individuals who have undergone hematopoietic cell transplantation (HCT) are at increased risk for secondary malignancy,1 most commonly nonmelanoma skin cancer (NMSC), including cutaneous squamous cell carcinoma (SCC) and basal cell carcinoma (BCC).2 Whereas both SCC and BCC are generally associated with low mortality, individuals who have undergone HCT are more likely to develop clinically aggressive forms of disease with locoregional metastasis, deep tissue spread, and perineural and lymphatic invasion associated with poorer prognosis and requiring chemoradiotherapy.3–5 In addition, the high morbidity and treatment costs of advanced NMSC can be significant.6,7 With increasing numbers of HCT survivors,8 the number of patients who have undergone HCT and are at risk for NMSC will continue to grow.9–11
Voriconazole has been available since 2002 for managing fungal infections and has contributed in 2 ways to improved clinical outcomes in patients who undergo HCT. First, it is an excellent treatment option for post-transplant fungal infections, including invasive fungi. Second, it is used as a prophylactic agent in severely immunocompromised patients, such as those who develop graft-versus-host disease (GVHD) after allogeneic HCT.12,13 However, voriconazole is associated with cutaneous toxicities, including photosensitivity,14 and along with its metabolite, voriconazole N-oxide (VNO), it sensitizes keratinocytes to ultraviolet A light and may induce DNA damage and inhibit repair mechanisms.15 Although the association between voriconazole and NMSC has been established in the population of solid organ transplant recipients,16,17 less is known about patients who have undergone HCT. Therefore, we sought to determine the effect of voriconazole on post-HCT risk for NMSC.
MATERIALS AND METHODS
Study cohort
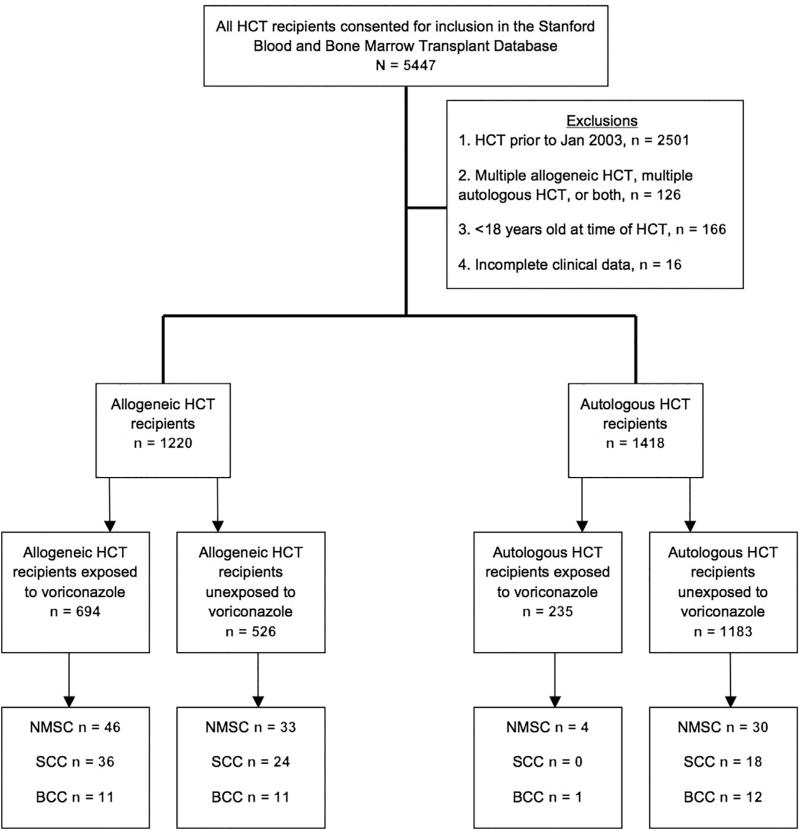
A total of 5447 individuals who had undergone HCT consented to inclusion in the Stanford Blood and Marrow Transplantation database at the time of this analysis. Transplant patients before January 2003 (n = 2501) were excluded owing to low use of voriconazole, as were the following patients: those who had undergone autologous HCT more than once, allogeneic HCT more than once, or both (n = 126); those younger than 18 years at the time of HCT (n = 166); and those with incomplete data (n = 16) (Fig 1).
