Abstract
Importance
Treatment of Crohn disease is rapidly evolving, with the induction of novel biologic therapies and newer, often more intensive treatment approaches. Knowing how to treat individual patients in this quickly changing milieu can be a challenge.
Objective
To review the diagnosis and management of moderate to severe Crohn disease, with a focus on newer treatments and goals of care.
Evidence Review
MEDLINE was searched from 2000 to 2011. Additional citations were procured from references of select research and review articles. Evidence was graded using the American Heart Association level-of-evidence guidelines.
Results
Although mesalamines are still often used to treat Crohn disease, the evidence for their efficacy is lacking. Corticosteroids can be effectively used to induce remission in moderate to severe Crohn disease, but they do not maintain remission. The mainstays of treatment are immunomodulators and biologics, particularly anti–tumor necrosis factor.
Conclusion and Relevance
Immunomodulators and biologics are now the preferred treatment options for Crohn disease.
Mr C: His View
I was first diagnosed with Crohn's disease a little over a year ago. During that time, I was having unexplained stomach issues and that's when I started going to my primary care physician. She prescribed Prilosec [omeprazole], but I wasn't getting better. One night we went out to eat and I thought I had food poisoning, as I was up all night in the bathroom. I went to the ER and after all these tests and finally meeting a GI doctor, I was diagnosed with Crohn's.
The initial management was 1000 mg of mesalamine twice a day. I had a flare-up and I was switched to budesonide and I felt better. Then I had another flare-up and I went back on the budesonide, and that's when my GI doctor was a little concerned about continuing budesonide. She started discussing other options if we weren't able to get the Crohn's into remission. We discussed adalimumab vs infliximab vs mercaptopurine and decided to try mercaptopurine and see how I did. For the 5 or 6 weeks I was on it, I felt good. Then I developed bad nausea and since this wasn't one of my normal symptoms, I called the gastroenterologist and we stopped mercaptopurine.
On days I don't feel well, I have an uncomfortable feeling, which makes me want to take it easy. So I cut back on how much I'm eating and wait for it to subside. I think that's just part of learning to live with a chronic disease.
Common Clinical Manifestations Of Crohn Disease
Dr Cheifetz
Crohn disease and ulcerative colitis are chronic idiopathic inflammatory disorders of the GI tract, commonly referred to as IBD. There are an estimated 1.2 million patients with IBD in the United States. As in Mr C's case, most patients present in late adolescence and early adulthood.1,2 Crohn disease and ulcerative colitis differ in a number of ways (Figure 1). Crohn disease is more complex because of its variable location and transmural inflammation. Additionally, it is characterized by skip lesions, which are diseased sections of bowel next to uninvolved areas. Although Crohn disease affects the terminal ileum and colon in 50% of patients, it can involve any portion of the GI tract but typically spares the rectum. Twenty percent of patients have colon involvement only, and 30% of patients, like Mr C, have disease confined to the small bowel. In addition, up to 25% of patients have perianal complications.
Figure 1. Differences in Disease Characteristics Between Crohn Disease and Ulcerative Colitis.
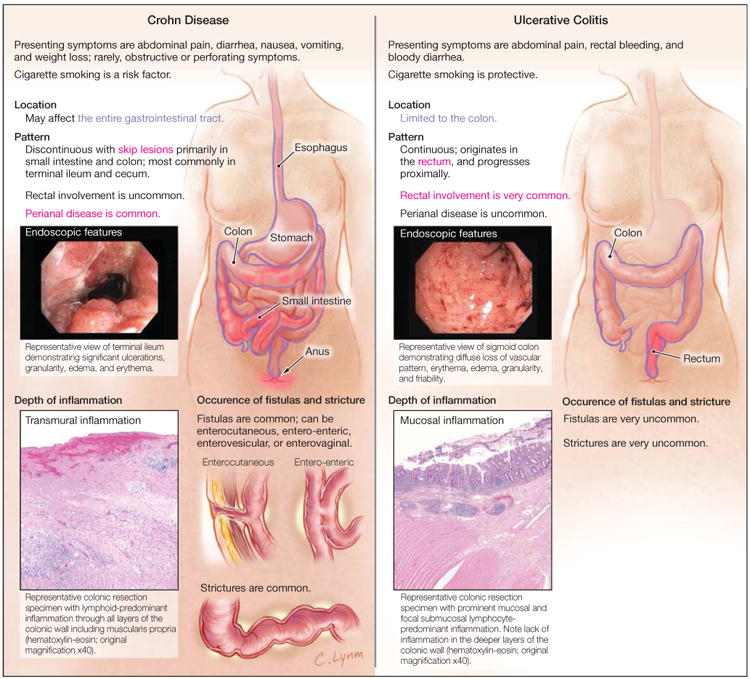
Because of the variability of severity and location of Crohn disease, as well as its transmural nature, patients can have wide-ranging presentations. Patients usually present with some combination of abdominal pain and diarrhea. Some patients, such as Mr C, may exhibit only intermittent abdominal discomfort. Systemic symptoms such as fatigue, anorexia, and weight loss may be present in more severe cases. Less commonly, patients may initially present with stricturing or perforating complications.3 In up to 10% of patients, perianal complications or extraintestinal manifestations may be the presenting symptoms.4 Axial and peripheral arthropathies are the most common extraintestinal manifestations, but uveitis, episcleritis, pyoderma gangrenosum, erythema nodosum, and primary sclerosing cholangitis are all associated with IBD.
The differential diagnosis for the signs and symptoms typical of Crohn disease is extremely broad. As a result, diagnosis may be delayed.5 Nine months between symptom onset and diagnosis, as occurred with Mr C, is not uncommon. After the diagnosis is made, clinicians should be aware that patients with Crohn disease are at risk of non-IBD diseases such as gallstones, renal stones, small intestinal bacterial overgrowth, and gastrointestinal infection.
