Abstract
The introduction of ionizing radiation in medicine revolutionized the diagnosis and treatment of disease and dramatically improved, and continues to improve, the quality of health care. Cardiovascular imaging and medical imaging in general are, however, associated with a range of radiobiologic effects, including, in rare instances, moderate to severe skin damage resulting from cardiac fluoroscopy. For the dose range associated with diagnostic imaging (corresponding to effective doses of the order of 10 mSv (1 rem)), the possible effects are stochastic in nature and largely theoretical. The most notable of these effects is, of course, a possible increase in cancer risk. The current review addresses radiobiology relevant to cardiovascular imaging, with particular emphasis on radiation induction of cancer including consideration of the linear non-threshold dose-response model and of alternative models such as radiation hormesis.
Keywords: cancer risks, hormesis, linear non-threshold model, linear-quadratic model, deterministic effects, radiation dosimetry, radiation effects, radiation genetic effects, radiobiology, reverse causation, stochastic effects
Graphical abstract
Introduction
The use of ionizing radiation in medicine has revolutionized the diagnosis and treatment of disease. Radiation-based imaging techniques continue to improve the quality of health care. As a result of the documented value of diagnostic imaging, the use of these techniques has grown dramatically over the last several decades (1). A recent American College of Radiology (ACR) “white paper” (2) reported that the annual number of nuclear medicine procedures increased three-fold (from 7 million to 20 million) and the annual number of computed tomography (CT) procedures increased twenty-fold (from 3 million to 60 million) between 1985 and 2005 in the United States. This increase in utilization has led to an increase in radiation exposure of the population, which, in turn, raised concern over the radiogenic risks associated with medical imaging. Reports of such risks, some alarmist in tone (3), in both the scientific and lay media has led to thoughtful critical evaluation of imaging procedures, with technical optimization, justification (ie elimination of truly unnecessary procedures), and minimization of imaging doses without compromising the diagnostic information being sought. However, the excessive emphasis on radiogenic cancer risk can create the misconception that not only is radiation the only risk to be considered in medical imaging but also that the benefit of imaging procedures may be outweighed by the risk. It is in this context that the current review addresses radiobiology relevant to cardiovascular imaging, with particular emphasis on radiation induction of cancer including consideration of the linear non-threshold dose-response model and of alternative models such as radiation hormesis. Additional recent articles on the radiobiologic effects of cardiovascular imaging include references (4–6).
Stochastic and Deterministic Effects of Radiation
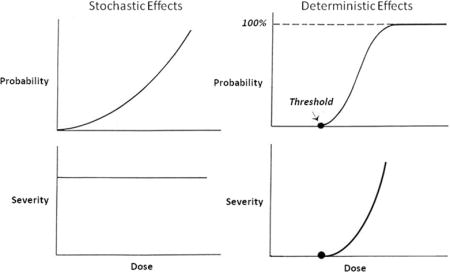
The radiobiologic effects of radiation are often distinguished as either stochastic (ie statistical) or non-stochastic (ie deterministic). The distinction between stochastic and deterministic effects is perhaps best understood in terms of their respective probability-dose and severity-dose relationships, illustrated in Figure 1. A stochastic effect is characterized by the absence of a threshold, meaning that any radiation dose above background is associated with a corresponding finite (or non-zero) increase in the probability above background of the effect occurring. As the dose increases above background this excess probability also increases. However, the severity of the effect does not increase with dose, that is, the severity of a stochastic effect is independent of dose. Stochastic effects include radiation-induced carcinogenesis and germ-cell mutagenesis and are generally associated with low-level (eg diagnostic) exposures. A deterministic effect is characterized by a well-defined threshold dose, meaning that the probability of the effect occurring does not increase above the background probability until the threshold is exceeded. However, once the threshold dose is exceeded, the severity as well as the excess probability of the effect increases with dose, with essentially all irradiated individuals exhibiting the effect (ie the probability reaches 100%) at sufficiently high doses; the dose-dependent probability increases in a sigmoidal fashion typical of pharmacological dose-response curves. The range of effects of radiation on skin typify deterministic effects, as discussed below. Deterministic effects are generally associated with high-level (eg therapeutic) radiation exposures.
Figure 1. Radiation effects.
Stylized probability-dose and severity-dose relationships for stochastic and deterministic effects.
At the cellular level, stochastic effects presumably result from non-lethal genetic mutations and, in principle, the clonogenic proliferation of a single mutated cell may progress to a tumor. Although a gross oversimplification (and one which ignores immunosurveillance and other homeostatic functions), this is mechanistically consistent with the presumed absence of a threshold dose for a stochastic effect such as cancer induction. Induction of a deterministic effect, on the other hand, requires elimination by apoptosis or other cell-killing mechanisms of a critical mass of cells within one or more functional cell compartments in order to induce a demonstrable clinical effect. This is consistent with a non-zero threshold for such an effect and with the dose dependency of the severity as well as the probability of deterministic effects.
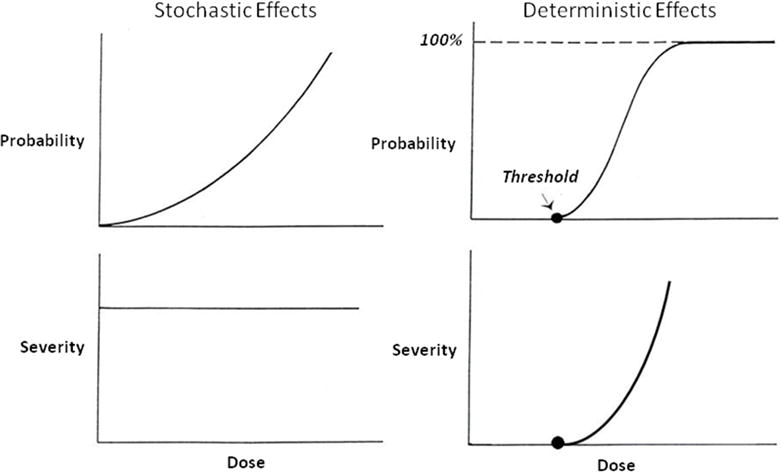
For cardiovascular imaging, the radiation doses, expressed in terms of effective dose, are typically less than 20 mSv (2 rem); organ absorbed doses range from 10 to 50 mGy (1 to 5 rad), with most organ doses at the lower end of this range (6) (Figure 2). For cardiovascular imaging, as for diagnostic imaging generally, the radiation effects of practical concern are therefore largely low-level stochastic effects, most notably, possible carcinogenesis. As discussed below, however, there are also potential risks of non-carcinogenic effects, and even of deterministic effects, associated with the cardiovascular imaging.
Figure 2. Radiation doses in cardiovascular imaging.
Effective doses for common cardiovascular imaging procedures with current instruments and techniques. For comparison, the average annual effective dose per capita from natural background radiation in the Unites States is also shown (6). Reproduced with permission.
Radiation Effects of Cardiovascular Imaging: Non-Carcinogenic
Reproductive Effects (including Germ-cell Mutagenesis)
Sex, or germ cells (ie sperm and eggs) are among the most radiosensitive cells in the body. As reviewed by Hall and Giaccia (7), gonadal irradiation can result in adverse effects on reproductive function, ranging from heritable mutations in sperm and eggs to impaired fertility and even permanent sterility at sufficiently high doses. Testicular doses as low as 0.15 Gy (15 rad) result in oligospermia and doses greater than 0.5 Gy (50 rad) in azoospermia and therefore temporary sterility, (with latent periods of several months after exposure). The duration of sterility is dose-dependent, ranging from less than 1 year at doses up to 1 Gy (100 rad) to several years at doses of 2–3 Gy (200–300 rad); sterility may be permanent at gonadal doses of 5–6 Gy (500–600 rad) or greater. Ovarian doses required to induce permanent sterility range from 12 Gy (1,200 rad) in pre-pubescent females to 2 Gy (200 rad) in sexually mature, pre-menopausal females. Because eggs are non-proliferating (in contrast to spermatozoa), there is no significant latent period associated with radiogenic infertility in females.
Aside from the issue of radiogenic impairment of fertility and as early as the 1920s, Hermann Muller, in his Nobel Prize-winning work, reported that exposure to x-rays could cause demonstrable germ-cell mutations resulting in heritable effects (ranging from change in eye color to recessive lethal mutations) in the offspring of irradiated fruit flies (Drosophila melanogaster) (8). A “doubling dose” (ie the gonadal radiation dose which increases the overall rate of mutations in a defined set of genes to twice the spontaneous rate) as low as 5 R (equivalent to 5 cGy or 5 rad) was estimated from these data; this finding was largely the original basis of establishing in the 1950s a maximum permissible dose limit of 5 cGy (5 rad) per year for occupationally exposed individuals (9–11). At about the same time, the husband-and-wife team of WL Russell and LB Russell at the Oak Ridge National Laboratory initiated what came to be known as the “megamouse project” to determine locus-specific mutation rates in mice under various irradiation conditions (including widely varying doses and dose rates) (12,13). This seminal work demonstrated: a wide (~30-fold) variation in the sensitivity of specific genes to radiogenic mutations; a pronounced dose-rate effect; and maximal elimination (ie repair) of radiogenic mutations in germ cells if conception were delayed at least 2 months post-irradiation (corresponding to ~6 months in humans). In addition to the foregoing non-human studies, germ-cell mutagenesis and its possible effects has been extensively studied among subsequently conceived offspring (a total of over 30,000 children) of A-bomb survivors who received significant but non-lethal doses of radiation (mean gonadal doses: 0.3–0.4 Gy (30–40 rad)). There was no significant increase among these children in the incidence of genetic abnormalities compared to that in a matched population born to unexposed Japanese parents (a total of over 40,000 control children) (14–16). Despite the absence of any demonstrable genetic effects, statistical analysis has been used to derive estimates of the mutation rate in human populations exposed to low-LET, chronic or low-dose-rate irradiation, with the risk per million live-born progeny in the first (F1)-generation as follows (17): autosomal dominant and x-linked diseases, 750–1,500 cases/Gy (7.5–15 cases/rad); autosomal recessive, ~0 cases/Gy (0 cases/rad); chronic multifactorial diseases, 250–1,200 cases/Gy (2.5–12 cases/rad); and congenital abnormalities, ~2000 cases/Gy (20 cases/rad). The total risk is therefore of the order of 3,000–4,700 cases/Gy (30 to 47 cases/rad), which represent 0.4– 0.6 %/Gy (0.004–0.006 %/rad) of the baseline frequency (738,000 per million). This corresponds to an overall doubling dose of about 1 Gy (100 rad).
