Abstract
Background
Polycystic ovary syndrome (PCOS), and one if its distinguishing characteristics, oligomenorrhea, have both been associated with ovarian cancer risk in some but not all studies. However, these associations have been rarely been examined by ovarian cancer histotypes which may explain the lack of clear associations reported in previous studies.
Methods
We analyzed data from 14 case-control studies including 16,594 women with invasive ovarian cancer (n=13,719) or borderline ovarian disease (n=2,875) and 17,718 controls. Adjusted study-specific odds ratios (ORs) were calculated using logistic regression and combined using random-effects meta-analysis. Pooled histotype-specific ORs were calculated using polytomous logistic regression.
Results
Women reporting menstrual cycle length >35 days had decreased risk of invasive ovarian cancer compared to women reporting cycle length ≤35 days (OR=0.70; 95% Confidence Interval [CI]=0.58-0.84). Decreased risk of invasive ovarian cancer was also observed among women who reported irregular menstrual cycles compared to women with regular cycles (OR=0.83; 95% CI=0.76-0.89). No significant association was observed between self-reported PCOS and invasive ovarian cancer risk (OR=0.87; 95% CI=0.65-1.15). There was a decreased risk of all individual invasive histotypes for women with menstrual cycle length >35 days, but no association with serous borderline tumors (pheterogeneity=0.006). Similarly, we observed decreased risks of most invasive histotypes among women with irregular cycles but an increased risk of borderline serous and mucinous tumors (pheterogeneity<0.0001).
Conclusion
Our results suggest that menstrual cycle characteristics influence ovarian cancer risk differentially based on histotype.
Impact
These results highlight the importance of examining ovarian cancer risk factors associations by histologic subtype.
Keywords: oligomenorrhea, menstrual cycle characteristics, polycystic ovary syndrome, ovarian cancer, histologic subtype
Introduction
Polycystic ovary syndrome (PCOS) is a complex endocrine disorder characterized by oligomenorrhea and abnormal hormone levels including hyperandrogenism, hyperinsulinemia, and gonadotropin imbalance. While PCOS has been examined as a risk factor for ovarian cancer in multiple studies(1–9), results have been inconsistent, due in part the inherent limitations in assessing PCOS in a population-based study(10). More objective measures, like measurement of circulating androgen levels, are not generally available. However, oligomenorrhea, defined as infrequent or irregular periods, is estimated to occur in 75-85% of women with PCOS and less than 18% of women without PCOS, and can be assessed by questionnaire (10–12). Oligomenorrhea has also been examined as an ovarian cancer risk factor but results from studies of this association are also inconsistent (2,4,7,13–19). Some studies have reported a reduced risk of ovarian cancer among women with oligomenorrhea (4,19), consistent with the incessant ovulation hypothesis (20) which proposes that repeated damage and repair to the ovarian surface increases ovarian cancer risk. Thus, among women with infrequent or irregular cycles the resulting reduction in lifetime ovulations would be expected to reduce ovarian cancer risk. However, other studies have observed increased risks of ovarian cancer among women with oligomenorrhea (7,14). Hormonal alterations in women with oligomenorrhea (21–23) including elevated androgen levels, a common characteristic of women with PCOS, have been hypothesized to explain this increased ovarian cancer risk (24,25). Small sample sizes in previous individual studies in combination with subtype heterogeneity may in part explain the inconsistent results in studies examining PCOS and oligomenorrhea with ovarian cancer risk. To address these issues, we sought to examine the associations between PCOS, menstrual cycle length and irregularity, and risk of ovarian cancer, overall and by histological subtype among 14 studies from the Ovarian Cancer Association Consortium.
Materials and Methods
Study Design and Population
Individual-level data were obtained from 14 case-control studies participating in the Ovarian Cancer Association Consortium (OCAC). To be included studies must have collected data on menstrual cycle irregularity, menstrual cycle length, and/or polycystic ovary syndrome. All studies had ethics approval and all study participants provided informed consent. More details on OCAC are provided elsewhere (26). Characteristics of the 14 studies included in the analysis are presented in Table 1. Nine studies were conducted in the United States: the Connecticut Ovarian Cancer Study (CON), the Diseases of the Ovary and their Evaluation Study (DOV), the Hawaii Ovarian Cancer Case-Control Study (HAW), the Hormones and Ovarian Cancer Prediction Study (HOP), the North Carolina Ovarian Cancer Study (NCO), the New England Case-Control Study of Ovarian Cancer (NEC), the New Jersey Ovarian Cancer Study (NJO), the University of California, Irvine Ovarian Cancer Study (UCI), and the University of Southern California Study of Lifestyle and Women’s Health (USC); two studies were conducted in Canada: the Southern Ontario Ovarian Cancer Study (SON) and Familial Ovarian Tumor Study (TOR); two were conducted in Europe: the Danish Malignant Ovarian Tumor Study (MAL) and Polish Ovarian Cancer Case-Control Study (POL); and one was conducted in Australia: the Australian Ovarian Cancer Study (AUS). Participants included 16,594 women with invasive ovarian cancer (n=13,719) or borderline ovarian disease (n=2,875) and 17,718 controls.
Table 1.
