Abstract
Diabetes is a progressive disease affecting millions of people worldwide. There are several medications and treatment options to improve the life quality of people with diabetes. One of the strategies for the treatment of diabetes could be the use of human pluripotent stem cells or induced pluripotent stem cells. The recent advances in differentiation of stem cells into insulin secreting beta-like cells in vitro make the transplantation of the stem cell-derived beta-like cells an attractive approach for treatment of type 1 diabetes. While stem cell-derived beta-like cells provide an unlimited cell source for beta cell replacement therapies, these cells can also be used as a platform for drug screening or modeling diseases.
Keywords: Diabetes, human pluripotent stem cells, induced pluripotent stem cells, differentiation, pancreatic beta cells
Introduction
The impact of diabetes both in the United States and worldwide is astounding; in the US alone, more than 29.1 million people (9.3% of the US population) have either diagnosed (21 million) or undiagnosed (8.1 million) diabetes, and as many as 86 million adults (more than 1 in 3) have prediabetes [1]. Among the various types of diabetes, type 2 diabetes is by far the most common, accounting for up to 95% of diagnosed diabetes in US adults. The other 5% of adults with diabetes have type 1 [1]. The true prevalence of maturity-onset diabetes of the young (MODY) is unknown, but it is thought to account for 1-5% of diabetes cases; patients in this category may be misdiagnosed as having either type 1 or type diabetes [2].
Although there are a multitude of therapies to treat type 2 diabetes, the progressive nature of the disease makes it difficult to control precisely. The United Kingdom Prospective Diabetes Study showed that, within 3 years of diagnosis, up to 50% of patients will require more than one pharmacologic agent to maintain target glycemic levels; this increases to 75% by 9 years [3]. Regeneration of β cells using stem cells is an attractive approach to potentially ameliorate many of the challenges inherent in type 2 diabetes treatment, and may not be so far in the future. In this review we will discuss both the promise and limitations in this approach to type 2 diabetes.
The Definition and Pathogenesis of Type 2 Diabetes
The diagnosis of diabetes is made when one of the following criteria are met: Hemoglobin A1c >6.5%, fasting plasma glucose ≥ 126 mg/dl, 2 hour post-prandial plasma glucose ≥ 200 mg/dl during an oral glucose tolerance test, or anyone with classic symptoms of hyperglycemia (polyuria, polydipsia) and a random plasma glucose ≥ 200 mg/dl [4]. While type 1 is a T-cell mediated destruction of pancreatic beta cell, type 2 diabetes is characterized by peripheral insulin resistance and pancreatic β cell dysfunction, often in the setting of excess weight or obesity. MODY is an inheritable form of diabetes characterized by mutations in a subset of genes, leading to β-cell dysfunction.
Both genetic and environmental factors play a role in the diagnosis of type 2 diabetes, with recent genome-wide association studies showing multiple loci contributing to increased susceptibility to type 2 diabetes [5–9]. Environmental factors include obesity, older age, and lack of exercise. An increase in type 2 diabetes frequency occurs in certain populations, such as women with a history of gestational diabetes, people with high blood pressure or dyslipidemia, and certain ethnicities (African Americans, American Indians, Latinos, and Asian Americans) [4].
The insulin resistance in type 2 diabetes is linked to increased adipocyte mass. Adipocytes release cytokines, non-essential fatty acids, and adipogenic hormones; these in turn alter insulin sensitivity in tissues and muscles. Insulin resistance increases demand on the β cell to produce more insulin; while initially the β cells are able to compensate, over time they become unable to keep up with insulin demands leading to overt diabetes. Ensuing glucolipotoxicity further promotes cell apoptosis [10]. More recent studies have focused on investigating the role of the gut microbiome in chronic diseases such as type 2 diabetes; intriguingly, patients with type 2 diabetes have been found to have different bacterial compositions and altered function as compared to controls [11–13].
Current Treatment Options for Type 2 Diabetes
The treatment of type 2 diabetes involves lifestyle modifications, oral antidiabetic agents, insulin, bariatric surgery, and treatment of co-morbidities such as obesity, dyslipidemia, hypertension, chronic kidney disease, cardiovascular disease, and depression. Intensive blood glucose control is paramount for prevention of microvascular complications [14, 15].