The diagnosis of Crohn disease is usually established from clinical findings and relies on a good history and physical examination. A Crohn disease diagnosis is suggested by a family history of IBD, recent use of nonsteroidal anti-inflammatory drugs, and cigarette smoking, all of which are risk factors for Crohn disease.6 As is the case for Mr C, most patients do not have any identifiable risk factors or relatives with IBD. The abdominal and perianal examinations are important, as are the presence of extraintestinal manifestations. The presence of abdominal fullness or mass or a perianal fissure, fistula, mass, or large skin tags may suggest Crohn disease. No laboratory tests specifically establish a Crohn disease diagnosis. Anemia, thrombocytosis, and increased levels of inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein are nonspecific markers of inflammation and can be seen in patients with Crohn disease. Fecal calprotectin and lactoferrin are increasingly used as indicators because they appear to be more sensitive in detecting inflammation, though, like the serum markers, they are nonspecific. Stool studies, including tests for bacterial and parasitic pathogens, are necessary to rule out gastrointestinal infection. Typically, the combination of ileocolonoscopy and small bowel imaging (Figure 2) establish the diagnosis, location, and extent of Crohn disease. Histopathology can confirm Crohn disease with findings of focal intestinal inflammation as was seen on Mr C's ileal biopsies (Figure 3). Although noncaseating granulomas are typically considered to be pathognomonic for Crohn disease, they are infrequently found on biopsy.7
Figure 2. Helical Computed Tomography Imaging of the Patient's Abdomen and Pelvis.
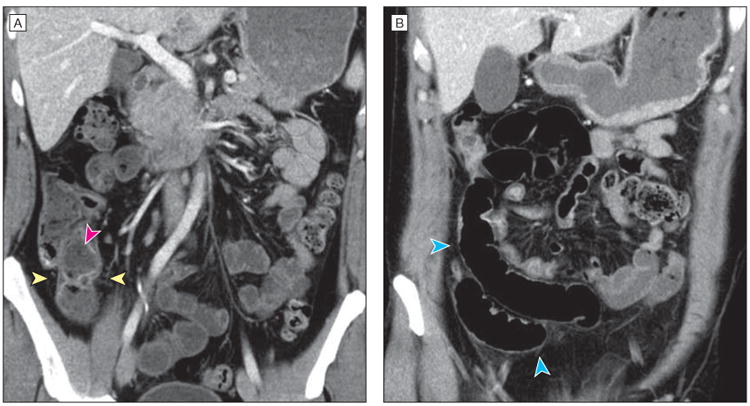
Coronal computed tomography views show slightly increased enhancement in the mucosa of the terminal ileum near the ileocecal valve with areas of slight wall thickening and mural stratification with predominantly intramural fat (yellow arrowheads), as well as feculent luminal contents (pink arrowhead). More proximal loops of ileum are slightly dilated (blue arrowheads).
Figure 3. Biopsy of the Patient's Ileal Mucosa.
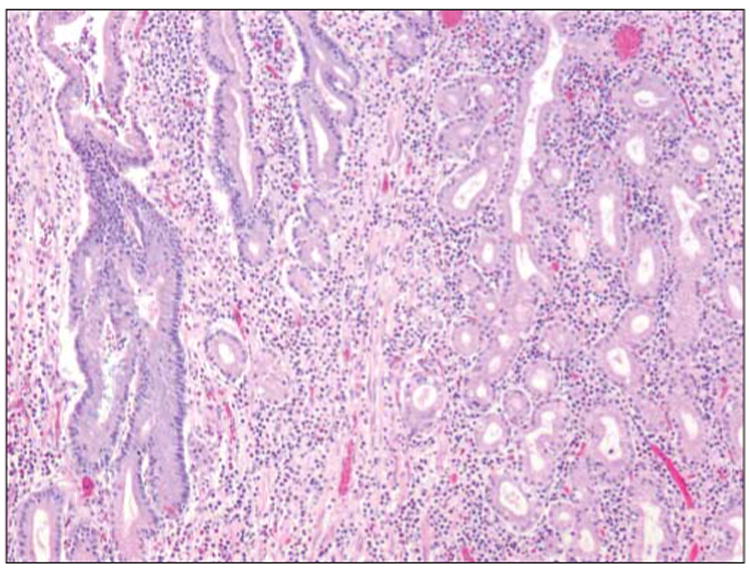
Biopsy demonstrating replacement of the normal intestinal-type epithelium, composed of goblet cells and enterocytes, with mucous-type glands normally seen in the gastric pylorus (hematoxylin-eosin stain, original magnification ×400). This histologic feature of pyloric gland metaplasia is indicative of chronic injury often seen in the terminal ileum of patients with Crohn disease.
The colon and distal ileum are readily accessible with colonoscopy. Adequate imaging of the small bowel is more challenging. Historically, barium studies were used, but other imaging tests are now more effective. Cross-sectional imaging techniques, including CT enterography and magnetic resonance enterography, evaluate all layers of the bowel wall and have the added advantage of identifying extraluminal complications.8,9 Magnetic resonance enterography is more expensive and time consuming than CT enterography but does not require ionizing radiation. Capsule endoscopy is a relatively noninvasive method that directly visualizes the small bowel mucosa and has the advantage of being highly sensitive for mucosal lesions, but it lacks specificity.10 Additionally, there is an increased risk of capsule retention in Crohn disease.11
Common Classifications Of Crohn Disease
The numerous classification systems available for Crohn disease are mainly used for research purposes. In clinical practice, a patient's disease is stratified by disease severity (mild, moderate, or severe), disease location (upper GI, ileal, ileocolonic, colonic, or perianal), extent of disease, and disease phenotype (penetrating, stricturing, or inflammatory). Disease severity is typically determined by clinical course and treatment history. Based on the available information, Mr C's Crohn disease appears limited to the ileum and of moderate to severe intensity because of his symptom severity and steroid dependency. His response to budesonide suggests an inflammatory component to his disease.