Characteristics of the studies included in the menstrual cycle charcteristics and PCOS analyses1
| Irregular cycles2 | Cycle length >353 | Self-reported PCOS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study4 | Diagnosis years |
Cases, n (%) |
Controls, n (%) |
Exposure ascertainment | Cases, n (%) |
Controls, n (%) |
Exposure ascertainment | Cases, n (%) |
Controls, n (%) |
Exposure ascertainment |
| Asia-Pacific | ||||||||||
| AUS | 2002-2005 | 351 (23.6%) | 358 (24.8%) | How regular were your periods? Very regular, Sometimes irregular, Very irregular | 22 (1.7%) |
29 (2.3%) |
What was the usually time between the beginning of one period and the beginning of the next period? | 29 (1.8%) | 25 (1.7%) |
Have you ever had polycystic ovary syndrome? |
| Canada | ||||||||||
| SON | 1989-1992 | 50 (11.2%) | 97 (17.2%) | Over the years of your periods, would you say that your periods were usually regular? Yes, No | 16 (3.6%) |
24 (4.4%) |
On average, how many days would there be between one period and the next? | data not collected | n/a | |
| TOR | 1995-2003 | data not collected | n/a | 18 (2.4%) |
13 (2.3%) |
On average, how many days would there be between the start of one period and the start of the next? | data not collected | n/a | ||
| Europe | ||||||||||
| MAL | 1994-1999 | 30 (5.4%) | 92 (5.9%) |
Were you (in the age of 20-30 years) normally able to predict within a couple of days when your period would start? Yes, No | 2 (0.4%) |
20 (1.3%) |
How long was your menstrual cycle normally when you were aged 20-30 years? (only periods where you did not receive any hormones) | data not collected | n/a | |
| POL | 2001-2003 | data not collected | n/a | 0 (0%) |
16 (4.7%) |
On average, how many days are there between your periods? | data not collected | n/a | ||
| United States | ||||||||||
| CON | 1998-2003 | 67 (13.5%) | 82 (14.9%) | How regular were your menstrual cycles (in your 20s)? Generally regular, Fairly irregular, Very irregular | 8 (1.7%) |
24 (4.4%) |
In generally, in your 20s, how many days long would a typical cycle be? | 2 (0.4%) |
3 (0.5%) |
Has a doctor ever told you that you had polycystic ovary syndrome? |
| DOV | 2002-2009 | 109 (7.0%) | 130 (7.0%) | Have your periods ever been regular during times when you were not using birth control pills, shots, or implants? Yes, No | 100 (6.6%) | 134 (7.5%) | In your 20s, how often on average did you have your menstrual period? That is, how many dyas were there between the first day of one period and the first day of the next? | 12 (0.8%) |
14 (0.8%) |
Did a doctor ever tell you that you had polycystic ovarian disease? |
| HAW | 1993-2008 | 81 (24.8%) | 85 (17.4%) | [During your 20s/30s when you were not using birth control pills] did you periods usually come about every [cycle length] days or did they often vary from this by two days or more? Regular, Variable | 20 (6.2%) |
44 (9.1%) |
[During your 20s and 30s when you were not using birth control pills] what was your cycle length; that is, the average number of days from the first day of one period to the first day of the next? | 3 (0.3%) |
4 (0.4%) |
Did a doctor ever tell you that you had polycystic ovaries or Stein Levanthal syndrome? |
| HOP | 2003-2008 | 185 (21.0%) | 404 (22.6%) | During your 20s and 30s (when you were not using birth control pills) were you usually able to predict when your periods would start within 2-3 days? No, Yes | data not collected | n/a | 7 (0.8%) |
26 (1.4%) |
Did a doctor or health professional ever tell you that you had polycystic ovaries or PCOS or Stein Leventhal Syndrome? | |
| NCO | 1999-2008 | 93 (8.1%) | 92 (8.5%) | What was your usual cycle length? <26 days, 26-30, 31-34, 35-60, Irregular: could not tell within 1 weeks when your period would come | 21 (2.1%) |
20 (2.1%) |
What was your usual cycle length? | 8 (0.7%) |
10 (0.9%) |
Have you ever had polycystic ovary syndrome (Stein-Levanthal’s disease)? |
| NEC | 1992-2008 | 153 (7.4%) | 175 (8.3%) | Were your periods regular from the start? Yes, No If no, how many months did it take before they became predictable [during your 20s and 30s]? X months, never became regular | 19 (1.0%) |
26 (1.3%) |
When your periods first became regular, what was the average number of days from the start of one period to the start of another [during your 20s and 30s]? | 41 (2.7%) |
37 (2.3%) |
Did you have polycystic ovaries? |
| NJO | 2004-2008 | 47 (20.1%) | 111 (24.5%) | Did you periods usually come regularly or did they often vary from [the number of days of your menstrual cycle from the prevous question] by two or more days? Regular, Variable | 3 (1.4%) |
16 (3.8%) |
Thinking about when you were in your 20s and 30s and not using birth control pills - what was the usual number of days of your menstrual cycle (from the first day of one period to the first day of the next)? | 6 (2.5%) |
9 (2.0%) |
Were you ever told by a health professional you had polycystic ovaries? |
| UCI | 1994-2004 | 89 (15.1%) | 42 (13.5%) | Which of these best describes the normal pattern of your menstrual cycle over most of your life? Regular (usually could predict when it would occur), Irregular (not predictable) | 13 (2.3%) |
12 (4.1%) |
What was the average number of days from the start of one period to the start of another? | 10 (1.7%) |
11 (2.0%) |
Have you ever been told by a physician you had polycystic ovary disease? |
| USC | 1993-2009 | 404 (17.1%) | 444 (17.3%) | [In your 20s and 30s when you were not using birth control pills] did you periods usually come about every [cycle length] days or did they often vary from this by two or more days? Regular, Variable | 9 (0.5%) |
27 (1.3%) |
[In your 20s and 30s when you were not using birth control] what was your usual cycle length: that is, the average number of days from the first day of one period to the first day of the next? | 17 (0.7%) |
12 (0.5%) |
Did a doctor ever tell you that you had polycystic ovaries or Stein Levanthal syndrome? |
Sample sizes vary according to the exposure of interest.
Ever reporting menstrual cycle irregularity (defined by the underlined response) was considered irregular.
When reported in categories the midpoint of the category was used to assign numerical menstrual cycle length.
Australia Ovarian Cancer Study (AUS), Connecticut Ovarian Cancer Study (CON), Disease of the Ovary and their Evaluation (DOV), Hawaii Ovarian Cancer Case-Control Study (HAW), Hormones and Ovarian Cancer Prediction Study (HOP), Danish Malignant Ovarian Tumor Study (MAL), North Carolina Ovarian Cancer Study (NCO), New England Case-Control Study of Ovarian Cancer (NEC), New Jersey Ovarian Cancer Study (NJO), Polish Ovarian Cancer Case-Control Study (POL), Southern Ontario Cancer Study (SON), Familial Ovarian Tumor Study (TOR), Univeristy of California-Irvine Ovarian Cancer Study (UCI), and University of Southern California Study of Lifestyle and Women’s Health (USC).
