Abstract
The Helicobacter pylori-induced burden of gastric cancer varies based on geographical regions and ethnic grouping. Vietnam is a multiethnic country with the highest incidence of gastric cancer in Southeast Asia, but previous studies focused only on the Kinh ethnic group. A population-based cross-sectional study was conducted using 494 volunteers (18–78 years old), from 13 ethnic groups in Daklak and Lao Cai provinces, Vietnam. H. pylori status was determined by multiple tests (rapid urease test, culture, histology, and serology). cagA and vacA genotypes were determined by PCR-based sequencing. The overall H. pylori infection rate was 38.1%. Multivariate analysis showed that variations in geographical region, age, and ethnicity were independent factors associated with the risk of H. pylori acquisition. Therefore, multicenter, multiethnic, population based study is essential to assess the H. pylori prevalence and its burden in the general population. Only the E De ethnicity carried strains with Western-type CagA (82%) and exhibited significantly lower gastric mucosal inflammation compared to other ethnic groups. However, the histological scores of Western-type CagA and East-Asian-type CagA within the E De group showed no significant differences. Thus, in addition to bacterial virulence factors, host factors are likely to be important determinants for gastric mucosal inflammation and contribute to the Asian enigma.
Keywords: Helicobacter pylori, molecular epidemiology, virulence factor, Asian enigma
1. Introduction
Gastric cancer (GC) is the third most common cause of cancer-associated death and the fifth common cancer worldwide (IARC, GLOBOCAN 2012, http://globocan.iarc.fr/). Hence, the malignant disease remains a significant, serious public health problem, as well as a burden in both developed and developing countries. Interestingly, the incidence of GC fluctuates widely dependent on the differences in geography and ethnicity, such as between different continents, countries, and even regions belong to the same country [1,2].
The finding of Marshall and Warren about a spiral, Gram-negative, urease-producing bacteria colonizing in human gastric mucosa, called Helicobacter pylori (H. pylori) published in The Lancet in the 1980s, gave a crucial milestone toward understanding the pathogenic mechanism of upper digestive diseases [3]. The bacterial species infects half of the world’s population and a causal relationship between H. pylori and severe gastro-duodenal diseases was well-demonstrated through epidemiological and basic studies [4,5]. Intriguingly, despite H. pylori’s ubiquitous dissemination, the infection rate differs according to differences in countries, races, socio-economic statuses, and living or hygiene conditions [2,6]. On this basis, a hypothesis was given that a high incidence of GC might be attributable to a high prevalence of H. pylori infection and vice versa. However, it is not always applied in all cases, especially in the case of South Asia and Africa that were so-called, respectively, as Asian and African enigmas [7], from which these areas had a high infection rate, but low incidence of GC.
Practically, not all H. pylori-infected subjects develop such severe upper digestive tract diseases, but only 3% and 15% of them were estimated to suffer GC and peptic ulcer (PU) during their whole life, respectively [8]. This suggested that H. pylori-caused clinical progress is not only dependent on the bacterium, but is a combination of many factors including bacterial virulence, and host and environmental factors; or H. pylori has different virulent strains contributing to expressing different phenotypes [2].
The incidence of GC in Vietnam was classified as an intermediate risk in Asia, but the highest in Southeast Asia (age-standardized rate (ASR) of GC, 16.3/100,000 in both sexes). Several studies conducted in Vietnam initially disclosed an intimate association between H. pylori and gastroduodenal diseases [9,10]. Nevertheless, it should be noticed that Vietnam is a multiethnic nation consisting of 54 ethnicities and the previous studies only focused on the Kinh people, a major ethnic group accounting for 87% of total population of Vietnam [11]. The rest of the minor ethnic groups is usually scattered on high mountainous areas spreading from the north to the south and has not been studied yet. Due to the difference of H. pylori prevalence in various areas, as well as ethnic groups, an association between the microorganism and clinical outcomes from minor ethnic groups contribute to supply the essential information for gaining an overview of H. pylori prevalence, as well as the burden of H. pylori-caused diseases on the Vietnamese population. It is necessary to assist in establishing a feasible, practical screening strategy of GC in Vietnam. Moreover, it is particularly meaningful to minor ethnic groups which have many difficulties in contacting healthcare services due to their low socioeconomic and living conditions. Therefore, the aim of the study was to reveal the H. pylori infection in minor ethnic groups residing in mountainous areas, Daklak and Lao Cai, Vietnam, and investigate the virulence factor involved.