Fig 1.
Study participant flowchart. BCC, Basal call carcinoma; HCT, hematopoietic cell transplantation; NMSC, nonmelanoma skin cancer; SCC, squamous cell cancer.
Incident post-transplant malignancies, including NMSC, were documented in the Stanford Blood and Marrow Transplantation database, as required by the Stem Cell Therapeutic and Research Act of 2005. To ensure that we had identified all cases of NMSC, we queried Stanford Hospital’s electronic medical and pathology records with the search terms basal cell carcinoma, squamous cell carcinoma, BCC, SCC, nonmelanoma skin cancer, and keratinocyte cancer. Medical records were reviewed to verify the cancer histology and date of diagnosis. SCC included either in situ or invasive disease. Those with SCC of the oral mucosa (n = 3) or anogenital region (n = 1) were excluded.
We built cohorts of those who had undergone HCTs by voriconazole use, which was identified through a query of Stanford Hospital’s electronic medical and pharmacy records. Patients were considered exposed if there was documented use of voriconazole either before or after HCT. Pretransplant use of voriconazole was included because the majority of exposures occurred during induction chemotherapy within a few months before HCT and the effect of voriconazole can be long lasting. Because of the difficulty of confirming doses and tracking the complete prescription history of individual patients, we documented exposure only as ever exposed or never exposed. Patients whose first documented exposure to voriconazole occurred after diagnosis of NMSC were considered never exposed (recipients of 6 allogeneic transplants and 1 autologous transplant). Additionally, 2 allogeneic transplant recipients whose last documented follow-up dates were before their first recorded exposure to voriconazole were considered unexposed (Fig 1).
Time to NMSC was defined as time between the day of HCT and first malignancy diagnosis. The diagnosis of GVHD was ascertained from the Stanford Blood and Marrow Transplantation database along with other covariates such as age at HCT, sex, race, and indication for transplant.
Statistical analysis
To compare demographic characteristics, the Student t test or Wilcoxon rank sum test was applied for continuous variables and the chi-square test was used for categorical variables. A Cox proportional hazards model was applied to estimate the hazard ratio of development of NMSC with voriconazole exposure, with adjustment for diagnosis of chronic GVHD, age at HCT (treated as a continuous variable), race, sex, and history of NMSC before transplantation. Voriconazole use and diagnosis of chronic GHVD were treated as time-dependent variables. Schoenfeld residuals were checked to evaluate the proportional hazards assumptions. An alternative model was fit using voriconazole exposure and nonexposure matched on age at HCT, sex, and follow-up time by the propensity score matching method, and a Cox proportional hazard model was applied. Incidence rates (IRs) and 95% exact confidence intervals (CIs) were calculated as number of skin cancers per 1000 person-years. All P values were 2 sided, and values less than .05 were considered statistically significant. All statistical analyses were conducted using the SAS statistical software package (version 9.4, SAS Institute, Inc, Cary, NC).
RESULTS
Patient population
The study cohort included a total of 2638 individuals who had undergone HCT (1220 allogeneic and 1418 autologous) (Fig 1 and Table I). There were more men than women in both the autologous and allogeneic groups, with white being the most represented race. Acute leukemia (51%) was the most common primary malignancy among those who had undergone allogeneic HCT, followed by non-Hodgkin lymphoma (17%). Autologous transplant recipients were the most likely to have either a plasma cell disorder (43%) or non-Hodgkin lymphoma (37%). Chronic GVHD occurred in 493 of those who had undergone allogeneic HCT (40%).
