Abstract
The design and development of novel methodologies and customized materials to fabricate patient-specific 3D printed organ models with integrated sensing capabilities could yield advances in smart surgical aids for preoperative planning and rehearsal. Here, we demonstrate 3D printed prostate models with physical properties of tissue and integrated soft electronic sensors using custom-formulated polymeric inks. The models show high quantitative fidelity in static and dynamic mechanical properties, optical characteristics, and anatomical geometries to patient tissues and organs. The models offer tissue-mimicking tactile sensation and behavior and thus can be used for the prediction of organ physical behavior under deformation. The prediction results show good agreement with values obtained from simulations. The models also allow the application of surgical and diagnostic tools to their surface and inner channels. Finally, via the conformal integration of 3D printed soft electronic sensors, pressure applied to the models with surgical tools can be quantitatively measured.
Keywords: 3D printing, organ models, 3D printed sensors, surgical aids, soft materials
One recent study suggests that medical errors are the third leading cause of death in the United States, resulting in a mean death rate of 251,454 patients annually.[1] In addition, over 4,000 surgical “never event” claims occur each year in the United States alone.[2] Although the complete elimination of errors in clinical procedures is impossible, effective preoperative planning and rehearsal could play a vital role in decreasing their occurrence. Medical professionals often rely on magnetic resonance imaging (MRI), computed tomography (CT), and/or 3D virtual visualization to develop an understanding of the unique anatomies and disease states of patient organs.[3,4] However, these approaches often fail to provide information regarding the intricate orientations, dimensions, and kinesthetic feedback within the organs.[3,4] Physical organ models offer an effective option for representing the three-dimensional structure of the organs. Yet, molded organ models can lack patient-specificity and exhibit inaccurate or poorly-adjustable physical properties.[5,6]
Recently, patient-specific 3D printed organ models have been introduced as tools for providing accurate anatomical details of the patient organs for preoperative planning, foreseeing intraoperative complications, and reducing the operation time for improving patient safety and surgical outcomes.[7–13] However, the application of 3D printed organ models as advanced surgical aids is currently hampered in two main aspects. First, current 3D printed organ models, while anatomically correct, lack precise mimicry of the physical properties of real tissue.[7] This limits their application for accurate prediction and replication of organ physical behavior, such as deformation and reaction force during surgical handling. The organ models are typically printed using commercial hard plastics and rubber-like materials (Polylactic acid, Polystyrene, NinjaFlex®, etc.).[14] There are significant differences in tactile sensation, mechanical properties and color of these materials compared to their biological counterparts, limiting their effectiveness in preoperative planning, rehearsal with surgical tools, and other surgical tasks such as pressing and cutting.[7,8] Ideally, a 3D printed organ model would match the mechanical and physical properties of the organ and tissue, including viscoelasticity and hardness. Second, current organ models lack the ability to collect quantitative feedback from organ and tissue handling. This important metric is an indicator of surgical performance and can provide important feedback for physical trainers or simulators. This feedback includes providing medical professionals with the ability to quantify and control the force ranges they apply to the organ during preoperative rehearsal and training.
Here we demonstrate a new concept in the development of 3D printed patient-specific prostate models using customized polymeric inks to address the abovementioned issues. The developed model has physical properties closely matching those of prostate tissue and integrated sensing capabilities that can be used for advanced surgical aid applications. We chose the prostate as a proof-of-principle organ model, due to its relatively simple geometry. In addition, prostate surgeries have inherent risks for damaging the urethral sphincter and neurovascular bundle (NVB) during prostate removal, and therefore, proper preoperative planning and rehearsal via a 3D printed prostate model may have implications for surgical outcomes in thousands of patients worldwide.[15]
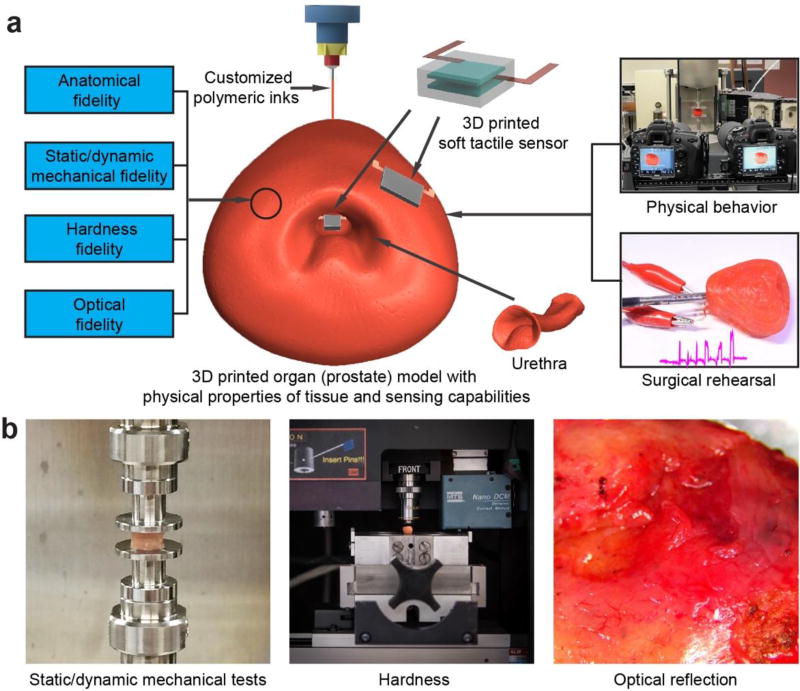
Figure 1a provides an overview of the concept: a patient-specific, 3D printed prostate model that exhibits the physical properties of tissue with high fidelity, and provides sensing capabilities via integrated soft tactile sensors. Two potential surgical aid examples of the proposed model are also depicted in Figure 1a: organ physical behavior prediction and quantitative surgical rehearsal. The following six steps were conducted for the development and application of the advanced 3D printed prostate models: (1) collection and analysis of organ anatomy and properties of prostate tissue, including static and dynamic mechanical properties, hardness, and optical reflection (Figure 1b); (2) design and development of customized polymeric inks based on the tissue data, and ink fidelity analysis with the physical properties of tissue; (3) 3D printing of prostate models and anatomical fidelity analysis; (4) investigating the application of 3D printed prostate models as advanced surgical aids for quantitative prediction of organ physical behavior and comparison with corresponding finite element method (FEM) simulations; (5) 3D printing soft tactile sensors for integration on the model surfaces and interiors; (6) investigating the practical and quantitative aspects of the 3D printed prostate models as advanced surgical aids via the application of diagnostic and surgical tools.
