Abstract
Proper management of an HIV infection requires that a patient be at least 80–95% adherent to a prescribed drug regimen to avoid poor health outcomes and the development of drug-resistant HIV strains. Clinicians generally monitor adherence habits indirectly through patient self-reporting, pill counting, and electronic drug monitoring. While direct measurement of patient samples like urine for monitoring drug levels is possible, it requires specialized equipment and training that is not readily available in resource-limited settings where the need is greatest. In this work we report the development of an antibody that binds to tenofovir (TFV), a key small molecule drug for both the treatment and prevention of HIV, and a competitive lateral flow assay that uses that antibody to monitor urine samples for the presence of the drug. TFV was conjugated to an immunogenic protein and injected into rabbits to raise polyclonal antibodies sensitive to the drug. The antibodies were verified for TFV-sensitivity by immunoprecipitation and HPLC. A gold nanoparticle-based competitive assay was developed to detect the presence of TFV in urine samples with a sensitivity of 1 μg mL−1. This TFV assay could be deployed as a point-of-care device for adherence monitoring in resource-limited settings as a low-cost, accurate, and speedy alternative to current methods to better inform changes in treatment.
Keywords: HIV, Adherence, Tenofovir, Antibodies, Lateral Flow, Phosphonate Bioconjugation
Graphical abstract
1. Introduction
Tenofovir (TFV) has become a cornerstone of HIV treatment since its approval for use in 2001 as tenofovir disoproxil fumarate (TDF). In 2015, the World Health Organization maintained its recommendation that TDF, which is metabolized into TFV in vivo, be part of the preferred first-line regimen for antiretroviral therapy to treat HIV patients [1]. In addition, the WHO recommends pre-exposure prophylaxis (PrEP) therapies containing TFV-derived medications be deployed to prevent the transmission of the virus among high-risk populations in both high- and low-resource settings [2]. As a result of these recommendations and the development of new formulations, such as tenofovir alafenamide (TAF), TFV will likely remain one of the most important tools for the treatment and prevention of HIV.
The number of people accessing antiretroviral medications to manage their HIV infections has risen to over 18 million worldwide as of June 2016 [3]. Around 10 million of these people are on treatment regimens containing TFV [4]. Mismanagement of HIV drug regimens routinely results in a heightened risk of transmission, decreased patient health and quality of life, and an increase in the incidence of HIV drug resistance [5]. The WHO cites poor adherence as the main reason for suboptimal clinical benefits managing chronic illnesses such as HIV/AIDS [6]. As a result, it is critical that clinicians monitor the adherence of HIV patients to their prescribed treatment regimens.
To manage HIV infections and keep viral loads low patients must be at least 80–95% adherent to their antiretroviral treatments [7, 8, 9, 10], and many populations of patients do not demonstrate adequate adherence rates [11, 12, 13]. There are many factors that diminish adherence rates: complexity of regimen, side effects, and patient psychological factors among others [14, 15, 16, 17, 18]. Fortunately, there are many interventions that have been shown to improve adherence behaviors and health outcomes [19].
Current methods for tracking adherence behaviors are mostly indirect such as pill counting, electronic drug monitoring, and patient self-reporting [5]. Pill counting and electronic monitoring are limited in their deployment and self-reporting, the most widely used method, is prone to overestimation [20, 21]. Current direct methods to measure drug levels in patient samples generally require expensive equipment [22] that is not easily accessible in resource-limited settings where the need is greatest.
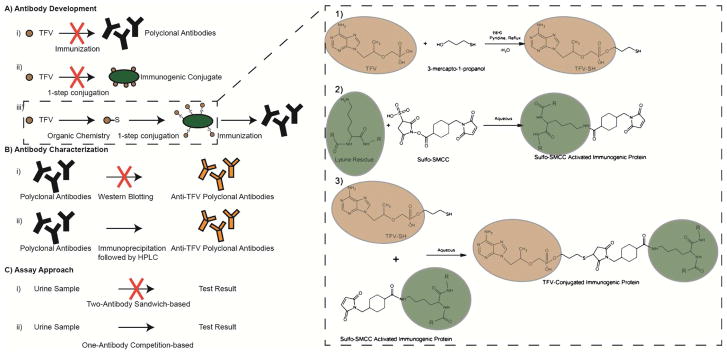
We report here the first antibody-based direct measurement of TFV in urine. Using a gold nanoparticle-based competitive lateral flow strip assay, we have detected TFV in spiked urine samples at clinically relevant concentrations. Raising antibodies against small molecule targets like TFV is not straightforward. Conjugation of TFV to a protein substrate is required, and in this case was not trivial (Figure 1). As such, we have included the details of our approach.