Table I.
Characteristics of hematopoietic cell transplant recipients, Stanford University Medical Center, January 2003 to March 2015
| Variable | Allogeneic HCT recipients, n (%) | Autologous HCT recipients, n (%) |
|---|---|---|
| Total | 1220 | 1418 |
| Age at HCT, mean (SD), y | 49.2 (14.1) | 53.0 (12.9) |
| Sex | ||
| Female | 539 (44.2) | 548 (38.7) |
| Male | 681 (55.8) | 870 (61.3) |
| Race | ||
| American Indian/Alaskan Native | 0 | 1 (0.1) |
| Asian | 145 (11.9) | 139 (9.8) |
| Black | 20 (1.6) | 64 (4.5) |
| Multiracial | 6 (0.5) | 4 (0.3) |
| Native Hawaiian/Pacific Islander | 12 (1.0) | 4 (0.3) |
| Other | 9 (0.7) | 10 (0.7) |
| Unknown/Not reported | 181 (14.8) | 171 (12.1) |
| White | 847 (69.4) | 1025 (72.3) |
| Indication for HCT | ||
| Acute leukemia | 621 (50.9) | 34 (2.4) |
| Chronic leukemia | 138 (11.3) | 0 |
| Hodgkin lymphoma | 6 (0.5) | 226 (15.9) |
| Non-Hodgkin lymphoma | 206 (16.9) | 518 (36.5) |
| Plasma cell disorders | 5 (0.4) | 602 (42.5) |
| All other | 244 (20.0) | 38 (2.7) |
| Chronic GVHD | 493 (40.4) | 0 |
| History of NMSC | 30 (2.5) | 12 (0.9) |
| Post-HCT NMSC* | 79 (6.5) | 34 (2.4) |
| Squamous cell carcinoma | 60 (4.9) | 18 (1.3) |
| BCC | 22 (1.8) | 13 (0.9) |
| NMSC 10-year incidence rate (95% CI) | 27.4 (21.8–33.9) | 6.5 (4.4–9.3) |
| Squamous cell carcinoma | 19.6 (15.0–25.3) | 3.8 (2.2–6.0) |
| BCC | 7.0 (4.4–10.6) | 2.7 (1.4–4.6) |
| Time from HCT to incident NMSC, median (range), y | 1.9 (0.1–9.9) | 1.6 (0.2–10.9) |
BCC, Basal cell carcinoma; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation; NMSC, nonmelanoma skin cancer; SD, standard deviation.
SCC and BCC were diagnosed simultaneously in 3 patients who had undergone allogeneic HCT; histology was unknown in 3 patients who had undergone autologous HCT.
NMSC risk in allogeneic HCT
Among patients who had undergone allogeneic HCT, 79 developed NMSC, with a 10-year IR of 27.4 (95% CI, 21.8–33.9) (Table I). The median time from HCT to first diagnosis of NMSC was 1.9 years (range, 0.1–9.9). Those with voriconazole use had a median time from HCT to NMSC diagnosis of 2.9 years (range, 0.1–9.9), whereas those no voriconazole use had a median time to diagnosis of 0.7 years (range, 0.1–8.2). SCC was more common than BCC. The 10-year IR for SCC was 19.6 (95% CI, 15.0–25.3) and 7.0 (95% CI, 4.4–10.6) for BCC (Table I). NMSC occurred more often on the head and neck in the exposed cohort than in the unexposed cohort (P = .020) (data not shown).
In multivariate analysis, voriconazole use was associated with an increased overall risk for NMSC (hazard ratio [HR], 1.82; 95% CI; 1.13–2.91; P = .013) among patients who had undergone allogeneic HCT (Table II). The association with voriconazole exposure was significant for SCC (HR, 2.25; 95% CI, 1.30–3.89; P = .004) but not for BCC (HR, 1.05; 95% CI, 0.44–2.52; P = .913). Older age at the time of HCT, male sex, white race, and history of NMSC were each associated with increased overall risk for NMSC and SCC (Table II). Older age at HCT and history of NMSC were found to be associated with increased risk for BCC. Chronic GVHD was associated with increased overall risk for NMSC.