Lifestyle modifications that lead to weight loss have been associated with an up to 58% risk reduction after 3 years for development of type 2 diabetes in those with impaired glucose tolerance; this risk reduction decreased but remained significant up to 10 years after intervention [16, 17].
Within the category of oral antidiabetic agents, first-line agents include biguanides such as metformin; these work by increasing the insulin sensitivity of muscle and decreasing hepatic glucose production. Sulfonylureas and meglitinides target the inward-rectifying potassium-adenosine triphosphate (ATP) channel on beta cells, leading to increased insulin secretion from remaining beta cells. Thiazolidinediones act on peroxisome proliferator-activated receptor-γ (PPARγ) receptors, transcription factors that regulate a variety of genes involved in glucose homeostasis and lipid metabolism, enhancing insulin sensitivity. Glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors increase levels of GLP-1, a gastric compound that promotes insulin release while suppressing glucagon and slowing gastric emptying. Alpha-glucosidase inhibitors act by competing with oligosaccharides for binding to the alpha-glucosidase enzyme on the brush border of the small intestine. Sodium-glucose transport-2 (SGLT2) inhibitors, the newest class of oral antidiabetic medications, work on the kidney to increase urine excretion of glucose.
The side effects of the many classes of oral anti-diabetic agents vary; ones that target the beta cell to increase insulin secretion (such as sulfonylureas and meglitinides) may lead to hypoglycemia and weight gain. Others (such as biguanides, GLP-1 receptor agonists and alpha-glucosidase inhibitors) have gastrointestinal side effects. Certain thiazolidinediones and DPP4-inhibitors are associated with an increased risk of cardiovascular side effects [18–20]. SGLT2 inhibitors may increase the risk of dehydration, urinary tract infections and bone fractures [21, 22].
Although traditionally used as a later treatment modality, insulin has been implemented earlier in the disease given improved glycemic control [23]. It often leads to weight gain, has the potential to cause profound hypoglycemia, and must be given subcutaneously either with twice daily injections or an insulin pump.
Perhaps the most effective treatment for type 2 diabetes is bariatric surgery. A recent meta-analysis found that a review of 26 studies showed improvement or remission of diabetes in 89.2% of patients [24]. Adverse events are related to surgical risks and post-operative hypoglycemia.
Although there are a multitude of options to treat type 2 diabetes, none address what lies at the core of the disease: the increased stress and progressive destruction of insulin-producing beta cells. The creation of beta cells from embryonic or induced pluripotent stem cells would therefore be the first bona-fide cure for this highly prevalent, difficult to manage disease.
The Promise of Stem Cells
Embryonic stem (ES) cells and induced pluripotent stem (iPS) cells have an unlimited proliferative capacity and the potential to differentiate into virtually every cell type in the body. Although they appear to be biologically very similar, ES cells and iPS cells originate from different sources. ES cells are derived from blastocysts, an early stage of the embryo, and iPS cells are derived from adult cells such as fibroblasts, adipocytes or blood cells by conversion of somatic cells into a pluripotency state. The first human ES cell lines were isolated from inner cell mass of embryos which were generated from in vitro fertilization and donated by individuals for research [25]. However, the use of human embryos for research raised ethical concerns. The reprogramming of adult cells circumvents these ethical issues, and thus iPS cells constitute a suitable alternative for ES cells. Additionally, using patient-derived iPS cells for regenerative therapy makes their use advantageous, because iPS cells avoid rejection by the host immune system as cells have the same genetic background as the donor. iPS cells were first generated in 2006 by Takahashi and Yamanaka by introducing four pluripotency genes, Oct3/4, Sox2, Klf4 and c-Myc into mouse fibroblast cells using retroviral vectors [26]. Soon thereafter human iPS cells were generated from dermal fibroblast cells using the same approach [27]. Utilization of retroviruses or lentiviruses for reprogramming brought safety concerns related to genomic integration and the risk of tumor formation. Several non-integrative approaches, such as reprogramming by adenoviral vectors, Sendai virus vectors, plasmid DNA, recombinant cell penetrating peptides, synthetic mRNA, and miRNA have all been reported to overcome the risk of permanent genetic modification to the cells and have made reprogramming more reliable. Another issue with using iPS cells in clinical trials is whether iPS cells are equivalent to ES cells, known as the gold standard of pluripotent cells. A recent study that compared genetically matched ES cells and iPS cells demonstrated that major differences between them were attributable to genetic background variation, but not to the cellular source (ES vs iPS cells) [28].