2. Results
2.1. Characteristics of the Study Population
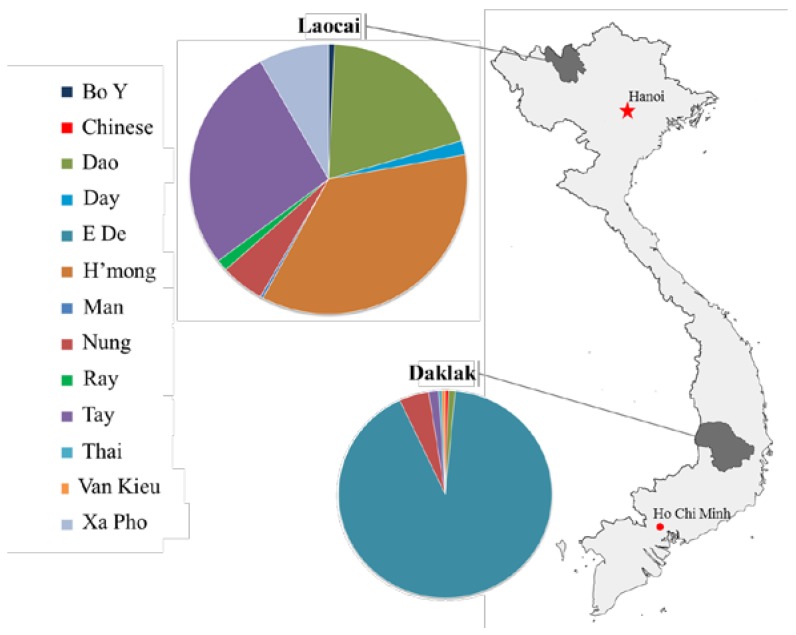
In total, 494 volunteers (210 males and 284 females), median age 38, interquartile range (IQR) 17 years old, were enrolled. Of which, there were 183 E De, nine Nung, three Tay, two Dao, one Van Kieu, one Chinese, one Thai in Daklak; and 105 H’mong, 15 Nung, 80 Tay, 58 Dao, 24 Xa Pho, five Day, four Ray, two Bo Y and one Man in Lao Cai province (Figure 1), of which the clinical manifestations included 465 (94.1%) with gastritis, 16 (3.2%) with gastric ulcer (GU), 12 (2.4%) with duodenal ulcer (DU), 19 (3.8%) with GERD, and 1 (0.2%) with GC.
Figure 1.
Map of Vietnam showing the geographical location and the frequency of the studied minority ethnic groups residing in Daklak and Lao Cai.
2.2. The Prevalence of H. pylori Infection in General as Well as Its Differences between Geographical Regions and Ethnicities
In general, 188/494 (38.1%) were H. pylori-positive by culture, or at least two positive tests in the case of negative cultures. This infection rate in Daklak was significantly higher than that in Lao Cai (51.0% vs. 29.3%, p < 0.001). In a more detailed analysis, Table 1 shows that E De and Nung ethnicities in Daklak had significantly higher prevalence of H. pylori infection compared to Nung, Tay, and Dao ethnicities in Lao Cai.
Table 1.
The prevalence of Helicobacter pylori infection in a population study, in general, and in Daklak and Lao Cai provinces, in particular.