Figure 1.
3D printed patient-specific prostate model with physical properties of tissue and integrated sensing capabilities for advanced surgical rehearsal. a) Schematic of the 3D printed prostate model highlighting the various components, properties, and applications. b) Mechanical and optical tests for obtaining the physical and optical properties of patient prostate tissue samples to guide ink development.
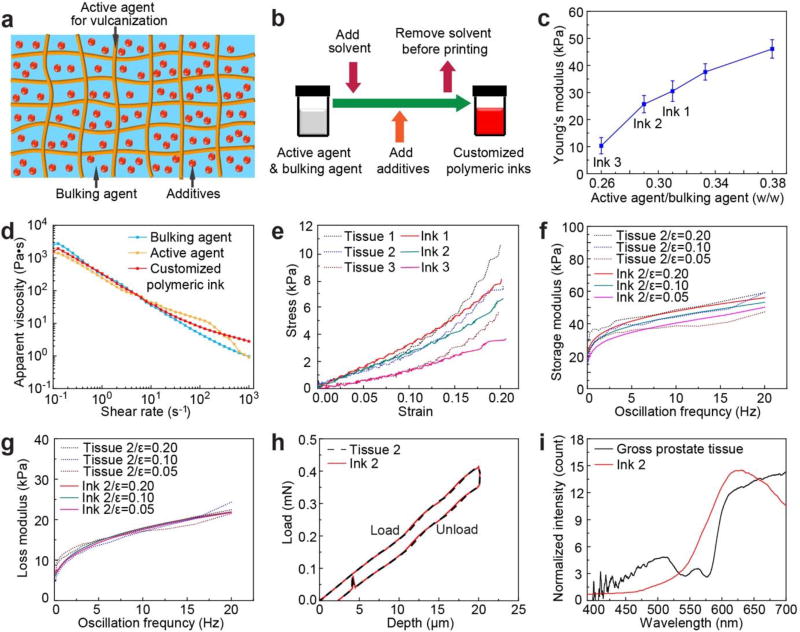
A critical step is to formulate customized polymeric 3D printing inks to adequately mimic patient prostate tissue. There are several requirements for an ideal ink formulation, including adjustable properties, good printability, maintaining stable structures and properties during and after printing, vulcanization at room temperature within a short time period, and convenient preparation. In general, the customized inks consisted of three main components: an active agent for vulcanization, a bulking agent, and additives (Figure 2a). The composite nature of the inks is analogous to human tissue.[16,17] We ultimately selected silicone sealant (room temperature vulcanization) as the active agent to stabilize the structures, silicone grease as a bulking agent to contribute softness and flexibility, and additives for fine-tuning of color and/or printability. The prime components in the ink system are all silicone-based materials, which enables their homogeneous mixture. Silicone-based materials exhibit appropriate shear thinning behavior, resist polymer creep before crosslinking, and have good elasticity after crosslinking.
Figure 2.
Design and development of customized polymeric inks based on patient-specific prostate tissue data, and resulting ink fidelity with physical properties of the tissue. a) Schematic of the composite structure of the customized polymeric inks. b) Preparation procedure for the customized polymeric inks. c) A plot of prime component weight ratios vs. Young’s moduli for the polymeric inks. d) Log-log plots of apparent viscosity versus shear rate for a customized polymeric ink (including its constituent components) used for printing the prostate model. e) Static compression fidelity via stress-strain curves between different patient prostate tissue samples (Tissues 1–3) and printed samples of customized polymeric inks (Ink 1, 2, 3). f) Dynamic compression fidelity of storage modulus between a patient prostate tissue sample (Tissue 2) and a sample of customized polymeric ink (Ink 2) at frequencies of 0.1–20 Hz and strains of 0.05, 0.10, and 0.20. g) Dynamic compression fidelity of loss modulus between a patient prostate tissue (Tissue 2) and a sample of customized polymeric ink (Ink 2) at frequencies of 0.1–20 Hz and strains of 0.05, 0.10, and 0.20. h) Hardness fidelity via load-depth curves between a patient prostate tissue sample (Tissue 2) and a sample of customized polymeric ink (Ink 2). i) Optical fidelity via reflection curves between patient prostate gross tissue and a customized polymeric ink (Ink 2).