Table II.
Multivariate analysis of risk factors for nonmelanoma skin cancer among allogeneic hematopoietic cell transplant recipients
| NMSC | SCC | BCC | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Variable | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| Voriconazole exposure*† | 1.82 (1.13–2.91) | .013 | 2.25 (1.30–3.89) | .004 | 1.05 (0.44–2.52) | .913 |
| Chronic GVHD† | 1.80 (1.03–3.12) | .038 | 1.88 (0.98–3.62) | .058 | 2.49 (0.89–6.98) | .084 |
| Age at HCT | 1.07 (1.04–1.10) | <.001 | 1.08 (1.04–1.11) | <.001 | 1.06 (1.02–1.12) | .010 |
| Male sex | 3.67 (2.00–6.78) | <.001 | 4.34 (2.03–9.26) | <.001 | 2.20 (0.78–6.19) | .137 |
| White race | 12.60 (3.02–52.52) | <.001 | 15.75 (2.17–114.48) | .007 | 5.85 (0.76–45.18) | .090 |
| History of NMSC | 10.95 (5.84–20.52) | <.001 | 4.64 (2.31–9.30) | <.001 | 8.35 (2.77–25.16) | <.001 |
BCC, Basal cell carcinoma; CI, confidence interval; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation; NMSC, nonmelanoma skin cancer; SD, standard deviation.
Voriconazole exposure included both pretransplant and post-transplant exposure.
Voriconazole exposure and diagnosis of chronic GVHD were calculated as time-dependent variables.
NMSC risk in autologous HCT
Among patients who had undergone autologous HCT, 34 developed NMSC, with a 10-year IR of 6.5 (95% CI, 4.4–9.3) and a median time from HCT to diagnosis of 1.6 years (range, 0.2 –10.9) (Table I). Those with a history of voriconazole use had a median time from HCT to NMSC diagnosis of 1.1 years (range, 0.2–5.7) and those with no voriconazole use had a median time to diagnosis of 1.8 years (range, 0.3–10.9). The 10-year IRs among individuals who had undergone autologous HCT for SCC and BCC, respectively, were 3.8 (95% CI, 2.2–6.0) and 2.7 (95% CI, 1.4–4.6) (Table I). In multivariate analysis, there was no relationship between voriconazole use and risk for NMSC (HR, 0.99; 95% CI, 0.35–2.84; P = .988) (Table III). Risk factors for NMSC among individuals who had undergone autologous HCT included older age at HCT, male sex, and history of NMSC. Our analysis using age- and sex-matched cohorts showed similar results, with slightly higher HRs than reported earlier in this article (data not shown).
Table III.
Multivariate analysis of risk factors for development of NMSC among autologous hematopoietic cell transplant recipients
| NMSC | ||
|---|---|---|
|
|
||
| Variable | Hazard ratio (95% CI) | P value |
| Voriconazole exposure*† | 0.99 (0.35–2.84) | .988 |
| Age at HCT | 1.10 (1.05–1.16) | <.001 |
| Male sex | 2.98 (1.23–7.25) | .016 |
| History of NMSC | 16.94 (5.07–56.64) | <.001 |
CI, Confidence interval; HCT, hematopoietic cell transplantation; NMSC, nonmelanoma skin cancer.
Voriconazole exposure included both pretransplant and post-transplant exposure.
Voriconazole exposurewas calculated as a time-dependent variable.