Figure 1.
Technical challenges. A) Antibody development: TFV alone cannot generate polyclonal antibodies (i) and there is not a straightforward method for direct conjugation onto an immunogenic protein (ii). TFV was conjugated to an immunogenic protein (iii). B) Antibody characterization: Antibodies targeting small molecules cannot be characterized by western blotting, the standard method (i). Immunoprecipitation followed by LC-MS analysis was used (ii). C) Assay Approach: A small molecule target is not suitable for standard sandwich-based assays (i). A competition-based assay was designed (ii).
This assay has the potential to facilitate objective monitoring of HIV adherence habits in all settings without the need for expensive equipment or long turnaround times allowing clinicians to intervene in cases of noncompliance and improve overall patient outcomes.
2. Methods
2.1 Materials
TFV was purchased from Ark Pharm, Inc. (Libertyville, IL). Bovine serum albumin (BSA), keyhole limpet hemocyanin (KLH), sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (sulfo-SMCC), 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), Pierce Protein G agarose beads, CarboxyLink Coupling Gel, Whatman chromatography paper, and pico chemiluminescent substrate were purchased from ThermoFisher (Waltham, MA). Amersham Protran nitrocellulose was purchased from GE Healthcare Life Sciences (Pittsburgh, PA). Anti-rabbit antibody conjugated to horseradish peroxidase (HRP) was purchased from GE Healthcare (Chicago, IL). Goat anti-rabbit antibody was purchased from Abcam (Cambridge, MA). N,N′-Dicyclohexylcarbodiimide (DCC), pyridine, 3-mercapto-1-propanol, silica gel Davisil grade 643, Hi-Flow Plus HF180 nitrocellulose sheets, adenosine monophosphate, Tween 20, sucrose, adenosine monophosphate (AMP), and all solvent and buffers were purchased from Sigma-Aldrich (St. Louis, MO). A 40nm InnovaCoat Gold Conjugation Kit was purchased from Innova Biosciences (Babraham, England).
2.2 Synthesis of tenofovir-thiol hapten
The synthesis of tenofovir-thiol (TFV-SH) is outlined in Figure 1a (inset). The synthesis was performed using a modified version of the protocol of Varal et al. [23] for the esterification of the tenofovir phosphonate group. In short, 270mg of TFV and 389mg of DCC were measured into a round-bottom flask to which 10mL of dry pyridine and 100μL of 3-mercapto-1-propanol was added. The mixture was stirred under argon and refluxed for 18–24 hours. The solution was dried and dissolved in 10mL of a 1:1 dichloromethane:methanol mixture. The solution was filtered and concentrated. Flash chromatography through a short silica gel column was performed on the concentrate with elution by a gradient from 0% methanol to 40% methanol in dichloromethane. The fractions of interest were dried by rotary evaporation followed by high vacuum overnight. The presence of TFV-SH was confirmed by liquid chromatography–mass spectrometry (LC-MS).
2.3 LC-MS characterization
LC-MS measurements were performed on an Agilent 1100 series LC/MSD with a Cortecs C18 column (90Å, 2.7 μm, 4.6 mm × 150 mm) from Waters (Milford, MA). Samples were dissolved in a solution of 975μL 0.6% tri-fluoroacetic acid in water and 25μL methanol. 20μL of sample was injected into a gradient mobile phase outlined in Table S1 at a flowrate of 0.6mL min−1 into the column equilibrated at 45°C and passed through the mass spectrometer set for positive polarity electrospray ionization (ESI+) at a range of 100–1500 m/z.
2.4 KLH-TFV and BSA-TFV conjugation
20mg of BSA and 20mg of KLH were each dissolved in 2mL of phosphate buffer saline (PBS). 4mL of 5mg mL−1 sulfo-SMCC in PBS was added to each protein solution. The samples were incubated for 1 hour at room temperature while rotating before being desalted into PBS. 50mg of TFV-SH was dissolved in 500μL methanol and added to each sample before being incubated 2 hours at room temperature while rotating. The samples were desalted into PBS. About 300μL of the BSA-TFV sample was then desalted into pure water and conjugation was confirmed by matrix-assisted laser desorption/ionization - time of flight (MALDI-TOF) and acrylamide gel electrophoresis. Mass measurements of BSA-TFV were taken in a Bruker Autoflex MALDI-TOF Mass Spectrometer with a sinapinic acid double layer matrix set to linear positive mode.