Exposure and covariate data
History of PCOS, menstrual cycle irregularity, and menstrual cycle length were self-reported in all studies. The wording of the questions for each of the three exposure variables varied between studies (Table 1). PCOS, polycystic ovary disease, Stein Levanthal syndrome, and polycystic ovaries were reported by the participants (n=10 studies). For menstrual cycle irregularity (n=12 studies) we defined women as having irregular cycles if they reported that their cycles were: “sometimes irregular”, “very irregular”, “fairly irregular”, “never regular”, “variable in length”, or were “unable to predict when next period would start”. Menstrual cycle length in days was reported by the participants as a continuous variable or in categories (n=13 studies). When menstrual cycle length was reported in categories the midpoint of the category was used to assign numerical menstrual cycle length. Menstrual cycle length was dichotomized into >35 days and ≤35 days (reference). Sensitivity analysis were also conducted excluding women with a cycle length <21 days from the reference group. When available, individual studies provided information on other characteristics associated with PCOS and hyperandrogenism (11) including: amenorrhea (absence of a menstrual period for 3 months or longer; n=6 studies), hirsutism/excess body hair (n=6 studies), and adult acne (n=8 studies). Information on known and suspected risk factors for ovarian cancer was collected in each study including age, race/ethnicity, oral contraceptive use, parity, tubal ligation, family history of breast or ovarian cancer, and body mass index (BMI).
Statistical analysis
For analysis including all cases we calculated study-specific adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the association between each exposure variable and ovarian cancer risk using logistic regression. Study-specific ORs were combined using random-effects meta-analysis to obtain a summary OR. Between study heterogeneity was assessed with Cochran’s Q statistic. All analyses were adjusted for age (continuous), oral contraceptive use (never use, <2, 2-<5, 5-<10, or 10+ years), parity (0, 1, 2, 3, 4+), history of tubal ligation (yes, no), family history of ovarian or breast cancer (yes, no), BMI (<20, 20-<25, 25-<30, 30+), and race/ethnicity (white, non-white). If an individual study did not have enough subject exposure variation (<5 cases and/or controls reporting a given exposure) to calculate study-specific ORs the study was excluded from the meta-analysis but included in the pooled analyses (described below).
For histology specific analyses the data from individual studies were pooled and polytomous logistic regression was used to simultaneously estimate separate ORs and 95% CIs for each histotype (serous borderline, mucinous borderline, low grade serous, high grade serous, mucinous invasive, clear cell, and endometrioid) (27). Likelihood ratio statistics were used to calculate p-values for heterogeneity comparing models allowing seperate associations for each histotype to models assuming the same association for all histotypes (28). All of the included covariates were allowed to vary across histotypes. In addition to adjustment by the covariates listed above, pooled analyses were additionally adjusted for study site. To evaluate effect modification by BMI (<25, ≥25), oral contraceptive use (never, <2 years, 2-<5 years, ≥5 years), age (<50, ≥50), and parity (nulliparous, parous), data from individual studies were pooled. Likelihood ratio statistics were used to compare models with interaction terms and main effects to a model with main effects only. We also conducted the following sensitivity analyses: 1) excluding studies (n=3) that only asked about diagnosis of polycystic ovaries and not PCOS; 2) limiting to studies (n=7) that asked specifically about menstrual cycle characteristics in women’s 20s and 30s; and 3) excluding the three OCAC studies that had previously published on menstrual cycle characteristics and/or PCOS by histotype (AUS, HAW, and NEC); 4) excluding women with a cycle length <21 days from the reference group. We also examined the association between amenorrhea (no period for > 3 months) (n=6 studies), hirsutism/excess body hair (n=6 studies), and adult acne (n=8 studies) and ovarian cancer among the studies with these data available. All p-value were based on two-sided statistics and were considered statistically significant if p<0.05. Statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc, Cary, NC) and Stata 9 (StataCorp, College Station, TX).
Results
Overall, 16,594 women with invasive ovarian cancer (n=13,719) or borderline ovarian disease (n=2,875) and 17,718 controls were included in the analysis. Irregular cycles was the most commonly reported of the three exposures followed by typical cycle length >35 days and self-reported PCOS (Table 1). The prevalence of these exposures among controls differed between study sites. For PCOS the prevalence was highest in the NEC study (2.3%) and lowest among women in the HAW study (0.4%). The percentage of women reporting irregular cycles was highest in the AUS study (24.8%) and lowest in MAL (5.9%). The highest prevalence of reported cycle lengths >35 days was in the HAW study (9.1%) while in three studies (MAL, NEC, and USC) the prevalence was only 1.3%. Women reporting irregular cycles were more likely to report longer cycle lengths (mean cycle length=32.3) than those reporting regular cycles (mean cycle length=28.3).
Self-reported PCOS was associated with a non-significant decrease in risk of invasive ovarian cancer (summary OR=0.87; 95% CI=0.65-1.15) (Figure 1). Menstrual cycle irregularity was associated with a 17% decrease in risk of invasive ovarian cancer compared to regular cycles (95% CI=0.76-0.89) (Figure 2). Reported typical menstrual cycle length >35 days was associated with a 30% decrease in risk of invasive ovarian cancer compared to women reporting cycle length ≤35 days (95% CI=0.58-0.84) (Figure 3). There was no evidence of study heterogeneity for PCOS, menstrual cycle length, or menstrual cycle irregularity (pstudy heterogeneity >0.46) (Figures 1–3). Among the studies six studies with information on periods of amenorrhea (no menstrual period for >3 months), we observed a decreased risk of ovarian cancer for women with this exposure (summary OR=0.88; 95% CI=0.78-0.99). No association was observed between hirsutism (summary OR=0.93; 95% CI=0.80-1.08; nstudies=6) or adult acne (summary OR=0.94; 95% CI=0.80-1.10; nstudies=8), and ovarian cancer risk. Results were similar in sensitivity analyses that excluded studies that only asked about diagnosis of polycystic ovaries and not PCOS, when restricted to studies that asked specifically about menstrual cycle characteristics in women’s 20s and 30s, when the two OCAC studies that had previously published on menstrual cycle characteristics were excluded, and when women with a cycle length <21 days were excluded from the reference group.
Figure 1.