By adjusting the component ratios as a general preparative procedure for different ink formulations (Figure 2b,c, Figure S1, and Table S1), the printability can be optimized and the properties of the inks can be tuned to match the different tissue mechanical properties. As shown in Figure 2c and Table S1, by increasing the weight ratios of the active agent to the bulking agent (from 0.26 (0.82/3.18) to 0.38 (1.10/2.90)), the corresponding values of Young’s moduli increase (from 10.3 ± 3.0 kPa to 46.1 ± 3.4 kPa). This trend correlates with the increase of the crosslinking density, which can be used to tailor the mechanical properties of the inks. Thus, this trend can be utilized as a reference for adjusting the composition of the polymeric inks to match the tissue mechanical properties. Rheological properties of the two main components of the custom inks were used as a reference for adjusting the printing conditions (Figure 2d). As the shear rate increased from 10−1 s−1 to 103 s−1, the apparent viscosity of the inks decreased from 103 Pa·s to 10 Pa·s or less, which confirms the shear thinning behavior of the developed inks. This facilitates the flow of the inks through fine nozzles during the 3D printing process.[18]
Following development, a quantitative analysis of the inks allowed us to quantify the fidelity of the physical properties to tissue, including a comparison of static and dynamic compression, hardness, and optical reflection characteristics between the printed inks and their corresponding tissue samples. For quantitative analysis of fidelity of static compression, stress-strain curves for cylindrical samples from patient prostate tissue were compared to printed samples of customized polymeric inks (Figure 2e). At 0–0.15 strain range (an acceptable range for most surgical tasks on prostate), the customized polymeric inks 1, 2 and 3, containing different ratios of active and bulking agents (Table S1), closely matched the general trends of stress-strain curves obtained from three prostate tissue samples, suggesting patient-specificity in ink composition. The Young’s moduli for strains less than 10% for representative samples of inks 1 (31.6 kPa), 2 (26.0 kPa) and 3 (12.4 kPa) (see Figure 2c and Table S1 for prime component ratios in the formulations, average modulus values and corresponding standard deviations) are analogous to tissue samples 1 (25.7 kPa), 2 (20.3 kPa) and 3 (10.9 kPa). At high strains, the modulus values increase with a nonlinear trend for tissue samples (due to viscoelastic, poroelastic and anisotropic properties of soft tissue[19–21]) and polymeric ink samples (due to viscoelastic behavior). The values obtained for Young’s moduli are also comparable to previous reports for the prostate tissue samples,[22] and are well below the values for typical 3D printed hard plastics and rubber-like materials. Indeed, the Young’s moduli for commercial Loctite® RTV silicone (595™ CL, Young’s modulus = 0.24 MPa) and NinjaFlex® (Young’s modulus = 15.20 MPa) are at least one order of magnitude higher than patient prostate tissue and our customized polymeric inks (Figure S2).
After demonstrating the capability of tailoring ink composition to match the properties of a specific tissue, we chose the ink 2/tissue 2 pair as a representative example for the remainder of the characterization, 3D printing and proof of concept demonstrations. For quantitative analysis of the fidelity of dynamic compression properties, the mechanical responses of cylindrical samples under applied dynamic compression were evaluated and compared for ink 2 and the corresponding tissue 2 (Figure 2f,g). Both storage (E’) and loss (E”) moduli for cylindrical samples of tissue 2 and ink 2 at 0.05, 0.10 and 0.20 strains showed high fidelity. For the storage modulus, the values for both tissue and ink increased with increasing oscillation frequency (0.1 Hz to 20 Hz) and strain (0.05 to 0.20). The trends of the obtained results are similar to previous reports for biological tissue (for example, human cervix tissue) exhibiting viscoelastic properties.[21] For the loss modulus, the values only indicate a clear increase with oscillation frequency.
For quantitative analysis of the fidelity of hardness, a nanoindentation methodology was applied.[23,24] The hardness properties of the cylindrical samples for both tissue and printed inks were tested and compared for tissue 2 and ink 2, showing that the load-depth curves for tissue 2 and ink 2 overlap (Figure 2h). The difference is ca. 0.1% of the tissue hardness at the maximum nanoindenter load for these two specific test results (the difference is 7.8% of the tissue hardness for the average hardness values), indicating high fidelity. For quantitative analysis of the optical fidelity, optical reflections at the outer surface of the gross prostate tissue and ink 2 were compared (Figure 2i).[25] Both the tissue and the ink possess the strongest reflection at wavelengths of 590–700 nm (orange and red), indicating good optical fidelity. Finally, the average density of the 3D printed model and inks was calculated to be 1.05 ± 0.07 g/cm3, which is identical with human prostate tissue (1.05 g/cm3).[26]
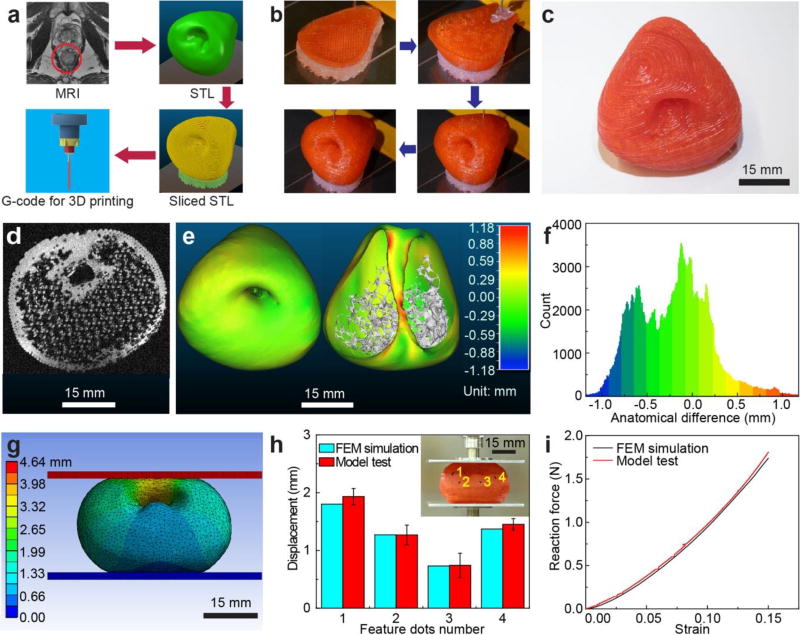
Next, information about the prostate anatomy extracted from MRI scans (Movie S1) was utilized to create a stereolithographic (STL) model and sliced into horizontal layers to generate G-code for the 3D printing process (Figure 3a) using our custom-built 3D printing system. Unlike conventional commercial 3D printers that use heating for extrusion of thermoplastic filaments, this setup uses adjustable pressure settings, which is better suited to handle the shear thinning properties of silicone-based polymeric inks at room temperature.[27] The 3D printing process (Figure 3b and Movie S2) follows the pathways dictated by the corresponding sliced STL model (Figure S3) in order to generate the final 3D printed prostate model (Figure 3c). The printed models were typically left untouched for at least 1–3 days prior to performing the remaining experiments. A quantitative analysis of the organ model allowed us to quantify the fidelity of the anatomy between the 3D printed prostate model and its corresponding patient prostate. First, a 3D registration technique was used for surface comparison[28] in order to analyze anatomical fidelity. The 3D printed prostate model was scanned by MRI, and then the MRI image stack (Figure 3d and Movie S3) was utilized to generate an STL model. A calibrated distance map (Figure 3e) and a histogram of the calibrated distances (Figure 3f) of the corresponding points on the surface of the patient prostate model and 3D printed prostate model were generated via 3D registration. The results indicated that the anatomical difference for the outer surface (Figure 3e (left)) and inner urethra surface (Figure 3e (right)) is trivial, and most of the calibrated distance points scatter from −0.8 to 0.3 mm, with peaks close to 0 mm (Figure 3f). The overall anatomical fidelity was found to be 98% (Supporting Information).