The ability of pluripotent stem cells to be differentiated towards specific cell types has drawn attention to stem cell based cell replacement therapy. Especially, the ability of pluripotent stem cells to differentiate into insulin-secreting beta cells has heightened the interest in using stem cells for the treatment of diabetes.
Generating Beta Cells from ES Cells or from iPS Cells
Elucidation of important signaling pathways and master regulators controlling cell fate commitment during developmental stages of pancreas paved the way for directed differentiation of pluripotent stem cells towards pancreatic lineage in vitro. There have been numerous attempts to derive pancreatic “beta-like” cells from pluripotent stem cells by mimicking normal pancreas development in vitro using a stepwise manner through definitive endoderm, primitive gut tube, foregut, ventral and dorsal pancreatic endoderm, pancreatic progenitors, and ultimately insulin-producing beta-like cells. These efforts benefited from small molecules and recombinant proteins for stimulation or inhibition of important development signaling pathways sequentially. Although initial attempts to derive pancreatic beta cells resulted in generation of polyhormonal endocrine cells minimally responsive to glucose [29, 30], subsequent studies reported the generation of insulin-secreting glucose-responsive endocrine cells several months after transplantation of pancreatic progenitors into mice [31]. These data suggest as yet unidentified factors in vivo in the mouse system were able to induce maturation of stem cell-derived pancreatic progenitors and gave rise to cells co-expressing insulin and key transcription factors of beta cells such as PDX1, NKX6.1, MAFA, PCSK1, and PCSK2. These in vivo differentiated cells were also capable of ameliorating type 1 diabetes [32] and type 2 diabetes in mice [33]. Subsequently, studies reported enrichment of cells expressing high levels of NKX6.1 from the pancreatic progenitor cell population accelerated in vivo maturation period [34]. Furthermore, transplantation of stem-cell derived endocrine cells into rats relative to mice hastened the in vivo maturation process [35]. However, several unanswered questions remain in the context of maturation of pancreatic progenitors in the rodent system and the relevance of the in vivo maturation process when translating the approach to human clinical trials.
Since most of our current knowledge for guiding differentiation of pluripotent stem cells into pancreatic beta-like cells has emerged from research in rodents, a lack of sufficient developmental knowledge together with the known differences between mouse and human pancreas development continues to be a substantial challenge in the field. Although early developmental stages, including definitive endoderm and pancreatic progenitor stage can be established efficiently, several studies have failed to further differentiate these pancreatic progenitors into mature pancreatic beta cells in vitro. Instead, they reported generation of polyhormonal cells that resemble fetal beta cells but not mature beta cells [36–38]. For this and other reasons several investigators in the field prefer labeling these cells “beta-like”.
Recently, two groups independently reported protocols for in vitro generation of pancreatic beta cells [39, 40]. The first protocol was published by BetaLogics Venture in collaboration with the Kieffer group, and the second one was subsequently reported by the Melton group by modifying their own previously published protocols and extending in vitro differentiation to mature beta cells. Both groups efficiently induced either ES cells or iPS cells into definitive endoderm and subsequently into pancreatic precursors. Further differentiation of pancreatic precursors using several small molecules and growth factors for 3-4 weeks resulted in generation of pancreatic beta-like cells. Unlike the previous studies yielding mostly non-functional polyhormonal cells with only a small percentage of insulin expressing cells, new protocols overcame these problems and generated monohormonal cells secreting insulin similar to that of human islets in response to glucose in static incubation experiments. Ultrastructural analysis of secretory granules showed presence of insulin-like endocrine granules in stem cell-derived beta-like cells generated by both protocols. Additionally, these beta-like cells were able to ameliorate hyperglycemia in a short time when transplanted into diabetic mice. However, the first paper (Rezania et. al.) demonstrated functional differences between stem cell-derived beta-like cells and human pancreatic islets by functional assessment of the cells. Insulin secretion dynamics and calcium oscillations in response to high glucose (20 mM) and incretin (exendin-4) showed delayed and weak response of stem cell-derived beta cells compared to human islets. The functional limitations indicated that stem cell-derived beta-like cells and human islets are not completely identical. Although stem cell-derived beta-like cells express most of the mature beta cell transcription factors similar or higher levels than that of human islets, expression of several genes remained lower than human islets (such as IAPP, CHGB, KCNK1, KCNK3, UCN3). The beta-like cells reported in the second paper (Pagliuca et. al.) also showed low level expression of some genes (KLF9, PCSK1, PCSK2) compared to human islets.