Patient gonadal doses in cardiovascular imaging are generally quite low, ranging from well under 1 cGy (1 rad) for cardiovascular fluoroscopy and cardiac CT to ~1 cGy (1 rad) for nuclear cardiology (18–20); the very low doses associated with the radiographic imaging procedures is, of course, due to the fact that the gonads receive are distant from the heart and receive only a scattered-radiation dose. Even for interventional radiologists performing cardiovascular fluoroscopy, occupational gonadal (ie under-apron) doses are of the order of only 10 Gy (1 mrad), or roughly 10 mGy (1 rad) per year (21,22). Thus, for patients undergoing cardiovascular imaging procedures and for personnel involved in performing such procedures, it is unlikely that there is any practically demonstrable risk of either radiogenic impairment of fertility or of germ-cell mutagenesis.
Teratogenesis
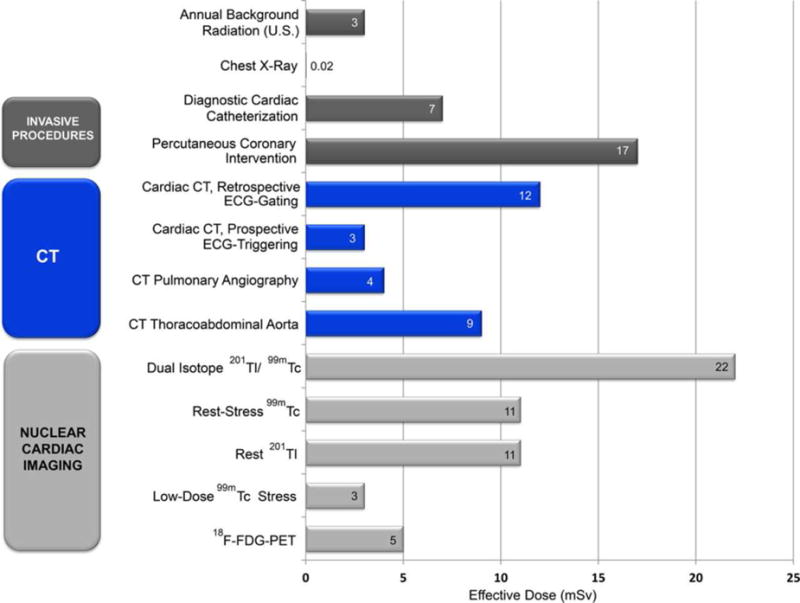
The embryo and fetus are highly radiosensitive, and radiation effects on the conceptus include lethality, congenital abnormalities, growth retardation, and childhood cancer (23). The probability of these effects depend on the dose (Figure 3), gestational age at the time of irradiation (Figures 3 and 4), and dose rate (Table 1) (24–26). The teratogenic effects of lethality, congenital abnormalities, and growth retardation are deterministic effects and thus characterized by a well-defined threshold dose. As illustrated, for example, in Figure 4 for the increased incidence of severe mental retardation among children irradiated in utero at the time of the atomic bombings of Hiroshima and Nagasaki, the threshold dose appears to be at least 0.23 Gy (23 rad) and possibly as high as 0.64 Gy (64 rad), with the maximal risk resulting when fetal irradiation is delivered at 8–15 weeks post-conception (24). Importantly, the radiation doses to the embryo or fetus associated with cardiovascular imaging (including fluoroscopy) are only 1 to several cGy (1 to several rad) (18,27,28) and thus at least an order of magnitude lower than the threshold dose for deterministic effects on the conceptus. These effects, therefore, are not a practical concern in the context of cardiovascular imaging.
Figure 3. Congenital abnormalities and perinatal death from in utero irradiation.
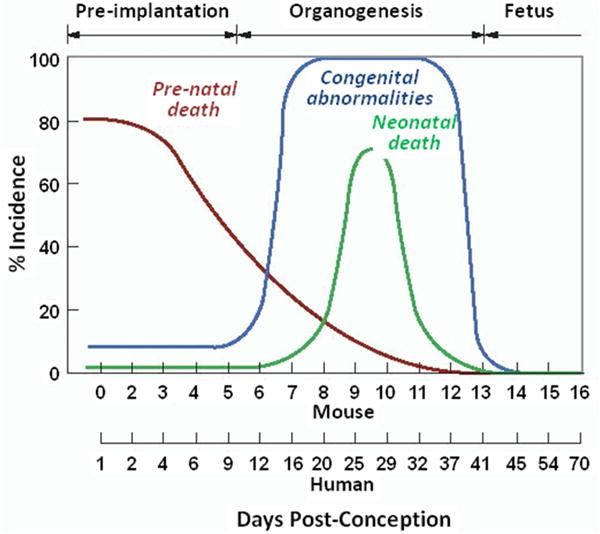
Incidence (percentage) of congenital abnormalities and of pre-natal and neonatal death in mice receiving an x-ray absorbed dose 2 Gy in utero as a function of gestational age (23, 26). Adapted from reference (23).
Figure 4. Mental retardation in children irradiated in utero.
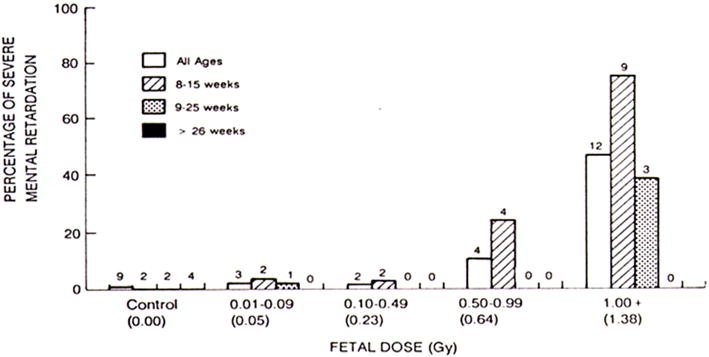
Incidence (percentage) of severe mental retardation in children irradiated in utero at the time of the atomic bombings of Hiroshima and Nagasaki (combined data) as a function of absorbed dose, stratified according to gestational age at the time of the bombings (24). Reproduced with permission.
Table 1.
The dose-rate effect in radiation teratogenesis. The percentage of rat pups with severe congenital abnormalities following a 1.5-Gy (150-rad) dose in utero decreases sharply, for some effects to 0, as the dose rate decreases from 1 to 0.005 Gy/min (100 to 0.5 rad/min) (25).
Cardiovascular effects
Although high (ie therapeutic) doses of ionizing radiation have long been associated with cardiovascular disease and circulatory disease in general (29,30), a causative relationship between such diseases and lower (ie diagnostic or occupational) exposures remains somewhat less apparent (31–34). In addition to that provided by the Life-Span Study (LSS) of atomic bomb survivors (31–34), a recent meta-analysis by Little et al appears to have found evidence for such a relationship (35). Using peer-reviewed publications reporting the incidence of circulatory disease (including ischemic heart disease) following cumulative mean whole-body doses less than 0.5 Sv (50 rem) and current mortality rates for nine developed countries, excess relative risks of circulatory disease were estimated. Excess population risks for all circulatory diseases combined ranged from 2.5 %/Sv (95% CI: 0.8–4.2 %/Sv) for France to 8.5 % /Sv (95% CI: 4.0–13.0 %/Sv) for Russia. There are still concerns with occupational studies because of confounding background risk factors for cardiovascular disease. Although much uncertainty still exists, should such associations between low-level radiation exposure and circulatory diseases actually reflect an underlying causal dose-response relationship that remains linear at low doses, the overall excess risk of mortality after low-dose exposure may be about twice that currently assumed based on estimated risks of mortality due to radiation-induced cancers alone. However, it should be noted that the current state of the relevant epidemiological evidence has led some (eg Akiba (36)) to conclude that the currently available findings “…do not constitute convincing evidence for an excess risk of circulatory disease in relation to low-level radiation exposure”. One of the difficulties is that there is an absence of a broadly accepted radiobiological mechanism to plausibly explain the apparent epidemiological associations between low-dose radiation and cardiovascular diseases. There is clearly a need for additional epidemiological and mechanistic studies in this area.
