Abstract
Plants of the genus Calathea possess many leaf colors, and they are economically important because they are widely used as ornamentals for interior landscaping. Physiological performances and photosynthetic capacities of C. insignis and C. makoyana were investigated. The photosynthetic efficiencies of C. insignis and C. makoyana were significantly increased when the photosynthetic photon flux density (PPFD) increased from 0 to 600 μmol photons·m−2·s−1 and became saturated with a further increase in the PPFD. The two Calathea species had lower values of both the light saturation point and maximal photosynthetic rate, which indicated that they are shade plants. No significant differences in predawn Fv/Fm values (close to 0.8) were observed between dark-green (DG) and light-green (LG) leaf sectors in all tested leaves. However, the effective quantum yield of photosystem II largely decreased as the PPFD increased. An increase in the apparent photosynthetic electron transport rate was observed in both species to a maximum at 600 μmol·m−2·s−1 PPFD, following by a decrease to 1500 μmol·m−2·s−1 PPFD. Compared to LG leaf extracts, DG leaf extracts contained higher levels of chlorophyll (Chl) a, Chl b, Chls a + b, carotenoids (Cars), anthocyanins (Ants), flavonoids (Flas), and polyphenols (PPs) in all plants, except for the Ant, Fla and PP contents of C. insignis plants. Calathea insignis also contained significantly higher levels of total protein than did C. makoyana. The adjusted normalized difference vegetation index (NDVI), photochemical reflectance index (PRI), red-green, and flavonol index (FlavI) were significantly correlated to leaf Chls a + b, Cars, Ants, and Flas in C. makoyana, respectively, and can be used as indicators to characterize the physiology of these plants.
Keywords: chlorophyll fluorescence, leaf sector, ornamental plant, photosynthesis, secondary metabolite, spectral reflectance
1. Introduction
Calathea plants belong to the Marantaceae family, which is native to South America and is mainly distributed in tropical regions of Africa and Asia [1]. It comprises 300 species; among them, Calathea insignis and C. makoyana are widely produced as ornamental foliage plants for interior landscaping due to their leaf patterns, colors, textures, and shapes [2]. Leaves of C. makoyana have lobed, dark-green (DG) regions extending from the mid vein, which sometimes merge with the DG margin [3]. However, C. insignis leaves are narrow, tapering, stiffly erect foliage with wavy margins, and look yellowish-green with alternating lateral large and small oval patches, with DG markings on the upper surface and a dark, purplish-red lower surface [4]. Moreover, C. insignis is an important source of nutrition, as it is known for the high quality of starch in its tubers and roots [5] and it is rich in both macro- and micronutrients [6]. The aerial parts of C. insignis and the residue after starch extraction can be used as a food source for animals or as raw material for the paper industry [7]. Both C. insignis and C. makoyana are perennial evergreen, herbaceous plants with potential sources of secondary metabolite compounds, and are considered to be economically plants [3,6]. However, there is limited information available regarding the photosynthetic physiology of these plants. Understanding the photosynthetic characteristics of C. insignis and C. makoyana would benefit field cultivation management.
Light intensity is one of the major factors that affect the growth, leaf form, floral morphology, and biochemical characterization of plants, and is also associated with their photosynthesis efficiency of plants [8]. At high light intensities, photosynthetic carbon fixation increases, but the excess light is a stressor and therefore causes depression of photosynthetic efficiency [9]. The study of photosynthesis-irradiance relationships is a basic aspect of plant ecophysiological research and is important for managing rare species; photosynthetic light-response curves are used to assess the ability to capture light and understand the optimal habitat light conditions of plants [10]. Insufficient light can limit photosynthesis, which causes reductions in net carbon gains and plant growth. Contrarily, under high irradiance, the light reaction can absorb more photons than can be used by carbon fixation reactions, e.g., by leaves in the upper canopy layer exposed to the sun and also by shade leaves exposed to sunflecks. The excessive absorbed energy can lead to reductions in the efficiency of photosystems (PSs), especially Photosystem II (PSII) [11]. Stress decreases the ability of photosynthetic systems to utilize incident photons, thus leading to photoinhibition, and reduced quantum yields of photochemistry and chlorophyll (Chl) fluorescence (ChlF). Photoinhibition causes inhibition of PSII, while also increasing thermal de-excitation of excited Chl [12]. Electrons transferred from PSII to Photosystem I PSI are used by downstream electron sink pathways, including photosynthetic carbon fixation and photorespiration. When carbon fixation becomes saturated, photosynthesis is unable to use all of the energy absorbed by the plants under high light irradiance [13]. Plants adapt photosynthesis to a certain degree in response to the prevailing environment, and the sensitivity of photosynthesis to stress varies among plant species and cultivars.
In the field, biochemical regulation of photosynthesis maintains a balance between rates of component processes and metabolite concentrations when exposed to environmental changes [14]. One study reported that the photosynthetic performance was related to dissipating excess radiation by plants, including interconversion of xanthophyll pigments, formation of thylakoid ∆pH, and conformational changes in chloroplasts [15]. Chloroplasts are the main target of many environmental stress factors. Plants respond to fluctuations in irradiance and temperature via their chloroplast molecular redox signaling transduction mechanisms that induce marked modulations in chloroplast components, ultimately leading to acclimation of the photosynthetic apparatus [16]. The photosynthetic light response varies by plant species, and ChlF measurement is a simple and reliable method for estimating photosynthesis [17,18]. Several ChlF parameters are highly sensitive markers of a plant’s physiological status, which can provide an expeditious way to clarify its physiological condition [19,20]. ChlF measurements were successfully used to study responses of plants to their environment; these included the status of the photosynthetic apparatus in plants and trees [21,22,23,24] in controlled environments and in nature. Knowledge of photosynthetic rates of plants under a broad range of environmental conditions and different light intensities is required for ecophysiological studies. However, photosynthesis by Calathea insignis and C. makoyana has not yet been examined. It is noteworthy that C. insignis and C. makoyana have a variety of leaf colors, and the literature has pointed out that leaf pigments affect photosynthesis and stress tolerance [25]. A better understanding of the photosynthesis of C. insignis and C. makoyana would aid the effective management of cultivation of those plants. The aim of this study was to investigate the physiological performance and photosynthetic capacities of C. insignis and C. makoyana. In addition, Chl, anthocyanin (Ant), carotenoid (Car), polyphenol (PP), and total flavonoid (Fla) contents of these plants were also examined. Precise management of the photosynthetic capacity in response to the photosynthesis photon flux density (PPFD) can potentially be used to maximize the efficiency of the growth, development, and metabolic potential of Calathea plants grown in controlled environments for economic benefits.