2.5 Anti-TFV antibody development
Antibody development was conducted by Covance Inc. (Denver, PA) in accordance with the NIH guidelines for the care and use of animals in research and under warranty that appropriate IRB approval was obtained. KLH-TFV was injected into rabbits, and serum containing anti-TFV polyclonal antibodies was collected and used for the current study.
2.6 Immunoprecipitation of TFV by anti-TFV antibody
Two samples were prepared containing a mixture of 100μL of 50μg mL−1 TFV and 100μL of 1mg mL−1 AMP. To one tube 375μL of anti-TFV serum (+IP) was added and to the other 375μL of PBS (−IP). The samples were incubated while rotating at 4°C overnight. 300μL of Protein G bead suspension was added to the samples and further incubated at 4°C for 1 hour. The samples were spun down for 1 minute at 3000rpm and decanted. The supernatant from the (−IP) sample (−IPsup) was set aside. The beads were then washed and decanted four times with yeast lysis buffer (50mM HEPES pH 7.6, 1mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 140mM NaCl), with the third wash being with a high salt yeast lysis buffer (500mM NaCl). The beads were then heat eluted for 5 minutes at 95°C into 100μL of TE buffer. The supernatant was set aside and heat elution was repeated to give a total volume of 200μL of supernatant per sample. The supernatants were then extracted by phenol:chloroform:isoamyl alcohol and the top aqueous phase kept in order to remove proteins. A second chloroform extraction was performed to remove as much phenol as possible from the upper aqueous phase. The samples were then tested by LC-MS to confirm the selective immunoprecipitation of TFV by the anti-TFV polyclonal antibody.
2.7 Antibody Purification
BSA-TFV protein was immobilized onto agarose beads using the CarboxyLink Coupling Gel protocol from ThermoFisher. In short, 2mL of gel slurry was packed into a disposable column and allowed to settle for 30 minutes. The column was washed with 10mL of 2-(N-morpholino)ethanesulfonic acid (MES) buffer. 1mL of approximate 1mg mL−1 BSA-TFV solution was desalted into MES buffer using a desalting column and added to the column along with 30mg EDC dissolved in 0.5mL MES buffer. The column was capped and the slurry was mixed end-over-end for 3 hours. The column was allowed to drain and washed with 1mL of 1M NaCl. The presence of protein in the flow-through was tested by Bradford assay against a BSA-TFV control sample to confirm protein remained on the beads. The column was washed with 5–10mL of 1M NaCl then equilibrated with 6mL PBS. 500μL of serum was added to the column which was then capped and allowed to incubate for 1 hour at room temperature. The column was washed with 12mL of PBS and eluted with 8mL of 100mM glycine buffer pH 3.0. Fractions were collected and immediately neutralized by addition of 100μL 1M Tris pH 7.5 buffer. Flow-through, wash, and elution fractions were checked for protein by Bradford assay. Elution fractions containing antibody were pooled and desalted into PBS. The presence of purified antibody was verified by acrylamide gel electrophoresis and functionality of the antibody was verified by immunoprecipitation.
2.8 Gold Nanoparticle Conjugation
Conjugation of purified antibody onto gold nanoparticles was performed using an InnovaCoat Gold Conjugation Kit from Innova Biosciences following the manufacturer’s instructions. The nanoparticles were suspended in 50μL of 10x diluted Innova quencher solution and stored at 4°C.
2.9 Competitive Lateral Flow Assay
Hi-Flow Plus HF180 nitrocellulose sheets were laser cut into lateral flow strips, and chromatography paper was adhered to the end of the strips as a waste reservoir. Samples of TFV were prepared at varying concentrations between 10ng mL−1 and 100μg mL−1 in urine alongside a no TFV control sample. For each sample, 1μL of antibody conjugated gold nanoparticles was added to 24μL of PBS and 80μL of the TFV solution was added. The mixture was allowed to incubate at room temperature on a rotator for at least 30 minutes. 0.3μL of anti-rabbit antibody was spotted on the strips as a control spot. The test spot, 0.3μL of 2.5μg mL−1 BSA-TFV, was spotted on the strips upstream of the control spot. The spots were allowed to dry and another 0.3μL of BSA-TFV was spotted on top. This spotted BSA-TFV acts as the test spot for the assay. After incubation, concentrated TBS-T was added to each sample such that the final Tween 20 concentration was 0.05%. One end of each of the lateral flow strips was dipped vertically into the sample tubes such that the solution ran up the nitrocellulose strip and into the waste reservoir via capillary action. The strips were incubated in solution for 25–30 minutes before being photographed with a Nikon DS-Ri2 camera and analyzed via ImageJ.