Lately, Russ and colleagues reported generation of functional beta-like cells exhibiting key features of bona fide human beta cells by improving published protocols [41]. They demonstrated that BMP inhibitors, which are used in most of the current differentiation protocols, induce pancreatic endoderm early to form immature polyhormonal cells expressing insulin but not NKX6.1, a critical beta cell transcription factor. By excluding the use of BMP inhibitors during endocrine commitment, they achieved differentiation of pluripotent stem cells towards glucose responsive monohormonal beta-like cells in vitro. Their simplified protocol generated 23% C-peptide positive beta-like cells within 3 weeks, which express critical beta cell genes and respond to high glucose concentration by secreting insulin. The ratio of insulin secreted in low glucose (2.8 mM) to high glucose (16.7 mM) concentrations was similar for beta-like cells and human islets.
In summary, the three protocols discussed above are examples of efforts to derive authentic beta cells. Although stem cell-derived beta cells display certain similarities to human beta cells regarding gene expression and secretory function, there continue to be challenges related to functional properties, and they cannot be considered mature bona fide human beta cells yet.
The Use of Stem Cell-Derived Pancreatic Cells in Investigating Disease Mechanisms
ES cells and iPS cells, owing to their ability to differentiate into virtually any cell type in the body, are valuable tools for the development of new drugs and for the assessment of potential toxic effects on cells (Figure 1). Thousands of small molecules can be tested rapidly in screening platforms of iPS cell-derived pancreatic progenitors and beta-like cells for finding antidiabetic drug candidates. Another application of human ES cells or iPS cells is in disease modeling to uncover novel cellular mechanisms underlying disease phenotypes. Although animal models are frequently used for studying diabetes, the use of patient specific cells is probably the most convenient model for studying human disease, considering that translating animal findings to humans sometimes might be challenging. iPS cells can also serve as a valuable resource in the absence of cellular or animal models mimicking human diseases. Various disease-specific human iPS cells have been generated including type 1 and type 2 diabetes, complex diseases such as diabetic cardiomyopathy, and several monogenic diseases which enabled investigators to explore human diseases in petri dishes [42–44]. A recent study from our group showed feasibility of using human iPS cells in modeling diabetic complications. iPS cells generated from patients with type 1 diabetes for more than fifty years with diabetic complications showed elevated miR200 expression and poor DNA repair suggesting that miR200 regulated DNA damage checkpoint pathway is a potential therapeutic target for treating diabetes complications [45].
Figure 1. The use of human ES cells and iPS cells for studying disease mechanisms and for the treatment of diabetes.
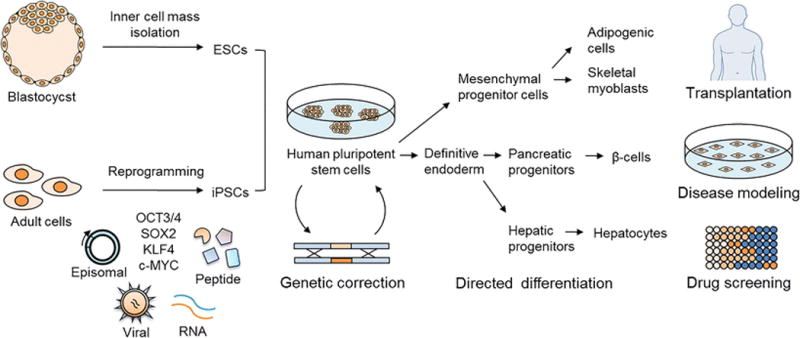
Embryonic stem (ES) cells are derived from isolated inner cell mass of blastocysts and induced pluripotent stem (iPS) cells are generated from adult cells by using reprogramming techniques including plasmid DNA (episomal), virus, synthetic mRNA or miRNA, and recombinant cell penetrating peptide-mediated transfer of the pluripotency factors (OCT3/4, SOX2, KLF4, c-MYC) into somatic cells. The genetic mutation causing diabetes has the potential to be corrected in patient-derived human pluripotent stem cells using gene editing technologies. Expanded human pluripotent stem cells can be directed towards definitive endoderm, pancreatic progenitors, and finally insulin secreting β-cells in vitro in a stepwise manner by mimicking human pancreas development in vivo. Pluripotent stem cells can also be differentiated into insulin responsive cells such as adipocytes, skeletal muscle cells and hepatocytes. These cells can be used for diabetes therapy, development of anti-diabetic drugs, and for disease modeling.