Cataractogenesis
The lens of the eye long been recognized as one of the most radiosensitive tissues in the body, with cataracts induced by doses of less than 2 Gy (200 rad) and protracted dose less than 5 Gy (500 rad) (37). The exact mechanism of radiation cataractogenesis remains unclear, however. Although cataractogenesis has historically been considered a deterministic effect with a threshold of ~2 Gy (200 rad), recent studies, including those of A-bomb survivors, American astronauts, and Chernobyl clean-up workers, suggest a much lower threshold dose, perhaps of the order of 0.5 Gy (50 rad) or less (37–39). Such studies further suggest that the dose-response relationship for radiation cataractogenesis may actually follow a linear, no-threshold model. Cataractogenesis is rather unique among radiation risks associated with medical imaging procedures in that there is a potentially significant risk to imaging personnel directly involved in some such procedures, specifically, fluoroscopy (40–44). Combined with the relatively high patient doses delivered by the primary x-ray beam and the close proximity of the fluoroscopist to the patient during the actual beam-on time, radiation scattered from the patient may deliver significant doses to the fluoroscopist’s eyes and thus pose a significant risk of radiation cataractogenesis. The results of several recent studies of interventional radiologists and other staff performing fluoroscopic cardiovascular imaging, in fact, suggest a causal relationship between cataracts (or at least lens opacifications) and occupational radiation exposure (37,41,43,45). In the 2010 study by Ciraj-Bjelac et al (43), for example, interventional radiologists and nurses and age- and gender-matched unexposed controls were screened for lens opacities by dilated slit-lamp examination and posterior lens changes graded using a modified Merriam-Focht technique. Individual cumulative lens x-ray exposure was calculated from responses to a questionnaire and personal interview. The prevalence of radiation-associated posterior lens opacities was 52% (29/56, 95% confidence interval (CI): 35–73%) for interventional radiologists, 45% (5/11, 95% CI: 15–100%) for nurses, and only 9% (2/22, 95% CI: 1–33%) for controls. The relative risks of lens opacity was therefore 5.7 (95% CI: 1.5–22) for interventional radiologists and 5.0 (95% CI: 1.2–21) for nurses. The estimated cumulative eye doses ranged from 0.01 to 43 Gy (1–4,300 rad) with mean and median values of 3.4 and 1.0 Gy (340 and 100 rad), respectively. Importantly, besides the relatively small number of study subjects, individual cumulative lens doses were derived by calculation based on responses to a questionnaire and personal interview rather than by direct measurement. These and other data nonetheless suggest a significant dose-response relationship between occupational exposure and the incidence of posterior lens changes.
Based on the foregoing data and other such data, the International Commission on Radiological Protection (ICRP) recently recommended reducing the annual dose limit to the lens of the eye for occupationally exposed individuals from its current value of 150 mSv (15 rem) to 20 mSv (2 rem) (46). Although this recommended lens-of-eye dose limit has not yet been implemented in the United States, the Electric Power Research Institute (EPRI) recently reviewed the available epidemiologic literature (47) and the National Council on Radiation Protection and Measurement (NCRP) is preparing an updated commentary addressing the issues of risk and dose limitation in radiation protection and including guidance on the lens of the eye (48). At the same time, various shielding strategies have been investigated to evaluate their impact in reducing the eye dose in fluoroscopy (48–53). For example, using an anthropomorphic phantom and a miniature solid-state dosimeter positioned at the phantom’s eye, Thornton et al (51) evaluated the impact of common shielding strategies, used alone and in combination, on the scattered dose to the fluoroscopy operator’s eye. The dose rate dose rate was recorded with and without a leaded table skirt, non-leaded and leaded (0.75-mm lead equivalent) eyeglasses, disposable tungsten-antimony drapes (0.25-mm lead equivalent), and suspended and rolling (0.5-mm lead equivalent) transparent leaded shields. Each strategy’s shielding efficacy was expressed as a reduction factor equal to the ratio dose rate without eye shielding to that with shielding. The results for an operator positioned at the patient’s upper abdomen and for posterior-anterior and left and right anterior oblique views are presented in Table 2. Use of leaded glasses alone reduced the lens dose rate by a factor of 5 to 10; scatter-shielding drapes alone reduced the dose rate by a factor of 5 to 25. Use of both together was always more protective than either used alone, reducing dose rate by a factor of 25 or more. Lens dose was routinely undetectably low when a suspended shield was the only barrier during low-dose fluoroscopy. Clearly, such practical shielding strategies, especially when used in combination, can dramatically reduce the eye dose and thus the risk of cataractogenesis (54).
Table 2.
Lens exposure while operating at patient’s upper abdomen during low-dose posterior-anterior, left anterior oblique, and right anterior oblique fluoroscopy and the impact of various shielding strategies (51).
| Posterior-Anterior
|
15° Left anterior oblique
|
15° Right anterior oblique
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Lens dose rate
|
Lens Dose Reduction Factor | Lens dose rate
|
Lens Dose Reduction Factor | Lens dose rate
|
Lens Dose Reduction Factor | ||||
| Shielding strategy | mSv/h | mR/h | mSv/h | mR/h | mSv/h | mR/h | |||
| Image intensifier at 3 cm (close) | 0.32 | 37 | – | 0.946 | 108 | – | 0.171 | 19.5 | – |
| plus leaded table skirt | 0.217 | 24.8 | RM | 0.79 | 90 | RM | 0.156 | 17.8 | RM |
| plus unleaded eyeglasses | 0.205 | 23.5 | 1 | 0.74 | 85 | 1.1 | 0.153 | 17.5 | 1 |
| plus leaded eyeglasses | 0.04 | 4.6 | 5.4 | 0.101 | 11.5 | 7.8 | 0.024 | 2.7 | 6.6 |
| plus scatter-shielding drape | 0.033 | 3.8 | 6.5 | 0.087 | 10 | 9 | 0.035 | 4 | 7.2 |
| plus leaded eyeglasses and scatter-shielding drape | 0.007 | 0.77 | 32.2 | 0.014 | 1.6 | 56.3 | LLD | LLD | >1,000 |
| plus ceiling-suspended shield | LLD | LLD | >1,000 | LLD | LLD | >1,000 | LLD | LLD | >1,000 |
Notes: Lens dose reduction factor = Lens dose rate reference measurement (RM)/Lens dose rate with shielding strategy.
LLD = below the low limit of detection, 0.001 mSv/h (0.1 mrem/h).
Skin Damage
In cardiovascular fluoroscopy and interventional imaging generally, skin absorbed doses can be up to several Gy (several hundred rad) (54–58). The acute threshold absorbed dose for skin effects (~2 Gy (200 rad) (Table 3)) may therefore be exceeded (55,56). In rare instances, skin doses may be considerably higher than 2 Gy (200 rad) and result in necrotic damage so severe as to necessitate surgical intervention (as described in Table 3 and illustrated in Figure 5) (55,56), particularly as fluoroscopically guided procedures have become more complex and beam-on times have increased. Largely for this reason the United States Food and Drug Administration (US FDA) in 1994 issued a health advisory entitled, “Avoidance of Serious X-ray Skin Injuries to Patients during Fluoroscopically Guided Procedures” (59), and established regulatory limits for the entrance dose rate, specifically, for the air kerma rate (AKR). With certain exceptions, the AKR shall not exceed of 88 mGy/minute (10 R/min) at the measurement point. (The foregoing regulatory limit applies specifically to fluoroscopic equipment manufactured on or after May 19, 1995. If the x-ray source is below the patient table, the AKR shall be measured at 1 cm above the tabletop. If the source is above the table, the AKR shall be measured at 30 cm above the tabletop with the end of the beam-limiting device or spacer positioned as closely as possible to the point of measurement. In a C-arm type of fluoroscope, the AKR shall be measured at 30 cm from the input surface of the fluoroscopic imaging.) The FDA subsequently amended its regulation entitled, “Performance Standards for Ionizing Radiation Emitting Products. Fluoroscopic Equipment” (60). The additions required that fluoroscopic equipment manufactured after June 10, 2006 display both AKR and cumulative air kerma in order to provide the fluoroscopist with real-time patient radiation dose data, with the intent that such information would result in reduced radiation doses.
Table 3.
| Approximate time of onset of effects post-irradiation
|
|||||
|---|---|---|---|---|---|
| Absorbed Dose (Gy (rad)) | National Cancer Institute grade | Prompt: <2 weeks | Early: 2–8 weeks | Mid-term: 6–52 week | Long-term: >40 weeks |
| 0–2 (0–200) |
Not applicable | No observable effects expected | No observable effects expected | No observable effects expected | No observable effects expected |
| 2–5 (200–500) |
1 | Transient erythema | Epilation | Recovery from hair loss | No observable effects expected |
| 5–10 (500–1,000) |
1 | Transient erythema | Erythema and epilation | Recovery; after higher doses, prolonged erythema and permanent partial epilation expected | Recovery; after higher doses, dermal atrophy and induration expected |
| 10–15 (1,000–1,500) |
1–2 | Transient erythema | Erythema and epilation; possible dry or moist desquamation; recovery from desquamation | Prolonged erythema; permanent epilation | Telangiectasia; dermal atrophy and induration expected; skin expected to be weak |
| >15 (1,500) | 3–4 | Transient erythema; after very high doses, edema and acute ulceration expected, with surgical intervention most likely required longer term | Erythema and epilation; moist desquamation | Dermal atrophy; secondary ulceration due to failure of moist desquamation to heal, with surgical intervention most likely required | Possible late skin breakdown; wound might persist and progress to deeper lesion, with surgical intervention most likely required |
Figure 5. Skin reactions from excessive fluoroscopic irradiation.