3. Results
3.1 Generation of IP-grade anti-TFV polyclonal antibodies from phosphonate-decorated protein complexes
To raise an antibody against TFV, the small molecule was attached to an immunogenic protein for rabbit immunization. TFV was modified to contain a reactive side group for conjugation. We added a thiol group to the phosphonate group of TFV utilizing a synthetic pathway similar to how TFV is converted to TDF [23, 24]. Here we reacted TFV with mercapto-alcohols in order to produce thiol-modified TFV molecules (TFV-SH) as shown in Figure S1.
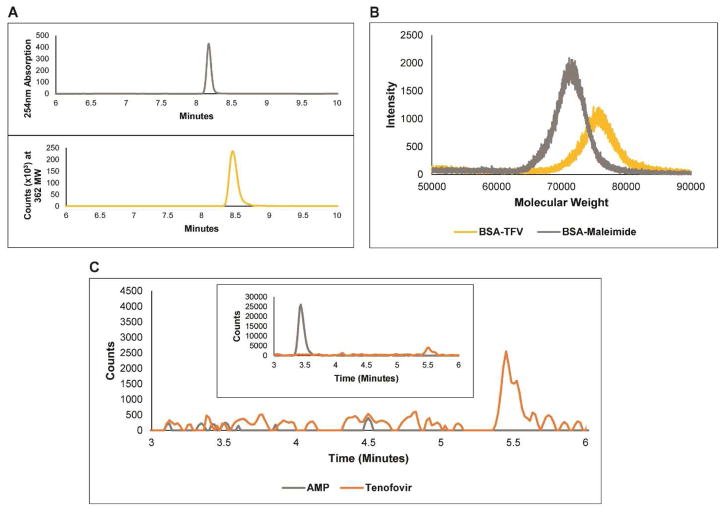
After reacting TFV (287MW) with 3-mercapto-1-propanol (92MW) the LC-MS data in Figure 2a confirmed the presence of TFV-SH. The synchronized increase in the UV absorbance at 254nm and the spike at 362MW at 8.4 minutes demonstrates the existence of TFV-SH indicating attachment of the thiol to the phosphonate side group the liberation of water.
Figure 2.
A) LC-MS characterization of the purified TFV-SH synthesis product. (top) UV Absorbance at 254nm. (bottom) Mass Spec signal of 362 MW. B) MALDI measurements of BSA-maleimide intermediate and BSA-TFV conjugate. BSA has a MW of 66,463. C) LC-MS characterization of TFV immunoprecipitation and (inset) pre-immunoprecipitation sample.
Once TFV-SH was synthesized, we attached the molecule to KLH and to BSA by adding a maleimide side group to each of the proteins and then conjugating the thiol group of the TFV-SH to the maleimide as outlined above. The increase in molecular weight of the BSA molecule is demonstrated by MALDI (Figure 2b) and confirms successful conjugation of TFV-SH to the proteins. The purified KLH-TFV conjugate was then injected into rabbits to generate polyclonal antibodies against TFV.
Serum from KLH-TFV immunized rabbits was analyzed to confirm the presence of TFV-sensitive antibodies. Western blotting and immunoprecipitation experiments were done to confirm the specific and sensitive antibody binding of the polyclonal antibody to BSA-TFV conjugates (Figure S3). While these tests were highly suggestive of the presence of TFV-binding polyclonal antibodies, they did not demonstrate direct binding of the antibody to native, unmodified TFV. This was expected, since TFV is a small molecule. As such, we performed a modified immunoprecipitation protocol as described above. In short, rather than transferring the immunoprecipitated sample to a gel for western blotting we removed proteins from the sample via phenol:chloroform:isoamyl alcohol purification and analyzed the resulting solution with LC-MS. The confirmed presence of TFV in this sample (Figure 2c) demonstrates the successful generation of TFV-sensitive polyclonal antibodies. The corresponding absence of AMP, a nucleotide analogue of TFV present at a 20x higher concentration than TFV in the initial immunoprecipitation sample (Figure 2c inset), establishes the high specificity the antibody has to TFV.
3.2 Lateral flow strip assays can detect 1μg mL−1 TFV in urine
After the specificity of the antibody was established, a dot blot assay was used to characterize its performance in TFV spiked urine samples (see supplemental materials). Development of a lateral flow strip assay required immobilization of the anti-TFV antibody to gold nanoparticles. The serum was processed by affinity purification to isolate anti-TFV antibodies and was validated by immunoprecipitation (Figure S4) to confirm that the purified antibodies were still functional. It was then conjugated to 40nm gold nanoparticles for use in the lateral flow assay.