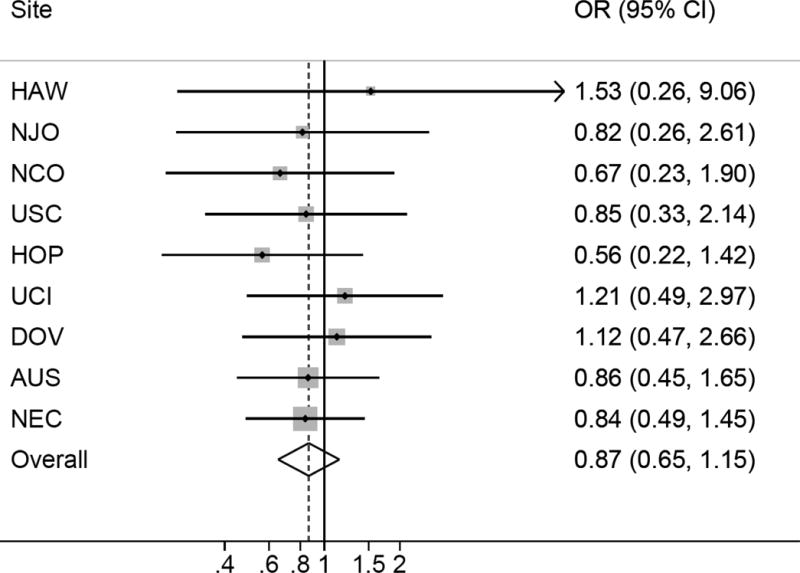
Self-reported polycystic ovary syndrome and invasive ovarian cancer risk. Figure 1 shows the association between self-reported polycystic ovary syndrome and invasive ovarian cancer risk among 9 studies. All models are adjusted for age (continuous), oral contraceptive use (never use, <2, 2-<5, 5-<10, or 10+ years), parity (0, 1, 2, 3, 4+), history of tubal ligation (yes, no), family history of ovarian or breast cancer (yes, no), BMI (<20, 20-<25, 25-<30, 30+), and race/ethnicity (white, non-white). There was no evidence for heterogeneity based on Cochran’s Q statistic (p=0.97).
Figure 2.
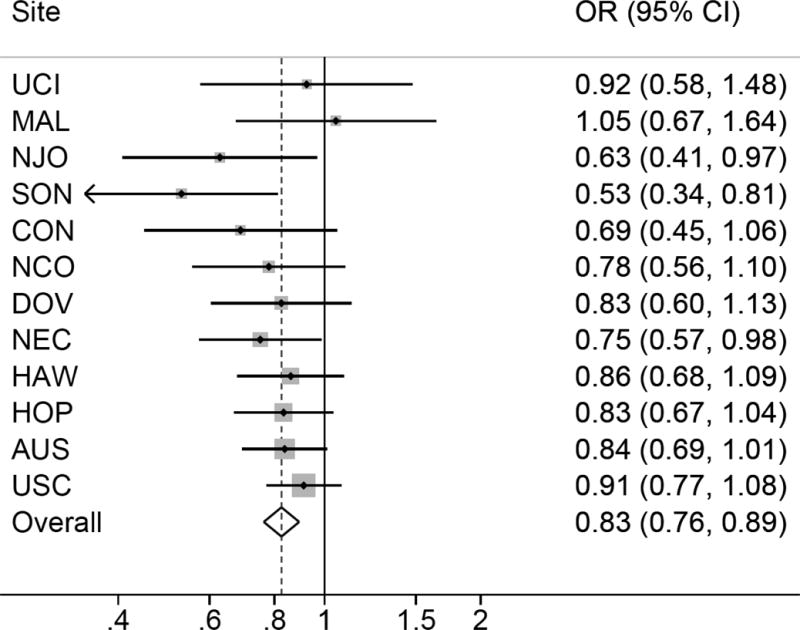
Menstrual cycle irregularity and invasive ovarian cancer risk. Figure 2 show the association between menstrual cycle irregularity and invasive ovarian cancer risk among 12 studies. All models are adjusted for age (continuous), oral contraceptive use (never use, <2, 2-<5, 5-<10, or 10+ years), parity (0, 1, 2, 3, 4+), history of tubal ligation (yes, no), family history of ovarian or breast cancer (yes, no), BMI (<20, 20-<25, 25-<30, 30+), and race/ethnicity (white, non-white). There was no evidence for heterogeneity based on Cochran’s Q statistic (p=0.55).
Figure 3.
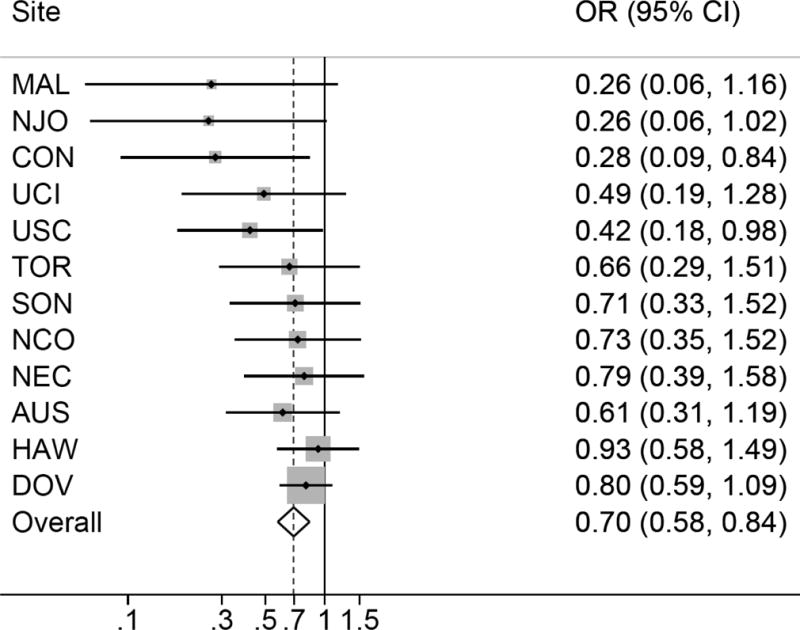
Menstrual cycle length >35 days and invasive ovarian cancer risk. Figure 3 show the association between menstrual cycle length > 35 days and invasive ovarian cancer risk among 12 studies. All models are adjusted for age (continuous), oral contraceptive use (never use, <2, 2-<5, 5-<10, or 10+ years), parity (0, 1, 2, 3, 4+), history of tubal ligation (yes, no), family history of ovarian or breast cancer (yes, no), BMI (<20, 20-<25, 25-<30, 30+), and race/ethnicity (white, non-white). There was no evidence for heterogeneity based on Cochran’s Q statistic (p=0.47).