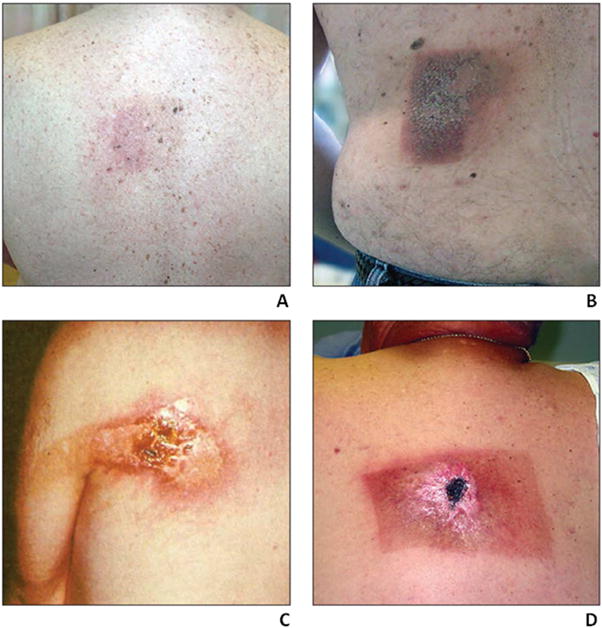
Photographs of representative skin reaction grades resulting from excessive fluoroscopic irradiation and corresponding to those described in Table 3: A. Grade 1, B. Grade 2, C. Grade 3, and D. Grade 4 (55, 56). Reproduced with permission.
Radiation Effects of Cardiovascular Imaging: Carcinogenic
Despite the various non-carcinogenic risks associated with cardiovascular imaging and medical imaging in general, the principal radiation risk of practical concern remains the possibility of cancer induction. The scale of this risk appears to differ sharply between pre- and post-natal irradiation. The actual risk, if any, of radiation carcinogenesis at diagnostic and other comparably low doses remains highly controversial, and some have argued that there is, in fact, a hormetic (or protective) effect against the development of cancer at doses at or below ~0.1 Sv (10 rem). The literature on radiation carcinogenesis and on radiation hormesis is vast and continues to grow, and even a cursory review of this literature is beyond the scope of the current article. A brief review of pre- and post-natal radiation carcinogenesis and of radiation hormesis is provided, however.
Pre-natal Irradiation
In contrast to the deterministic, high-dose effects of irradiation of the embryo and fetus (discussed above), the increased risk of childhood cancer, a stochastic effect, associated with in utero irradiation is worrisome even at diagnostic radiation doses. The NCRP recently published a detailed review and evaluation of preconception and pre-natal radiation exposure health effects (61). Originally, the Oxford Survey of Childhood Cancers in the 1950s suggested an association between an increased risk of childhood cancer, principally leukemia, and exposure in utero to diagnostic x-rays (62). In this retrospective case-controlled study, of 7,649 children who died of cancer, 1,141 had been x-rayed in utero; among the controls (ie children who did not develop cancer), only 774 had been irradiated in utero. This yields a crude hazards ratio of 1,141/774 = 1.47 (ie a 47% increase) for childhood cancer per obstetric x-ray examination. The irradiated children received 1 to 5 films, with a fetal dose per film of ~3 mGy (0.3 rad); the estimated doses have a large uncertainty, however. The apparent carcinogenicity of in utero irradiation at diagnostic levels remains controversial. For example, in a more recent population-based study (63) in Ontario, Canada from 1991 to 2008, 5,590 mothers underwent diagnostic imaging studies (73% were CT scans and 27% nuclear medicine scans) and 1,829,927 mothers did not. After a median follow-up of 8.9 years, 4 childhood cancers developed in the exposed group (1.13 per 10,000 person-years) and 2,539 in the unexposed group (1.56 per 10,000 person-years), yielding a hazard ratio of 0.68 (95% CI: 0.26–1.8) and therefore suggesting that diagnostic imaging in pregnancy is not carcinogenic. Although the association between in utero irradiation and childhood cancer appears to be incontrovertible, the question remains whether this is a causative relationship or rather an example of “reverse causation” (64): because pregnant women were referred for imaging for some medical problem, their children may have been at a naturally increased risk for cancer due to their mother’s underlying medical condition rather than as a result of any diagnostic irradiation. The preponderance of evidence, however, suggests to many a causative relationship between diagnostic irradiation in utero and an increased risk of childhood cancer. The relevant literature was reviewed in detail by Doll and Wakeford (65), who concluded that a typical obstetric x-ray examination (corresponding to a fetal radiation doses of ~10 mGy (1 rad)) results in about a 40% increase in the incidence of childhood cancer, with an excess absolute risk of ~5 %/Gy (0.05 %/rad). In light of such a significant risk of childhood cancer associated with (and possibly causatively related to) diagnostic imaging studies, prudence dictates that proceeding with such a study in a pregnant (or possibly pregnant) female should be based on a considered and documented decision as to its medical necessity despite the foregoing risk. This decision should include consideration not only of alternative procedures not involving irradiation of the conceptus but also of the timeliness (or not) of the availability of such procedures relative to the urgency of obtaining the diagnostic information being sought. For fluoroscopic and CT examinations of the heart, the dose (ie the scattered-radiation dose) to the embryo or fetus may be so low, well below 1 cGy (1 rad), that the decision to proceed with the examination will be justified in almost all cases; for nuclear cardiology studies, however, where doses to the conceptus may be as high as 1 to several cGy (1 to several rad), the decision to proceed may be less clear-cut but nonetheless generally justified.
Post-natal Irradiation
Although the possibility of induction of a subsequent cancer in an unborn child from in utero diagnostic irradiation is understandably worrisome, a more frequently encountered concern is the risk of radiation carcinogenesis resulting from imaging studies in pediatric and adult patients. The challenge in assessing this risk is that there are very few reliable data in humans quantifying an increased cancer incidence, if any, following diagnostic radiation doses (ie less than ~100 mSv (10 rem)). Unfortunately, no prospective epidemiologic studies with appropriate non-irradiated controls have definitely demonstrated either the adverse (or hormetic (ie beneficial)) effects of radiation doses less than 100 mSv (10 rem) in man, and current estimates of the risks of low-dose radiation suggest that very large-scale epidemiological studies with long-term follow-up would be needed to actually quantify any such risk or benefit; such studies may be logistically and financially prohibitive.
The most creditable dose-response data for radiation carcinogenesis in man mainly involve doses one to two orders of magnitude greater than those encountered in diagnostic imaging studies - of the order of 1 Sv (100 rem) and greater - including, most notably, the A-bomb survivor follow-up data. A handful of high-profile studies have, however, reported cancer risks derived from relatively low-dose exposures. Pierce and Preston (66), for example, published an analysis of the A-bomb Radiation Effects Research Foundation data on cancer risks among survivors receiving doses less than 0.5 Sv (50 rem), with ~7,000 cancer cases among ~50,000 low-dose survivors. They concluded that cancer risks are not overestimated by linear risk estimates computed over the dose range 0.05–0.1 Sv %-10 rem), with a statistically significant risk in the range 0–0.1 Sv (0–10 rem) and an upper confidence limit on any possible threshold of 0.06 Sv (6 rem). The United Kingdom CT study (67,68), a record-linkage study of leukemia and myelodysplastic syndrome (MDS) and of brain cancer incidence following CT scans of 178,000 pediatric patients (0–21 years of age), reported excess relative risks (see below) of 36 /Gy (0.36 /rad) for leukemia and MDS and of 23 /Gy (0.23 /rem). These values are quite high, and critical evaluation of this study cited absence of individual scan parameters and therefore organ doses for individual patients and, again, the possible confounding effect of reverse causation (64,69). In the INWORKS study (70–73), an international cohort study of over 300,000 workers (over 8.2 million person-years) in the nuclear industry with detailed external dose data (mean dose: 21 mGy (2.1 rad)), the reported excess relative risk for all cancers was 0.51 /Gy (95% CI: 0.23–0.82 /Gy) (0.0051 /rad (95% CI: 0.0023–0.0082 /rad)). In addition to possible uncertainty in personnel dose estimates, smoking and occupational asbestos exposure were identified as potential confounding factors; however, exclusion of deaths from lung cancer and pleural cancer did not affect the association of cancer risk and occupational radiation exposure.
Estimation of the excess cancer risk from imaging studies and other low-dose exposures requires mathematical extrapolation of high-dose dose-response data to the lower diagnostic-dose range. There are at least several distinct dose-response models for radiation carcinogenesis which can be used for this extrapolation: the supra-linear model, the linear no-threshold (LNT) model, the sub-linear (or linear-quadratic (LQ)) model, and the hormesis model (Figure 6).
Figure 6. Dose-response curves for radiation carcinogenesis.
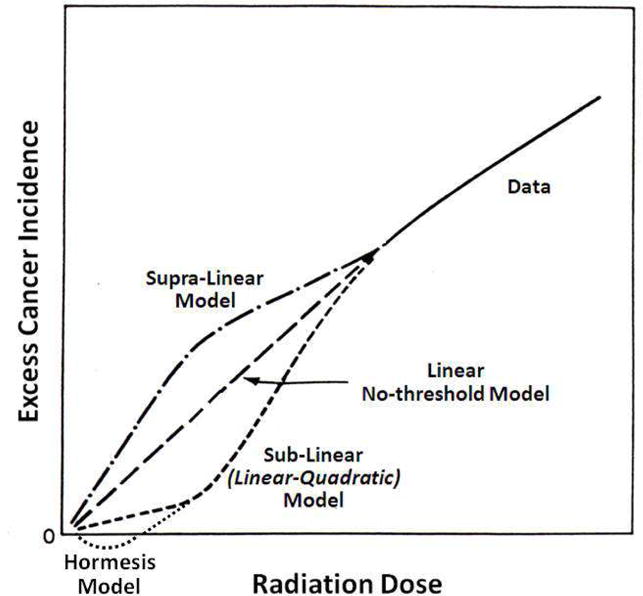
Stylized dose-response curves for radiation carcinogenesis for the supra-linear, linear no-threshold (LNT), sub-linear (or linear-quadratic), and hormetic models. Note that for the hormetic model the excess incidence becomes negative at low radiation doses, indicating a cancer incidence less than the naturally occurring incidence and thus a radioprotective effect.