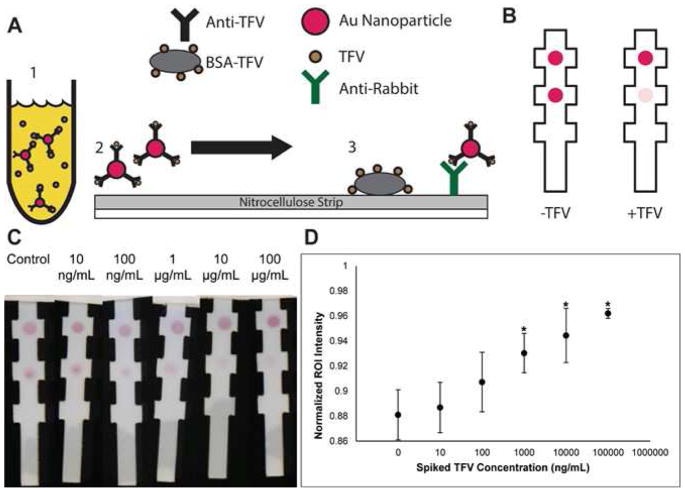
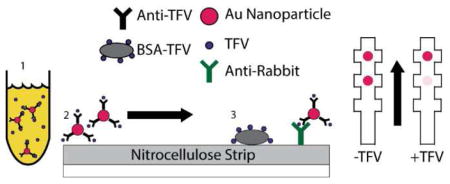
The lateral flow assay is outlined schematically in Figure 3a and Figure 3b. Anti-TFV antibody conjugated to gold beads was incubated with TFV-spiked urine samples and allowed to flow through a nitrocellulose strip containing spots of BSA-TFV and anti-rabbit antibody control spots. The results of the assay in Figure 3c, Figure 3d, and Figure S5 show sensitive, statistically significant, and specific detection of TFV in spiked urine samples with a preliminary lower limit of detection of 1μg mL−1. The assay takes about 55 minutes to perform given the two incubation steps required. Our initial attempts at shortening these incubation times resulted in unacceptably poor signal for a colorimetric read.
Figure 3.
A) Schematic of competitive gold nanoparticle lateral flow assay. B) Expected output of competitive gold nanoparticle lateral flow assay. As the amount of TFV increases in the sample, the test spot intensity decreases. C) Sensitivity test of competitive gold nanoparticle lateral flow assay in TFV-spiked urine samples. D) Competitive gold nanoparticle lateral flow assay statistical analysis of sensitivity test. Strips were imaged and processed using ImageJ to get background-normalized intensity measurements of test spots for varying concentrations of TFV spiked into urine. Images were converted to grayscale and intensity measurements were made with normalization to the control spots. Points with asterisks are significantly different than the no-TFV control strips as determined by one-way ANOVA (F(5,24) = 15.0, p=1.02 × 10^-6 and a Tukey test (Data from 5 Biological Replicates).
4. Discussion
Raising and deploying an antibody against a small molecule presents several challenges (Figure 1). Most often the small molecule is not immunogenic on its own. As a result, the desired antigen must be conjugated to a carrier protein to coax an immune response from the host (Figure 1a) [25, 26, 27]. Another challenge is characterization of the host serum after immunization (Figure 1b). For protein targets, gel electrophoresis is a common technique to isolate and visualize antibody-protein interactions. However, since small molecules cannot be characterized by gel electrophoresis, other techniques such as quartz crystal microbalance [28], surface plasmon resonance [29], or mass spectrometry [30] must be used to confirm antibody-target binding.
Additionally, small molecule targets present challenges for use in standard lateral flow assays (Figure 1c). The most common lateral flow configuration uses antibodies in a sandwich assay to both capture and visualize the same molecule using two antibodies, one immobilized to the lateral flow substrate and the other linked to a visualization moiety. This method relies on large targets such as peptides and proteins that have multiple antibody-binding sites. Small molecules, however, often only have a single antibody-binding site and this precludes the possibility of using a sandwich assay. For small molecules, competitive ELISAs have become a common technique [31, 32, 33]. In this work, we were able to overcome many of these challenges to develop an assay for the detection of TFV.