Since diabetes is a complex disorder secondary to environmental and genetic risk factors, several laboratories have pursued modeling monogenic forms of diabetes mellitus using iPS cells. Salvatore and colleagues recently generated iPS cells from individuals with insulin receptor mutations causing insulin resistance syndromes, and reported altered insulin signaling, reduced proliferation and altered gene expression in mutant iPS cells [46]. Besides disease modeling, iPS cell-derived specialized cells could provide a platform for uncovering poorly understood genetic influences. For instance, iPS cell-derived pancreatic beta cells from patients with maturity-onset of diabetes of the young (MODY) can provide a valuable tool to understand the importance of disease-relevant genes in the development and function of pancreatic beta cells, considering that all MODY genes contribute to beta cell dysfunction. Recently, it has been shown that iPS cell lines were generated from skin fibroblasts of patients with several different types of MODY including MODY1, MODY2, MODY3, MODY5, and MODY8 [47]. The impact of mutations in the HNF1B gene on pancreas development which result in MODY5 phenotype was investigated in MODY5 pancreatic progenitors derived from patient specific iPS cells. Perturbations in the transcriptional network during early pancreas development due to the mutation in the HNF1B gene were observed [48]. MODY2 iPS cells, which express mutations in the glucokinase gene, were used to recapitulate MODY2 disease in iPS cells [49]. MODY2-iPS cells were successfully differentiated into pancreatic beta cells with an efficiency that was comparable to that of healthy-iPS cells. Beta cells derived from MODY2-iPS cells exhibited decreased glucose sensitivity that was comparable to the phenotype of MODY2 patients, confirming the feasibility of iPS-differentiated cells in disease modeling. Another study reported the use of iPS cell-derived beta cells to model Wolfram Syndrome which results from mutations in the WFS1 (Wolfram Syndrome 1) gene. iPS cells were generated from four Wolfram Syndrome patients, and then differentiated into C-peptide positive beta cells with efficiency similar to controls. Reduced insulin secretion and elevated cell stress were observed in mutant iPS cell-derived beta cells. Importantly, a chemical chaperone (4-phenyl-butyric acid) reverted stress-associated phenotype in beta cells and provides an example of therapeutic relevance of this molecule for treatment of Wolfram Syndrome patients [50].
iPS cell-derived pancreatic exocrine cells also represent an important model for studying diseases of exocrine pancreas. Tulpule and colleagues utilized exocrine cells differentiated from iPS cells of Shwachman-Diamond syndrome patients displaying exocrine insufficiency and hematopoietic dysfunction due to mutations in the SBDS (Shwachman-Bodian-Diamond syndrome) gene. The phenotype was reversed by using protease inhibitors pointing to a potential therapeutic strategy for treating Shwachman-Diamond syndrome [51].
Another way to generate in vitro model of diabetes could be the use of iPS cell derived non-pancreatic cells, such as hepatocytes, myotubes and adipocytes which are the important cells for glucose utilization and become insulin resistant in type 2 diabetes. The use of these differentiated cells in order to investigate the ways of improvement of glucose uptake in peripheral tissues could be an option of treating type 2 diabetes. Recently, modeling of genetic insulin resistance in iPS cell-derived myotubes was reported [52]. Although differentiation of human pluripotent stem cells to adipocyte [53] and hepatocyte [54] is well established, their use in investigating mechanisms of insulin resistance has not been reported yet.