The Crohn Disease Activity Index is the gold standard for defining Crohn disease clinical activity and evaluating clinical response and remission in randomized controlled trials12 (Table 1). However, because it is cumbersome, uses subjective variables, and has high interobserver variability, the Crohn Disease Activity Index is significantly limited.13,14 These limitations have motivated the recent use of endoscopic healing as a preferred end point for clinical trials. However, the use of endoscopic healing also has limitations. The Montreal Classification is often used in clinical trials and classifies patients based on age of diagnosis, location of disease, and disease phenotype.15
Table 1. Crohn Disease Activity Index (CDAI)a.
| Variable | Description | Score | Multiplier |
|---|---|---|---|
| No. of liquid stools | Sum of 7 days | 2 | |
| Abdominal pain | Sum of 7 days | 0 = none; 1 = mild; 2 = moderate; 3 = severe | 5 |
| General well-being | Sum of 7 days | 0 = generally well; 1 = slightly under par; 2 = poor; 3 = very poor; 4 = terrible | 7 |
| Extraintestinal complications | Number of listed complications | Arthritis, arthralgia, iritis, uveitis, erythema nodosum, pyoderma gangrenosum, aphthous stomatitis, anal fissure/fistula/abscess, fever >37.8°C (100°F) | 20 |
| Antidiarrheal drugs | Use in the previous 7 days | 0 = no; 1 = yes | 30 |
| Abdominal mass | 0 = no; 2 = questionable; 5 = definite | 10 | |
| Hematocrit | Expected minus observed level | Male: 47% – observed; female: 42% – observed | 6 |
| Body weight | Use in the previous 7 days | 1 – (ideal observed) × 100 | 1 (not if <10) |
Remission: CDAI score <150. Response: decrease in CDAI score of >70 or >100 (depending on trial). Moderate to severe Crohn disease: CDAI score of 220-450. Severe Crohn disease: CDAI score >450.12
Natural History
The pattern of Mr C's symptoms is not unusual. Like many chronic diseases, the course of Crohn disease tends to be relapsing and remitting. Population-based studies from Scandinavia suggest that after initial diagnosis, 50% of patients with Crohn disease relapse within 1 year. Over 4 years, 22% of patients are in remission, 25% have chronic active symptoms, and the majority of patients (53%) have a fluctuating course.16,17
Although most patients with Crohn disease initially present with inflammatory disease, with time the disease progresses such that most patients develop stricturing or penetrating complications.3 These complications result in a high surgery rate in Crohn disease; in some studies, surgery is as high as 50% within 10 years of initial diagnosis.18 Surgery for Crohn disease is not curative. Within 5 years of an operation, about half of patients relapse and many ultimately require a repeat resection.19
Goals Of Treatment
The conventional approach for Crohn disease treatment is administration of mesalamines and multiple courses of corticosteroids prior to escalation to immunosuppressive therapy. This is what Mr C received. However, this approach does not decrease complication or surgery rates.20 Long-term outcomes may be improved by earlier use of bio-logics and induction of mucosal healing.21 Treatment paradigms and goals have changed as these newer and more effective therapies have become available. Anti–tumor necrosis factor (TNF) therapies are being used much earlier in the course of disease, mesalamines are no longer recommended, and the goals of therapy are being expanded to include mucosal healing.22
Treatment goals include improving patient symptoms, inducing and maintaining a steroid-free clinical remission, improving quality of life, and minimizing toxicity.22 Accumulating evidence suggests that mucosal healing is an important treatment goal because it may be associated with decreased likelihood of a flare, progression to complications, and need for hospitalization and surgery.21,23 Outcome studies regarding endoscopic healing are not definitive as most of the evidence is indirect and methodological concerns limit these studies. Prospective studies assessing the effect of treating to “deep remission” (clinical plus endoscopic remission) are ongoing and will hopefully further clarify the role of mucosal healing. The main treatment goal is steroid-free clinical remission, but deep remission may become the ultimate therapeutic target. Currently, there is not enough evidence to support more intensive therapy for all patients already in clinical remission who continue to have endoscopic evidence of ongoing inflammation. Use of the end point of mucosal healing may be appropriate for some patients with a more aggressive disease phenotype or extensive disease.
Recently issued national and international guidelines for the treatment of Crohn disease continue to emphasize inducing and maintaining clinical remission as the main treatment goal.22,24 However, for the first time, endoscopic healing is mentioned as a treatment goal in the American College of Gastroenterology adult Crohn disease management guidelines: “Newer goals of therapy include induction and maintenance of mucosal (and histologic) healing.” But they also note that “novel endpoints for successful medical therapy … are needed.”22 Certainly, there are a number of questions that need to be answered before there is a paradigm shift in therapeutic objectives. However, the treatment of Crohn disease is rapidly evolving, and paradigms may change before the guidelines do. Recent data suggest that IBD guidelines are, on average, 4 years old and may contain dated recommendations. Additionally, the recommendations are not always consistent across societies.25
Medical Therapies
As shown in Table 2, a number of medical therapies are available for the treatment of Crohn disease. In the treatment of Crohn disease, it is important to differentiate between induction and maintenance of remission. It is also essential to take into account the severity and location of the disease.
Table 2. Medications Used for the Treatment of Crohn Disease and Evidence-Based Dataa.
| Medications | Induction of Remission, % of Patients | Level of Evidenceb | Maintenance of Remission, % of Patients | Level of Evidenceb | Common Adverse Effects | Rare Adverse Effects (<1% of Patients) |
|---|---|---|---|---|---|---|
| Aminosalicylates (sulfasalazine, mesalamine) | Limited or no role for mesalamine26,27 Sulfasalazine may be considered for mild Crohn colitis | III, A IIb, B | No role26,27 | III, B | Nausea, vomiting; headache; reversible male infertility (sulfasalazine) | Interstitial nephritis |
| Corticosteroids | 70-8028,29 | I, A | No evidence of benefit | III, A | Steroid adverse effects | |
| Budesonide | 50-7030,31 | I, A | No evidence of benefit beyond 6 mo | III, A | Steroid adverse effects | |
| Thiopurines (azathioprine, mercaptopurine) | 55 (Slower onset of action)32 | IIa, B | 50-7033 | I, A | Nausea, vomiting; pancreatitis; infection; bone marrow suppression; liver toxicity | Lymphoma |
| Methotrexate (intramuscular) | 40 (Slower onset of action)34 | IIa, B | 6535 | I, B | Nausea, vomiting; infection; liver toxicity; contraindicated in pregnancy | Pulmonary |
| Anti-TNFs (infliximab, adalimumab, certolizumab) | 60-8036-38 | I, A | 40-6036-41 | I, A | Infection; infusion/injection reactions | Lymphoma; heart failure; demyelination |
| Natalizumab | 61 (Indicated in anti-TNF failures)42 | IIa, B | 4442 | IIa, A; depends on JC virus antibody status | Infection | Progressive multifocal leukoencephalopathy; JC virus antibody test should be performed prior to initiation |
Abbreviation: TNF, tumor necrosis factor.