| Ethnic | H. pylori Infection | Total | |
|---|---|---|---|
| No. of participant (%) | 188 (38.1%) | 494 | |
| Daklak province | |||
| E De | 94 (51.4%) | 183 | |
| Nung | 6 (66.7%) | 9 | |
| Tay | 0 (0%) | 3 | |
| Dao | 0 (0%) | 2 | |
| Van Kieu | 1 (100%) | 1 | |
| Thai | 1 (100%) | 1 | |
| Chinese | 0 (0%) | 1 | |
| Lao Cai province | |||
| H′mong | 40 (38.1%) a | 105 | |
| Nung | 2 (13.3%) a,b | 15 | |
| Tay | 15 (18.8%) a,b | 80 | |
| Dao | 12 (20.7%) a,b | 58 | |
| Xa Pho | 13 (54.2%) | 24 | |
| Day | 1 (20%) | 5 | |
| Ray | 2 (50%) | 4 | |
| Bo Y | 0 (0%) | 2 | |
| Man | 1 (100%) | 1 |
a indicates statistically significant differences when compared with E De at p < 0.05; b indicates statistically significant differences when compared with Nung in Daklak province at p < 0.05.
Since the sample size is equal or smaller than 5, some ethnic groups, such as Tay, Dao, Van Kieu, Thai, Chinese, Day, Ray, Bo Y, and Man were excluded from the analysis.
2.3. Risk of H. pylori Infection
Table 2 presents the associations between H. pylori acquisition and potential risk factors, including age group, gender, geographical location, ethnicities, and marital, smoking, and drinking status. The results showed that subjects living in Daklak had a significantly higher risk of H. pylori infection than those in Lao Cai (crude OR, 2.52; 95% CI, 1.73–3.65). With respect to minor ethnic groups, E De (crude OR, 2.44; 95% CI, 1.64–3.62) was at significantly higher risk, whereas Tay (crude OR, 0.30; 95% CI, 0.16–0.56) and Dao (crude OR, 0.37; 95% CI, 0.17–0.73) were at significantly lower risk of H. pylori acquisition.
Table 2.
Risk factors of H. pylori infection in the study population.
| Risk Factor | H. pylori Positive/Total Number (%) | Crude OR | 95% CI | p-Value |
|---|---|---|---|---|
| Age group | ||||
| ≤ 29 | 38/118 (32.2%) | 0.72 | 0.45–1.13 | 0.16 |
| 30–39 | 63/156 (40.4%) | 1.15 | 0.77–1.73 | 0.49 |
| 40–49 | 38/107 (35.5%) | 0.87 | 0.54–1.39 | 0.58 |
| 50–59 | 33/67 (49.3%) | 1.70 | 0.98–2.95 | 0.06 |
| ≥ 60 | 16/46 (34.8%) | 0.86 | 0.42–1.68 | 0.75 |
| Gender | ||||
| Male | 90/210 (42.9%) | 1.42 | 0.98–2.05 | 0.06 |
| Female | 98/284 (34.5%) | 1.00 | ||
| Geographical location | ||||
| Daklak | 102/200 (51.0%) | 2.52 | 1.73–3.65 | <0.001 |
| Lao Cai | 86/294 (29.3%) | 1.00 | ||
| Ethnicities | ||||
| E De | 94/183 (51.4%) | 2.44 | 1.64–3.62 | <0.001 |
| H′mong | 40/105 (38.1%) | 1.00 | 0.62–1.59 | 1.00 |
| Nung | 8/24 (33.3%) | 0.81 | 0.29–2.04 | 0.67 |
| Tay | 15/83 (18.1%) | 0.30 | 0.16–0.56 | <0.001 |
| Dao | 12/60 (20%) | 0.37 | 0.17–0.73 | 0.002 |
| Xa Pho | 13/24 (54.2%) | 1.99 | 0.87–4.54 | 0.13 |
| Other* | 6/15 (40%) | 0.64 | 0.24–1.68 | 0.5 |
| Marital status | ||||
| Single | 1/3 (33.3%) | 1.00 | ||
| Married | 187/491 (38.1%) | 1.23 | 0.06–73.0 | 1.00 |
| Smoking | ||||
| Yes | 28/81 (34.6%) | 0.84 | 0.49–1.41 | 0.53 |
| No | 160/413 (38.7%) | 1.00 | ||
| Drinking | ||||
| Yes | 55/121 (45.5%) | 1.50 | 0.97–2.32 | 0.07 |
| No | 133/373 (35.7%) | 1.00 |
* include the ethnicities (including Day, Ray, Bo Y, Van Kieu, Thai, Chinese, and Man) with a sample size that is equal or smaller than 5.