Gene Editing and Relevance to Therapy
Advances in DNA editing technology have enabled targeted modification of the human genome. Numerous studies have shown that patient derived iPS cells can be genetically modified by employing genome editing tools such as zinc finger nucleases (ZFN), transcription activator-like effector nucleases (TALEN) or clustered regularly interspaced short palindromic repeat (CRISPR) systems [55]. These gene editing nucleases have been utilized for disease modeling by generating cell lines carrying disease specific mutation or by generating isogenic controls by correcting the mutation in patient-derived iPS cells. Recently, CRISPR, has been used to edit the genome of a human embryo [56, 57] and can potentially be used to treat patients with monogenic diabetes. For example, disease specific mutations could be corrected in the patient-derived iPS cells and then these corrected cells differentiate to disease relevant cell type which could provide a non-immunogenic and healthy cell source for the treatment of the patients with monogenic diabetes.
Clinical Trials
Remarkably, in under a decade, human clinical trials have started using human embryonic stem cell therapy to treat type 1 diabetes. Using the human ES cell line CyT49, researchers at ViaCyte, Inc, have successfully developed a scalable, controlled, and regulated differentiation process to create islet-like cells capable of producing insulin in response to glucose stimulation [58, 59]. They have also patented a macroencapsulation device (Encaptra) for delivery of these stem cells in patients, thereby preventing immune rejection and allowing easy retrieval if needed [58]. In July 2014 the company filed an Investigational New Drug application with the Food and Drug Administration for this combination product, VC-01. This was approved in August 2014. Phase I/II human clinical trials in type 1 diabetics began in September 2014; trials are anticipated to end August 2017 [60]. The scientific community awaits the outcome of these trials with anticipation.
Challenges to Stem Cell Therapy
Although there have been monumental advances in human stem cell-based therapy, using this approach in medical management of disease remains in its infancy. There remain a multitude of issues to consider in adopting this technology. At the cellular level, one must decide whether to use human embryonic stem cells versus inducible pluripotent stem cells. Embryonic stem cells have the ability to differentiate into any and all cell types without the need for viral transformation, but carry ethical concerns. On the other hand iPS cells can arise from any cell, but require viral transformation that could lead to genetic instability, teratoma formation, and variability in differentiation [61–63].
Another challenge to overcome in stem cell therapy is host immune rejection. Employing immunosuppressive regimens to combat this reaction would likely make the benefits of stem cells negligible. ViaCyte and others have circumvented the immune system by inventing macroencapsulation devices with semipermeable barriers [58, 64, 65]. An additional benefit to macroencapsulation is the ability to retrieve the stem cells if needed. Other researchers are working on immune system modulation [66, 67]. Autologous stem cells would not be targeted as “foreign” by the body, but the harvesting, cultivating, and delivery of these is too costly and labor-intensive to be a feasible option at this time. A further issue to debate is whether patients with a common disease can benefit from using beta-like cells or any other cells for therapy that have been generated from a “common” bank of iPS cells derived from populations of different ethnic backgrounds and maintained centrally in key areas in a geographical location.