Figure 3.
3D printing of prostate model, anatomical fidelity analysis and organ physical behavior prediction using the 3D printed prostate model. a) Procedure for converting patient-specific MRI to G-code for the 3D printing process. b) 3D printing process of the prostate model using the customized polymeric ink. c) Photograph of the 3D printed prostate model. d) An MRI image obtained via scanning a 3D printed prostate model. e) Calibrated distance map via 3D registration for comparison of anatomical fidelity between patient prostate and 3D printed prostate model at the outer surface (left) and urethra surface (right). f) Histogram of the calibrated distances of the surface points for comparison of anatomical fidelity between the patient prostate model and 3D printed prostate model. g) Total deformation results after compression of the FEM model (15% of model height). h) Displacement comparison for feature dots between results from compression of the 3D printed prostate model (with standard deviation error bars) and the FEM simulated model. Inset: Displacement of the feature dots on the 3D printed prostate model after compression with the displacement trajectories. i) Reaction force comparison between results from compression of the 3D printed prostate model and the FEM simulated model.
Next, considering the high fidelity of the ink properties and anatomical structure, the 3D printed prostate model can be used to predict the physical behavior of patient organs during surgical handling, which can help avoid the application of excessive deformation and force, and thus tissue damage during operation procedures. Our evaluation of organ physical behavior includes both FEM simulations of the patient organ and compression tests on the 3D printed prostate model (Figure S4a). This procedure evaluates the predicted deformation of the 3D printed model both geometrically and mechanically.[29–31] We selected an Ogden 3rd order model[32,33] that fits the stress-strain curves of both the prostate tissue 2 and customized ink 2 for FEM simulation (Supporting Information) within the strain range of 0–0.15 (Figure S4b and Table S2). We then simulated a compression process (15% of entire model height, about 4.64 mm) via lowering the plate on top of the patient prostate model from its original state (Figure S4c) and fixing the bottom plate. The different parts of the model showed varying deformation under the same applied compression (Figure 3g and Movie S4). The reaction force of the model during compression was also predicted. The results from the FEM simulation provide a reference for organ physical behavior.
To demonstrate that the 3D printed prostate model can accurately and directly predict the organ physical behavior under compression, we designed a compression test by employing the 3D printed prostate model (Figure S4d) under the same conditions as the simulation. A customized stereo system was designed to track the deformation of the prostate model (Figure S5 and Supporting Information).[34–39] The displacement trajectories of four randomly selected feature dots were tracked on the model before (Figure S4d) and after (Figure 3h Inset) the compression test process (Movie S5). We then accurately located the feature dots on the FEM simulated model. A 3D scanned model (with surface texture) of the 3D printed prostate was matched to the original computer-aided design (CAD) model in order to register the feature dots in the FEM simulation (Figure S6). Then, we read the displacement values for the dots from the simulated model. Finally, we compared the displacements of the dots in the compression test with the ones in the FEM simulation, and found that the differences in average displacement for each dot were within 10% of simulation results (Figure 3h). In addition, the reaction force versus strain in the compression test was similar to the FEM simulation results (1.82 ± 0.11 N for the compression test and 1.74 N for the simulation at a strain of 0.15, and 1.04 ± 0.06 N for the compression test and 1.02 N for the simulation at a strain of 0.10) (Figure 3i). These results demonstrate the feasibility of utilizing the 3D printed prostate model for organ physical behavior prediction.
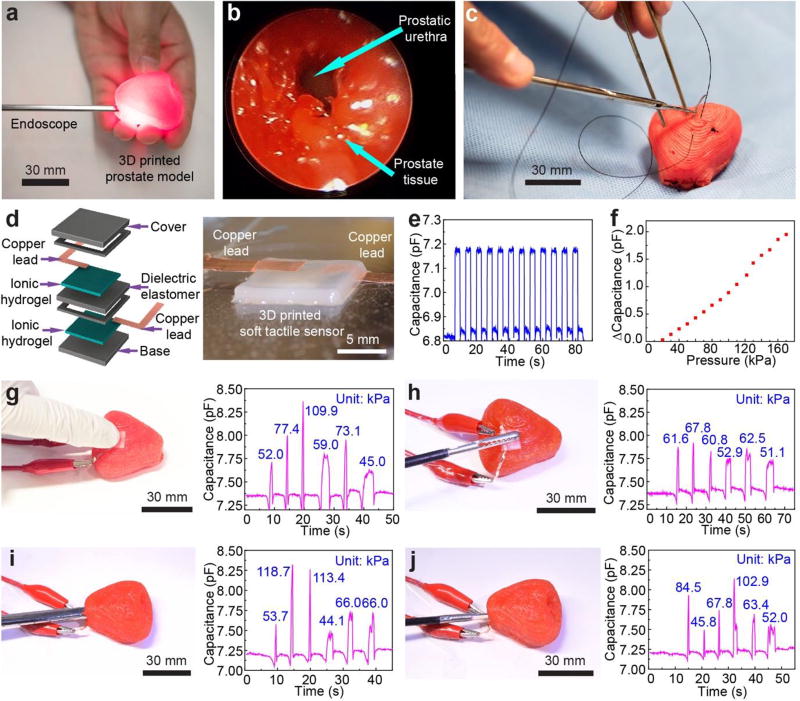
We next sought to investigate the use of the 3D printed prostate models in surgical rehearsal simulations. We first applied an endoscope to enter the urethra of the 3D printed prostate model (Figure 4a and Figure S7). To further enhance the effectiveness of the model as a surgical aid, we repeated this application with the prostate model embedded in a kidney-urethra-bladder (KUB) model from the Center for Research in Education and Simulation Technologies (CREST)[40] (Figure S8). Due to the matching physical properties of the model with tissue, the endoscope can be easily inserted into the urethra to obtain an endoscopic view for any region of the surface, even under conditions of pressing or squeezing (Figure 4b and Movie S6). The endoscopic view from the 3D printed prostate model showed the unfilled prostatic urethra in the patient’s MRI, which was neither dilated by the endoscope nor filled by irrigating fluid and urine. Thus, this application suggests the effectiveness of these organ models in assisting medical professionals for more efficient planning and rehearsal from organ inner channels via the use of an endoscope. In addition, we conducted a suturing experiment on the 3D printed prostate model with the aid of a surgeon (Figure 4c and Movie S7). Although this is not a common practice for the prostate organ, it indicated that the 3D printed models exhibited sufficiently good strength to avoid excessive damage during invasive surgical procedures, such as needle penetration. Furthermore, feedback from the surgeon indicated that 3D printed prostate model remained robust during suturing, it did not exhibit any tearing, and the surgical knot did not pull through.