DISCUSSION
Voriconazole has previously been associated with cutaneous SCC in solid organ transplant recipients,16–18 but little is known about its effects in the post-HCT setting. In this study, we found a high rate of post-HCT NMSC, which was more pronounced among those who had undergone allogeneic HCT than among those who had undergone autologous HCT. Voriconazole use increased the risk for NMSC, particularly SCC, among those who had undergone allogeneic but not autologous HCT, and it had no effect on the risk for BCC. These data are in contrast to those of a prior study that did not find an increased risk for SCC among those who had undergone allogeneic HCT with voriconazole use.10 The difference in these results may be due to the larger sample size in our study, as well to a population residing in a region with higher ambient ultraviolet radiation.
Our findings also support a multifactorial etiology in the post-HCT pathogenesis of NMSC. We identified increasing age, male sex, white race, and history of NMSC as risk factors. These are known risk factors for primary NMSC in other populations,19,20 and our results confirm these associations in the HCT population as well. Impaired immune function also appeared to play an important etiologic role. Individuals who had undergone allogeneic HCT had a higher risk for NMSC than those who had undergone autologous HCT (10-year IR 25.6 vs 6.3), likely because of prolonged immune dysregulation and the presence of chronic GVHD in 40% of cases. This is consistent with the results of previous studies and likely related to prolonged systemic immunosuppression, as well as to chronic inflammation of the skin.21 In contrast, immune suppression is thought to play a less important role in the pathogenesis of BCC, and the lack of association between chronic GVHD and BCC is in accordance with this hypothesis.
The mechanism by which voriconazole leads to SCC is not well understood. Voriconazole and its metabolite, VNO, have been shown to increase the susceptibility of keratinocytes to ultraviolet A damage.15 Interestingly, ultrarapid metabolizers of voriconazole with higher levels of VNO appear to be especially susceptible to development of SCC,22 suggesting that VNO may have greater carcinogenic effects than voriconazole itself. Photosensitivity occurs more often among voriconazole users who develop SCC,23 and in our study population, more NMSCs occurred on sun-exposed areas of the body (eg, head and neck) than on unexposed areas in the voriconazole cohort, suggesting that photosensitization may indeed be a contributor. However, our failure to detect an association between voriconazole and BCC indicates that phototoxicity is not the sole pathway, as previous studies have shown somewhat stronger associations of photosensitizing medications with BCC than with SCC.24
In contrast to what might be expected, the time to incident post-HCT NMSC was shorter among allogeneic hematopoietic cell transplant recipients who were not exposed to voriconazole than among those who received voriconazole. This difference was mainly due to cases with SCC (a median of 0.5 years [range, 0.1–8.7] in the nonexposed group versus a median of 3.0 years [range, 0.1–8.6] in the voriconazole-exposed group). This could be explained by voriconzole inducing additional de novo SCC,25 which would develop at a later time and account for delayed first SCC diagnosis in the voriconazole-exposed group. Consistent with our data, a recent study found a mean time to SCC diagnosis after voriconazole exposure of 35 months among those who had undergone HCT.22 It is also possible that the difference was due to the relatively small number of SCCs that developed in our study.
There are several limitations of this retrospective study. First, we did not have information on the skin type and sensitivity to sun exposure of individual patients. Patients with lighter skin types and patients with skin that is more sensitive to ultraviolet radiation have a higher propensity to development of skin cancer and were found to have increased risk for SCC in solid organ transplant recipients.26 Second, information on history of sun exposure was not available. Third, rather than considering the cumulative duration or dose, which have been shown to be independent risk factors for development of SCC in other studies, our analysis was limited to binary ever versus never exposure to voriconazole.10,27 Future prospective studies are needed to address all of these shortcomings.
Our data indicate that history of voriconazole exposure increases the risk for SCC in those who have undergone allogeneic HCT. Although other factors have greater impact on the risk for NMSC in those who have undergone HCT, the widely accepted use of voriconzaole as either a therapeutic or prophylactic antifungal agent in patients who have undergone HCT makes it an important risk factor to consider.28 On the basis of our data, several recommendations should be considered in patients who have undergone HCT with high risk for development of NMSC. First, patients should have a pretransplant consultation with an experienced dermatologist to be screened for any skin cancer or precursor lesions and receive a thorough overall risk assessment. Second, alternative antifungal agents should be considered in high-risk patients. It is important to note, however, that the effect of newer azoles on the risk for development of NMSC remains unknown. Third, patient education with a focus on avoiding further sun damage and awareness of early detection should be implemented. Fourth, regular, long-term surveillance should be conducted by a dermatologist after HCT.