Attaching TFV onto a carrier protein was not trivial. Unmodified TFV cannot be attached to a protein in a simple one-step reaction like peptides or other biological molecules. The primary amine on the adenine base is less reactive since it is stabilized by the aromatic rings. The phosphonate group is also less reactive than typical phosphates on nucleotides. As such, we modified the TFV molecule to contain a more reactive side group. We added a thiol group to the TFV molecule via phosphonate chemistry to enable the use of Sulfo-SMCC to link thiol-containing molecules to proteins [34]. While there are many ways to modify phosphonates [35], it does not appear to have been done to attach TFV onto an immunogenic protein for the purpose of raising an antibody. The schematic in Figure 2b shows the two-step reaction. It is important to note that the MALDI data presented in Figure 2b shows the results of TFV conjugation onto BSA, not the results of conjugation onto KLH. KLH is too large of a protein (390kDa) for convenient MALDI measurements, so often the BSA conjugated analog is used for characterization, as we have done here. It is assumed that the KLH-TFV reaction proceeds with similar efficiency [36, 37, 38].
After immunization, the serum was analyzed to confirm that the polyclonal antibodies generated were both sensitive and specific to TFV. Figure S3 and Figure 2c show that the anti-TFV serum is sensitive and specific to BSA-TFV and to TFV, respectively. Immunoprecipitations were done in the presence of excess whole cell lysate or AMP to demonstrate the specificity of the antibody. AMP was chosen because of its structural similarities to TFV and because adenosine in general is present in urine. Since TFV cannot be characterized by standard western blotting, we eluted the Protein G beads and purified the sample using phenol:chloroform:isoamyl alcohol mixture before LC-MS measurements were made
With the anti-TFV antibody in hand, we developed a competitive assay to detect TFV. Like other competitive ELISAs, the principle of the assay is that as the concentration of TFV in the sample increases and more of the anti-TFV antibody is bound to free floating TFV, less of the anti-TFV antibody is unbound and available to bind to the BSA-TFV immobilized onto a nitrocellulose substrate (Figure 3a).
TFV-spiked urine was our model biological sample. Urine provides several advantages in monitoring TFV adherence. Urine tests are non-invasive compared to assays that measure plasma. For patients on TFV regimens, the urinary concentration of TFV is usually over 100x more concentrated than in plasma [39]. Also, urine does not require additional sample preparation before testing.
The assay to detect TFV in urine needs to be sensitive enough to distinguish between patients that are properly adhering to their regimens and not so sensitive that it can identify those who are not. For a patient taking 300mg TDF daily, the mean urine concentration of TFV was 23μg mL−1 [40]. When 300mg of TFV is ingested only once, the mean concentration of TFV in urine after 24–72 hours was reported to be 1–10μg mL−1 [41]. The increased use of TAF as an alternative to TDF in drug regimens will shift these sensitivity windows. TAF is dosed at 25mg per day and thus results in about a 10x decrease in TFV plasma concentrations [42]. Assuming this also leads to a 10x decrease in TFV urine concentrations, it would suggest that daily doses of 25mg TAF would result in about 2.3μg mL−1 of TFV in urine as a gross estimate. The 24–72 hour TFV urine concentration after a single 25mg dose of TAF would similarly be estimated to be around 0.1–1μg mL−1. Use of a competitive ELISA assay allows us to tailor the dynamic range of the assay to reflect current dosing recommendations and new formulations that may shift the excretion profile of the drug.
The gold nanoparticle lateral flow strip assay generates a clear colorimetric readout requiring less time, training, and equipment than more complex detection methods such as HPLC and mass spectrometry. The strip architecture was chosen as a way to facilitate faster prototyping to allow hand-spotting of the BSA-TFV spots rather than using an automated spotter [43]. The sensitivity down to 1ug mL−1 and the high specificity of the anti-TFV antibody suggests the lateral flow assay is appropriate for assaying urine samples of HIV patients prescribed TFV-containing drug regimens. A major limitation of the current assay in this format is the time to result of 55 minutes. However, time in clinic for an individual patient visit can and usually does exceed one hour. If urine is collected and tested upon check-in (a common intake procedure), results can be obtained discussed before discharge. The overall assay time for the gold-based lateral flow device was determined by varying the two key incubation steps of the assay: incubating the spiked urine sample with the gold-conjugated anti-TFV antibodies, and incubating the resulting mixture with the lateral flow strip. A number of strategies can be deployed to reduce these incubation times. Though on the high end of acceptable times to result, the simplicity of the lateral flow assay shows potential for deployment in settings where such measurements are now completely lacking.
5. Conclusion
The assay is flexible enough to be immediately paired with other low-cost monitoring methods in the treatment of diseases that are commonly comorbid with HIV such as hepatitis [44] and tuberculosis [45].