Figure 4.
Quantitative surgical rehearsal using the 3D printed prostate model. a) Surgical rehearsal involving applying an endoscope in the urethra of the 3D printed prostate model. b) Endoscopic view of the urethra inside of the 3D printed prostate model. c) Surgical suturing on the 3D printed prostate model. d) Schematic of the structure of the 3D printed soft tactile sensor (left) and photograph of the corresponding 3D printed sensor (right). e) Characterization of the response repeatability for the soft tactile sensor via capacitance changes with an applied cyclic pressure of 50 kPa. f) Calibration of the 3D printed sensor based on the correlation between capacitance change and the applied pressure. g,h) Quantitative surgical rehearsal involving the 3D printed prostate model upon applying a finger (g) and a surgical grasper (h), respectively, on the sensor integrated on the outer surface of the model and their corresponding pressure responses (indicated at each of the peaks) from the capacitance changes of the sensor. i,j) Quantitative surgical rehearsal involving the 3D printed prostate model when applying an endoscope (i) and surgical scissors (j) on the sensor integrated on the urethra surface inside of the model, and their corresponding pressure responses (indicated at each of the peaks) from the capacitance changes of the sensor.
Next, we 3D printed soft capacitive tactile sensors[41] for incorporation onto the 3D printed prostate model with the purpose of offering quantitative sensing capabilities via conformal integration. The sensor consists of a polyacrylamide-based ionic hydrogel and a silicone-based dielectric elastomer (Figure 4d), which were used as the electrodes and electroactive component of the sensor, respectively.[41,42] The hydrogel electrodes and the dielectric elastomer layer have elastic moduli of 11.05 ± 2.97 kPa and 75.47 ± 12.65 kPa at 100% strain rate, respectively. In addition, they possess shear thinning properties, which facilitates the 3D printing process. We also added silicone layers on the top and bottom of the device to facilitate its handling and longevity. The final 3D printed device has dimensions of 10 × 10 × 1.2 mm (L × W × H). Upon the application of external pressure to the sensor, the dielectric elastomer experiences a deformation (compression of thickness and expansion in area), which results in a change in device capacitance.[41,42] After printing, the sensor was calibrated using pin compression under various applied pressures (Figure S9). The response of the tactile sensor exhibited excellent repeatability in terms of capacitance change under applied pressure (Figure 4e, under 50 kPa of applied pressure as a demonstration example). The capacitance change and the applied pressure showed a linear correlation at a pressure range of 20 to 120 kPa (Figure 4f). This calibration data can be used to calculate the pressure applied to the tactile sensor via corresponding changes in capacitance.
We designed and conducted quantitative surgical rehearsal applications using the 3D printed prostate model with the integrated 3D printed sensors. These applications aim to train medical professionals to quantitatively realize and control the amount of applied pressure and its duration within reasonable ranges before operating on patient organs. For each application, we applied three quick press-release and three press-hold-release cycles using either a finger or surgical and diagnostic tools on the sensor. For the first application example, we integrated the sensor on the outer surface of the model. Then we applied finger pressing, a surgical grasper, and surgical scissors to the sensor and deduced their corresponding pressure responses from the capacitance changes (Figure 4g,h and Figure S10a). For the second application example, we integrated the sensor on the urethra surface of the model. Then, we used an endoscope, surgical grasper and surgical scissors on the sensor and deduced their corresponding pressure responses from the capacitance changes (Figure 4i,j and Figure S10b). The real-time applications and corresponding capacitance changes of the sensor in the first and second application examples are shown in Figure S11 and Movie S8–S13.
In summary, we have designed and developed a series of novel methodologies and customized inks for fabricating a 3D printed prostate model with physical properties of tissue and integrated sensing capabilities that can be used for quantitative, advanced surgical rehearsal. The 3D printed prostate model demonstrated high fidelity with patient organ and tissue in anatomical, static and dynamic mechanical, hardness, and optical properties. Therefore, the prostate model could aid medical professionals to perform more effective preoperative planning and rehearsal, and predict organ physical behavior more accurately. The tissue-like tactile sensation can help to hone surgical skills for training purposes. In addition, the flexible urethra provides the possibility of practicing with tools within organ channels. Finally, conformal integration of 3D printed soft tactile sensors on the surface of the 3D printed prostate model allows the model to exhibit quantitative feedback. Future studies will focus on several different directions, including: 1) fabrication of organ models with heterogeneous properties, durometers, and dynamic functionalities; 2) direct integration of 3D printed electronics for multi-dimensional feedback; 3) incorporation of virtual and assisted reality tools; 4) evaluation of this work in real use cases for patient safety and surgical outcomes; and 5) manipulation of anisotropic properties of the different organ models, since previous work has shown that by controlling the orientation of printing pathways[43,44] and imbedding fillers[45,46], anisotropic properties can be introduced into 3D printed materials and models. Overall, we believe that the concept of creating 3D printed patient-specific organ models with anatomical accuracy, physical properties of tissue, and integrated 3D printed soft electronics suggests a new paradigm in preoperative practice.