CAPSULE SUMMARY.
Voriconazole increases the risk for cutaneous squamous cell carcinoma (SCC) among solid organ transplant recipients.
We identify voriconazole as a risk factor for cutaneous SCC following allogeneic hematopoietic cell transplantation.
Allogeneic hematopoietic cell transplant patients exposed to voriconazole should be educated about skin cancer risk reduction and screened regularly for cutaneous SCC.
Acknowledgments
Support for this project was provided by National Cancer Institute Cancer Center Support Grant 5P30CA124435 and Stanford National Institutes of Health/National Center for Research Resources Clinical and Translational Science Award UL1 RR025744.
Abbreviations used
- BCC
basal cell carcinoma
- CI
confidence interval
- HCT
hematopoietic cell transplantation
- HR
hazard ratio
- IR
incidence rate
- NMSC
nonmelanoma skin cancer
- SCC
squamous cell carcinoma
- VNO
voriconazole N-oxide
Footnotes
Conflict of interest: None declared.
Presented at the 58th American Society of Hematology Annual Meeting and Exposition, San Diego, CA; December 2–4, 2016.
References
- 1.Rizzo JD, Curtis RE, Sobocinski KA, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–1184. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leisenring W, Friedman DL, Flowers ME, et al. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24(7):1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 3.Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol. 2010;62(1):31–37. doi: 10.1016/j.jaad.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lott DG, Manz R, Koch C, et al. Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation. 2010;90(6):683–687. doi: 10.1097/TP.0b013e3181ec7228. [DOI] [PubMed] [Google Scholar]
- 5.Gmeinhart B, Hinterberger W, Greinix HT, et al. Anaplastic squamous cell carcinoma (SCC) in a patient with chronic cutaneous graft-versus-host disease (GVHD) Bone Marrow Tranplant. 1999;23(11):1197–1199. doi: 10.1038/sj.bmt.1701772. [DOI] [PubMed] [Google Scholar]
- 6.Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527–1534. doi: 10.1038/jid.2013.446. [DOI] [PubMed] [Google Scholar]
- 7.Mudigonda T, Pearce DJ, Yentzer BA, et al. The economic impact of non-melanoma skin cancer: a review. J Natl Compr Canc Netw. 2010;8(8):888–896. doi: 10.6004/jnccn.2010.0066. [DOI] [PubMed] [Google Scholar]
- 8.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa W, Pond GR, Rifkind JT, et al. Long-term follow-up of secondary malignancies in adults after allogeneic bone marrow transplantation. Bone Marrow Tranplant. 2005;35(1):51–55. doi: 10.1038/sj.bmt.1704706. [DOI] [PubMed] [Google Scholar]
- 10.Wojenski DJ, Bartoo GT, Merten JA, et al. Voriconazole exposure and the risk of cutaneous squamous cell carcinoma in allogeneic hematopoietic stem cell transplant patients. Transpl Infect Dis. 2015;17(2):250–258. doi: 10.1111/tid.12367. [DOI] [PubMed] [Google Scholar]
- 11.Inamoto Y, Shah NN, Savani BN, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Tranplant. 2015;50(8):1013–1023. doi: 10.1038/bmt.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin T, Sharma M, Damon L, et al. Voriconazole is safe and effective as prophylaxis for early and late fungal infections following allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2010;12(1):45–50. doi: 10.1111/j.1399-3062.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 13.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 14.Haylett AK, Felton S, Denning DW, et al. Voriconazole-induced photosensitivity: photobiological assessment of a case series of 12 patients. Br J Dermatol. 2013;168(1):179–185. doi: 10.1111/j.1365-2133.2012.11196.x. [DOI] [PubMed] [Google Scholar]