Antibiotics are used in the treatment of perianal Crohn disease but are not included because they have no proven benefit in luminal disease. It is difficult to provide a single value for rates of induction and maintenance of remission because of variations in populations, studies, and trial design, but this figure represents an overall estimate of response.
American Heart Association levels of evidence43:I: Benefit ⋙ risk. Treatment should be administered. IIa: Benefit ≫ risk, but additional studies with focused objectives are needed. It is reasonable to administer treatment. IIb: Benefit ≥ risk, but additional studies with broad objectives are needed; additional registry data would be helpful. Treatment may be considered. III: No benefit or harm. Procedure not helpful or harmful. Treatment has no proven benefit or is harmful. A: Multiple populations evaluated. Data derived from multiple randomized controlled trials or meta-analysis. B: Limited populations evaluated. Data derived from a single randomized controlled trial or nonrandomized studies. C: Very limited populations evaluated. Only consensus opinions of experts, case studies, or standard of care.
Mesalamines and Antibiotics
Although high-quality evidence exists supporting use of 5-aminosalicylates in ulcerative colitis, this is not the case for Crohn disease.26 These agents are no longer recommended for Crohn disease treatment, as only sulfasalazine has shown modest efficacy for treating active Crohn disease, though it has not been shown to maintain remission. Despite this, Mr C's treatment course exemplifies that mesalamine products remain among the most commonly prescribed drugs for Crohn disease.5 Most likely this is because of their low toxicity and physician comfort. The latest American College of Gastroenterology and European treatment guidelines recommend against use of 5-aminosalicylates to treat Crohn disease.22,27 Using ineffective treatments may negatively affect the course of Crohn disease by allowing the disease process to progress with the potential for structuring and penetrating complications and eventual surgery. There is also no clear role for the use of antibiotics in treatment of active Crohn disease, although they are used to treat suppurative complications and perianal disease.22,27
Corticosteroids
Systemically acting corticosteroids have been the mainstay therapy for Crohn disease. They very effectively induce remission of moderate to severe Crohn disease.44 These drugs do not maintain remission. Systemic corticosteroids are associated with significant long-term complications including an increased risk of serious infections and mortality in patients with Crohn disease.45 Enteric-coated budesonide, as used in Mr C's care, is an alternative to conventional steroids in patients with ileal and right-sided colonic Crohn disease.46 Because of its substantial first-pass metabolism in the liver, budesonide is associated with less toxicity than systemically absorbed steroids. Budesonide may delay relapse for up to 9 months, but it is not effective in maintaining remission of Crohn disease.46 Once steroids are given to a patient with moderate to severe Crohn disease, the physician should develop a plan to maintain steroid-free remission. This typically entails use of an immunomodulator or biologic.
Immunomodulators
Immunomodulators, thiopurines (azathioprine, mercaptopurine), and methotrexate are used primarily for maintenance of remission in patients with moderate to severe or steroid-dependent Crohn disease.33,34,47 Although these drugs may induce remission, they are suboptimal because of their slow onset of action.47 Typically, immunomodulators must be used together with a more rapid-onset medication. Adverse effects associated with immunomodulators include increased risk of infection, bone marrow suppression, and liver toxicity.24,48 They are also associated with an increased risk of nonmelanoma skin cancers.49 There is a 4- to 5-fold increased risk of non-Hodgkin lymphoma with thiopurines in this patient population.50 Patients taking immunomodulators require continual surveillance for complications, including skin examinations and routine monitoring of complete blood count and liver function tests.24,48
Biologics
The introduction of biologics has substantially improved Crohn disease treatment. Monoclonal antibodies directed against TNF effectively induce and maintain remission in patients with moderate to severe Crohn disease.36-39 Anti-TNFs are also effective for the treatment of perianal fistulas. There are 3 anti-TNFs available for the treatment of Crohn disease in the United States: infliximab, adalimumab, and certolizumab pegol. These compounds differ based on their chemical structure and mechanism of delivery. The adverse effects of the various anti-TNFs appear similar to each other, with infection being the most concerning. These drugs have also been associated with paradoxical psoriasis.51 There also is a slight increased risk of non-Hodgkin lymphoma, particularly in patients currently or previously exposed to thiopurines.52 Monotherapy with anti-TNFs does not appear to be associated with lymphoma. Melanoma is more common in patients receiving anti-TNFs.49 However, the most common adverse effect is infusion (infliximab) or injection site (adalimumab, certolizumab pegol) reactions, which are usually minor and easily controlled.53 As with corticosteroids and immunomodulators, close monitoring of patients receiving anti-TNFs is required.