2. Results and Discussion
2.1. Photosynthetic Capacity and Fluorescence Parameters of C. insignis and C. makoyana
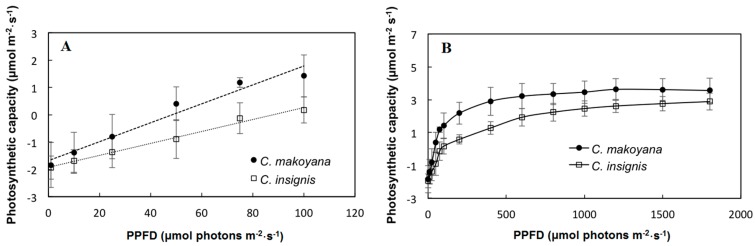
Environmental conditions are generally considered to be the main constraints to the growth, productivity, and distribution of plants. Figure 1 shows the photosynthetic capacities of C. insignis and C. makoyana in response to the PPFD. Photosynthetic rates of both C. insignis and C. makoyana were below 1.3 μmol·m−2·s−1 at 0–100 μmol·m−2·s−1 PPFD, and increased from 1.3 to 3.0 μmol·m−2·s−1 when the PPFD was 200–600 μmol·m−2·s−1 PPFD. However, the photosynthetic capacity became saturated with a further increase (600–1800 μmol·m−2·s−1 PPFD) in the PPFD. The photosynthetic light compensation points of C. insignis and C. makoyana were 100 and 50 μmol·m−2·s−1 PPFD, respectively. The photosynthetic light saturation points of C. insignis and C. makoyana were 600 and 400 μmol·m−2·s−1 PPFD, respectively. Thus, the photosynthetic capacity of C. makoyana was somewhat higher than that of C. insignis. By increasing the efficiency of photosynthesis, sun plants and sun leaves can efficiently use light energy, thus presenting higher light-saturation points and maximal photosynthetic rates. On the other hand, shade plants and shaded leaves tend to have contrasting behaviors [26,27]. Because these two Calathea species presented lower values for both the light saturation point and maximal photosynthetic rate, they are probably shade plants.
Figure 1.
Photosynthetic capacity of Calathea makoyana and C. insignis under 0–100 (A) and 0–1800 (B) μmol·m−2·s−1 photosynthetic photon flux density (PPFD). Vertical bars indicate the standard deviation (n = 5).
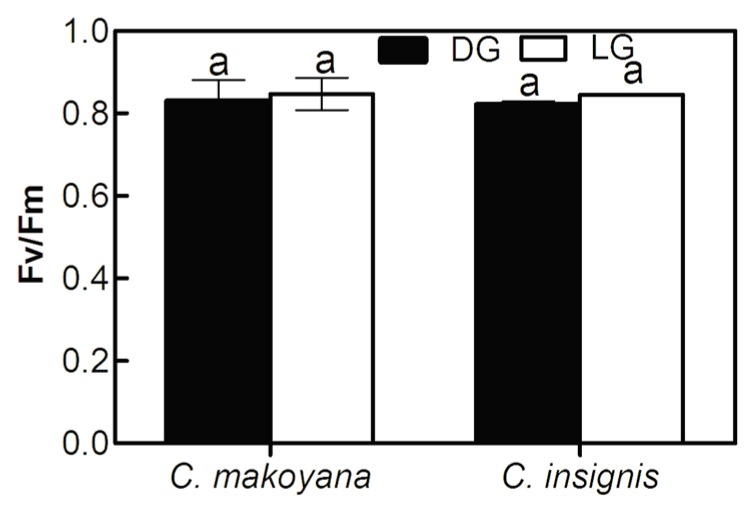
Photosynthesis is biochemically regulated to maintain a balance between the rates of component processes and metabolite concentrations. Photoinhibition of photosynthesis is indicated by reduced quantum yields of photochemistry and ChlF, including both inhibition of PSII and higher thermal de-excitation of excited Chl [12]. Moreover, the effective quantum yield of PSII was determined by measuring various ChlF parameters; these are highly sensitive indicators representing the physiological status of stressed plants [28]. In this study, the Fv/Fm values of C. insignis and C. makoyana were measured at predawn and compared between the DG and LG leaf sectors. As shown in Figure 2, predawn Fv/Fm values showed no significant differences (close to 0.8) between DG and LG leaves in either species. Under suitable growth conditions, a plant’s Fv/Fm value is around 0.83, but this value may be strongly depressed after exposure to stresses, which precipitates suppression of the electron transfer chain [29]. This index indicates the photosynthetic potential and the potential for photochemical dissipation, and it shows the PSII percentage that is open and also the effectiveness of capturing photo energy from light-harvesting complexes and subsequent quantum transfer. If the light energy that plants absorb is incompletely quenched, the activity of the photosynthesis system is deactivated by the excessive energy; this causes termination of the PSII reaction because of its partially reduced state [30]. Predawn Fv/Fm values of both C. insignis and C. makoyana obtained in this study were close to 0.8, indicating that there was no long-term photoinhibition, and a light intensity of 600 μmol·m−2·s−1 was suitable for growth of these plants.
Figure 2.
Fv/Fm value of dark green (black bar, DG) and light green (white bar, LG) leaf sectors in Calathea makoyana and C. insignis. Vertical bars indicate the standard deviation (n = 5). The same characters represent no significant difference (p < 0.05).
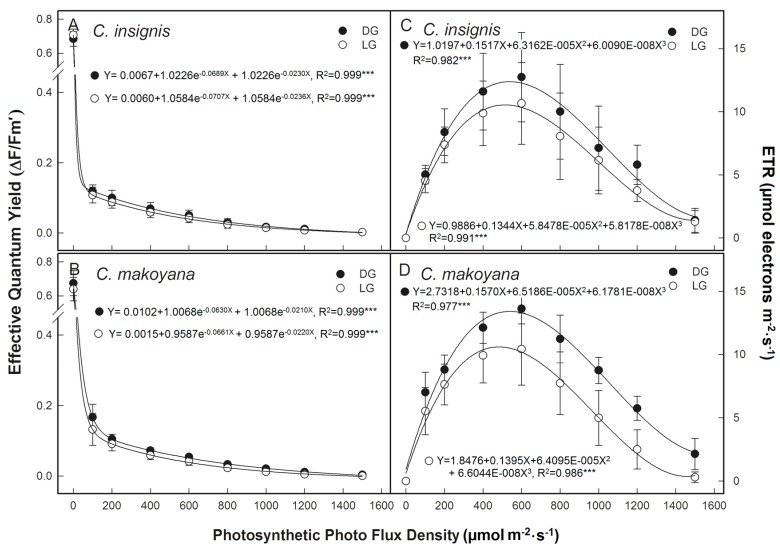
Under high irradiance, the light reaction is able to absorb more photons than the carbon reaction can use. This excess absorbed energy can lead to reduced efficiency of PSs, especially PSII [11,12]. To avoid damage caused by excessive absorbed energy, plants can use photoprotective mechanisms to counteract the harmful effects of excess photon absorption. Non-photochemical quenching (NPQ) quenches excess energy and downregulates the efficiency of the PS. It plays an important role in the photoprotection of the PS [31,32]. Figure 3A,B shows that when overnight dark-adapted leaves were suddenly exposed to light, ΔF/Fm’ sharply decreased in all leaves in a low light condition (<100 μmol·m−2·s−1 PPFD). Thereafter, ΔF/Fm’ slowly decreased with an increasing PPFD. This study used the rapid light-curve program, where the actinic light intensity was measured for only 30 s at each luminosity. The transient decline in ∆F/Fm’ in the initial stage of light induction was mainly due to downregulation of PSII efficiency [33,34,35,36]. This phenomenon indicates that both species had low photosynthetic rates (Figure 1), and needed to dissipate any excessive energy to protect the PS; therefore, both species exhibited high downregulation of PSII efficiency [36,37].