As the use of TFV-containing drugs continues to expand in the treatment and prevention of HIV, so too does the need increase for accurate monitoring techniques in order to improve patient outcomes and reduce the proliferation of TFV-resistant HIV strains. In this work, we have developed an anti-TFV antibody and a competitive gold nanoparticle lateral flow assay to test urine as an objective tool for monitoring patient adherence habits. The assay does not need an external power source, is affordable to develop and deploy in both resource-rich and resource-limited settings, and non-invasively gives an accurate colorimetric output measuring clinically relevant concentrations of TFV in urine in less than 1 hour.
Supplementary Material
Lateral flow strip is created to test HIV patients for drug adherence to Tenofovir
Anti-Tenofovir antibody is raised as a key component of the urine-based test
Gold nanoparticle-based competitive assay detects clinically relevant concentrations
New assay potentially deployable in resource-limited settings where need is greatest
Acknowledgments
This work was supported by NIH (4U54EB015403-05) (5UL1TR001430-03). Analytical work was performed with the assistance of the Boston University Chemical Instrumentation Center (CIC) and NSF (CHE-1337811). In particular, thank you to Dr. Norman Lee for instrumentation assistance. Thank you to Jose Gomez-Marquez for helpful discussions.
Footnotes
Author Contributions
All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Fact Sheet: HIV treatment and care: what’s new in HIV treatment. 2015 [Google Scholar]
- 2.Baggaley R, Dalal S, Johnson C, Macdonald V, Mameletzis I, Rodolph M, Figueroa C, Samuelson J, Verster A, Doherty M, Hirnschall G. J Int AIDS Soc. 2016;19(1):21348. doi: 10.7448/IAS.19.1.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS(UNAIDS) Fact Sheet: Global HIV statistics. 2016. [Google Scholar]
- 4.Clinton Health Access Initiative. ARV Market Report. 2016. [Google Scholar]
- 5.AIDSinfo. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2017. [Google Scholar]
- 6.Dunbar-Jacob J, Erlen JA, Schlenk EA, Ryan CM, Sereika SM, Doswell WM. Annu Rev Nurs Res. 2000;18:48–90. [PubMed] [Google Scholar]
- 7.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 8.Montaner JS, Reiss P, Cooper D, Vella S, Harris M, Conway B, Wainberg MA, Smith D, Robinson P, Hall D, Myers M, Lange JM. JAMA. 1998;279(12):930–937. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- 9.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa aR, Holodniy M, Sheiner L, Bamberger JD, Chesney Ma, Moss a. AIDS. 2000;14(4):357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 10.Viswanathan S, Detels R, Mehta SH, Macatangay BJC, Kirk GD, Jacobson LP. AIDS Behav. 2015;19(4):601–611. doi: 10.1007/s10461-014-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNabb JJ, Nicolau DP, Stoner Ja, Ross J. AIDS. 2003;17(12):1763–1767. doi: 10.1097/00002030-200308150-00005. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, Christian J, Maldonado T, Duran D, Kaplan AH, Wenger NS, Inhibitors HIVP. Ann Intern Med. 2001;134(10):968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 13.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, Buono D, Eckholdt H, Howard AA, Schoenbaum EE. Clin Infect Dis. 2001;33(8):1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr A. Clin Infect Dis. 2000;30(Suppl 2):S135–42. doi: 10.1086/313854. [DOI] [PubMed] [Google Scholar]
- 15.Chesney MA. Improv Manag HIV Dis. 1997;5(12) [Google Scholar]
- 16.Malow R, Devieux JG, Rosenberg R, et al. Psychol AIDS Exch. 2001;30:23–26. [Google Scholar]
- 17.d’Arminio Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, Angarano G, Colangeli V, De Luca A, Ippolito G, Caggese L, Soscia F, Filice G, Gritti F, Narciso P, Tirelli U, Moroni M. Aids. 2000;14(5):499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 18.Kaul DR, Cinti SK, Carver PL, Kazanjian PH. Pharmacotherapy. 1999;19:281–298. doi: 10.1592/phco.19.4.281.30937. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Adherence to long-term therapies: Evidence for action. 2003;2 [Google Scholar]