Experimental Section
Fabrication of customized polymeric inks with physical properties of tissue for 3D printing
Silicone sealant (acetoxy-based RTV sealant Loctite SI 595™ CL), silicone grease (#LP20, Trident®), Procyinyl Red GS (ICI America Inc.) and fumed silica (7 nm, Aldrich) were combined in the formulation as an active agent for vulcanization, a bulking agent (softness after vulcanization), a coloring agent, and a thickening agent, respectively. The active agent and the bulking agent were mixed at proper weight ratios (Figure 2c and Table S1) to achieve different values of Young’s modulus via a mixer (ARE-310, Thinky) to form the primary component of the customized polymeric inks.
3D printing of organ models
The MRI image pack (1 mm resolution) of the patient prostate organ was processed to generate G-code for printing using a custom-built 3D printing system (AGS 100, Aerotech). The customized polymeric ink and supporting ink (Loctite SI 595™ CL) were deposited from two dispensing apparatuses. The supporting ink was removed mechanically after the inks in the model were fully cured. For more complex organ models, the supporting ink can be replaced with 33 wt% Pluronic® F-127 (Sigma-Aldrich) in water. The supporting ink can then be easily removed via flushing with water at 4 °C.
Characterization of prostate tissue and customized polymeric inks
The human prostate was collected using radical prostatectomy. The tissue was cut into cylindrical samples for testing. The customized polymeric inks were 3D printed into cylindrical samples for direct comparison of results. Static and dynamic compression tests were carried out using a mechanical analyzer (RSA-G2, TA Instruments). The hardness tests were conducted on a nanoindentation system (Nanoindenter XP, MTS). For optical reflection tests, the gross tissue and colored 3D printed samples were evaluated using fiber optic equipment (Ocean Optics).
Rheological characterization
The rheology of the customized polymeric ink and its corresponding active and bulking agents was characterized on a magnetic bearing rheometer (AR-G2, TA Instruments).
MRI of 3D printed prostate model
Imaging of the 3D printed prostate model was carried out using an MRI system (9.4 Tesla) while the 3D printed prostate model was placed in a 31 cm bore (Magnex Scientific).
3D registration for anatomical fidelity
3D registration of the STL files between the 3D printed prostate model and the patient prostate model was achieved using CloudCompare software.
FEM simulation
The FEM software employed for simulation was ANSYS Workbench 17.1 and the component was Static Structural. An Ogden third model was used to fit the measured strain-stress data. The simulation setup was configured to be identical to the compression test. The contacts between the FEM model and plates were defined as frictional with a friction coefficient of 10, and 137,905 nodes were generated for the model with a size of 45.14 mm × 41.70 mm × 30.95 mm (L × W × H). In the mesh section, the element size of the prostate model was set to be 3 mm, and the surface size of the contacting areas with the top and bottom plates was set to be 1 mm. The top and bottom plates were meshed by sweeping in the Z-axis with one division separately, and the edges were divided into 20 segments each with a bias factor of 5. The element types were determined via ANSYS Workbench.
3D displacement measurement using stereo system with feature dots during model compression
A 3D displacement measurement procedure based on a stereo system was designed and applied to track the 3D trajectories of the feature dots on the 3D printed prostate model (ink 2, 100% fill density) during model compression by a mechanical analyzer (RSA-G2, TA Instruments). The reaction force was also read from the mechanical analyzer.
Mapping of the feature dots to the corresponding locations on the FEM simulation model
A 3D printed prostate model with feature dots was coated with a thin layer of baby powder for 3D scanning (HDI 109, GoMeasure3D). The scanned model and the FEM simulation model were then imported into the CloudCompare software for 3D registration with a uniform coordinate system. The location coordinates (x, y, z) of the feature dots on the scanned model were used to map the corresponding locations with the same coordinates on the FEM simulation model.
3D printing and calibration of soft tactile sensor
A soft capacitive sensor device was 3D printed by alternately depositing layers of two different materials (polyacrylamide-based ionic hydrogel and silicone-based dielectric elastomer), followed by their photopolymerization via exposure to a UV system (Omnicure® S1500, Excelitas Technologies). The soft sensor was then calibrated by applying varying pressures to the device and measuring the changes in capacitance.
Advanced surgical rehearsal using the 3D printed prostate model
(1) An endoscope was inserted into the urethra of the model and the endoscopic view was observed from the surgical display in the endoscopic tower station (Stryker). (2) Surgical suturing was conducted on the surface of the 3D printed prostate model with the assistance of a surgeon and by utilizing a surgical needle for penetration and surgical thread (ETHICON) for suturing. (3) Finger, surgical, and diagnostic tools were applied on the sensors integrated on the surface and interior of the 3D printed prostate model. For each application, three quick press-release and three press-hold-release cycles were applied. The signal responses of capacitance changes of the sensor were then converted into values of applied pressures via sensor calibration data.
Supplementary Material
Acknowledgments
Prof. M. C. McAlpine acknowledges the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (Award No. 1DP2EB020537), and the Army Research Office for the development of the 3D printed soft sensor (Award No. W911NF-15-1-0469). Prof. B. M. Ogle acknowledges support by the National Heart, Lung, And Blood Institute of the National Institutes of Health (Award No. R01HL137204). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Prof. R. M. Sweet and Prof. B. R. Konety acknowledge support from the Department of Urology at the University of Minnesota (UMN). Prof. C. C. Chu acknowledges support from the Rebecca Q. Morgan Foundation. The authors thank D. Sciacca and S. Krishna from the UMN Medical School for their assistance in acquiring prostate tissue. The authors thank Dr. D. Giles from the UMN Polymer Characterization Facility and L. M. Tenorio from UMN Biomedical Engineering for mechanical test support. The authors also thank Dr. J. Nelson from the UMN Characterization Facility and Dr. D. Nedrelow from the UMN Tissue Mechanics Lab for assistance with hardness tests. The authors thank J. Lee from UMN Biomedical Engineering for optical test support. The authors thank T. Reihsen, L. H. Poniatowski and D. Burke from the University of Washington (UW) Department of Urology for tissue testing, organ MRI processing, and medical application and material suggestions. The authors thank Dr. D. Idiyatullin from UMN Center for Magnetic Resonance Research (CMRR), Department of Radiology for assistance with acquiring the MRI for the 3D printed prostate model. The authors thank J. Grafft from UMN SimPORTAL for assistance with using the endoscope tower station. The authors thank N. J. Bibus from the UMN Medical Center for assistance with acquiring surgical and diagnostic tools. The authors thank Dr. J. Gorman from UMN Mechanical Engineering and J. Malluege from ANSYS, Inc. for FEM simulation assistance. The authors thank S. Thomalla from the Medical Device Center for help with commercial material printing for comparison purposes. The authors also thank Dr. D. Joung, D. Yang and B. Bogdalek from UMN Mechanical Engineering, for their valuable comments and suggestions during manuscript preparation.