Figure 3.
Light response changes in effective quantum yield (ΔF/Fm’, (A,B)) and electron transport rate (ETR, (C,D)) from dark green leaf sector (black circle, DG) and light green leaf sector (white circle, LG) in Calathea makoyana and C. insignis. Vertical bars indicate the standard deviation (n = 5). *** p < 0.001.
As the light intensity changed from 0 to 600 μmol·m−2·s−1 PPFD, the ETR of both Calathea species continued to increase as indicated by the light response curves, followed by a decrease. Maximum values of electron transport rate (ETR) were 12.7 and 10.6 μmol e−1·m−2·s−1 for DG and LG of C. insignis, and were 13.6 and 10.4 μmol e−1·m−2·s−1 for DG and LG of C. makoyana, respectively. In addition, these ETR values at 1500 μmol·m−2·s−1 PPFD were nearly 0 μmol e−1·m−2·s−1 for both DG and LG of C. insignis, respectively, and were around 2 μmol e−1·m−2·s−1 for DG and 0 μmol e−1·m−2·s−1 for LG of C. makoyana (Figure 3C,D). The both values of ΔF/Fm’ and ETR were not significant different between DG and LG for C. makoyana and C. insignis. In addition, photosynthetic rates of the two Calathea species reached saturation at around 600 μmol·m−2·s−1 (Figure 1). These results indicated that Calathea plants appear to be adapted to low light intensities.
In these plants, the development of chloroplasts may be particularly sensitive to light intensity, or perhaps there are protective mechanisms which prevent the leaves from undergoing extreme reductions in PSII acceptors. PSI photoinhibition can be caused by an over-reduction of PSI acceptors and electron transport from PSII to PSI [38]. Strong incident light can be very harmful to PSI if the number of electrons entering the electron transfer chain by PSII surpasses the capacity of electron acceptors on the reducing side of PSI. Control of photoinhibition in PSII is the decisive regulator of the electron transfer chain, by providing a photoprotective mechanism against the formation of reactive oxygen species (ROS) and photodamage to PSI [39]. PSI uses contrasting strategies to deal with photo-oxidative stress.
2.2. Photosynthetic Pigments, Secondary Metabolite Compounds, and Total Protein Contents
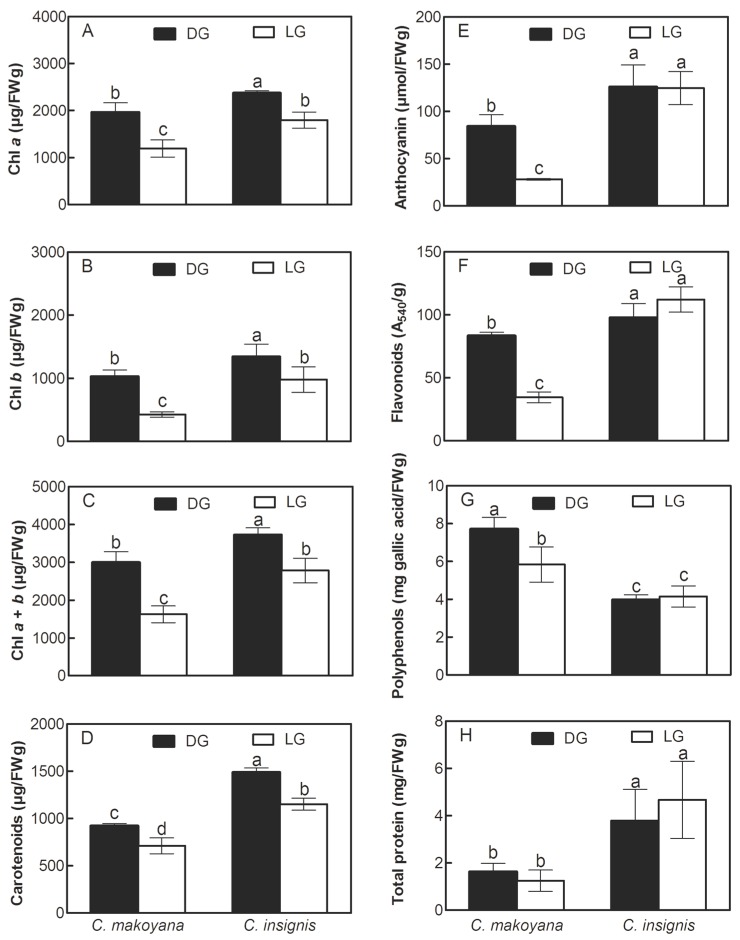
Calathea plants are very popular ornamentals in China and Taiwan, being used indoors for their beautiful foliage [7], and they are also treated as herbaceous plants with potential sources of metabolite compounds [3]. In this study, two leaf sectors of C. insignis and C. makoyana showed wide variations in pigments, secondary metabolite compounds, and total protein contents. Figure 4 presents contents of Chl a, Chl b, Chls a + b, Cars, Ants, Flas, PPs, and total protein in DG and LG leaf extracts of C. insignis and C. makoyana. Levels of Chl a, Chl b, total Chl, Cars, Ants, Flas, and PPs in DG leaves of C. makoyana were significantly higher than those of LG leaves. In C. insignis, Chl a, Chl b, total Chl, and Car contents in DG leaves were also found to be significantly higher than those in LG leaves, whereas Ant, Fla, PP, and total protein levels showed no significant differences between the two leaf sectors of this plant. Compared to C. makoyana, C. insignis contained significantly higher levels of Chl a, Chl b, Car, Ants, Flas, and total protein. However, the PP content of C. makoyana was significantly higher than that of C. insignis.
Figure 4.