The associations between menstrual cycle characteristics differed by histotype (Table 2). Menstrual cycle irregularity was associated with increased risk of the serous borderline (OR=1.34; 95% CI=1.16-1.55) and mucinous borderline (OR=1.21; 95% CI=1.02-1.43) histotypes but with decreased risk of high grade serous, endometrioid, and clear cell histotypes, with corresponding ORs (95% CI) of 0.86 (0.78-0.95), 0.84 (0.72-0.98), and 0.68 (0.55-0.84), respectively (pheterogeneity<0.001). No associations were observed between cycle irregularity and low grade serous and invasive mucinous histotypes. Associations with menstrual cycle length also showed heterogeneity by histotype (pheterogeneity=0.006). Menstrual cycle length >35 days was associated with the lowest risk for low grade serous (OR=0.48; 95% CI=0.25-0.92), invasive mucinous (OR=0.38; 95% CI=0.19-0.76), and high grade serous (OR=0.62; 95% CI=0.48-0.80) histotypes, but only a suggestion of decreased risk among the other invasive histotypes (Table 2). No significant associations were found between self-reported PCOS and individual ovarian cancer histotypes (Table 2).
Table 2.
Multivariable1 odds ratios (OR) and 95% confidence intervals (95% CI) for the association between menstrual cycle characteristics and polycystic ovary syndrome and ovarian cancer by histologic subtype
|
|
||||||
|---|---|---|---|---|---|---|
| Irregular Cycles | Menstrual cycle length > 35 days | Polycystic ovary syndrome | ||||
| Cases (n) | OR (95% CI) | Cases (n) | OR (95% CI) | Cases (n) | OR (95% CI) | |
| Borderline | ||||||
| Serous | 1424 | 1.34 (1.16-1.55) | 1252 | 1.18 (0.87-1.59) | 1298 | 1.28 (0.84-1.96) |
| Mucinous | 985 | 1.21 (1.02-1.43) | 898 | 0.63 (0.41-0.98) | 867 | 0.51 (0.24-1.10) |
| Invasive | ||||||
| Low grade serous | 881 | 1.00 (0.81-1.25) | 770 | 0.48 (0.25-0.92) | 310 | 1.07 (0.43-2.66) |
| High grade serous | 5216 | 0.86 (0.78-0.95) | 4357 | 0.62 (0.48-0.80) | 4588 | 0.88 (0.62-1.26) |
| Mucinous | 840 | 0.95 (0.78-1.16) | 763 | 0.38 (0.19-0.76) | 530 | 0.61 (0.22-1.66) |
| Endometrioid | 1749 | 0.84 (0.72-0.98) | 1648 | 0.75 (0.54-1.06) | 1374 | 1.03 (0.63-1.67) |
| Clear cell | 895 | 0.68 (0.55-0.84) | 810 | 0.70 (0.43-1.13) | 673 | 0.77 (0.34-1.78) |
| Pheterogeneity2 | <0.0001 | 0.006 | 0.32 | |||
| Pheterogeneity3 | 0.08 | 0.32 | 0.86 | |||
Adjusted for age (continuous), oral contraceptive use (never use, <2, 2-<5, 5-<10, or 10+ years), parity (0, 1, 2, 3, 4+), history of tubal ligation (yes, no), family history of ovarian or breast cancer (yes, no), BMI (<20, 20-<25, 25-<30, 30+), race/ethnicity (white, non-white), and study site (AUS, CON, DOV, HAW, HOP, MAL, NCO, NEC, NJO, POL, SON, TOR, UCI, and USC).
Determined using polytomous logistic regression comparing all subtypes.
Determined using polytomous logistic regression comparing only invasive subtypes.
In analyses stratified by BMI, the association between irregular menstrual cycles and risk of the serous borderline histotype was significantly stronger among women who were overweight (BMI ≥25: OR=1.66; 95% CI=1.36-2.03) compared to normal weight women (BMI<25: OR=1.14; 95% CI=0.88-1.47) (pinteraction=0.009). The inverse associations observed between menstrual cycle irregularity and invasive histotypes were generally stronger among overweight women; however, the p-values for interaction did not reach statistical significance (all pinteraction>0.15) (Supplemental Table 1). No differences in effect estimates by BMI were observed for cycle length (Supplemental Table 1) or PCOS. Generally, the PCOS stratified analyses were limited by small numbers in many of the histotype groups.
Significant differences by age were observed for the clear cell histotype, with stronger inverse associations for both irregular cycles and cycle length >35 for women under age 50 years compared to women 50 years and older. Women with a cycle length of >35 days who were <50 years had an 87% (95% CI=0.02-0.92) decreased risk of clear cell ovarian cancer while women ages 50 years and older had a 7% (0.53-1.66) decreased risk (pinteraction=0.01) (Supplemental Table 2). No differences were observed by age for PCOS with ORs for the association between PCOS and invasive ovarian cancer of 0.88 (95% CI=0.58-1.35) and 0.93 (95% CI=0.64-1.35) for <50 and ≥50, respectively. When the associations were examined stratified by oral contraceptive use (never use, < 2 years, 2 - <5 years, ≥5 years) no consistent patterns emerged (Supplemental Table 3). No significant differences by parity (nulliparous vs parous) were observed.
Discussion
In this large consortium study including over 16,000 women with invasive ovarian cancer or borderline ovarian disease and 17,000 controls we observed that both irregular and longer (>35 days) menstrual cycles were associated with decreased risks of invasive ovarian cancer. There was also an inverse association between self-reported PCOS and risk of invasive ovarian cancer, but this result was not statistically significant. Among overweight women who reported irregular cycles, an increased risk of the serous borderline histotype was noted.
Our results for menstrual cycle irregularity are consistent with two prior studies, both of which are included in the present analyses, that examined the associations by histotype. The population-based NEC study of ovarian cancer reported that irregular cycles were associated with a decreased risk of the high grade serous (OR=0.68; 95% CI=0.49-0.95; ncases=846) and clear cell histotypes (OR=0.39; 95% CI=0.56-1.36) although the small numbers of women with clear cell cancer (ncases=116) meant the result did not reach customary statistical significance. An increased risk of serous borderline tumors with menstrual cycle irregularity that was stronger among women who were overweight (OR=2.29; 95% CI=1.32-3.98) was also observed (4). In a population-based case-control study conducted in Hawaii and Southern California, that overlapped with the HAW study in the current analyses, Tung, et al. also reported a significant inverse association between menstrual cycle irregularity and invasive ovarian cancer overall (OR=0.7; 95% CI=0.5-0.9; ncases=431). When examined by histotype, this association remained significant for only clear cell disease (OR=0.3; 95% CI=0.1-0.7; ncases=48) (19). In contrast, a cohort analysis (Child Health and Development Studies (CHDS)) found the risk of ovarian cancer was increased for women who reported irregular menstrual cycles, a result that was statistically significant among women age 70 years and older. For high grade serous disease, the only histotype examined in this study, a non-significant increased risk was observed for women reporting irregular cycles (HR=2.1; 95% CI=0.9-5.0; ncases=30). The CHDS cohort enrolled women that were currently pregnant, including 60% of subjects who were multiparous at study enrollment. CHDS women were relatively young (median enrollment age 26 years), had not used oral contraceptives in the period prior to their pregnancy (96%), and investigators did not have information on established ovarian cancer risk factors that occurred after the study observation period (e.g., tubal ligation, oral contraceptive use) (14). In contrast, the 14 studies included in our analyses included nulliparous and parous women among whom oral contraceptive use was not uncommon. In addition, we were able to adjust for tubal ligation, family history of cancer, and lifetime oral contraceptive use. How such differences in study population characteristics and covariate-adjustment could have produced results with opposite directions of association is unclear.