To estimate the effect of each studied independent factor on the risk of H. pylori infection, as well as the complex interplay between them, multivariate logistic regression was performed. Among all the evaluated characteristics, the final model showed that people residing in Daklak (adjusted OR, 2.84; 95% CI,1.93–4.20), age group (adjusted OR, 1.79; 95% CI,1.05–3.07), and Xa Pho ethnicity (adjusted OR, 3.04; 95% CI,1.29–7.16) were significantly independent associated with the risk of H. pylori infection.
2.4. H. pylori and the Gastroduodenal Diseases
Among H. pylori-infected participants, gastritis is the most prevalent diagnosis; 171/188 (91%), followed by GU 9/188 (4.8%), DU 8/188 (4.3%), and GERD 4/188 (2.1%). However, a high prevalence of H. pylori was observed in subjects suffering from GU (58.3%) and DU (64.3%), while subjects with gastritis were only 36.8% H. pylori-positive. When considering DU and GU as PU, the prevalence of H. pylori infection in PU was significantly higher than that in gastritis (60.7% vs. 36.8%, p = 0.02). The results from univariate analysis also showed that H. pylori positivity was significantly associated with PU (OR = 2.7, 95% CI 1.2–5.8). There were 19 cases with GERD diagnosed by endoscopic observation and no association with H. pylori infection was found.
2.5. H. pylori and Histological Evaluation
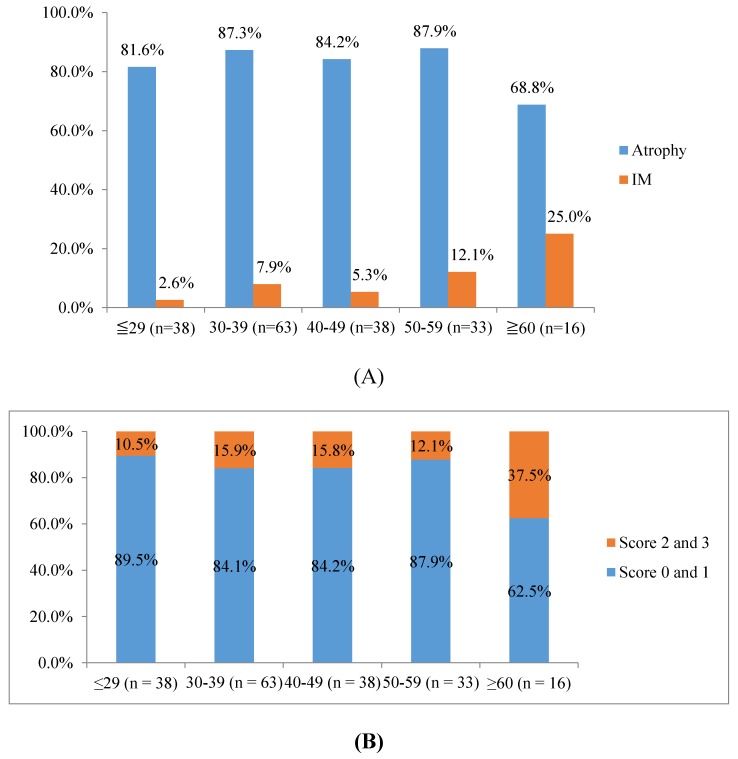
Among H. pylori-infected subjects, the intestinal metaplasia (IM) significantly increased with aging (p trend = 0.016) (Figure 2A), but no association was found in the case of atrophy. However, when the level of atrophic inflammation was classified by OLGA staging for gastritis criteria, our results showed that participants with high levels of atrophy, corresponding with an OLGA score of 2 or 3, significantly increased with aging (p trend = 0.017) (Figure 2B).
Figure 2.
H. pylori and histology. (A) The distribution of atrophic gastritis (atrophy) and intestinal metaplasia (IM) based on age groups within H. pylori infected subjects. (B) The distribution of OLGA score (high risk: OLGA 2 and 3, and low risk: OLGA 0 and 1) based on age groups within H. pylori-infected subjects.