Content of Chl a (A), Chl b (B), Chl a + b (C), Carotenoids (Cars) (D), Anthocyanins (Ant) (E), Flavonoids (Fla) (F), Polyphenol (PP) (G), and total protein (H) of dark green leaf sector (black bar, DG) and light green leaf sector (white bar, LG) in Calathea makoyana and C. insignis. Vertical bars indicate the standard deviation (n = 5). Different lowercase letters among levels are significantly different at p ≤ 0.05 by Fisher’s least significant difference (LSD) test.
Chls have high light absorption at 400–500 and 630–680 nm, and Cars have high light absorption at 400–500. Meanwhile, both Chls and Cars have low light absorption at 530–610 nm. In general, DG leaves of both plants contained more pigments and secondary metabolite compounds than LG leaves, except for Ant, Fla, and PP contents in C. insignis. High contents of pigments in leaves of these plants can be attributed to the wide existence of those plants even under high environmental light and radiation conditions, since those compounds were reported to play important roles protecting against stressful conditions [40]. The PPFD level influences the growth, morphology, and photosynthetic potential of Calathea plants, and different responses to pigments, metabolic compounds, and total protein contents in leaves depend on the variety of Calathea, which may optimize growth and development of plants in climate-controlled settings. Different PPFD culture systems may achieve commercial Calathea plant production by utilizing rapid, large-scale, precise management practices.
2.3. Correlations between Pigment Contents and Non-Destructive Measurements of Different Leaf Sectors
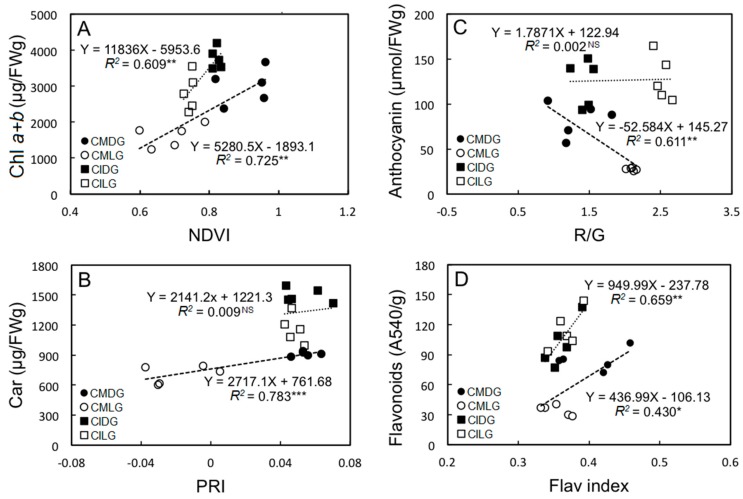
Various theoretical models based on leaf reflectance have been used to calculate a series of vegetation indices for monitoring plant growth [41,42]. Figure 5A shows relationships between the NDVI and total Chl from C. insignis and C. makoyana leaves. The slopes of regression lines were 11,836 and 5280.5. Correlation values (R2) of those relationships were 0.609 and 0.725, respectively, indicating significant correlations between the NDVI and total Chl in C. insignis and C. makoyana. The NDVI is a sensitive indicator of the canopy structure, green leaf area index, and Chl content [43], and it offers a simple, rapid, nondestructive, and precise method to characterize the ecophysiology of plants [44]. This index is correlated with net primary production [45] and photosynthetic rates [46,47]. Therefore, the NDVI is more comprehensively applicable to nondestructively estimate Chl contents of plant leaves.
Figure 5.
Correlations between Chl a + b and NDVI (A), Car content and PRI (B), Ant content and red-green ratio (R/G) (C), and Fla content and FlavI (D) of different leaf sectors in Calathea makoyana and C. insignis. Black circle (dark green leaf sector in C. makoyana; CMDG) and white circle (light green leaf sector in C. makoyana; CMLG) represent dark green and light green leaf sector in C. makoyana, respectively. Black square (dark green leaf sector in C. insignis; CIDG) and white square ((light green leaf sector in C. insignis; CILG) represent dark green and light green leaf sector in C. insignis, respectively. NS represents not significant, * p < 0.05, ** p < 0.01, *** p < 0.001.
Pigment conversion to their de-epoxidized (photoprotective) form changes leaf reflectance within a tight wave-band centered around 531 nm [48]. At this bandwidth, the PRI incorporates reflectance, and there are correlations with xanthophyll cycle pigment contents and activities and with the photochemical efficiency of PSII; therefore the PRI varies with light-use efficiency [48,49]. To better understand the ecophysiology of Calathea plants, the relationship of the photosynthetic efficiency with pigment contents was analyzed by determining the reflectance index. Figure 5B illustrates the relationship between the PRI and Car contents of C. insignis and C. makoyana leaves with respective R2 values of 0.009 and 0.783, slope values of 2141.2 and 2717.1. Reflectance indices respond to slight changes in Cars and also give accurate estimates of changes in the photosynthetic flux of evergreen canopies [50], since Cars of the xanthophyll cycle are closely related to PSII’s photochemical efficiency [51] and dissipate light energy not used in photosynthesis [52].
Figure 5C shows the relationship between R/G and Ant contents of C. insignis and C. makoyana leaves with respective correlation values of 0.002 and 0.611, slope values of 1.7871 and −52.584. There were significant correlations of Ants with R/G, Cars with the PRI, and total Chl with the NDVI in C. makoyana, indicating that these reflectance indexes can be useful for non-destructive estimations of leaf Chl, Car, and Ant contents in C. makoyana. However, no correlations of Ants with R/G or Cars with the PRI in C. insignis were observed. This was because the color of adaxial parts were all purple, which affected the composition of the reflected spectrum and caused the low correlations.
Figure 5D shows relationships between FlavI and Fla contents from C. insignis and C. makoyana leaves, with respective R2 values of 0.658 and 0.430, slope values of 949.99 and 436.99. Flavonoids, synthesized in response to light density [53,54,55], are able to effectively scavenge reactive oxygen forms [56,57]. In our study, FlavI was useful for non-destructive estimation of leaf Fla contents, since these indices were significantly correlated with Fla contents in leaves of both C. insignis and C. makoyana. To ecophysiologically characterize a plant’s photosynthetic capacity, light-response curves and consequent cardinal points yield very interesting parameters. However, steady-state conditions, which are strictly required, can often not be achieved in nature. So, instantaneous light-response curves, with quick alterations in the PPFD but with the clear risk of encountering non-steady-state conditions, can be used as an alternative way to achieve insights into light-response characteristics of plants in the field. Our findings suggest that the NDVI, PRI, R/G, and FlavI are respectively reliable descriptors of leaf Chl, Car, Ant, and Fla contents in C. makoyana, and these indices can be used for ecophysiological research in C. makoyana. In addition, Flav and NDVI indices can be used as indicators to characterize the physiology of C. insignis plants.