Natalizumab is a monoclonal antibody directed against α4 integrins. It is the first of a class of biological selective adhesion molecule inhibitors that prevent recruitment and trafficking of leukocytes across the vascular endothelium. Natalizumab is effective for the induction and maintenance of remission in patients with moderate to severe Crohn disease.42 Because of a risk of progressive multifocal leukoencephalopathy, the US Food and Drug Administration has restricted the use of this drug to patients in whom therapy with anti-TNFs has failed.54 Fear of progressive multifocal leukoencephalopathy has limited the use of this drug in Crohn disease. A blood test for the JC virus antibody was recently introduced that facilitates risk stratification and may make natalizumab therapy more practical. Patients receiving natalizumab must be closely monitored for toxic effects and may continue the drug only if efficacy is demonstrated.55
Current Controversies
Controversy exists regarding which patients should be treated with a combination therapy of immunomodulators and anti-TNFs and which should be treated with monotherapy. Good evidence suggests that biologic and immunomodulator-naive patients with moderate to severe Crohn disease have improved outcomes with combination therapy.56 In the SONIC trial, combination therapy was more effective than infliximab, which, in turn, was more effective than azathioprine at maintaining clinical and endoscopic remission at 26 weeks and 54 weeks.56 Azathioprine appears to decrease anti-infliximab antibodies and increase infliximab levels. It is not clear if azathioprine confers additional antiinflammatory effects. The benefits of combination therapy beyond 1 year are unknown in this group of patients with moderate to severe Crohn disease previously naive to immunomodulators and anti-TNFs. Questions remain about optimal induction strategies and whether combination therapy should be continued indefinitely or eventually changed to monotherapy.57,58 For patients in whom immunomodulator therapy fails, the benefit of combination therapy is less clear.36-38,40 Some, but not all, studies show an association between combination therapy and adverse events, particularly for non-Hodgkin lymphoma and opportunistic infections.52,59 More information is needed regarding which patients benefit from combination therapy and what is the optimal duration of treatment.
Risk factors for debilitating disease, including ileal Crohn disease, perianal fistulas, and steroid requirement, may be used when weighing the risks and benefits of combination therapy vs monotherapy.60 Patients with severe endoscopic lesions and those with more extensive disease and high-risk anatomy (lesions in the foregut and rectal areas) may benefit from combination therapy. In Mr C's situation, the CT scan should be reviewed to assess the extent of ileal disease.
Controversy exists regarding which patients with Crohn disease require “early aggressive therapy” vs those who can be treated with more conventional management. Early data for aggressive therapy suggest it may improve the natural history of Crohn disease.61 Predictors of aggressive Crohn disease may be helpful in risk stratification and deciding who would benefit from early aggressive therapy. Improved understanding of Crohn genetics and biomarker identification will hopefully facilitate risk stratification and identification of patients who will benefit from aggressive and earlier treatment.62,63
Surgery
The majority of patients with Crohn disease require surgery over the course of their lifetimes. A subgroup of patients require multiple operations.18 Surgery is not curative and the disease recurs in most patients within 5 years.19,64 Surgery is typically used to treat the penetrating and stricturing complications of Crohn disease. It is also used when medical therapy fails or patients remain steroid dependent. Uncontrolled bleeding, toxic megacolon, and dysplasia and malignancy are also indications for surgery.
Recommendations For Mr C
Mr C is a 41-year-old man with moderate to severe ileal Crohn disease whose response to budesonide suggests an inflammatory component of his disease. Because he required multiple courses of budesonide over a short period, he should be considered steroid dependent. It is unclear from his history if he was truly responsive to mercaptopurine, since it was discontinued soon after initiation. It is also unclear if his nausea was related to the mercaptopurine.
At this time, Mr C has active Crohn disease. Treatment options for Mr C include azathioprine/mercaptopurine, anti-TNF, methotrexate, combination therapy, or a limited ileal resection. Prior to making this decision, I would recommend a magnetic resonance enterography to better assess the extent and activity of his Crohn disease and to rule out stricturing of the terminal ileum. The potential risks and benefits of the various options should be discussed with Mr C. With the data available, several different approaches could be supported, though I would favor another trial of mercaptopurine. I am not convinced that mercaptopurine failed in Mr C. Even if the nausea was believed to be an adverse effect (and this is not clear), he is still a candidate for azathioprine. A significant percentage of patients who do not tolerate mercaptopurine do tolerate azathioprine and vice versa.65 The recommended dose of mercaptopurine is 1 to 1.5 mg/kg, significantly higher than what Mr C was receiving.22,27 The recommended dose of azathioprine is 2 to 3 mg/kg. Methotrexate is another option in steroid-dependent Crohn disease if thiopurines fail or are not tolerated. However, I would recommend an anti-TNF drug based on its efficacy. This could be considered either alone or in combination with an immunomodulator. If combination therapy is chosen, consideration could be given to stopping one of the medications after a year as no data exist that it is beneficial after this time.57,58 Because removing the ileocecal valve has long-term consequences including an increased risk of small intestinal bacterial overgrowth and altered bowel function, I would try medical therapy before recommending a resection unless there was clear evidence of a tight ileal stricture.
Future Management Of Crohn Disease
The management of Crohn disease is rapidly changing. A number of promising drugs are in development that will cause treatment approaches to evolve further. Vedolizumab, a selective adhesion molecule inhibitor, is a humanized monoclonal IgG1 antibody directed at integrin α4β7 that appears to be effective in treating Crohn disease.66,67 As its activity is focused on mucosal addressin cell adhesion molecule 1, which is virtually exclusively expressed in the gastrointestinal tract, vedolizumab likely has advantages over natalizumab in terms of the risk of progressive multifocal leukoencephalopathy. Ustekinumab is a monoclonal antibody that blocks interleukins 12 and 23 through their common p40 subunit and is currently approved by the US Food and Drug Administration for psoriasis; early studies in Crohn disease are hopeful.68 Other therapeutic pathways are being explored, including inhibition of janus kinase.
Hopefully, the research on the genetics of Crohn disease and the human microbiome will help uncover the cause of Crohn disease and result in new treatment options and/or Crohn disease prevention strategies. Because these research areas are in their infancy, current emphasis is on greater understanding of new treatment paradigms and their potential to improve patient outcomes. Further studies of genetic, serologic, and clinical markers predicting disease severity and identification of patients most likely to benefit from early aggressive or combination therapy are ongoing. Additionally, studies determining whether the goal of therapy should be clinical remission or deep remission are under way.
Questions And Discussion
Question: What is the known about the pathogenesis of Crohn disease?
Dr Cheifetz
We are making headway in determining the pathogenesis of Crohn disease. There seems to be interplay between host genetics, the immune system, and the environment. The current hypothesis is that following an environmental hit that likely alters the commensal flora or mucosal barrier, a genetically susceptible individual develops an abnormal immune response to the commensal gastrointestinal flora.69,70 More than 100 susceptibility genes and loci have already been identified in Crohn disease.71 Further studies on the genetics of IBD and the microbiome are ongoing and will hopefully further elucidate what causes Crohn disease.