Footnotes
Experimental details for procedures and parameter settings, as well as theoretical models, can be found in the Supporting Information.
Supporting Information is available from the Wiley Online Library or from the author.
Additional Information
An Institutional Review Board (IRB) review at the University of Minnesota determined that this experiment does not meet the federal definition of human subjects research and, therefore, an IRB review was not required.
Competing financial interests
The authors declare no competing financial interests.
Contributor Information
Kaiyan Qiu, Department of Mechanical Engineering, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Zichen Zhao, WWAMI Institute for Simulation in Healthcare, University of Washington, Seattle, Washington 98195, United States; Department of Urology, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Ghazaleh Haghiashtiani, Department of Mechanical Engineering, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Shuang-Zhuang Guo, Department of Mechanical Engineering, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Mingyu He, Fiber Science & Biomedical Engineering Programs, Cornell University, Ithaca, New York 14853, United States.
Ruitao Su, Department of Mechanical Engineering, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Zhijie Zhu, Department of Mechanical Engineering, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Didarul B. Bhuiyan, Department of Biomedical Engineering, University of Minnesota, Minneapolis, Minnesota 55455, United States
Paari Murugan, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Fanben Meng, Department of Mechanical Engineering, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Sung Hyun Park, Department of Mechanical Engineering, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Chih-Chang Chu, Fiber Science & Biomedical Engineering Programs, Cornell University, Ithaca, New York 14853, United States.
Brenda M. Ogle, Department of Biomedical Engineering, University of Minnesota, Minneapolis, Minnesota 55455, United States
Daniel A. Saltzman, Department of Surgery, University of Minnesota, Minneapolis, Minnesota 55455, United States
Badrinath R. Konety, Department of Urology, University of Minnesota, Minneapolis, Minnesota 55455, United States
Robert M. Sweet, WWAMI Institute for Simulation in Healthcare, University of Washington, Seattle, Washington 98195, United States Department of Urology, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Michael C. McAlpine, Department of Mechanical Engineering, University of Minnesota, Minneapolis, Minnesota 55455, United States
References
- 1.Makary MA, Daniel M. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139. [DOI] [PubMed] [Google Scholar]
- 2.Thiels CA, Lal TM, Nienow JM, Pasupathy KS, Blocker RC, Aho JM, Morgenthaler TI, Cima RR, Hallbeck S, Bingener J. Surgery. 2015;158:515. doi: 10.1016/j.surg.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGhee J. J. Anat. 2010;216:264. doi: 10.1111/j.1469-7580.2009.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An G, Hong L, Zhou X-B, Yang Q, Li M-Q, Tang X-Y. Ann. Anat. 2017;210:76. doi: 10.1016/j.aanat.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Adams F, Qiu T, Mark A, Fritz B, Kramer L, Schlager D, Wetterauer U, Miernik A, Fischer P. Ann. Biomed. Eng. 2017;45:963. doi: 10.1007/s10439-016-1757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadoya N, Miyasaka Y, Nakajima Y, Kuroda Y, Ito K, Chiba M, Sato K, Dobashi S, Yamamoto T, Takahashi N, Kubozono M, Takeda K, Jingu K. Med. Phys. 2017;44:1445. doi: 10.1002/mp.12168. [DOI] [PubMed] [Google Scholar]
- 7.Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor H-U, Giesel FL. Int. J. Comput. Assist. Radiol. Surg. 2010;5:335. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 8.Farooqi KM, Lengua CG, Weinberg AD, Nielsen JC, Sanz J. Pediatr. Cardiol. 2016;37:1028. doi: 10.1007/s00246-016-1385-8. [DOI] [PubMed] [Google Scholar]
- 9.Wake N, Chandarana H, Huang WC, Taneja SS, Rosenkrantz AB. Clin. Radiol. 2016;71:610. doi: 10.1016/j.crad.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Zein NN, Hanouneh IA, Bishop PD, Samaan M, Eghtesad B, Quintini C, Miller C, Yerian L, Klatte R. Liver Transpl. 2013;19:1304. doi: 10.1002/lt.23729. [DOI] [PubMed] [Google Scholar]
- 11.Komai Y, Sugimoto M, Gotohda N, Matsubara N, Kobayashi T, Sakai Y, Shiga Y, Saito N. Urology. 2016;91:226. doi: 10.1016/j.urology.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 12.Kusaka M, Sugimoto M, Fukami N, Sasaki H, Takenaka M, Anraku T, Ito T, Kenmochi T, Shiroki R, Hoshinaga K. Transplant. Proc. 2015;47:596. doi: 10.1016/j.transproceed.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 13.Mottl-Link S, Hübler M, Kühne T, Rietdorf U, Krueger JJ, Schnackenburg B, De Simone R, Berger F, Juraszek A, Meinzer H-P, Karck M, Hetzer R, Wolf I. Ann. Thorac. Surg. 2008;86:273. doi: 10.1016/j.athoracsur.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Ciriza G, Hussain T, Gómez-Cía T, Valverde I. Interv. Cardiol. 2015;7:343. [Google Scholar]