To date, eight studies and one meta-analysis have assessed the association between PCOS and ovarian cancer with primarily null and non-significant increased risks reported (29). Only two studies, which are included in the present analysis (AUS and NEC), have examined the association by histotype subtype with both studies reporting an increased risk with the serous borderline histotype which is consistent with our results (4,5). We also observed an increased risk of the serous borderline hisotype among women with both long and irregular menstrual cycles however this association was only statistically significant for women reporting irregular cycles. Androgen excess among women with oligomenorrhea may explain these observations. Women with menstrual cycle irregularity often have higher total and free testosterone levels (30), with potential effects on ovarian tissue. Serous borderline ovarian tumors can express androgen receptors (31) indicating that androgens could exert physiologic effects on these tumors. We observed that the increase in risk associated with irregular cycles was greatest among women with who were overweight or obese (BMI≥25). In addition to elevated androgen levels women who have irregular cycles and are additionally overweight or obese are more likely to suffer other metabolic abnormalities including insulin resistance, and limited cross-sectional evidence suggests that elevated insulin levels may be associated with ovarian cancer risk (32,33).
Oligomenorrhea was associated with decreased risk of most invasive histologic subtypes. Fewer ovulatory cycles or more anovulatory cycles among women with long and irregular menstrual cycles is a possible explanation for the observed decreased risks. While an association between calculated number of ovulatory cycles and ovarian cancer risk has been established for decades (34–43), few previous studies have examined the association by histologic subtypes. Those studies all found an inverse association that was strongest for the endometrioid histotype (38,39,43). While we observed a decreased risk for endometrioid tumors for both menstrual cycle irregularity and longer length (>35 days), the strongest histotype specific associations were observed with clear cell (for both cycle irregularity and length) and mucinous invasive (for menstrual cycle length). To our knowledge, only the NEC study has examined ovulatory cycles separately for serous borderline and serous invasive cases, reporting a significant increased risk of serous invasive ovarian cancer with increasing number of ovulatory cycles, and a non-significant decreased risk of serous borderline disease with increasing cycles (43); this is consistent with the opposite direction of associations that we observed for the serous borderline and serous invasive histotypes for both measures of oligomenorrhea.
In this analyses, PCOS and menstrual cycle characteristics were self-reported. Estimates of PCOS prevalence in reproductive age women range from 4-21%, influenced by both population characteristics and the diagnostic criteria used (44–50). Percentage estimates for PCOS in the included studies ranged from 0.4% to 2.3%, well below the expected population levels. While PCOS was initially described in 1935 (51), modern clinical definitions did not emerge until 1990 (NIH criteria), 2003 (Rotterdam criteria), and 2006 (Androgen Excess and PCOS Society criteria) (52). Given the majority of the women participating in our studies were of reproductive age prior to 1990, before PCOS was as commonly clinically recognized, it is likely that PCOS was under-diagnosed in our study population. Among women with clinically apparent PCOS, approximately 80% will have oligomenorrhea (11), thus many women who reported irregular and/or long menstrual cycles may have PCOS.
In case-control studies, recall bias is a common concern with self-reported exposures. Cases could be more likely to recall PCOS or menstrual cycle irregularities than controls. However, given the differing results we observed by histological subtype, recall bias is an unlikely explanation for our results, particularly for ORs less than unity. More plausible is that underdiagnosis of PCOS in both cases and controls attenuated the results. Another limitation of our study was the differences between the studies in how the question on menstrual cycle irregularity was worded and how answers were categorized. Some studies include a “yes” or “no” response to menstrual cycle irregularity, while others offered more than two answer choices (e.g., very regular, sometimes irregular, very irregular). This likely resulted in some non-differential misclassification of this exposure.
Strengths of our study include the large number of participants, which provided us with greater statistical power than previous studies to examine the associations by histologic subtype. In addition, previous harmonization of the covariates allowed for consistent adjustment for confounders across the included studies. The associations observed were generally consistent across studies providing further support for our results.
In conclusion, in this large consortium analysis of case-control studies we observed a decreased risk of most invasive epithelial ovarian cancer histological subtypes among women reporting longer or irregular menstrual cycles. In contrast, such cycles were associated with an apparent increased risk of serous borderline tumors. Further research is needed to understand the mechanisms underlying these bi-directional associations.
Supplementary Material
Acknowledgments
This work was supported by grant K22 CA193860 from the National Cancer Institute, National Institutes of Health (to H.R. Harris) and Department of Defense grant W81XWH-10-1-0280 (to K.L. Terry). Support for the Ovarian Cancer Association Consortium was provided by donations from family and friends of the Kathryn Sladek Smith to the Ovarian Cancer Research Fund. In addition, these studies were supported by the National Institutes of Health (R01-CA054419 D.W. Cramer, P50-CA105009 D.W. Cramer, R01-CA063678 S.A. Narod, R01-CA074850 H.A. Risch, R01-CA080742 H.A. Risch, R01-CA112523 M.A. Rossing, R01-CA087538 M.A. Rossing, R01-CA058598 M.T. Goodman, N01-CN-55424 M.T. Goodman, N01-PC-67001 M.T. Goodman, R01-CA095023 R.B. Ness, P50-CA159981 K.B. Moysich, K07-CA080668 F. Modugno, R01-CA061107 S.K. Kjær, R01-CA076016 JMS, K07-CA095666 E.V. Bandera, R01-CA083918 SHO, K22-CA138563 E.V. Bandera, P30-CA072720, R01-CA063678 S.A. Narod, R01-CA063682 H.A. Risch, R01-CA058860 H. Anton-Culver, P01-CA17054 A.H. Wu, P30-CA14089 A.H. Wu, R01-CA061132, N01-PC67010, R03-CA113148 C.L. Pearce, R03-CA115195 C.L. Pearce, N01-CN025403, R01-CA149429 C.M. Phelan, Intramural Research Program N. Wentzensen), the U.S. Department of Defense (DAMD17-01-1-0729, DAMD17-02-1-0669 F. Modugno, DAMD17-02-1-0666 A. Berchuck), National Health & Medical Research Council of Australia (199600 P.M. Webb and 400281 P.M. Webb), Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania, Cancer Foundation of Western Australia (multi-state applications 191, 211, and 182 P.M. Webb); Danish Cancer Society (94 222 52 S.K. Kjær), the Mermaid I project (S.K. Kjær), the Cancer Institute of New Jersey, National Health Research and Development Program Health Canada (6613-1415-53 H.A. Risch), Lon V Smith Foundation (LVS-39420 H. Anton-Culver), and the California Cancer Research Program (00-01389V-20170 C.L. Pearce, 2II0200 A.H. Wu).