Conclusions
Type 2 diabetes is a progressive disease which involves the dysfunction and eventual loss of insulin-producing beta cells on a background of peripheral insulin resistance. It often requires multiple medications to control, and management is often suboptimal. The differentiation of stem cells into pancreatic beta-like cells offers a means to replace the beta cell mass lost by the body, and is an attractive approach for treatment. While several critical issues remain to be addressed prior to this therapy reaching the type 2 diabetes population, the initiation of stem cell based clinical trials for type 1 diabetics appears promising.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Sevim Kahraman, Erin R. Okawa, and Rohit N. Kulkarni declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers or particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Centers for Disease Control and Prevention. Diabetes Report Card 2015. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2015. [Google Scholar]
- 2.Ledermann HM. Is maturity onset diabetes at young age (MODY) more common in Europe than previously assumed? Lancet. 1995;345(8950):648. doi: 10.1016/s0140-6736(95)90548-0. [DOI] [PubMed] [Google Scholar]
- 3.Turner RC, et al. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281(21):2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 4.2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39(Suppl 1):S13–22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 5.Cho YS, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet. 2012;44(1):67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaulton KJ, et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet. 2015;47(12):1415–25. doi: 10.1038/ng.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kooner JS, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet. 2011;43(10):984–9. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahajan A, et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46(3):234–44. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris AP, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–90. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson RP, et al. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–24. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 11.Larsen N, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson FH, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 14.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowler WC, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 17.Holman RR, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 18.Stein SA, Lamos EM, Davis SN. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin Drug Saf. 2013;12(2):153–75. doi: 10.1517/14740338.2013.752813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351(11):1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 20.Scirica BM, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz SS, Ahmed I. Sodium-glucose cotransporter 2 inhibitors: an evidence-based practice approach to their use in the natural history of type 2 diabetes. Curr Med Res Opin. 2016:1–13. doi: 10.1185/03007995.2016.1151774. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. 2015 [Last accessed February 19, 2016]; Available from: http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm.
- 23.Hanefeld M, et al. Early Treatment with Basal Insulin Glargine in People with Type 2 Diabetes: Lessons from ORIGIN and Other Cardiovascular Trials. Diabetes Ther. 2016 doi: 10.1007/s13300-016-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, et al. The long-term effects of bariatric surgery for type 2 diabetes: systematic review and meta-analysis of randomized and non-randomized evidence. Obes Surg. 2015;25(1):143–58. doi: 10.1007/s11695-014-1460-2. [DOI] [PubMed] [Google Scholar]
- 25.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 28•.Choi J, et al. A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nat Biotechnol. 2015;33(11):1173–81. doi: 10.1038/nbt.3388. Extensive study comparing several iPS and ES lines using bioinformatic tools. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.D’Amour KA, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–401. doi: 10.1038/nbt1259. These authors were the first to differentiate human ES cells into endocrine cells; these cells had an insulin content similar to adult islet cells and released insulin in response to multiple secretory stimuli. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25(8):1940–53. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 31••.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. doi: 10.1038/nbt1393. In this paper, human ES cells were differentiated into endocrine cells that were capable of secreting insulin in response to glucose. [DOI] [PubMed] [Google Scholar]
- 32.Rezania A, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–29. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruin JE, et al. Treating diet-induced diabetes and obesity with human embryonic stem cell-derived pancreatic progenitor cells and antidiabetic drugs. Stem Cell Reports. 2015;4(4):605–20. doi: 10.1016/j.stemcr.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezania A, et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31(11):2432–42. doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- 35.Bruin JE, et al. Accelerated Maturation of Human Stem Cell-Derived Pancreatic Progenitor Cells into Insulin-Secreting Cells in Immunodeficient Rats Relative to Mice. Stem Cell Reports. 2015;5(6):1081–96. doi: 10.1016/j.stemcr.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basford CL, et al. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012;55(2):358–71. doi: 10.1007/s00125-011-2335-x. [DOI] [PubMed] [Google Scholar]
- 37.Nostro MC, et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138(5):861–71. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruin JE, et al. Characterization of polyhormonal insulin-producing cells derived in vitro from human embryonic stem cells. Stem Cell Res. 2014;12(1):194–208. doi: 10.1016/j.scr.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 39••.Rezania A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33. doi: 10.1038/nbt.3033. This is the first study to describe the in vitro generation of functional “beta-like” cells from human pluripotent stem cells. [DOI] [PubMed] [Google Scholar]