The supra-linear model implies that the cancer risk per unit dose (ie the risk per Sv or per rem) is greater at lower than at higher doses. There really are no data or mechanistic considerations which support such a model, and it is thus not a creditable option for extrapolation of high-dose cancer risk estimates to diagnostic doses.
The LNT model, which implies a uniform cancer risk per unit dose from higher to lower doses, is the model currently recommended by authoritative advisory bodies such as the ICRP (74) the NCRP (75,76), UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) (77) and adopted by regulatory agencies such as the NRC (78). As its name indicates, also implicit in the LNT model is that there is no threshold dose for radiation carcinogenesis, that is, there is no radiation dose above background below which there is not a finite increase in cancer risk. The excess absolute risk (EAR) is the number of excess fatal cancers per number of irradiated individuals (excess above the naturally occurring incidence) predicted by the model in a large (and therefore gender- and age-averaged) population exposed to a uniform whole-body dose (or effective dose) of radiation. The ERR is the EAR divided by the naturally occurring incidence of fatal cancer, and may be expressed as either a fraction or a percentage per unit effective dose. The excess number of fatal cancers in an irradiated population using the LNT model can be then calculated as the number of persons exposed • effective dose (rem or mSv) per person • ERR (/rem or /mSv). A widely cited ERR value is that recommended by the NCRP Report No 115 (79), 5 × 10−5 per person per mSv (or 5 × 10−4 per person per rem). Thus, if a population of a million people each received an effective dose of 10 mSv (1 rem), the expected number of excess fatal cancers observed in this population over the balance of the lifetimes of the individuals in this population would be 1×106 persons • 10 mSv • 5×10−5 person/mSv = 500. This compares to the spontaneous, or background, lifetime incidence of ~300,000 (or 30%) otherwise occurring in such a population; this correspondence to an increase in overall incidence of only (500/300,000) • 100% = 0.17%. Importantly, even if one concedes the accuracy of the LNT model, it cannot be applied reliably to individuals but only to large populations (74), that is, populations sufficiently large that differences in radiation sensitivity related to gender, age, diet and other lifestyle effects, and intrinsic biology are effectively averaged out.
The sub-linear, or LQ, model implies that the excess cancer risk per unit dose is lower at lower than at higher does and further implies the possibility of at least a practical threshold dose for radiation carcinogenesis, that is, a non-zero dose below which is there is no demonstrable increase in cancer incidence.
According to the hormesis model, individuals exposed to low radiation doses actually have a lower subsequent risk of cancer than unexposed individuals (Figure 6), presumably as a result of radiogenic upregulation of cellular repair mechanisms or other adaptive response(s) (80,81). Although radiation hormesis had been largely dismissed for many years, there are mounting creditable data in the peer-reviewed scientific literature supporting this phenomenon (82). Feinendegen, for example, recently reviewed a number of pre-clinical studies demonstrating radiation hormesis and, in particular, radioprotective adaptive responses to low-dose irradiation (as summarized in Figure 7) and concluded that radiation doses less than about 600 mGy (60 cGy) induced a pronounced (~50%) protective effect against a variety of molecular, cellular, and whole-animal radiation effects (83).
Figure 7. Radiation hormesis in pre-clinical models.
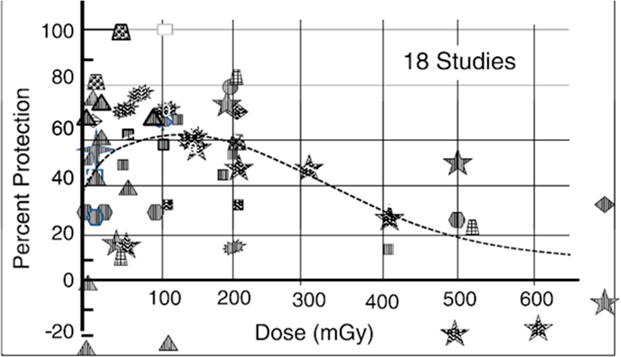
Dose dependence of the radioprotective, or hormetic, adaptive response of acute low-dose irradiation in pre-clinical models. Each observation point of protection per study was plotted individually as a function of dose, yielding a total of 54 observation points. The categories of response include molecular-level, cellular-level, and cancer-level responses and included enzyme inactivation, DNA repair changes, chromosomal changes such as micronuclei formation, cell death, immune response, experimental cancer and metastasis induction, and by-stander protection (83). Reproduced with permission.
Some argue that the data and associated analyses supporting the LNT model are further refuted by epidemiologic and experimental studies and that this model over-states the risk of radiation carcinogenesis at doses of the order of 100 mSv (10 rem) and less and does not account for creditable evidence for a threshold for cancer induction, that is, a non-zero radiation dose below which there is no increased risk of cancer (84–87). Others argue that the preponderance of data, especially epidemiology data, support, or at least are consistent with an LNT dose-response model down to the low-dose range (76,88). The validity, applicability, and utility of the LNT model and of alternative models this remains controversial and subject to often contentious debate (86,89–92). As John Boice, President of the NCRP, has aptly stated (93), “LNT is not TNT, but differences in opinions sometimes appear explosive!” For example, the attitude of a number of investigators with respect to the LNT model was recently reflected by Calabrese (94).
“…the evidence used by the US National Academy of Sciences (NAS) Biological Effects of Atomic Radiation (BEAR) I Committee, Genetics Panel, in 1956 to usher LNT into its dominant position in cancer risk assessment is highly problematic. The Genetics Panel did not base its assessment on appropriate studies, and the research record was falsified and fabricated to enhance acceptance of its LNT recommendation. The Genetics Panel also failed to provide a justification for the switch from the threshold to the linear model. However, as a regulatory tool, LNT had two attractive features: namely, ease of application and likelihood to consistently overestimate risk (U. S. Interagency Staff Group 1986). Therefore, once the NAS recommendation for LNT was accepted, risk estimates became highly sensitive to the LNT model, making the latter a regulatory gold standard without adequate validation.”
The dilemma over the LNT model and alternative models in deducing reliable low-dose factors for radiation carcinogenesis from high-dose data is illustrated in Figure 8, summarizing the dose-response data and different model fits to these data for solid-cancer incidence in the A-bomb Life-Span Study (LSS) (32). Several points are apparent upon examining these data. First, even for an exposed cohort as thoroughly well-characterized as the LSS cohort, the uncertainties (in terms of the 95% confidence intervals) of the dose-dependent ERRs and of the model fits to these data are rather large (of the order of 50%). This suggests, at least to some, that risk factors derived from such data may be unreliable. Second, statistical rigor aside, one is challenged to discern any sort of practically meaningful distinction between the fits of the linear (L) and the linear-quadratic (LQ) models to these dose-response data, especially in the low-dose range. Third, one is likewise challenged to confirm or refute a non-zero threshold. Based on a rigorously derived linear-model fit to these data, the gender-averaged ERR per Gy was 0.42 (95% CI: 0.32–0.53) (ERR per rad: 0.0042 (95% CI: 0.0032–0.0053)) for all solid cancers at age 70 years after exposure at age 30 years. The estimated lowest dose range with a significant nonzero ERR for all solid cancer was 0 to 0.20 Gy (0 to 20 rad), and a formal dose-threshold analysis indicated no threshold (ie zero dose was the best estimate of the threshold). However, an alternative analysis of the LSS data showed that a linear-quadratic model was the best fit for dose-response data restricted to doses less than 2 Gy, as the risk estimates for doses up to ~0.5 Gy were lower than those predicted by the linear model (95). Using a non-parametric statistical procedure, this re-analysis derived a threshold 200 mSv (20 rem) with a negative ERR, suggesting a radiation hormesis effect.
Figure 8. Risk of cancer among A-bomb survivors.
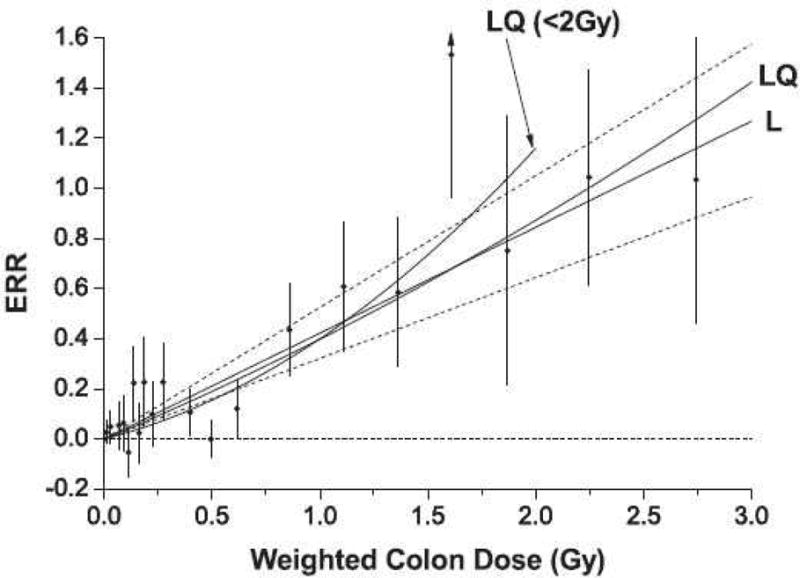
Excess relative risk (ERR) for all solid cancers as a function of colon absorbed dose (as a surrogate of effective dose) in the A-bomb Life-Span Study (LSS). The black circles represent ERR and 95% CI for the dose categories and the solid lines the fits of the linear (L) (with 95% confidence intervals (CI) (dotted lines)) and linear-quadratic (LQ) models to these data. The fit of the LQ model for the data restricted to doses less than 2 Gy (LQ< 2 Gy) is also shown (32). Reproduced with permission.