Question: What is the risk of colon cancer in this population and how should patients be screened?
Dr Cheifetz
Patients with extensive Crohn colitis (involving at least one-third of the colon) are at increased risk of colorectal cancer.72 More recent studies suggest that while not as high as previously thought, the risk is approximately 2 to 3 times that of the general population.73 Further risk modifiers include duration of disease, extent of disease, activity of disease, family history of colon cancer, and primary sclerosing cholangitis.74 The most recent US guidelines74 recommend a screening colonoscopy after 8 years of disease and then every 1 to 3 years based on their specific risk factors for colorectal cancer. Patients with primary sclerosing cholangitis should have annual colonoscopies from the time of diagnosis. Although recent studies suggest that most dysplasia is visible, the typical practice is to obtain 4-quadrant biopsies every 10 cm (>32 biopsies) to assess for microscopic dysplasia and targeted biopsies of abnormal areas. The current guidelines allow those with expertise to perform chromoendoscopy with dilute indigo carmine or methylene blue in addition to targeted biopsies of abnormal-appearing mucosa, as this has been associated with a 2- to 3-fold increased detection rate of dysplasia.74-76
Question: One problem is the cost of medication, particularly the biologics. How will this affect future management of Crohn disease?
Dr Cheifetz
As far as biologics are concerned, I agree they are quite expensive. However, studies suggest that the majority of the cost of treating Crohn disease comes from hospitalizations and surgeries.77 Cost-effectiveness analyses suggest that anti-TNFs are cost-effective or cost-saving because they prevent hospitalizations and surgeries.78,79 Additionally, keeping patients in remission and at work allows for cost savings elsewhere. Prescribing drugs such as mesalamine and antibiotics, though they are less expensive, but for which there is little evidence, may allow the disease to progress and lead to complications and overall higher costs.
Dr Libman
Mr C is a 41-year-old man who experiences intermittent abdominal pain that is sometimes generalized and other times localized to the right lower quadrant. There is no association of the pain with fever, weight loss, or other gastrointestinal (GI) symptoms. Empirical treatment with omeprazole temporarily improved his symptoms. With time, his pain has worsened, and an abdominal computed tomography (CT) scan revealed partial obstruction and thickening of the distal ileum. In November 2010, Mr C was referred to a gastroenterologist, who performed a colonoscopy that demonstrated ileitis and a stricture 2 cm proximal to the ileocecal valve. Histological examination of an ileal biopsy showed chronic, focally active ileitis. A diagnosis of Crohn disease was established.
Mesalamine was started, followed by budesonide, with some improvement. Mr C's pain recurred on completion of the budesonide course. Two more courses of budesonide were given, with temporary improvement. Mercaptopurine, 75 mg/d, was given and produced an indeterminate response. The mercaptopurine was stopped after 2 months because of nausea. A year after his diagnosis, Mr C is at a therapeutic crossroads, having had several medical treatments fail for his Crohn disease. He wants to know his treatment options.
He is taking mesalamine, 1000 mg orally twice per day, a multivitamin, a vitamin D supplement, and a probiotic. He has no known allergies.
Mr C is married with 2 children. He works as a researcher, drinks alcohol occasionally, and does not smoke cigarettes. He has no family history of inflammatory bowel disease (IBD) or colon cancer.
Footnotes
The conference on which this article is based took place at the General Medicine Grand Rounds at Beth Israel Deaconess Medical Center, Boston, Massachusetts, on January 5, 2012.
Clinical Crossroads at Beth Israel Deaconess Medical Center is produced and edited by Risa B. Burns, MD, series editor; Tom Delbanco, MD, Howard Libman, MD, Eileen E. Reynolds, MD, Marc Schermerhorn, MD, Amy N. Ship, MD, and Anjala V. Tess, MD.
Clinical Crossroads Section Editor: Edward H. Livingston, MD, Deputy Editor, JAMA.
Conflict of Interest Disclosures: The author has completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Cheifetz reports participation in advisory boards for UCB, Janssen (previously Centocor), Abbott, Warner-Chillcot (previously Procter & Gamble), Shire, Given Imaging, and Prometheus. He reports research support from Pfizer and the National Institute of Diabetes and Digestive and Kidney Diseases (grant 1R01DK092235-01A1).
Additional Contributions: We thank the patient for sharing his story and for providing permission to publish it. Pathology images were provided by Robert M. Najarian, MD, and radiology images by Martin P. Smith, MD, both of Beth Israel Deaconess Medical Center and Harvard Medical School.