- 40••.Pagliuca FW, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–39. doi: 10.1016/j.cell.2014.09.040. This landmark paper was the first to report a scalable differentiation protocol to create glucose-responsive “beta-like” cells from human induced pluripotent stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russ HA, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34(13):1759–72. doi: 10.15252/embj.201591058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kudva YC, et al. Transgene-free disease-specific induced pluripotent stem cells from patients with type 1 and type 2 diabetes. Stem Cells Transl Med. 2012;1(6):451–61. doi: 10.5966/sctm.2011-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drawnel FM, et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9(3):810–21. doi: 10.1016/j.celrep.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 45••.Bhatt S, et al. Preserved DNA Damage Checkpoint Pathway Protects against Complications in Long-Standing Type 1 Diabetes. Cell Metab. 2015;22(2):239–52. doi: 10.1016/j.cmet.2015.07.015. This study is focused on using fibroblasts and iPS cells to define the signficance of miR200 in regulating pathways that have the potential to protect some patients with long-standing type 1 diabetes from developing complications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iovino S, et al. Genetic insulin resistance is a potent regulator of gene expression and proliferation in human iPS cells. Diabetes. 2014;63(12):4130–42. doi: 10.2337/db14-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teo AK, et al. Derivation of human induced pluripotent stem cells from patients with maturity onset diabetes of the young. J Biol Chem. 2013;288(8):5353–6. doi: 10.1074/jbc.C112.428979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teo AK, et al. Early Developmental Perturbations in a Human Stem Cell Model of MODY5/HNF1B Pancreatic Hypoplasia. Stem Cell Reports. 2016 doi: 10.1016/j.stemcr.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hua H, et al. iPSC-derived beta cells model diabetes due to glucokinase deficiency. J Clin Invest. 2013;123(7):3146–53. doi: 10.1172/JCI67638. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Shang L, et al. beta-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes. 2014;63(3):923–33. doi: 10.2337/db13-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tulpule A, et al. Pluripotent stem cell models of Shwachman-Diamond syndrome reveal a common mechanism for pancreatic and hematopoietic dysfunction. Cell Stem Cell. 2013;12(6):727–36. doi: 10.1016/j.stem.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iovino S, et al. Myotubes derived from human-induced pluripotent stem cells mirror in vivo insulin resistance. Proc Natl Acad Sci U S A. 2016;113(7):1889–94. doi: 10.1073/pnas.1525665113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahfeldt T, et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 2012;14(2):209–19. doi: 10.1038/ncb2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siller R, et al. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Reports. 2015;4(5):939–52. doi: 10.1016/j.stemcr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teo AK, et al. Dissecting diabetes/metabolic disease mechanisms using pluripotent stem cells and genome editing tools. Mol Metab. 2015;4(9):593–604. doi: 10.1016/j.molmet.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang P, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6(5):363–72. doi: 10.1007/s13238-015-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Callaway E. UK scientists gain licence to edit genes in human embryos. Nature. 2016;530(7588):18. doi: 10.1038/nature.2016.19270. [DOI] [PubMed] [Google Scholar]
- 58•.Agulnick AD, et al. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Transl Med. 2015;4(10):1214–22. doi: 10.5966/sctm.2015-0079. This article was written by the researchers at Viacyte, the company conducting the first clinical trials of “beta-like” differentiated human embryonic stem cells in type 1 diabetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulz TC, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7(5):e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Viacyte. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. A safety, tolerability, and efficacy study of VC-01 combination product in subjects with type 1 diabetes mellitus. cited 2016 Jan, 21. Available from: http://clinicaltrials.gov/ct2/show/NCT02239354. [Google Scholar]
- 61.Ramos-Mejia V, et al. iPSC lines that do not silence the expression of the ectopic reprogramming factors may display enhanced propensity to genomic instability. Cell Res. 2010;20(10):1092–5. doi: 10.1038/cr.2010.125. [DOI] [PubMed] [Google Scholar]
- 62.Laurent LC, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8(1):106–18. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miura K, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27(8):743–5. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 64.Bruin JE, et al. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013;56(9):1987–98. doi: 10.1007/s00125-013-2955-4. [DOI] [PubMed] [Google Scholar]
- 65.Motte E, et al. Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts. Am J Physiol Endocrinol Metab. 2014;307(9):E838–46. doi: 10.1152/ajpendo.00219.2014. [DOI] [PubMed] [Google Scholar]
- 66.Szot GL, et al. Tolerance induction and reversal of diabetes in mice transplanted with human embryonic stem cell-derived pancreatic endoderm. Cell Stem Cell. 2015;16(2):148–57. doi: 10.1016/j.stem.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Lui KO, et al. Tolerance induction to human stem cell transplants with extension to their differentiated progeny. Nat Commun. 2014;5:5629. doi: 10.1038/ncomms6629. [DOI] [PubMed] [Google Scholar]