3. Materials and Methods
3.1. Plant Materials and Cultural Practice
For these experiments, C. insignis and C. makoyana plants were obtained from local shops in Taipei, Taiwan. Plants were 16–20 cm tall, were transplanted into 12.7 cm plastic pots (917 mL) containing commercial potting soil with a 4:1 (v/v) mixture of peat moss and perlite, and were placed in an environment-controlled greenhouse. Plants were evenly spaced to promote similar growth rates and sizes. Plants were watered twice each week, and an optimal amount of a compound fertilizer solution (20N-8.7P-16.6K water-soluble fertilizer at 0.5 g·L−1) was applied biweekly. Plants were grown for 1 month, and those with a uniform size were selected and randomly separated into 14 groups for subsequent experiments.
3.2. Determination of Photosynthetic Capacity
Photosynthetic light response curves were determined with a portable, open-flow gas exchange system connected to a leaf chamber and light emitting diode (LED) light source at 30 °C, a CO2 concentration of 400 μmol·mol−1, and a relative humidity of 80%. The PPFD was set from high to low levels (1800, 1500, 1200, 1000, 800, 600, 400, 200, 100, 75, 50, 25, 10, and 0 μmol·m−2·s−1). All measurements were taken before 11:00 a.m. to avoid the midday depression in photosynthesis. Values of CO2 exchange was recorded every 2 min, until the CO2 exchange was stable (about 10 min under each level of illumination). Portions of both DG and LG leaves of plant were measured together in the chamber.
3.3. Determination of ChlF
The potted plants were moved to the shade under a cottage before sunrise at 05:30–06:00, and then the ChlF parameters of dark-adapted leaves were measured at ambient temperature after adaptation to the dark for 20 min with a portable fluorometer (Mini-Pam; Heinz Walz, Effeltrich, Germany) [58]. The middle portions of both DG and LG leaves of a plant were used for the measurements. Values of the minimal ChlF (Fo) and maximal ChlF (Fm) of dark-adapted samples were respectively determined using modulated irradiation of a weak LED beam (measuring light) and saturating pulse. We then calculated the maximum photochemical quantum yield (Fv/Fm), where Fv, the yield of variable fluorescence, was calculated as (Fm − Fo). When measuring Fv/Fm, samples were first acclimated to dark conditions to ensure that all reaction centers were in an open state, and there was minimal non-photochemical dissipation of excitation energy. Instantaneous light-response curves of the effective quantum yield of PSII (ΔF/Fm’) were determined with the rapid light-curve program of the Mini-PAM; this was achieved by increasing the actinic light intensity in 4 min by a step each 30 s for a total of nine steps. The leaves measured were dark-adapted 30 min before the rapid light-curve runs. In the following, effective quantum yields measured using the instant light-response curve program were notated. The ΔF/Fm’ ratio was calculated as (Fm’ − F)/Fm’, where F is the fluorescence yield of the light-adapted sample, and Fm’ is the maximum light-adapted fluorescence yield upon superimposition of a saturating light pulse. The apparent photosynthetic electron transport rate (ETR) of PSII was determined as ETR = ΔF/Fm’ × PPFD × 0.5 × 0.84; the factor 0.5 was assumed to equal excitation of both PSII and PSI. An ETR correction factor of 0.84 was used as the two PSs actually only absorb a fraction of the incident light [24].
3.4. Determination of Chl, Car, Ant, Total Fla, PP, and Total Protein Contents
Contents of Chls and Cars in DG and LG leaves were determined using methods described by Porra et al. [59]. In brief, 0.01 g of dry power and 5 mL of 80% acetone were mixed at 4 °C overnight; then the supernatant was obtained by centrifuging the mixture at 13,000× g for 5 min. Supernatants were tested for the absorbance of Chl a and Chl b in acetone at 663.6 and 646.6 nm, respectively, with a U-2000 type spectrophotometer (Hitachi, Tokyo, Japan). Chl a and Chl b concentrations and Cars were calculated with the following equations:
| Chl a = (12.25 × OD663.6 − 2.55 × OD646.6) × volume of supernatant (mL)/sample weight (g); | (1) |
| Chl b = (20.31 × OD646.6 − 4.91 × OD663.6) × volume of supernatant (mL)/sample weight (g) | (2) |
| Car = [(4.69 × OD440.5 × volume of supernatant (mL)/sample weight (g)] − 0.267 × (Chl a + Chl b) | (3) |
Ant contents of the extracts were measured based on the protocol of Mancinelli et al. [60]. A mixture of 99% methanol containing 1% HCl was added to powdered samples and incubated for 1 hr at room temperature. The mixture was then centrifuged at 4 °C and 3000 rpm for 5 min to obtain the supernatant, followed by measuring the absorbances at 530 and 657 nm on a spectrophotometer. The following Equation (4) was used:
| Ant (μmol·g−1 DW) = (A530 − 0.33 × A657/31.6) × volume of supernatant (mL)/sample weight (g), with DW being the dry weight. | (4) |
Total Flas in the extracts were determined by the method of Djeridane et al. [61]. The methanol extract (1 mL; 0.5 mg·mL−1) was mixed with 1 mL of 2% aluminum chloride. The mixture was stirred and allowed to sit for 15 min at room temperature, after which the absorbance was measured at 430 nm on a spectrophotometer. Quercetin was used as a reference standard, and the total Fla content was expressed as milligrams of quercetin equivalent (QE) per gram of DW (mg QE·g−1 DW).
The PP content was determined according to the method of Taga et al. [62]. Briefly, standard gallic acid (GA) and an aliquot of the acidic methanolic extract were diluted with an acidified methanol solution containing 1% HCl. Two milliliters of 2% Na2CO3 were mixed into each sample of 100 µL and allowed to equilibrate for 2 min before adding 50% Folin-Ciocalteau reagent (Sigma, St. Louis, MO, USA). The absorbance at 750 nm was measured at room temperature using a Varioskan Flash Multimode Reader (Thermo Scientific, Rockford, IL, USA). The standard curve for GA was used to calculate PP levels. Total phenolics were expressed as the mg GA equivalent (GAE)·g−1 DW. The standard curve equation was y = 0.4995x − 0.011, where R2 = 0.9944.
The total protein content was determined using the method of Bradford [63]. Samples of 0.05 g fresh weight of leaves were ground with liquid nitrogen in a mortar, to which was added 3 mL of a phosphate-buffered solution (pH 7.0). After the extract was centrifuged at 13,000× g for 15 min at 4 °C, 0.1 mL of the supernatant was combined with 4.9 mL of a Coomassie brilliant blue G-250 solution (0.1 g·L−1). The soluble protein content was measured after 2 min at a 595-nm wavelength.