To comprehensively compare histological scores of H. pylori culture-positive subjects in different ethnicities, data from the Kinh people of a previous study were used (Table 3). In our previous study, all populations studied were Kinh and there were 103 H. pylori culture-positive cases. Overall, the histological scores in E De were significantly lower than those in the remaining ethnicities (Table 3). Namely, the active (neutrophil) and chronic (monocyte) inflammation scores were significantly lower in E De compared to H’mong, Tay, Dao, and Kinh groups (all p < 0.05). Additionally, IM scores in the antrum in E De were significantly lower than that in H’mong, Tay, and Xa Pho groups; and the IM scores in the corpus in E De were also significantly lower than that in Kinh people (all p < 0.05). The OLGIM score in E De was significantly lower than that in H’mong, Tay, Xa Pho, and Kinh.
Table 3.
Comparison of histological scores between H. pylori infected ethnic groups.
| E De (n = 90) | Nung (n = 6) | H’mong (n = 39) | Tay (n = 12) | Dao (n = 9) | Xa Pho (n = 11) | Kinh (n = 103) a | ||
|---|---|---|---|---|---|---|---|---|
| Antrum | ||||||||
| Neutrophil | 1.2 (1) | 2.0 (2) b | 1.7 (2) c | 1.7 (2) d | 1.7 (2) e | 2.0 (2) f | 1.2 (1) | |
| Monocyte | 1.6 (2) | 2.3 (2.5) b | 2.1 (2) c | 2.2 (2) d | 2.0 (2) | 2.2 (2) f | 1.7 (2) | |
| Atrophy | 0.9 (1) | 1.5 (1) | 1.0 (1) | 1.2 (1) | 0.9 (1) | 1.0 (1) | 0.9 (1) | |
| IM | 0.02 (0) | 0.0 (0) | 0.2 (0) c | 0.2 (0) d | 0.0 (0) | 0.4 (0) f | 0.1 (0) | |
| Corpus | ||||||||
| Neutrophil | 0.8 (1) | 0.8 (1) | 1.0 (1) c | 0.9 (1) | 1.1 (1) | 0.9 (1) | 1.0 (1) g | |
| Monocyte | 0.6 (1) | 0.5 (0.5) | 1.2 (1) c | 1.1 (1) d | 1.2 (1) e | 0.6 (1) | 1.5 (1) g | |
| Atrophy | 0.02 (0) | 0.0 (0) | 0.2 (0) c | 0.2 (0) d | 0.2 (0) e | 0.0 (0) | 0.6 (1) g | |
| IM | 0.01 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.1 (0) g | |
| OLGA | 0.9 (1) | 1.5 (1) | 1.1 (1) | 1.2 (1) | 0.9 (1) | 1.0 (1) | 1.0 (1) | |
| OLGIM | 0.03 (0) | 0.0 (0) | 0.2 (0) c | 0.2 (0) d | 0.0 (0) | 0.4 (0) f | 0.2 (0) g | |
a Data obtained from our previous study. b indicates a statistically significant difference between E De and Nung. c indicates a statistically significant difference between E De and H’mong. d indicates a statistically significant difference between E De and Tay. e Indicates a statistically significant difference between E De and Dao. f indicates a statistically significant difference between E De and Xa Pho. g indicates a statistically significant difference between E De and Kinh. Statistically significant difference was determined on the basis of the Mann–Whitney test (p < 0.05).
2.6. The Distribution of cagA and vacA Genotypes
The general frequency of cagA was 170/171 (99.4%). Among cagA positive strains, it was consisted of 97/170 (57.1%) East Asian-type CagA and 73/170 (42/9%) Western-type CagA (Table 4). Interestingly, Western-type CagA was only found in E De with high proportion, 73/89 (82%), whereas all the rest of minor ethnic groups, as well as the Kinh people, isolates possessed East Asian-type CagA, irrespective of geographical regions. Among 73 Western-type CagA strains (all from the E De group), 50 (68.5%), 15 (20.5%), 1 (1.4%), 1 (1.4%), and 6 (8.2%) were of ABC, ABCC, ABCCC, BC, and AB type, respectively. Out of 97 East Asian-type CagA strains, 90 (92.8%), 6 (6.2%), and 1 (0.01%) were of ABD, AB′BD, and AB type, respectively. Regarding the EPIYA motif, a total of 527 EPIYA motifs were obtained from the 170 cagA sequences. The distribution of EPIYA and EPIYA-like motifs were 490/527 (93%) EPIYA, 28/527 (5.3%) EPIYT, 6/527 (1.1%) ESIYA, and 2/527 (0.4%) and 1/527 (0.1%) ESIYT, respectively.