References
- 1.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: A case–control analysis. Gynecol Oncol. 2011;123:200–4. doi: 10.1016/j.ygyno.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Coulam C, Annegers J, Kranz J. Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol. 1983;61:403–7. [PubMed] [Google Scholar]
- 3.Gottschau M, Kjaer S, Jensen A, Munk C, Mellenmkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136:99–103. doi: 10.1016/j.ygyno.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Harris H, Titus L, Cramer D, Terry K. Long and irregular menstrual cycles, polycystic ovary syndrome, and ovarian cancer risk in a population-based case-control study. Int J Cancer. 2016 doi: 10.1002/ijc.30441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen CM, Green AC, Nagle CM, Jordan SJ, Whiteman DC, Bain CJ, et al. Epithelial ovarian cancer: testing the ‘androgens hypothesis’. Endocr Relat Cancer. 2008;15:1061–8. doi: 10.1677/ERC-08-0075. [DOI] [PubMed] [Google Scholar]
- 6.Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of Women with Polycystic Ovary Syndrome at Long-term Follow-up. J Clin Epidemiol. 1998;51:581–6. doi: 10.1016/s0895-4356(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 7.Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian Tumors in a Cohort of Infertile Women. N Engl J Med. 1994;331:771–6. doi: 10.1056/NEJM199409223311204. [DOI] [PubMed] [Google Scholar]
- 8.Schildkraut J, Schwingl P, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88:554–9. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- 9.Shen C-C, Yang AC, Hung J-H, Hu L-Y, Tsai S-J. A Nationwide Population-Based Retrospective Cohort Study of the Risk of Uterine, Ovarian and Breast Cancer in Women With Polycystic Ovary Syndrome. Oncologist. 2015;20:45–9. doi: 10.1634/theoncologist.2014-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon C. The epidemiology of polycystic ovary syndrome. Prevalence and associated disease risks. Endocrinol Metab Clin North Am. 1999;28:247–63. doi: 10.1016/s0889-8529(05)70069-4. [DOI] [PubMed] [Google Scholar]
- 11.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale H, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, et al. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87:2013–7. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- 13.Chiaffarino F, Pelucchi C, Parazzini F, Negri E, Franceschi S, Talamini R, et al. Reproductive and hormonal factors and ovarian cancer. Ann Oncol. 2001;12:337–41. doi: 10.1023/a:1011128408146. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo PM, Wang ET, Cedars MI, Chen L-m, Cohn BA. Irregular menses predicts ovarian cancer: Prospective evidence from the Child Health and Development Studies. Int J Cancer. 2016;139:1009–17. doi: 10.1002/ijc.30144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merritt MA, De Pari M, Vitonis AF, Titus LJ, Cramer DW, Terry KL. Reproductive characteristics in relation to ovarian cancer risk by histologic pathways. Hum Reprod. 2013;28:1406–17. doi: 10.1093/humrep/des466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parazzini F, La Vecchia C, Negri E, Gentile A. Menstrual factors and the risk of epithelial ovarian cancer. J Clin Epidemiol. 1989;42:443–8. doi: 10.1016/0895-4356(89)90134-0. [DOI] [PubMed] [Google Scholar]
- 17.Tavani A, Negri E, Franceschi S, Parazzini F, La Vecchia C. Risk factors for epithelial ovarian cancer in women under age 45. Eur J Cancer. 1993;29A:1297–301. doi: 10.1016/0959-8049(93)90077-s. [DOI] [PubMed] [Google Scholar]
- 18.Titus-Ernstoff L, Perez K, Cramer D, Harlow B, Baron J, Greenberg E. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer. 2001;84:714–21. doi: 10.1054/bjoc.2000.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tung K-H, Goodman MT, Wu AH, McDuffie K, Wilkens LR, Kolonel LN, et al. Reproductive Factors and Epithelial Ovarian Cancer Risk by Histologic Type:A Multiethnic Case-Control Study. Am J Epidemiol. 2003;158:629–38. doi: 10.1093/aje/kwg177. [DOI] [PubMed] [Google Scholar]
- 20.Fathalla M. Incessant ovulation–a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 21.Van Anders SM, Watson NV. Menstrual cycle irregularities are associated with testosterone levels in healthy premenopausal women. American journal of human biology: the official journal of the Human Biology Council. 2006;18:841–4. doi: 10.1002/ajhb.20555. [DOI] [PubMed] [Google Scholar]
- 22.Wei S, Jones G, Thomson R, Otahal P, Dwyer T, Venn A. Menstrual irregularity and bone mass in premenopausal women: cross-sectional associations with testosterone and SHBG. BMC musculoskeletal disorders. 2010;11:288. doi: 10.1186/1471-2474-11-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei S, Schmidt MD, Dwyer T, Norman RJ, Venn AJ. Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obesity (Silver Spring, Md) 2009;17:1070–6. doi: 10.1038/oby.2008.641. [DOI] [PubMed] [Google Scholar]
- 24.Risch HA. Hormonal Etiology of Epithelial Ovarian Cancer, With a Hypothesis Concerning the Role of Androgens and Progesterone. J Natl Cancer Inst. 1998;90:1774–86. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 25.Ose J, Poole EM, Schock H, Lehtinen M, Arslan AA, Zeleniuch-Jacquotte A, et al. Androgens are differentially associated with ovarian cancer subtypes in the Ovarian Cancer Cohort Consortium. Cancer Res. 2017 doi: 10.1158/0008-5472.CAN-16-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berchuck A, Schildkraut J, Pearce CL, Chenevix-Trench G, Pharoah P. Role of Genetic Polymorphisms in Ovarian Cancer Susceptibility: Development of an International Ovarian Cancer Association Consortium. In: Coukos G, Berchuck A, Ozols R, editors. Ovarian Cancer. Springer; New York: 2008. pp. 53–67. [DOI] [PubMed] [Google Scholar]