- 15.Ona K, Oh DH. Voriconazole N-oxide and its ultraviolet B photoproduct sensitize keratinocytes to ultraviolet A. Br J Dermatol. 2015;173(3):751–759. doi: 10.1111/bjd.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer JP, Boker A, Metchnikoff C, et al. High cumulative dose exposure to voriconazole is associated with cutaneous squamous cell carcinoma in lung transplant recipients. J Heart Lung Transpl. 2013;31(7):694–699. doi: 10.1016/j.healun.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansh M, Binstock M, Williams K, et al. Voriconazole exposure and risk of cutaneous squamous cell carcinoma, aspergillus colonization, invasive aspergillosis and death in lung transplant recipients. Am J Transpl. 2016;16(1):262–270. doi: 10.1111/ajt.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feist A, Lee R, Osborne S, et al. Increased incidence of cutaneous squamous cell carcinoma in lung transplant recipients taking long-term voriconazole. J Heart Lung Transpl. 2012;31(11):1177–1181. doi: 10.1016/j.healun.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Gray DT, Suman VJ, Su WP, et al. Trends in the population-based incidence of squamous cell carcinoma of the skin first diagnosed between 1984 and 1992. Arch Dermatol. 1997;133(6):1735–1740. [PubMed] [Google Scholar]
- 20.Lovatt TJ, Lear JT, Bastrilles J, et al. Associations between ultraviolet radiation, basal cell carcinoma site and histology, host characteristics, and rate of development of further tumors. J Am Acad Dermatol. 2005;52(3 Pt 1):468–473. doi: 10.1016/j.jaad.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 21.Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: nn international case-control study. Blood. 2005;105(10):3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams K, Arron ST. Association of CYP2C19 *17/*17 genotype with the risk of voriconazole-associated squamous cell carcinoma. JAMA Dermatol. 2016;152(6):719–720. doi: 10.1001/jamadermatol.2016.0351. [DOI] [PubMed] [Google Scholar]
- 23.Epaulard O, Villier C, Ravaud P, et al. A multistep voriconazole-related phototoxic pathway may lead to skin carcinoma: results from a French nationwide study. Clin Infect Dis. 2012;57(12):182–188. doi: 10.1093/cid/cit600. [DOI] [PubMed] [Google Scholar]
- 24.Robinson SN, Zens MS, Perry AE, et al. Photosensitizing agents and the risk of non-melanoma skin cancer: a population-based case-control study. J Invest Dermatol. 2013;133(8):1950–1955. doi: 10.1038/jid.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansh M, Ing L, Dimon M, et al. Voriconazole exposure regulates distinct cell-cycle and terminal differentiation pathways in primary human keratinocytes. Br J Dermatol. 2017;176(3):816–820. doi: 10.1111/bjd.14838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mclaughlin JM, Equils O, Somerville KT, et al. Risk-adjusted relationship between voriconazole utilization and non-melanoma skin cancer among lung and heart/lung transplant patients. Transpl Infect Dis. 2013;15(4):329–343. doi: 10.1111/tid.12063. [DOI] [PubMed] [Google Scholar]
- 27.Vadnerkar A, Nguyen MH, Mitsani D, et al. Voriconazole exposure and geographic location are independent risk factors for squamous cell carcinoma of the skin among lung transplant recipients. J Heart Lung Transpl. 2010;29(11):1240–1244. doi: 10.1016/j.healun.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Neoh CF, Snell GI, Kotsimbos T, et al. Antifungal prophylaxis in lung transplantation-a world-wide survey. Am J Transpl. 2011;11(2):361–366. doi: 10.1111/j.1600-6143.2010.03375.x. [DOI] [PubMed] [Google Scholar]