Table 4.
The distribution of cagA and vacA among minor ethnic group in Daklak and Lao Cai province.
| H. pylori Culture Positive Cases | cagA Positive | CagA Type | vacA s and m | ||
|---|---|---|---|---|---|
| Western | East Asian | s1m1 | s1m2 | ||
| Daklak (n = 96) | 95 (99%) | 73 (76.8%) | 22 (23.2%) | 78 (81.3%) | 18 (18.8%) |
| E De | 89 (98.9%) | 73 (82%) | 16 (18%) | 74 (82.2%) | 16 (17.8%) |
| Nunga | 4 (100%) | 0 (0%) | 4 (100%) | 2 (50%) | 2 (50%) |
| Van Kieu | 1 (100%) | 0 (0%) | 1 (100%) | 1 (100%) | 0 (0%) |
| Thai | 1 (100%) | 0 (0%) | 1 (100%) | 1 (100%) | 0 (0%) |
| Lao Cai (n = 75) | 75 (100%) | 0 (0%) | 75 (100%) | 34 (45.3%) | 39 (52%) |
| H’mong | 39 (100%) | 0 (0%) | 39 (100%) | 17 (43.6%) | 20 (51.3%) |
| Nung a | 2 (100%) | 0 (0%) | 2 (100%) | 2 (100%) | 0 (0%) |
| Tay a | 12 (100%) | 0 (0%) | 12 (100%) | 7 (41.7%) | 7 (58.3%) |
| Dao a | 9 (100%) | 0 (0%) | 9 (100%) | 4 (44.4%) | 5 (55.6%) |
| Xa Pho | 11 (100%) | 0 (0%) | 11 (100%) | 4 (36.4%) | 7 (63.6%) |
| Ray | 1 (100%) | 0 (0%) | 1 (100%) | 1 (100%) | 0 (0%) |
| Man | 1 (100%) | 0 (0%) | 1 (100%) | 1 (100%) | 0 (0%) |
| Total (n = 171) | 170 (99.4%) | 73 (42.9%) | 97 (57.1%) | 112 (65.5%) b | 57 (33.3%) b |
a These groups are present in both Daklak and Lao Cai provinces. b There were four cases with the genotype s1m1m2, and the cases were not listed.
An association between CagA type and histological scores within ethnic groups was presented in Table 5. Within the E De group, there was no significant difference in histological findings between individuals infected with Western-type CagA and those infected with East Asian-type CagA. In addition, among subjects infected with East Asian-type CagA, the histological scores were also significantly lower in the E De group than in the other minor groups and the Kinh group.
Table 5.
Comparison of histological scores between Western-type CagA and East–Asian-type CagA strains of different ethnic groups.
| Cell Infiltration | Western-Type CagA E De (n = 73) |
East Asian-Type CagA E De (n = 16) |
East Asian-Type CagA Non-E De (n = 81) |
East Asian-Type CagA Kinh (n = 103) |
|---|---|---|---|---|
| Histological scoresMean (median) | ||||
| Antrum | ||||
| Neutrophil | 1.2 (1) | 1.2 (1) | 1.7 (2) a | 1.2 (1) |
| Monocyte | 1.5 (2) | 1.7 (2) | 2.1 (2) a | 1.7 (2) |
| Atrophy | 0.9 (1) | 1.1 (1) | 1.1 (1) | 0.9 (1) |
| IM | 0.03 (0) | 0.0 (0) | 0.2 (0) a | 0.1 (0) |
| Corpus | ||||
| Neutrophil | 0.8 (1) | 0.6 (1) | 0.9 (1) | 1.0 (1) |
| Monocyte | 0.6 (1) | 0.5 (1) | 1.0 (1) a | 1.5 (2) a |
| Atrophy | 0.02 (0) | 0.0 (0) | 0.2 (0) a | 0.6 (1) a |
| IM | 0.01 (0) | 0.0 (0) | 0.0 (0) | 0.1 (0) |
| OLGA | 0.9 (1) | 1.1 (1) | 1.1 (1) | 1.0 (1) |
| OLGIM | 0.04 (0) | 0.0 (0) | 0.2 (0) a | 0.2 (0) a |
a p < 0.05 by Mann–Whitney test when compared with Western type CagA E De.