Benefit-Risk Analysis in Cardiovascular Imaging
Application with certitude of the LNT dose-response model for radiation carcinogenesis has led some to derive alarming estimates of excess numbers of cancers as a result of medical imaging, for example, in the thousands annually among the United States population (3). Such exercises largely ignore the considerable uncertainties (including the possibility of zero excess risk and even a hormetic effect) in deriving such estimates at diagnostic doses. Non-critical application of the LNT model in the context of medical imaging thus undermines a reasonable benefit-risk calculus and may thus adversely impact the patients’ medical management. Further, while the point is often made that the benefits of the uses of radiation in medicine are much greater than any theoretical risks, quantitative estimates of the benefits are not cited alongside any quantitative estimates of risk. This alone - expression of benefit in purely qualitative terms versus expression of risk in quantitative, and therefore seemingly more certain, terms - may well contribute to a skewed sense of the relative benefits and risks of diagnostic imaging among healthcare providers as well as patients. One benefit of a diagnostic imaging procedure may be expressed as the lives saved, that is, the number of lives lost by not performing the procedure or by performing an alternative, invasive procedure. (There may, of course, be other metrics of benefit such as improvements in the quality of life, shortening of hospital stays, and reduction of medical care costs.)
One example of such a quantitative benefit-risk analysis is use of scintigraphic myocardial perfusion imaging to predict and thereby avoid peri-operative cardiac events and associated mortality in non-cardiac surgery (96,97). The most important cause of peri-operative cardiac mortality and morbidity is myocardial infarction due to occult coronary artery disease. In a Veterans Administration (VA) series (98), the incidence and mortality of such events associated with vascular surgery (most commonly, carotid endarterectomy) was 13% and 40–70%, respectively. Based on pre-operative dipyridamole thallium-201 (201Tl) imaging, the incidence of peri-operative cardiac events was 2% for a severity level and an extent of 0 and 100% for a severity level of 3 and extent of 5–6, with 22% of patients have reversible perfusion defects. Thus, perfusion imaging was highly accurate for prediction of peri-operative cardiac events. The number of vascular surgeries (from the VA database) is ~9,500 per year and the number of peri-operative cardiac deaths (ie fatal myocardial infarctions) is thus estimated as 9,500 • 0.13 • 0.40 = 494 per year. Of these 494 peri-operative cardiac deaths annually, 0.22 • 494 = 109 were detectable pre-operatively and therefore avoidable, that is, the gross benefit of preoperative perfusion imaging with 201Tl is 109 lives saved per year in the VA system. The effective dose from the 201Tl study is 24 mSv = 0.024 Sv (2.4 rem) (6,20). Using the LNT-based lifetime risk factor (ie ERR) of 0.05 /Sv, a total of 9,500 • 0.05 /Sv • 0.024 Sv = 11 excess cancer deaths per year is predicted, yielding a net benefit of pre-operative myocardial perfusion imaging of 109 – 11 = 98 lives saved per year. If one considers a rest/stress myocardial perfusion study using 99mTc-MIBI, the ED is ~12 mSv (1.2 rem) (6,20), leading to only 6 excess cancer deaths per year and possible greater clinical benefit. Performing cardiac PET with rubidium-82 (82Rb)-rubidium chloride would result in an even lower ED of ~7.5 mSv (0.75 rem) (6,20), thus resulting in a theoretical risk of only 3 cancer deaths and a net savings of 106 lives per year.
The foregoing analysis and similar benefit-risk analyses demonstrate that cardiovascular imaging and diagnostic imaging in general saves many thousands of lives every year, while the theoretical, and possibly overestimated, cancer risks predicted by the LNT model are typically much lower.
Concluding Remarks
Cardiovascular imaging and medical imaging in general are associated with a range of radiobiologic effects, including, in rare instances, moderate to severe skin damage resulting from cardiac fluoroscopy. However, for the dose range associated with diagnostic imaging (corresponding to effective doses of the order of 10 mSv (1 rem)), the possible effects are stochastic in nature and appear to be largely theoretical. The most notable of these effects is, of course, a possible increase in cancer risk. The actual risk, if any, of radiation carcinogenesis at diagnostic and other comparably low doses remains highly controversial. Although the LNT model is the prevailing dose-response model, some have argued that there is a non-zero threshold dose for cancer induction and, in fact, a hormetic (or protective) effect against the development of cancer at doses at or below ~0.1 Sv (10 rem). In any case, application with certitude of the LNT or any alternative dose-response model is unjustified and has led some to derive alarming and possibly misleading estimates of excess numbers of cancers as a result of medical imaging. Application of the LNT or alternative dose response model to estimate radiogenic risks of medical imaging individual patients or to defined cohorts of patients, in particular, is inadvisable.
The International Organization for Medical Physics issued a policy statement highlighting the substantial uncertainties in estimating population cancer risk and noting the dangers of extrapolating risk estimates for radiation doses less than 100 mSv (10 rem) (99). The use of risk factors to estimate public-health consequences from individual or population exposures must be considered in the context of the attendant uncertainties. These include uncertainties related to dosimetry, epidemiology, low statistical power, modeling radiation risk data, and generalization of risk estimates across different populations and dose rates (100). Uncertainties in such risk estimates have been suggested as being up to a factor of 3 lower or higher than the estimate value itself (101). Such large uncertainties render projections of radiation-induced cancers or other detriment highly susceptible to biases and confounding influences that may be unidentifiable.
Several other professional societies and scientific bodies have provided guidance on the assessment of risk at diagnostic and other comparably low doses. The ICRP states, “There is…general agreement that epidemiological methods used for the estimation of cancer risk do not have the power to directly reveal cancer risks in the dose range up to around 100 mSv” (74). The Health Physics Society advises against estimation of health risks below an individual dose of 50 mSv in one year or a lifetime dose of 100 mSv above that received from natural sources (102), noting that “…below this level, only dose is credible and statement of associated risks are more speculative than credible”. Finally, the American Association of Physicists in Medicine (AAPM) has stated that, “…risks of medical imaging at effective doses below 50 mSv for single procedures or 100 mSv for multiple procedures over short time periods are too low to be detectable and may be non-existent” (103).
Supplementary Material
Abbreviations
- AAPM
American Association of Physicists in Medicine
- ACR
American College of Radiology
- BEIR
Biological Effects of Ionizing Radiation
- EPRI
Electric Power Research Institute
- ICRP
International Commission on Radiological Protection
- ICRU
International Commission on Radiation Units and Measurements
- INWORKS
International Nuclear Workers Study
- NCRP
National Council on Radiation Protection and Measurements
- NRC
Nuclear Regulatory Commission
- UNSCEAR
United Nations Scientific Committee on the Effects of Atomic Radiation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors have nothing to disclose.