- 20.Waterhouse DM, Calzone KA, Mele C, Brenner DE. J Clin Oncol. 1993;11(6):1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Nau DP. Ann Pharmacother. 2000;34(10):1117–1122. doi: 10.1345/aph.19339. [DOI] [PubMed] [Google Scholar]
- 22.Simiele M, Carcieri C, De Nicolò A, Ariaudo A, Sciandra M, Calcagno A, Bonora S, Di Perri G, D’Avolio A. J Pharm Biomed Anal. 2015;114:8–11. doi: 10.1016/j.jpba.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Varal D, Joshi M, Panmand D, Jadhav V. Der Pharma Chem. 2016;8(1):338–343. [Google Scholar]
- 24.Hostetler KY, Kini GD, Beadle JR. Phosphonate ester antiviral compounds. 2014 [Google Scholar]
- 25.Murphy K. In: In Janeway’s Immunobiology. 8. Lawrence E, editor. Garland Science; New York: 2012. pp. 718–719. [Google Scholar]
- 26.Karu AE, Goodrow MH, Schmidt DJ, Hammock BD, Bigelowl1 MW. J Agric Food Chem. 1994;42:301–309. [Google Scholar]
- 27.Weltzien HU, Moulon C, Martin S, Padovan E, Hartmann U, Kohler J. Toxicology. 1996;107:141–151. doi: 10.1016/0300-483x(95)03253-c. [DOI] [PubMed] [Google Scholar]
- 28.Ertekin O, Ozturk S, Ozturk ZZ. Sensors (Switzerland) 2016;16(8):1–12. doi: 10.3390/s16081274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittenberg NJ, Wootla B, Jordan LR, Denic A, Warrington AE, Oh S-H, Rodriguez M. Expert Rev Neurother. 2014;14(4):449–463. doi: 10.1586/14737175.2014.896199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar V, Barnidge DR, Chen L-S, Twentyman JM, Cradic KW, Grebe SK, Singh R. J Clin Chem. 2010;56(2):306–313. doi: 10.1373/clinchem.2009.134643. [DOI] [PubMed] [Google Scholar]
- 31.Afshar A, Thomas FC, Wright PF, Shapiro JL, Anderson J. Vet Rec. 1989;124(6):136–141. doi: 10.1136/vr.124.6.136. [DOI] [PubMed] [Google Scholar]
- 32.Perrett LL, McGiven JA, Brew SD, Stack JA. Croat Med J. 2010;51(4):314–319. doi: 10.3325/cmj.2010.51.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng S, Shi F, Jiang X, Wang L, Chen W, Zhu C. Anal Chem. 2012;84(5):2129–2132. doi: 10.1021/ac3001463. [DOI] [PubMed] [Google Scholar]
- 34.Hermanson GT. In Bioconjugate Techniques. Elsevier Inc; Amsterdam: 2008. pp. 284–285. [Google Scholar]
- 35.Horsman GP, Zechel DL. Chem Rev. 2017;117(8):5704–5783. doi: 10.1021/acs.chemrev.6b00536. [DOI] [PubMed] [Google Scholar]
- 36.Nagano M, Carrillo N, Otsubo N, Hakamata W, Ban H, Fuller RP, Bashiruddin NK, Barbas CF. Bioorganic & Medicinal Chemistry. 2017;25(21):5952–5961. doi: 10.1016/j.bmc.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Gascon J, et al. Analytica Chimica Acta. 1997;347:149–162. [Google Scholar]
- 38.Oubina A, Ballesteros B, Galve R, Marco MP. Analytica Chimica Acta. 1999;387:255–266. [Google Scholar]
- 39.Calcagno A, Cusato J, Marinaro L, Trentini L, Alcantarini C, Mussa M, Simiele M, D’Avolio A, Di Perri G, Bonora S. Pharmacogenomics J. 2016;16(6):514–518. doi: 10.1038/tpj.2015.71. [DOI] [PubMed] [Google Scholar]
- 40.Koenig H, Mounzer K, Daughtridge G, Sloan C, Lalley-Chareczko L, Moorthy G, Conyngham S, Zuppa A, Montaner L, Tebas P. HIV Med. 2017 doi: 10.1111/hiv.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray AS, Fordyce MW, Hitchcock MJM. Antiviral Res. 2016;125:63–70. doi: 10.1016/j.antiviral.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Uneke CJ, Ogbu O, Inyama PU, Anyanwu GI, Njoku MO, Idoko JH. Mem Inst Oswaldo Cruz. 2005;100(1):13–16. doi: 10.1590/s0074-02762005000100002. [DOI] [PubMed] [Google Scholar]
- 43.Bosch, et al. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aan1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann CJ, Thio CL. Lancet Infect Dis. 2007;7(6):402–409. doi: 10.1016/S1473-3099(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 45.Tiberi S, Carvalho ACC, Sulis G, Vaghela D, Rendon A, Mello FC, de Q, Rahman A, Matin N, Zumla A, Pontali E. Presse Med. 2017;46(2):e23–e39. doi: 10.1016/j.lpm.2017.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.