- 15.Hassanipour-Azgomi S, Mohammadian-Hafshejani A, Ghoncheh M, Towhidi F, Jamehshorani S, Salehiniya H. Prostate Int. 2016;4:118. doi: 10.1016/j.prnil.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster GA, Schuster TG. J. Urol. 1999;161:1168. [PubMed] [Google Scholar]
- 17.Huijing PA. J. Biomech. 1999;32:329. doi: 10.1016/s0021-9290(98)00186-9. [DOI] [PubMed] [Google Scholar]
- 18.Muth JT, Vogt DM, Truby RL, Mengüç Y, Kolesky DB, Wood RJ, Lewis JA. Adv. Mater. 2014;26:6307. doi: 10.1002/adma.201400334. [DOI] [PubMed] [Google Scholar]
- 19.Wells PNT, Liang H-D. J. R. Soc. Interface. 2011;8:1521. doi: 10.1098/rsif.2011.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Ultrason. Imaging. 1998;20:260. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 21.DeWall RJ, Varghese T, Kliewer MA, Harter JM, Hartenbach EM. Ultrason. Imaging. 2010;32:214. doi: 10.1177/016173461003200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, Nigwekar P, Castaneda B, Hoyt K, Joseph JV, di Sant'Agnese A, Messing EM, Strang JG, Rubens DJ, Parker KJ. Ultrasound Med. Biol. 2008;34:1033. doi: 10.1016/j.ultrasmedbio.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Ebenstein DM, Pruitt LA. J. Biomed. Mater. Res. A. 2004;69A:222. doi: 10.1002/jbm.a.20096. [DOI] [PubMed] [Google Scholar]
- 24.Zysset PK, Edward Guo X, Edward Hoffler C, Moore KE, Goldstein SA. J. Biomech. 1999;32:1005. doi: 10.1016/s0021-9290(99)00111-6. [DOI] [PubMed] [Google Scholar]
- 25.Bruno TJ, Svoronos PDN. CRC handbook of fundamental spectroscopic correlation charts. CRC Press; Boca Raton, FL, USA: 2005. [Google Scholar]
- 26.Karademir I, Shen D, Peng Y, Liao S, Jiang Y, Yousuf A, Karczmar G, Sammet S, Wang S, Medved M, Antic T, Eggener S, Oto A. AJR. Am. J. Roentgenology. 2013;201:1041. doi: 10.2214/AJR.13.10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo S-Z, Qiu K, Meng F, Park SH, McAlpine MC. Adv. Mater. 2017;29:1701218. doi: 10.1002/adma.201701218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shui W, Zhou M, Chen S, Pan Z, Deng Q, Yao Y, Pan H, He T, Wang X. Int. J. Comput. Assist. Radiol. Surg. 2017;12:13. doi: 10.1007/s11548-016-1461-9. [DOI] [PubMed] [Google Scholar]
- 29.McCurry MR, Evans AR, McHenry CR. PeerJ. 2015;3:e988. doi: 10.7717/peerj.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holzapfel GA, Gasser TC, Stadler M. Eur. J. Mech. A-Solid. 2002;21:441. [Google Scholar]
- 31.Brock KK, Sharpe MB, Dawson LA, Kim SM, Jaffray DA. Med. Phys. 2005;32:1647. doi: 10.1118/1.1915012. [DOI] [PubMed] [Google Scholar]
- 32.Ogden RW. Proc. R. Soc. A. 1972;326:565. [Google Scholar]
- 33.Kim B, Lee SB, Lee J, Cho S, Park H, Yeom S, Park SH. Int. J. Precis. Eng. Man. 2012;13:759. [Google Scholar]
- 34.Bouguet J-Y. Camera Calibration Toolbox for MATLAB. California Institute of Technology; 2004. http://www.vision.caltech.edu/bouguetj/calib_doc/ [Google Scholar]
- 35.Heikkila J, Silven O. presented at Proc. 13th Int. Conf. Pattern Recogn; Vienna, Austria. Aug, 1996. [Google Scholar]
- 36.Heikkila J, Silvén O. presented at IEEE Comp. Soc. Conf. Comp. Vision Pattern Recogn; San Juan, Puerto Rico, USA. Jun, 1997. [Google Scholar]
- 37.Zhang Z. presented at Proc. 7th IEEE Int. Conf. Comp. Vision; Kerkyra, Greece. Sep, 1999. [Google Scholar]
- 38.Duane CB. Photogramm. Eng. 1971;37:855. [Google Scholar]
- 39.Fryer JG, Brown DC. Photogramm. Eng. Remote Sens. 1986;52:51. [Google Scholar]
- 40.Sweet RM. J. Endourol. 2016;31:S69. doi: 10.1089/end.2016.0613. [DOI] [PubMed] [Google Scholar]
- 41.Robinson SS, O’Brien KW, Zhao H, Peele BN, Larson CM, Mac Murray BC, Van Meerbeek IM, Dunham SN, Shepherd RF. Extreme Mech. Lett. 2015;5:47. [Google Scholar]
- 42.Sun J-Y, Keplinger C, Whitesides GM, Suo Z. Adv. Mater. 2014;26:7608. doi: 10.1002/adma.201403441. [DOI] [PubMed] [Google Scholar]
- 43.Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL, Engel EA, Krick KD, Ju A, Meng F, Enquist LW, Jia X, McAlpine MC. Adv. Funct. Mater. 2015;25:6205. doi: 10.1002/adfm.201501760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou R, Xia Y, Liu S, Hu P, Hou W, Hu Q, Shan C. Composites Part B. 2016;99:506. [Google Scholar]
- 45.Gnanasekaran K, Heijmans T, van Bennekom S, Woldhuis H, Wijnia S, de With G, Friedrich H. Appl. Mater. Today. 2017;9:21. [Google Scholar]
- 46.Valentine AD, Busbee TA, Boley JW, Raney JR, Chortos A, Kotikian A, Berrigan JD, Durstock MF, Lewis JA. Adv. Mater. 2017;29:1703817. doi: 10.1002/adma.201703817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.