References
- 1.Ionizing Radiation NCRP. NCRP Report 160. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 2009. Exposure of the Population of the United States. [Google Scholar]
- 2.Amis ES, Jr, Butler PF, Applegate KE, et al. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4:272–84. doi: 10.1016/j.jacr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Redberg R, Smith-Bindman R. We are giving ourselves cancer. New York Times (Op-Ed) 2014 [Google Scholar]
- 4.Einstein AJ. Effects of radiation exposure from cardiac imaging: how good are the data? J Am Coll Cardiol. 2012;59:553–65. doi: 10.1016/j.jacc.2011.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einstein AJ, Knuuti J. Cardiac imaging: does radiation matter? Eur Heart J. 2012;33:573–8. doi: 10.1093/eurheartj/ehr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meinel FG, Nance JW, Jr, Harris BS, De Cecco CN, Costello P, Schoepf UJ. Radiation risks from cardiovascular imaging tests. Circulation. 2014;130:442–5. doi: 10.1161/CIRCULATIONAHA.113.005340. [DOI] [PubMed] [Google Scholar]
- 7.Hall E, Giaccia A. Radiobiology for the Radiologist. Vol. 2012. Philadelphia, PA: Lippincott, Williams & Wilkins; pp. 160–173. [Google Scholar]
- 8.Muller H. Artificial transmutation of the gene. Science. 1927;66:84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- 9.Sankaranarayanan K. Estimation of the hereditary risks of exposure to ionizing radiation: history, current status, and emerging perspectives. Health Phys. 2001;80:363–9. doi: 10.1097/00004032-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Sankaranarayanan K, Chakraborty R. Ionizing radiation and genetic risks. XIII. Summary and synthesis of papers VI to XII and estimates of genetic risks in the year 2000. Mutat Res. 2000;453:183–97. doi: 10.1016/s0027-5107(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 11.Sankaranarayanan K, Chakraborty R. Ionizing radiation and genetic risks. XI. The doubling dose estimates from the mid-1950s to the present and the conceptual change to the use of human data on spontaneous mutation rates and mouse data on induced mutation rates for doubling dose calculations. Mutat Res. 2000;453:107–27. doi: 10.1016/s0027-5107(00)00108-1. [DOI] [PubMed] [Google Scholar]
- 12.Russell WL. Mutation frequencies in female mice and the estimation of genetic hazards of radiation in women. Proc Natl Acad Sci U S A. 1977;74:3523–7. doi: 10.1073/pnas.74.8.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell WL, Kelly EM. Mutation frequencies in male mice and the estimation of genetic hazards of radiation in men. Proc Natl Acad Sci U S A. 1982;79:542–4. doi: 10.1073/pnas.79.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schull WJ. The children of atomic bomb survivors: a synopsis. J Radiol Prot. 2003;23:369–84. doi: 10.1088/0952-4746/23/4/r302. [DOI] [PubMed] [Google Scholar]
- 15.Schull WJ, Neel JV. Atomic bomb exposure and the pregnancies of biologically related parents. A prospective study of the genetic effects of ionizing radiation in man. Am J Public Health Nations Health. 1959;49:1621–9. doi: 10.2105/ajph.49.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schull WJ, Otake M, Neel JV. Genetic effects of the atomic bombs: a reappraisal. Science. 1981;213:1220–7. doi: 10.1126/science.7268429. [DOI] [PubMed] [Google Scholar]
- 17.UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Hereditary Effects of Radiation: The UNSCEAR 2001 Report to the General Assembly with Scientific Annex. New York: United Nations; 2001. [Google Scholar]
- 18.Behroozi H, Davoodi M, Aghasi S. Radiation Dose to the Thyroid and Gonads in Patients Undergoing Cardiac CT Angiography. Iranian journal of radiology: a quarterly journal published by the Iranian Radiological Society. 2015;12:e20619. doi: 10.5812/iranjradiol.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 20.Stabin MG. Radiopharmaceuticals for nuclear cardiology: radiation dosimetry, uncertainties, and risk. J Nucl Med. 2008;49:1555–63. doi: 10.2967/jnumed.108.052241. [DOI] [PubMed] [Google Scholar]
- 21.Kim KP, Miller DL, Balter S, et al. Occupational radiation doses to operators performing cardiac catheterization procedures. Health Phys. 2008;94:211–27. doi: 10.1097/01.HP.0000290614.76386.35. [DOI] [PubMed] [Google Scholar]
- 22.Kim KP, Miller DL, Berrington de Gonzalez A, et al. Occupational radiation doses to operators performing fluoroscopically-guided procedures. Health Phys. 2012;103:80–99. doi: 10.1097/HP.0b013e31824dae76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall E, Giaccia A. Radiobiology for the Radiologist. Philadelphia, PA: Lippincott, Williams & Wilkins; 2012. pp. 175–188. [Google Scholar]
- 24.BEIR. Health Effects of Exposure to Low Levels of Ionizing Radiation (BEIR V) Biological Effects of Ionizing Radiation (BEIR) Committee, National Research Council. Washington, DC: National Academies Press; 1990. [PubMed] [Google Scholar]
- 25.Brent RL. The response of the 9 and one-half-day-old-rat embryo to variations in exposure rate of 150 R x-irradiation. Radiat Res. 1971;45:127–36. [PubMed] [Google Scholar]
- 26.Russell L, Russell W. An analysis of the changing radiation response of the developing mouse embryo. J Cell Physiol. 1954;43(suppl 1):130–149. doi: 10.1002/jcp.1030430407. [DOI] [PubMed] [Google Scholar]
- 27.Russell JR, Stabin MG, Sparks RB. Placental transfer of radiopharmaceuticals and dosimetry in pregnancy. Health Phys. 1997;73:747–55. doi: 10.1097/00004032-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Russell JR, Stabin MG, Sparks RB, Watson E. Radiation absorbed dose to the embryo/fetus from radiopharmaceuticals. Health Phys. 1997;73:756–69. doi: 10.1097/00004032-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Lee PJ, Mallik R. Cardiovascular effects of radiation therapy: practical approach to radiation therapy-induced heart disease. Cardiology in review. 2005;13:80–6. doi: 10.1097/01.crd.0000131188.41589.c5. [DOI] [PubMed] [Google Scholar]
- 30.Stewart JR, Fajardo LF, Gillette SM, Constine LS. Radiation injury to the heart. Int J Radiat Oncol Biol Phys. 1995;31:1205–11. doi: 10.1016/0360-3016(94)00656-6. [DOI] [PubMed] [Google Scholar]
- 31.Ozasa K, Shimizu Y, Sakata R, et al. Risk of cancer and non-cancer diseases in the atomic bomb survivors. Radiat Prot Dosimetry. 2011;146:272–5. doi: 10.1093/rpd/ncr168. [DOI] [PubMed] [Google Scholar]
- 32.Ozasa K, Shimizu Y, Suyama A, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177:229–43. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- 33.Ozasa K, Takahashi I, Grant EJ. Radiation-related risks of non-cancer outcomes in the atomic bomb survivors. Ann ICRP. 2016 doi: 10.1177/0146645316629318. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu Y, Kodama K, Nishi N, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ. 2010;340:b5349. doi: 10.1136/bmj.b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little MP, Tawn EJ, Tzoulaki I, et al. Review and meta-analysis of epidemiological associations between low/moderate doses of ionizing radiation and circulatory disease risks, and their possible mechanisms. Radiat Environ Biophys. 2010;49:139–53. doi: 10.1007/s00411-009-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akiba S. Circulatory disease risk after low-level ionizing radiation exposure. Radiation Emergency Medicine. 2013;2:13–22. [Google Scholar]
- 37.Ainsbury EA, Bouffler SD, Dorr W, et al. Radiation cataractogenesis: a review of recent studies. Radiat Res. 2009;172:1–9. doi: 10.1667/RR1688.1. [DOI] [PubMed] [Google Scholar]
- 38.Blakely EA, Kleiman NJ, Neriishi K, et al. Radiation cataractogenesis: epidemiology and biology. Radiat Res. 2010;173:709–17. doi: 10.1667/RRXX19.1. [DOI] [PubMed] [Google Scholar]
- 39.Shore RE, Neriishi K, Nakashima E. Epidemiological studies of cataract risk at low to moderate radiation doses: (not) seeing is believing. Radiat Res. 2010;174:889–94. doi: 10.1667/RR1884.1. [DOI] [PubMed] [Google Scholar]
- 40.Antic V, Ciraj-Bjelac O, Rehani M, Aleksandric S, Arandjic D, Ostojic M. Eye lens dosimetry in interventional cardiology: results of staff dose measurements and link to patient dose levels. Radiat Prot Dosimetry. 2013;154:276–84. doi: 10.1093/rpd/ncs236. [DOI] [PubMed] [Google Scholar]
- 41.Ciraj-Bjelac O, Rehani M, Minamoto A, Sim KH, Liew HB, Vano E. Radiation-induced eye lens changes and risk for cataract in interventional cardiology. Cardiology. 2012;123:168–71. doi: 10.1159/000342458. [DOI] [PubMed] [Google Scholar]
- 42.Ciraj-Bjelac O, Rehani MM. Eye dosimetry in interventional radiology and cardiology: current challenges and practical considerations. Radiat Prot Dosimetry. 2014;162:329–37. doi: 10.1093/rpd/nct291. [DOI] [PubMed] [Google Scholar]
- 43.Ciraj-Bjelac O, Rehani MM, Sim KH, Liew HB, Vano E, Kleiman NJ. Risk for radiation-induced cataract for staff in interventional cardiology: is there reason for concern? Catheter Cardiovasc Interv. 2010;76:826–34. doi: 10.1002/ccd.22670. [DOI] [PubMed] [Google Scholar]
- 44.Rehani MM, Vano E, Ciraj-Bjelac O, Kleiman NJ. Radiation and cataract. Radiat Prot Dosimetry. 2011;147:300–4. doi: 10.1093/rpd/ncr299. [DOI] [PubMed] [Google Scholar]
- 45.Bitarafan Rajabi A, Noohi F, Hashemi H, et al. Ionizing radiation-induced cataract in interventional cardiology staff. Research in cardiovascular medicine. 2015;4:e25148. doi: 10.5812/cardiovascmed.25148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ICRP. Statement on Tissue Reactions. International Commission on Radiological Protection (ICRP); 2011. (ICRP ref 4825-3093-1464). [Google Scholar]
- 47.EPRI. Epidemiology and Mechanistic Effects of Radiation on the Lens of the Eye: Review and Scientific Appraisal of the Literature. Palo Alto, CA: Electric Power Research Institute (EPRI); 2016. (Report No 3002003162). http://www.epri.com/abstracts/Pages/ProductAbstract.aspx?productId=000000003002003162. [Google Scholar]
- 48.Dauer LT, Ainsbury EA, Dynlacht J, et al. Status of NCRP Scientific Committee 1–23 Commentary on Guidance on Radiation Dose Limits for the Lens of the Eye. Health Phys. 2016;110:182–4. doi: 10.1097/HP.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burns S, Thornton R, Dauer LT, Quinn B, Miodownik D, Hak DJ. Leaded eyeglasses substantially reduce radiation exposure of the surgeon’s eyes during acquisition of typical fluoroscopic views of the hip and pelvis. The Journal of bone and joint surgery American volume. 2013;95:1307–11. doi: 10.2106/JBJS.L.00893. [DOI] [PubMed] [Google Scholar]