References
- 1.Kurata JH, Kantor-Fish S, Frankl H, et al. Crohn's disease among ethnic groups in a large health maintenance organization. Gastroenterology. 1992;102(6):1940–1948. doi: 10.1016/0016-5085(92)90317-r. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV, Jr, Silverstein MD, Sandborn WJ, et al. Crohn's disease in Olmsted County, Minnesota, 1940-1993. Gastroenterology. 1998;114(6):1161–1168. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 3.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8(4):244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Singh B, Mortensen NJ, Jewell DP, George B. Perianal Crohn's disease. Br J Surg. 2004;91(7):801–814. doi: 10.1002/bjs.4613. [DOI] [PubMed] [Google Scholar]
- 5.Vavricka SR, Spigaglia SM, Rogler G, et al. Swiss IBD Cohort Study Group. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(3):496–505. doi: 10.1002/ibd.21719. [DOI] [PubMed] [Google Scholar]
- 6.Silverstein MD, Lashner BA, Hanauer SB, et al. Cigarette smoking in Crohn's disease. Am J Gastroenterol. 1989;84(1):31–33. [PubMed] [Google Scholar]
- 7.Heresbach D, Alexandre JL, Branger B, et al. ABERMAD (Association Bretonne d'Etude et de Recherche sur les Maladies de l'Appareil Digestif) Frequency and significance of granulomas in a cohort of incident cases of Crohn's disease. Gut. 2005;54(2):215–222. doi: 10.1136/gut.2004.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombel JF, Solem CA, Sandborn WJ, et al. Quantitative measurement and visual assessment of ileal Crohn's disease activity by computed tomography enterography. Gut. 2006;55(11):1561–1567. doi: 10.1136/gut.2005.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann EM, Al-Hawary MM. MRI of the small bowel in patients with Crohn's disease. Curr Opin Gastroenterol. 2011;27(2):132–138. doi: 10.1097/MOG.0b013e3283438e5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty GA, Moss AC, Cheifetz AS. Capsule endoscopy for small-bowel evaluation in Crohn's disease. Gastrointest Endosc. 2011;74(1):167–175. doi: 10.1016/j.gie.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 11.Cheifetz AS, Kornbluth AA, Legnani P, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn's disease. Am J Gastroenterol. 2006;101(10):2218–2222. doi: 10.1111/j.1572-0241.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 12.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. Gastroenterology. 1976;70(3):439–444. [PubMed] [Google Scholar]
- 13.Sands BE, Ooi CJ. A survey of methodological variation in the Crohn's disease activity index. Inflamm Bowel Dis. 2005;11(2):133–138. doi: 10.1097/00054725-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lahiff C, Safaie P, Awais A, et al. The Crohn's disease activity index (CDAI) is similarly elevated in patients with Crohn's disease and in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2013;37(8):786–794. doi: 10.1111/apt.12262. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease. Can J Gastroenterol. 2005;19(suppl A):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 16.Moum B, Ekbom A, Vatn MH, et al. Clinical course during the 1st year after diagnosis in ulcerative colitis and Crohn's disease. Scand J Gastroenterol. 1997;32(10):1005–1012. doi: 10.3109/00365529709011217. [DOI] [PubMed] [Google Scholar]
- 17.Munkholm P, Langholz E, Davidsen M, Binder V. Disease activity courses in a regional cohort of Crohn's disease patients. Scand J Gastroenterol. 1995;30(7):699–706. doi: 10.3109/00365529509096316. [DOI] [PubMed] [Google Scholar]
- 18.Romberg-Camps MJ, Dagnelie PC, Kester AD, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009;104(2):371–383. doi: 10.1038/ajg.2008.38. [DOI] [PubMed] [Google Scholar]
- 19.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99(4):956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 20.Cosnes J, Nion-Larmurier I, Beaugerie L, et al. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005;54(2):237–241. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baert F, Moortgat L, Van Assche G, et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010;138(2):463–468. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein GR, Hanauer SB, Sandborn WJ Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009;104(2):465–483. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 23.Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis. 2009;15(9):1295–1301. doi: 10.1002/ibd.20927. [DOI] [PubMed] [Google Scholar]
- 24.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130(3):935–939. doi: 10.1053/j.gastro.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 25.Feuerstein JD, Akbari M, Gifford AE, et al. Systematic review: the quality of the scientific evidence and conflicts of interest in international inflammatory bowel disease practice guidelines. Aliment Pharmacol Ther. 2013;37(10):937–946. doi: 10.1111/apt.12290. [DOI] [PubMed] [Google Scholar]
- 26.Hanauer SB, Strömberg U. Oral Pentasa in the treatment of active Crohn's disease. Clin Gastroenterol Hepatol. 2004;2(5):379–388. doi: 10.1016/s1542-3565(04)00122-3. [DOI] [PubMed] [Google Scholar]
- 27.Dignass A, Van Assche G, Lindsay JO, et al. European Crohn's and Colitis Organisation. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management [published correction appears. J Crohns Colitis. 2010;4(3):353. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]; J Crohns Colitis. 2010;4(1):28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Malchow H, Ewe K, Brandes JW, et al. European Cooperative Crohn's Disease Study (ECCDS) Gastroenterology. 1984;86(2):249–266. [PubMed] [Google Scholar]
- 29.Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;(2):CD006792. doi: 10.1002/14651858.CD006792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomsen OO, Cortot A, Jewell D, et al. International Budesonide-Mesalamine Study Group. A comparison of budesonide and mesalamine for active Crohn's disease. N Engl J Med. 1998;339(6):370–374. doi: 10.1056/NEJM199808063390603. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg GR, Feagan BG, Martin F, et al. Canadian Inflammatory Bowel Disease Study Group. Oral budesonide for active Crohn's disease. N Engl J Med. 1994;331(13):836–841. doi: 10.1056/NEJM199409293311303. [DOI] [PubMed] [Google Scholar]
- 32.Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2009;(4):CD000545. doi: 10.1002/14651858.CD000545.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2009;(1):CD000067. doi: 10.1002/14651858.CD000067.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Feagan BG, Rochon J, Fedorak RN, et al. North American Crohn's Study Group Investigators. Methotrexate for the treatment of Crohn's disease. N Engl J Med. 1995;332(5):292–297. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- 35.Feagan BG, Fedorak RN, Irvine EJ, et al. North American Crohn's Study Group Investigators. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. N Engl J Med. 2000;342(22):1627–1632. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 36.Hanauer SB, Feagan BG, Lichtenstein GR, et al. ACCENT I Study Group. Maintenance infliximab for Crohn's disease. Lancet. 2002;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 37.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease. Gastroenterology. 2006;130(2):323–333. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 38.Sandborn WJ, Feagan BG, Stoinov S, et al. PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357(3):228–238. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 39.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn's disease. Gut. 2007;56(9):1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease. Gastroenterology. 2007;132(1):52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 41.Sands BE, Blank MA, Diamond RH, Barrett JP, Van Deventer SJ. Maintenance infliximab does not result in increased abscess development in fistulizing Crohn's disease. Aliment Pharmacol Ther. 2006;23(8):1127–1136. doi: 10.1111/j.1365-2036.2006.02878.x. [DOI] [PubMed] [Google Scholar]