- 27.Marshall R, Chisholm E. Hypothesis testing in the polychotomous logistic model with an application to detecting gastrointestinal cancer. Stat Med. 1985;4:337–44. doi: 10.1002/sim.4780040313. [DOI] [PubMed] [Google Scholar]
- 28.Glynn RJ, Rosner B. Methods to evaluate risks for composite end points and their individual components. J Clin Epidemiol. 2004;57:113–22. doi: 10.1016/j.jclinepi.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Harris H, Terry K. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: a systematic review. Fertility Research and Practice. 2016;2:1–9. doi: 10.1186/s40738-016-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mu F, Eliassen A, Hankinson S, Tworoger S, Barbieri R, Dowsett M, et al. Menstrual cycle characteristics in relation to plasma steroid hormone, prolactin, and growth factor concentrations in premenopausal women. TBD. 2017 doi: 10.1007/s10552-017-0971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler MS, Ricciardelli C, Tilley WD, Hickey TE. Androgen Receptor Protein Levels Are Significantly Reduced in Serous Ovarian Carcinomas Compared with Benign or Borderline Disease but Are Not altered by Cancer Stage or Metastatic Progression. Horm Cancer. 2013;4:154–64. doi: 10.1007/s12672-013-0135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otokozawa S, Tanaka R, Akasaka H, Ito E, Asakura S, Ohnishi H, et al. Associations of Serum Isoflavone, Adiponectin and Insulin Levels with Risk for Epithelial Ovarian Cancer: Results of a Case-control Study. Asian Pacific journal of cancer prevention: APJCP. 2015;16:4987–91. doi: 10.7314/apjcp.2015.16.12.4987. [DOI] [PubMed] [Google Scholar]
- 33.Sun W, Lu J, Wu S, Bi Y, Mu Y, Zhao J, et al. Association of insulin resistance with breast, ovarian, endometrial and cervical cancers in non-diabetic women. American journal of cancer research. 2016;6:2334–44. [PMC free article] [PubMed] [Google Scholar]
- 34.Casagrande JT, Louie EW, Pike MC, Roy S, Ross RK, Henderson BE. “Incessant ovulation” and ovarian cancer. Lancet. 1979;2:170–3. doi: 10.1016/s0140-6736(79)91435-1. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Wu PC, Lang JH, Ge WJ, Hartge P, Brinton LA. Risk factors for epithelial ovarian cancer in Beijing, China. Int J Epidemiol. 1992;21:23–9. doi: 10.1093/ije/21.1.23. [DOI] [PubMed] [Google Scholar]
- 36.Moorman PG, Schildkraut JM, Calingaert B, Halabi S, Vine MF, Berchuck A. Ovulation and ovarian cancer: a comparison of two methods for calculating lifetime ovulatory cycles (United States) Cancer Causes Control. 2002;13:807–11. doi: 10.1023/a:1020678100977. [DOI] [PubMed] [Google Scholar]
- 37.Pelucchi C, Galeone C, Talamini R, Bosetti C, Montella M, Negri E, et al. Lifetime ovulatory cycles and ovarian cancer risk in 2 Italian case-control studies. Am J Obstet Gynecol. 2007;196:83.e1–7. doi: 10.1016/j.ajog.2006.06.088. [DOI] [PubMed] [Google Scholar]
- 38.Peres LC, Moorman PG, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy M, et al. Lifetime number of ovulatory cycles and epithelial ovarian cancer risk in African American women. Cancer Causes Control. 2017 doi: 10.1007/s10552-017-0853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purdie DM, Bain CJ, Siskind V, Webb PM, Green AC. Ovulation and risk of epithelial ovarian cancer. Int J Cancer. 2003;104:228–32. doi: 10.1002/ijc.10927. [DOI] [PubMed] [Google Scholar]
- 40.Tung KH, Wilkens LR, Wu AH, McDuffie K, Nomura AM, Kolonel LN, et al. Effect of anovulation factors on pre- and postmenopausal ovarian cancer risk: revisiting the incessant ovulation hypothesis. Am J Epidemiol. 2005;161:321–9. doi: 10.1093/aje/kwi046. [DOI] [PubMed] [Google Scholar]
- 41.Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136:1184–203. doi: 10.1093/oxfordjournals.aje.a116427. [DOI] [PubMed] [Google Scholar]
- 42.Yang HP, Murphy KR, Pfeiffer RM, George N, Garcia-Closas M, Lissowska J, et al. Lifetime Number of Ovulatory Cycles and Risks of Ovarian and Endometrial Cancer Among Postmenopausal Women. Am J Epidemiol. 2016;183:800–14. doi: 10.1093/aje/kwv308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terry KL, Titus-Ernstoff L, McKolanis JR, Welch WR, Finn OJ, Cramer DW. Incessant ovulation, mucin 1 immunity, and risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:30–5. doi: 10.1158/1055-9965.EPI-06-0688. [DOI] [PubMed] [Google Scholar]
- 44.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The Prevalence and Features of the Polycystic Ovary Syndrome in an Unselected Population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 45.Dokras A, Witchel SF. Are Young Adult Women with Polycystic Ovary Syndrome Slipping through the Healthcare Cracks? J Clin Endocrinol Metab. 2014:jc.2013–4190. doi: 10.1210/jc.2013-4190. [DOI] [PubMed] [Google Scholar]
- 46.March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 47.Mehrabian F, Khani B, Kelishadi R, Ghanbari E. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol. 2011;62:238–42. [PubMed] [Google Scholar]
- 48.Sirmans S, Pate K. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tehrani F, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol. 2011;9:39. doi: 10.1186/1477-7827-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27:3067–73. doi: 10.1093/humrep/des232. [DOI] [PubMed] [Google Scholar]
- 51.Stein I, Leventhal M. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–5. [Google Scholar]
- 52.National Institutes of Health. Evidence-based Methodology Workshop on Polycystic Ovary Syndrome. In: Health NIo, editor. Executive Summary. 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