3.5. Determination of the Fla Index and Spectral Reflectance
The Fla index (FlavI) was used to assess Fla contents, and was measured with a Plaquette Dualex Scientific™ instrument (Force-A, Orsay Cedex, France) [64]. The spectral reflectance was measured at wavelengths of 200–900 nm, using an integrating sphere fitted to a scanning spectrophotometer (U-3010, Hitachi, Tokyo, Japan). The following indices were calculated from the reflectance spectrum: (1) the adjusted normalized difference vegetation index (NDVI), calculated as (R750 − R705)/(R750 + R705 – 2 × R445), was used to assess the Chl content [65]; (2) the photochemical reflectance index (PRI), calculated as (R531 − R570)/(R531 + R570), was used to assess xanthophyll cycle pigments [66]; and (3) the red-green (R/G) ratio R500–599/R600–699 was calculated to assess the Ant content [67].
3.6. Statistical Analysis
Data were determined in triplicate, and results are expressed as the mean ± standard deviation (SD). An analysis of variance (ANOVA) with the least significant difference (LSD) test at p ≤ 0.05 was performed using SAS vers. 9 (SAS Institute, Cary, NC, USA). The experiment was performed twice independently with a randomized design for the growth environment, sampling day, and physiological analyses.
4. Conclusions
Yields and costs are the two most important criteria in agricultural production for which environmental factors should be optimized. In the present study, we investigated the effective and sufficient intensity for growing plants of two Calathea species with a variety of leaf colors. Responses of the photosynthetic rate and ETR to light indicated that two tested Calathea species appear to be adapted to low light intensities. Relationships of reflectance indices with pigments and flavonoids were established and can be used for ecophysiological research in C. insignis and C. makoyana. This study provides a better understanding of the photosynthetic characteristics of C. insignis and C. makoyana to promote the effective management and cultivation of these plants.
Author Contributions
Meng-Yuan Huang designed the experiments. Tung-Chuan Hsiung, Ming-Huang Hsu, Po-Yen Chen and Kai-Chieh Chang performed the experiments measurement. Meng-Yuan Huang, Hoang Chinh Nguyen and Chi-Ming Yang analyzed the data. Meng-Yuan Huang, Kuan-Hung Lin, Hoang Chinh Nguyen and Jen-Hsien Weng wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chao C.C.T., Devanand P.S., Chen J. AFLP analysis of genetic relationships among Calathea species and cultivars. Plant Sci. 2005;168:1459–1469. doi: 10.1016/j.plantsci.2005.01.012. [DOI] [Google Scholar]
- 2.Rout G.R., Senapati S.K., Aparajita S. Studies on the genetic relationship among 13 cultivars of Calathea (Marantaceae) using RAPD and ISSR markers. Adv. Hortic. Sci. 2007;21:147–155. [Google Scholar]
- 3.Hogewoning S.W., Harbinson J. Insights on the development, kinetics, and variation of photoinhibition using chlorophyll fluorescence imaging of a chilled, variegated leaf. J. Exp. Bot. 2007;58:453–463. doi: 10.1093/jxb/erl219. [DOI] [PubMed] [Google Scholar]
- 4.Kumari A., Lahiri K., Mukhopadhyay M.J., Mukhopadhyay S. Genome analysis of species of Calathea utilizing chromosomal and nuclear DNA parameters. Nucleus. 2014;57:203–208. doi: 10.1007/s13237-014-0121-0. [DOI] [Google Scholar]
- 5.Tran P.L., Lee J.S., Park K.H. Molecular structure and rheological character of high-amylose water caltrop (Trapa bispinosa Roxb.) starch. Food Sci. Biotechnol. 2013;22:979–985. doi: 10.1007/s10068-013-0173-y. [DOI] [Google Scholar]
- 6.Zhang L., Sun X.Y., Tian Y., Gong X.Q. Composted green waste as a substitute for peat in growth media: Effects on growth and nutrition of Calathea insignis. PLoS ONE. 2013;8:e78121. doi: 10.1371/journal.pone.0078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Sun X.Y., Tian Y., Gong X.Q. Biochar and humic acid amendments improve the quality of composted green waste as a growth medium for the ornamental plant Calathea insignis. Sci. Hortic. 2014;176:70–78. doi: 10.1016/j.scienta.2014.06.021. [DOI] [Google Scholar]
- 8.Montgomery R.A., Givnish T.J. Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: Dynamic photosynthetic responses. Oecologia. 2008;155:455. doi: 10.1007/s00442-007-0936-3. [DOI] [PubMed] [Google Scholar]
- 9.Powles S.B. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 1984;35:15–44. doi: 10.1146/annurev.pp.35.060184.000311. [DOI] [Google Scholar]
- 10.Aleric K.M., Kirkman L.K. Growth and photosynthetic responses of the federally endangered shrub, Lindera melissifolia (Lauraceae), to varied light environment. Am. J. Bot. 2005;92:682–689. doi: 10.3732/ajb.92.4.682. [DOI] [PubMed] [Google Scholar]
- 11.Adams W.W., Zarter C.R., Ebbert V., Demmig-Adams B. Photoprotective strategies of overwintering evergreens. BioScience. 2004;54:41–49. doi: 10.1641/0006-3568(2004)054[0041:PSOOE]2.0.CO;2. [DOI] [Google Scholar]
- 12.Demmig-Adams B., Adams W.W., Barker D.H., Logan B.A., Bowlong D.R., Verhoeven A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996;98:253–264. doi: 10.1034/j.1399-3054.1996.980206.x. [DOI] [Google Scholar]
- 13.Dewir Y.H., El-Mahrouk M.E.S., Al-Shmgani H.S., Rihan H.Z., Teixeira da Silva J.A., Fuller M.P. Photosynthetic and biochemical characterization of in vitro-derived African violet (Saintpaulia ionantha H. Wendl) plants to ex vitro conditions. J. Plant Interact. 2015;10:101–108. doi: 10.1080/17429145.2015.1018967. [DOI] [Google Scholar]
- 14.Singsaas E.L., Ort D.R., Delucia E.H. Diurnal regulation of photosynthesis in understory saplings. New Phytol. 2000;145:39–49. doi: 10.1046/j.1469-8137.2000.00556.x. [DOI] [Google Scholar]
- 15.Demmig-Adams B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta Bioenerg. 1990;1020:1–24. doi: 10.1016/0005-2728(90)90088-L. [DOI] [Google Scholar]
- 16.Anderson J.M., Park Y.I., Chow W.S. Photoinactivation and photoprotection of photosystem II in nature. Physiol. Plant. 1997;100:214–223. doi: 10.1111/j.1399-3054.1997.tb04777.x. [DOI] [Google Scholar]
- 17.Molina-Bravo R., Arellano C., Sosinski B.R., Fernandez G.E. A protocol to assess heat tolerance in a segregating population of raspberry using chlorophyll fluorescence. Sci. Hortic. 2011;130:524–530. doi: 10.1016/j.scienta.2011.07.022. [DOI] [Google Scholar]