- 42.Sandborn WJ, Colombel JF, Enns R, et al. ENACT-1 Trial Group; ENACT-2 Trial Group. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005;353(18):1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 43.Gibbons RJ, Smith S, Antman E American College of Cardiology; American Heart Association. American College of Cardiology/American Heart Association clinical practice guidelines, I: where do they come from? Circulation. 2003;107(23):2979–2986. doi: 10.1161/01.CIR.0000063682.20730.A5. [DOI] [PubMed] [Google Scholar]
- 44.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121(2):255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 45.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn's disease. Clin Gastroenterol Hepatol. 2006;4(5):621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Seow CH, Benchimol EI, Griffiths AM, et al. Budesonide for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;(3):CD000296. doi: 10.1002/14651858.CD000296.pub3. [DOI] [PubMed] [Google Scholar]
- 47.Sandborn W, Sutherland L, Pearson D, et al. Azathioprine or 6-mercaptopurine for inducing remission of Crohn's disease. Cochrane Database Syst Rev. 2000;(2):CD000545. doi: 10.1002/14651858.CD000545. [DOI] [PubMed] [Google Scholar]
- 48.Travis SP, Stange EF, Lémann M, et al. European Crohn's and Colitis Organisation. European evidence based consensus on the diagnosis and management of Crohn's disease. Gut. 2006;55(suppl 1):i16–i35. doi: 10.1136/gut.2005.081950b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390–399. doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beaugerie L, Brousse N, Bouvier AM, et al. CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease. Lancet. 2009;374(9701):1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 51.Cullen G, Kroshinsky D, Cheifetz AS, Korzenik JR. Psoriasis associated with anti-tumour necrosis factor therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34(11-12):1318–1327. doi: 10.1111/j.1365-2036.2011.04866.x. [DOI] [PubMed] [Google Scholar]
- 52.Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease. Clin Gastroenterol Hepatol. 2009;7(8):874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab. Am J Gastroenterol. 2003;98(6):1315–1324. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 54.Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med. 2005;353(4):362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 55.Van Assche G, Lewis JD, Lichtenstein GR, et al. The London position statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organisation: safety. Am J Gastroenterol. 2011;106(9):1594–1602. doi: 10.1038/ajg.2011.211. [DOI] [PubMed] [Google Scholar]
- 56.Colombel JF, Sandborn WJ, Reinisch W, et al. SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N EnglJ Med. 2010;362(15):1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 57.Treton X, Bouhnik Y, Mary JY, et al. Groupe D'Etude Thérapeutique Des Affections Inflammatoires Du Tube Digestif. Azathioprine withdrawal in patients with Crohn's disease maintained on prolonged remission. Clin Gastroenterol Hepatol. 2009;7(1):80–85. doi: 10.1016/j.cgh.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 58.Louis E, Mary JY, Vernier-Massouille G, et al. Groupe D'Etudes Thérapeutiques Des Affections Inflammatoires Digestives. Maintenance of remission among patients with Crohn's disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142(1):63–70. doi: 10.1053/j.gastro.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 59.Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn's disease. Gastroenterology. 2006;130(3):650–656. doi: 10.1053/j.gastro.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 61.D'Haens G, Baert F, van Assche G, et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease. Lancet. 2008;371(9613):660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 62.Vasiliauskas EA, Kam LY, Karp LC, et al. Marker antibody expression stratifies Crohn's disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut. 2000;47(4):487–496. doi: 10.1136/gut.47.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mow WS, Vasiliauskas EA, Lin YC, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology. 2004;126(2):414–424. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 64.McLeod RS, Wolff BG, Steinhart AH, et al. Risk and significance of endoscopic/radiological evidence of recurrent Crohn's disease. Gastroenterology. 1997;113(6):1823–1827. doi: 10.1016/s0016-5085(97)70001-5. [DOI] [PubMed] [Google Scholar]
- 65.Lees CW, Maan AK, Hansoti B, et al. Tolerability and safety of mercaptopurine in azathioprine-intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;27(3):220–227. doi: 10.1111/j.1365-2036.2007.03570.x. [DOI] [PubMed] [Google Scholar]
- 66.Feagan BG, Greenberg GR, Wild G, et al. Treatment of active Crohn's disease with MLN0002, a humanized antibody to the α4β7 integrin. Clin Gastroenterol Hepatol. 2008;6(12):1370–1377. doi: 10.1016/j.cgh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the α4β7 integrin. N Engl J Med. 2005;352(24):2499–2507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 68.Sandborn WJ, Feagan BG, Fedorak RN, et al. Ustekinumab Crohn's Disease Study Group. A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology. 2008;135(4):1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 69.Korzenik JR. Is Crohn's disease due to defective immunity? Gut. 2007;56(1):2–5. doi: 10.1136/gut.2006.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stappenbeck TS, Rioux JD, Mizoguchi A, et al. Crohn disease: a current perspective on genetics, autophagy and immunity. Autophagy. 2011;7(4):355–374. doi: 10.4161/auto.7.4.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140(6):1704–1712. doi: 10.1053/j.gastro.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Canavan C, Abrams KR, Mayberry J. Colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006;23(8):1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 73.Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143(2):375–381. doi: 10.1053/j.gastro.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 74.Farraye FA, Odze RD, Eaden J, et al. AGA Institute Medical Position Panel on Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138(2):738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 75.Biancone L, Michetti P, Travis S, et al. European Crohn's and Colitis Organisation. European evidence-based consensus on the management of ulcerative colitis. J Crohns Colitis. 2008;2(1):63–92. doi: 10.1016/j.crohns.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Kornbluth A, Sachar DB Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults (update) Am J Gastroenterol. 2004;99(7):1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 77.Odes S. How expensive is inflammatory bowel disease? World J Gastroenterol. 2008;14(43):6641–6647. doi: 10.3748/wjg.14.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bodger K, Kikuchi T, Hughes D. Cost-effectiveness of biological therapy for Crohn's disease. Aliment Pharmacol Ther. 2009;30(3):265–274. doi: 10.1111/j.1365-2036.2009.04033.x. [DOI] [PubMed] [Google Scholar]
- 79.Ananthakrishnan AN, Korzenik JR, Hur C. Can mucosal healing be a cost-effective endpoint for biologic therapy in Crohn's disease? Inflamm Bowel Dis. 2013;19(1):37–44. doi: 10.1002/ibd.22951. [DOI] [PubMed] [Google Scholar]