- 50.Dauer LT, Thornton RH, Solomon SB, St Germain J. Unprotected operator eye lens doses in oncologic interventional radiology are clinically significant: estimation from patient kerma-area-product data. J Vasc Interv Radiol. 2010;21:1859–61. doi: 10.1016/j.jvir.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Thornton RH, Dauer LT, Altamirano JP, Alvarado KJ, St Germain J, Solomon SB. Comparing strategies for operator eye protection in the interventional radiology suite. J Vasc Interv Radiol. 2010;21:1703–7. doi: 10.1016/j.jvir.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 52.Vano E, Gonzalez L, Fernandez JM, Haskal ZJ. Eye lens exposure to radiation in interventional suites: caution is warranted. Radiology. 2008;248:945–53. doi: 10.1148/radiol.2482071800. [DOI] [PubMed] [Google Scholar]
- 53.Schueler BA, Balter S, Miller DL. Radiation protection tools in interventional radiology. J Am Coll Radiol. 2012;9:844–5. doi: 10.1016/j.jacr.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 54.NCRP. Radiation dose management for fluoroscopically-guided interventional medical procedures. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 2010. (NCRP Report 168). [Google Scholar]
- 55.Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326–41. doi: 10.1148/radiol.2542082312. [DOI] [PubMed] [Google Scholar]
- 56.Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335–42. doi: 10.2214/AJR.13.12029. [DOI] [PubMed] [Google Scholar]
- 57.Balter S, Miller DL, Schueler BA. Radiation dose measurements and monitoring for fluoroscopically guided interventional procedures. J Am Coll Radiol. 2012;9:595–7. doi: 10.1016/j.jacr.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 58.Miller DL, Balter S, Cole PE, et al. Radiation doses in interventional radiology procedures: the RAD-IR study: part II: skin dose. J Vasc Interv Radiol. 2003;14:977–90. doi: 10.1097/01.rvi.0000084601.43811.cb. [DOI] [PubMed] [Google Scholar]
- 59.FDA. Avoidance of X-Ray-Induced Serious Skin Injuries to Patients During Fluoroscopically-Guided Procedures. Food and Drug Administration; Silver Spring, MD: 1994. [Google Scholar]
- 60.FDA. Performance Standards for Ionizing Radiation Emitting Products. In: Administration FaD, editor. Fluoroscopic Equipment. Silver Spring, MD: 2015. (21CFR8.1020.32). [Google Scholar]
- 61.NCRP. Preconception prenatal radiation exposure: health effects and protective guidance. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 2013. (NCRP Report 174). [Google Scholar]
- 62.Stewart A, Hewitt D. Oxford Survey of Childhood Cancers: Progress Report I. Monthly bulletin of the Ministry of Health and the Public Health Laboratory Service. 1963;22:182–92. [PubMed] [Google Scholar]
- 63.Ray JG, Schull MJ, Urquia ML, You JJ, Guttmann A, Vermeulen MJ. Major radiodiagnostic imaging in pregnancy and the risk of childhood malignancy: a population-based cohort study in Ontario. PLoS medicine. 2010;7:e1000337. doi: 10.1371/journal.pmed.1000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boice JD., Jr Radiation epidemiology and recent paediatric computed tomography studies. Ann ICRP. 2015;44:236–48. doi: 10.1177/0146645315575877. [DOI] [PubMed] [Google Scholar]
- 65.Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–9. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- 66.Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000;154:178–86. doi: 10.1667/0033-7587(2000)154[0178:rrcral]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 67.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berrington de Gonzalez A, Salotti JA, McHugh K, et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br J Cancer. 2016;114:388–94. doi: 10.1038/bjc.2015.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walsh L, Shore R, Auvinen A, Jung T, Wakeford R. Risks from CT scans–what do recent studies tell us? J Radiol Prot. 2014;34:E1–5. doi: 10.1088/0952-4746/34/1/E1. [DOI] [PubMed] [Google Scholar]
- 70.Cardis E, Vrijheid M, Blettner M, et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ. 2005;331:77. doi: 10.1136/bmj.38499.599861.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cardis E, Vrijheid M, Blettner M, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167:396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- 72.Leuraud K, Richardson DB, Cardis E, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. The Lancet Haematology. 2015;2:e276–e281. doi: 10.1016/S2352-3026(15)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS) BMJ. 2015;351:h5359. doi: 10.1136/bmj.h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.ICRP. 2007 Recommendations of the International Commission on Radiological Protection. International Commission on Radiological Protection (ICRP) Publication 103. Annals of the ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 75.NCRP. Limitation of exposure to ionizing radiation. Bethesda, MD: National Council on Radiation Protection and Measurement (NCRP); 1993. (NCRP Report No. 116). [Google Scholar]
- 76.NCRP. Evaluation of the linear-nonthreshold dose-response model for ionizing radiation. Bethesda, MD: National Council on Radiation Protection and Measurement (NCRP); 2001. (NCRP Report No 136). [Google Scholar]
- 77.UNSCEAR. Annex I: Epidemiological Evaluation of Radiation-induced Cancer. New York: United Nations; United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2000 Report: Sources and Effects of Ionizing Radiation. [Google Scholar]
- 78.Commission NR, editor. NRC. Standards for Protection against Radiation. Rockville, MD: 1991. (21CFR20). [Google Scholar]
- 79.NCRP. Risk estimates for radiation protection. Bethesda, MD: National Council on Radiation Protection and Measurement (NCRP); 1993. (NCRP Report No 115). [Google Scholar]
- 80.Vaiserman AM. Radiation hormesis: historical perspective and implications for low-dose cancer risk assessment. Dose-response: a publication of International Hormesis Society. 2010;8:172–91. doi: 10.2203/dose-response.09-037.Vaiserman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaiserman AM. Hormesis, adaptive epigenetic reorganization, and implications for human health and longevity. Dose-response: a publication of International Hormesis Society. 2010;8:16–21. doi: 10.2203/dose-response.09-014.Vaiserman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calabrese EJ, Blain RB. The hormesis database: the occurrence of hormetic dose responses in the toxicological literature. Regulatory toxicology and pharmacology: RTP. 2011;61:73–81. doi: 10.1016/j.yrtph.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 83.Feinendegen LE. Quantification of Adaptive Protection Following Low-dose Irradiation. Health Phys. 2016;110:276–80. doi: 10.1097/HP.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 84.Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251:13–22. doi: 10.1148/radiol.2511080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siegel JA, Stabin MG. Radar commentary: Use of linear no-threshold hypothesis in radiation protection regulation in the United States. Health Phys. 2012;102:90–9. doi: 10.1097/HP.0b013e318228e5b4. [DOI] [PubMed] [Google Scholar]
- 86.Cuttler JM. Commentary on Using LNT for Radiation Protection and Risk Assessment. Dose-response: a publication of International Hormesis Society. 2009;8:378–83. doi: 10.2203/dose-response.10-003.Cuttler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tubiana M, Aurengo A, Averbeck D, Bonnin D, LeGuen B, Masse R, Monier R, Valleron AJ, de Vathaire F. Academy of Medicine (Paris) and Academy of Science (Paris) Paris: 2005. Dose-Effect Relationships and the Estimation Estimation of the carcinogenic effects of low doses of ionizing radiation. (Joint Report No 2). [Google Scholar]
- 88.Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington de Gonzalez A. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology. 2009;251:6–12. doi: 10.1148/radiol.2511081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Calabrese EJ. Getting the dose-response wrong: why hormesis became marginalized and the threshold model accepted. Archives of toxicology. 2009;83:227–47. doi: 10.1007/s00204-009-0411-5. [DOI] [PubMed] [Google Scholar]
- 90.Calabrese EJ. How the US National Academy of Sciences misled the world community on cancer risk assessment: new findings challenge historical foundations of the linear dose response. Archives of toxicology. 2013;87:2063–81. doi: 10.1007/s00204-013-1105-6. [DOI] [PubMed] [Google Scholar]
- 91.Calabrese EJ. Cancer risk assessment foundation unraveling: new historical evidence reveals that the US National Academy of Sciences (US NAS), Biological Effects of Atomic Radiation (BEAR) Committee Genetics Panel falsified the research record to promote acceptance of the LNT. Archives of toxicology. 2015;89:649–50. doi: 10.1007/s00204-015-1455-3. [DOI] [PubMed] [Google Scholar]
- 92.Calabrese EJ. An abuse of risk assessment: how regulatory agencies improperly adopted LNT for cancer risk assessment. Archives of toxicology. 2015;89:647–8. doi: 10.1007/s00204-015-1454-4. [DOI] [PubMed] [Google Scholar]
- 93.Boice JD., Jr . The Boice Report #40: LNT 101. Bethesda, MD: 2015. [Google Scholar]
- 94.Calabrese EJ, Shamoun DY, Hanekamp JC. The Integration of LNT and Hormesis for Cancer Risk Assessment Optimizes Public Health Protection. Health Phys. 2016;110:256–9. doi: 10.1097/HP.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 95.Sasaki MS, Tachibana A, Takeda S. Cancer risk at low doses of ionizing radiation: artificial neural networks inference from atomic bomb survivors. Journal of radiation research. 2014;55:391–406. doi: 10.1093/jrr/rrt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zanzonico P, Stabin MG. Quantitative benefit-risk analysis of medical radiation exposures. Semin Nucl Med. 2014;44:210–4. doi: 10.1053/j.semnuclmed.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 97.Zanzonico PB. The neglected side of the coin: quantitative benefit-risk analyses in medical imaging. Health Phys. 2016;110:301–4. doi: 10.1097/HP.0000000000000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gojer B, Williams KA. The role of scintigraphic perfusion imaging for predicting ischemic cardiac events in noncardiac surgery. American journal of cardiac imaging. 1995;9:213–25. [PubMed] [Google Scholar]
- 99.Hendee WR. Policy statement of the International Organization for Medical Physics. Radiology. 2013;267:326–327. doi: 10.1148/radiol.13130567. [DOI] [PubMed] [Google Scholar]
- 100.NCRP. Uncertainties in internal radiation dose assessment. Bethesda, MD: National Council on Radiation Protection and Measurements (NCRP); 2010. (NCRP Report 164). [Google Scholar]
- 101.UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) New York: United Nations; 2012. The UNSCEAR 2012 Report to the General Assembly. [Google Scholar]
- 102.Society HP. Radiation Risk in Perspective. Position Statement PS010-2 of the Health Physics Society. McLean, VA: 1996. adopted January 1996, revised July 2010. [Google Scholar]
- 103.AAPM. Position Statement on Radiation Risks from Medical Imaging Procedures. Alexandria, VA: American Association of Physicists in Medicine; 2011. (Policy PP 25-A). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