- 18.Banks J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practitioners. Tree Physiol. 2017;37:1128–1136. doi: 10.1093/treephys/tpx059. [DOI] [PubMed] [Google Scholar]
- 19.D’Ambrosio N., Arena C., De Santo A.V. Temperature response of photosynthesis, excitation energy dissipation and alternative electron sinks to carbon assimilation in Beta vulgaris L. Environ. Exp. Bot. 2006;55:248–257. [Google Scholar]
- 20.Hirotsu N., Makino A., Yokota S., Mae T. The photosynthetic properties of rice leaves treated with low temperature and high irradiance. Plant Cell Physiol. 2005;46:1377–1383. doi: 10.1093/pcp/pci149. [DOI] [PubMed] [Google Scholar]
- 21.Kościelniak J., Biesaga-Kościelniak J. Photosynthesis and non-photochemical excitation quenching components of chlorophyll excitation in maize and field bean during chilling at different photon flux density. Photosynthetica. 2006;44:174–180. doi: 10.1007/s11099-006-0003-z. [DOI] [Google Scholar]
- 22.Melkonian J., Owens T.G., Wolfe D.W. Gas exchange and co-regulation of photochemical and nonphotochemical quenching in bean during chilling at ambient and elevated carbon dioxide. Photosynth. Res. 2004;79:71–82. doi: 10.1023/B:PRES.0000011921.64567.b2. [DOI] [PubMed] [Google Scholar]
- 23.Wong S.L., Chen C.W., Huang H.W., Weng J.H. Using combined measurements of gas exchange and chlorophyll fluorescence to investigate the photosynthetic light responses of plant species adapted to different light regimes. Photosynthetica. 2012;50:206–214. doi: 10.1007/s11099-012-0027-5. [DOI] [Google Scholar]
- 24.Wong S.L., Huang M.Y., Chen C.W., Weng J.H. Light induction of nonphotochemical quenching, CO2 fixation, and photoinhibition in woody and fern species adapted to different light regimes. Photosynthetica. 2014;52:272–280. doi: 10.1007/s11099-014-0023-z. [DOI] [Google Scholar]
- 25.Weng J.H., Chien L.F., Jiang C.Y., Shih F.C., Chen H.Y. A comparison between yellow-green and green cultivars of four vegetable species in pigments, ascorbate, photosynthesis, energy dissipation, and photoinhibition. Photosynthetica. 2011;49:361–370. doi: 10.1007/s11099-011-0046-7. [DOI] [Google Scholar]
- 26.Hölscher D., Leuschner C., Bohman K., Hagemeier M., Juhrbandt J., Tjitrosemito S. Leaf gas exchange of trees in old growth and young secondary forest stands in Sulawesi, Indonesia. Trees. 2006;20:278–285. doi: 10.1007/s00468-005-0040-4. [DOI] [Google Scholar]
- 27.Huang J., Boemer R.E.J., Rebbeck J. Ecophysiological responses of two herbaceous species to prescribed burning, alone or in combination with overstory thinning. Am. J. Bot. 2007;94:755–763. doi: 10.3732/ajb.94.5.755. [DOI] [PubMed] [Google Scholar]
- 28.Špunda V., Kalina J., Urban O., Luis V.C., Sibisse I., Puertolas J., Šprtová M., Marek M.V. Diurnal dynamics of photosynthetic parameters of Norway spruce trees cultivated under ambient and elevated CO2: The reasons of midday depression in CO2 assimilation. Plant Sci. 2005;168:1371–1381. doi: 10.1016/j.plantsci.2005.02.002. [DOI] [Google Scholar]
- 29.Wu C.W., Lin K.H., Lee M.C., Peng Y.L., Chou T.Y., Chang Y.S. Using chlorophyll fluorescence and vegetation indices to predict the timing of nitrogen demand in Pentas lanceolata. Korean J. Hortic. Sci. Technol. 2015;33:845–853. doi: 10.7235/hort.2015.15043. [DOI] [Google Scholar]
- 30.Fracheboud Y., Leipner J. The application of chlorophyll fluorescence to study light, temperature, and drought stress. In: DeEll J.R., Toivonen P.M.A., editors. Practical Applications of Chlorophyll Fluorescence in Plant Biology. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2003. pp. 125–150. [Google Scholar]
- 31.Müller P., Li X.P., Niyog K.K. Non-photochemical quenching: A response to excess light energy. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murchie E.H., Niyogi K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011;155:86–92. doi: 10.1104/pp.110.168831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finazzi G., Johnson G.N., Dallosto L., Joliot P., Wollman F., Bassi R. A zeaxanthinin dependent nonphotochemical quenching mechanism localized in the photosystem II core complex. Proc. Natl. Acad. Sci. USA. 2004;101:12375–12380. doi: 10.1073/pnas.0404798101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalituho L., Beran K.C., Jahns P. The transiently generated nonphotochemical quenching of excitation energy in Arabidopsis leaves is modulated by zeaxanthin. Plant Physiol. 2007;143:1861–1870. doi: 10.1104/pp.107.095562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng J.H., Wong S.L. Photosynthetic light responses of fern species adapted to different light regimes. In: Tuft J., editor. Ferns and Shrubs: Diversity, Cultivation and Implications for the Environment. Nova Science Publishers; New York, NY, USA: 2015. pp. 57–76. [Google Scholar]
- 36.Zulfugarov I.S., Ham O.K., Mishra S.R., Kim J.Y., Nath K., Koo H.Y., Kim H.S., Moon Y.H., An G., Lee C.H. Dependence of reaction center-type energy-dependent quenching on photosystem II antenna size. Biochim. Biophys. Acta Bioenerg. 2007;1767:773–780. doi: 10.1016/j.bbabio.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Demmig-Adams B., Ebbert V., Mellman D.L., Muech K.E., Schaffer L., Funk C., Zarter C.R., Adamska I., Jansson S., Adams W.W., III Modulation of PsbS and flexible vs sustained energy dissipation by light environment in different species. Physiol. Plant. 2006;127:670–680. doi: 10.1111/j.1399-3054.2006.00698.x. [DOI] [Google Scholar]
- 38.Sonoike K. Photoinhibition of photosystem I. Physiol. Plant. 2011;142:56–64. doi: 10.1111/j.1399-3054.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 39.Tikkanen M., Mekala N.R., Aro E.M. Photosystem II photoinhibition-repair cycle protects photosystem I from irreversible damage. Biochim. Biophys. Acta Bioenerg. 2014;1837:210–215. doi: 10.1016/j.bbabio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 40.De la Rosa-Manzano E., Andrade J.L., García-Mendoza E., Zotz G., Reyes-García C. Photoprotection related to xanthophyll cycle pigments in epiphytic orchids acclimated at different light microenvironments in two tropical dry forests of the Yucatan Peninsula, Mexico. Planta. 2015;242:1425–1438. doi: 10.1007/s00425-015-2383-4. [DOI] [PubMed] [Google Scholar]
- 41.Devitt D.A., Morris R.L., Fenstermaker L.K. Foliar damage, spectral reflectance, and tissue ion concentrations of trees sprinkle irrigated with waters of similar salinity but different chemical composition. HortScience. 2005;40:819–826. [Google Scholar]
- 42.Strachan I.B., Pattey E., Boisvert J.B. Impact of nitrogen and environmental conditions on corn as detected by hyperspectral reflectance. Remote Sens. Environ. 2002;80:213–224. doi: 10.1016/S0034-4257(01)00299-1. [DOI] [Google Scholar]
- 43.Bajwa S.G., Mishra A.R., Norman R.J. Canopy reflectance response to plant nitrogen accumulation in rice. Precis. Agric. 2010;11:488–506. doi: 10.1007/s11119-009-9142-0. [DOI] [Google Scholar]
- 44.Field C.B., Gamon J.A., Peñuelas J. Remote sensing of terrestrialphotosynthesis. In: Schulze E.D., Caldwell M.M., editors. Ecophysiology of Photosynthesis. Springer; Berlin, Germany: 1995. pp. 511–528. [Google Scholar]
- 45.Prince S.D. A model of regional primary production for use with coarse resolution satellite data. Int. J. Remote Sens. 1991;12:1313–1330. doi: 10.1080/01431169108929728. [DOI] [Google Scholar]
- 46.McMichael C.E. Estimating CO2 exchange at two sites in Arctic tundra ecosystems during the growing season using a spectral vegetation index. Int. J. Remote Sens. 1999;20:683–698. doi: 10.1080/014311699213136. [DOI] [Google Scholar]
- 47.Yoder B.J., Waring R.H. The normalized difference vegetation index of small Douglas-fir canopies with varying chlorophyll concentrations. Remote Sens. Environ. 1994;49:81–91. doi: 10.1016/0034-4257(94)90061-2. [DOI] [Google Scholar]
- 48.Gamon J., Serrano L., Surfus J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia. 1997;112:492–501. doi: 10.1007/s004420050337. [DOI] [PubMed] [Google Scholar]
- 49.Nichol C.J., Huemmrich K.F., Black T.A., Jarvis P.G., Walthall C.L., Grace J., Hall F.G. Remote sensing of photosynthetic-light-use efficiency of boreal forest. Agric. For. Meteorol. 2000;101:131–142. doi: 10.1016/S0168-1923(99)00167-7. [DOI] [Google Scholar]
- 50.Gitelson A., Merzlyak M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994;143:286–292. doi: 10.1016/S0176-1617(11)81633-0. [DOI] [Google Scholar]
- 51.Björkman O., Demmig-Adams B. Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In: Schulze E.D., Caldwell M.M., editors. Ecophysiology of Photosynthesis. Springer; Berlin, Germany: 1995. pp. 17–47. [Google Scholar]
- 52.Frank H.A., Cua A., Chynwat V., Young A., Gosztola D., Wasielewski M.R. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth. Res. 1994;41:389–395. doi: 10.1007/BF02183041. [DOI] [PubMed] [Google Scholar]
- 53.Babu T.S., Akhtar T.A., Lampi M.A., Tripuranthakam S., Dixon D.G., Greenberg B.M. Similar stress responses are elicited by copper and ultraviolet radiation in the aquatic plant Lemna gibba: Implication of reactive oxygen species as common signals. Plant Cell Physiol. 2003;44:1320–1329. doi: 10.1093/pcp/pcg160. [DOI] [PubMed] [Google Scholar]
- 54.Bidel L.P.R., Meyer S., Goulas Y., Cadot Y., Cerovic Z.G. Responses of epidermal phenolic compounds to light acclimation: In vivo qualitative and quantitative assessment using chlorophyll fluorescence excitation spectra in leaves of three woody species. J. Photochem. Photobiol. B Biol. 2007;88:163–179. doi: 10.1016/j.jphotobiol.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Kotilainen T., Tegelberg R., Julkunen-Tiitto R.I.I.T.T.A., Lindfors A., Aphalo P.J. Metabolite specific effects of solar UV-A and UV-B on alder and birch leaf phenolics. Glob. Chang. Biol. 2008;14:1294–1304. doi: 10.1111/j.1365-2486.2008.01569.x. [DOI] [Google Scholar]
- 56.Agati G., Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010;186:786–793. doi: 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- 57.Ma H., Johnson S.L., Liu W., DaSilva N.A., Meschwitz S., Dain J.A., Seeram N.P. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. Int. J. Mol. Sci. 2018;19:461. doi: 10.3390/ijms19020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weng J.H., Jhaung L.H., Lin R.J., Chen H.Y. Relationship between photochemical efficiency of photosystem II and the photochemical reflectance index of mango tree: Merging data from different illuminations, seasons and leaf colors. Tree Physiol. 2010;30:469–478. doi: 10.1093/treephys/tpq007. [DOI] [PubMed] [Google Scholar]
- 59.Porra R., Thompson W., Kriedemann P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989;975:384–394. doi: 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]
- 60.Mancinelli A.L., Yang C.P.H., Lindquist P., Anderson O., Rabino I. Photocontrol of anthocyanin synthesis III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiol. 1975;55:251–257. doi: 10.1104/pp.55.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Djeridane A., Yousfi M., Nadjemi B., Boutassouna D., Stocker P., Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. doi: 10.1016/j.foodchem.2005.04.028. [DOI] [Google Scholar]
- 62.Taga M.S., Miller E.E., Pratt D.E. Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 1984;61:928–931. doi: 10.1007/BF02542169. [DOI] [Google Scholar]
- 63.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 64.Cerovic Z.G., Masdoumier G., Ghozlen N.B., Latouche G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012;146:251–260. doi: 10.1111/j.1399-3054.2012.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitehead D., Boelman N.T., Turnbull M.H., Griffin K.L., Tissue D.T., Barbour M.M., Hunt J.E., Richardson S.J., Peltzer D.A. Photosynthesis and reflectance indices for rainforest species in ecosystems undergoing progression and retrogression along a soil fertility chronosequence in New Zealand. Oecologia. 2005;144:233–244. doi: 10.1007/s00442-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 66.Stylinski C., Gamon J., Oechel W. Seasonal patterns of reflectance indices, carotenoid pigments and photosynthesis of evergreen chaparral species. Oecologia. 2002;131:366–374. doi: 10.1007/s00442-002-0905-9. [DOI] [PubMed] [Google Scholar]
- 67.Sims D.A., Gamon J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002;81:337–354. doi: 10.1016/S0034-4257(02)00010-X. [DOI] [Google Scholar]