All strains, 171/171 (100%), possessed vacA s1. In the m region, the distribution was 112/171 (65.5%) with m1, 57/171 (33.3%) with m2, and 2/171 (1.2%) with m1m2, of which vacA m1 was predominant in E De, 74/90 (82.2%), and Nung, 4/6 (66.7%), while vacA m2 was dominant in H’mong, 20/39 (51.3%), Tay, 7/12 (58.3%), Dao, 5/9 (55.6%), and Xa Pho, 7/11 (63.6%) (Table 4). When combining cagA and vacA, the frequency of East Asian-type CagA/vacA s1m1 in DU was significantly higher than that in gastritis (66.7% vs. 25.8%, p = 0.048) (Table 6).
Table 6.
The association between H. pylori virulence factors and clinical outcomes.
| No. of Samples | |||||
|---|---|---|---|---|---|
| Type | GU | DU | PU | Gastritis | Total |
| No of culture positive cases | 10 | 6 | 16 | 155 | 171 |
| cagA positive | 10 (100%) | 6 (100%) | 16 (100%) | 154 (99.4%) | 170 (99.4%) |
| Western-type CagA | 4 (40%) | 2 (33.3%) | 6 (37.5%) | 67 (43.2%) | 73 (42.9%) |
| East-Asian-type CagA | 6 (60%) | 4 (66.7%) | 10 (62.5%) | 87 (56.1%) | 97 (57.1%) |
| vacA s1 | 10 (100%) | 6 (100%) | 16 (100%) | 155 (100%) | 171 (100%) |
| vacA m1 | 8 (80%) | 5 (83.3%) | 13 (81.3%) | 99 (63.9%) | 112 (65.5%) |
| vacA m2 | 2 (20%) | 1 (16.7%) | 3 (18.7%) | 54 (34.8%) | 57 (33.3%) |
| vacA m1m2 | 2 (20%) | 0 (0%) | 2 (12.5%) | 2 (1.3%) | 2 (1.2%) |
| vacA s1m1 | 8 (80%) | 5 (83.3%) | 13 (81.3%) | 99 (63.9%) | 112 (65.5%) |
| vacA s1m2 | 2 (20%) | 1 (16.7%) | 0 (0%) | 54 (34.8%) | 57 (33.3%) |
| vacA s1m1m2 | 2 (20%) | 0 (0%) | 0 (0%) | 2 (1.3%) | 2 (1.2%) |
| Western-type CagA/vacA s1m1 | 4 (40%) | 1 (16.7%) | 5 (31.3%) | 58 (37.4%) | 63 (36.8%) |
| Western-type CagA/vacA s1m2 | 0 (0%) | 1 (16.7%) | 1 (6.3%) | 9 (5.8%) | 10 (5.8%) |
| East-Asian-type CagA/vacA s1m1 | 4 (40%) | 4 (66.7%) a | 8 (50%) | 40 (25.8%) a | 48 (28.1%) |
| East-Asian-type CagA/vacA s1m2 | 2 (20%) | 0 (0%) | 0 (0%) | 45 (29%) | 47 (27.5%) |
| East-Asian-type CagA/vacA s1m1m2 | 0 (0%) | 0 (0%) | 0 (0%) | 2 (1.3%) | 2 (1.2%) |
a p = 0.048 by Fisher exact test when compared between DU and gastritis.
3. Discussion
This is the first population-based cross-sectional study about H. pylori focusing on minor ethnic groups in Vietnam. Overall, the prevalence of H. pylori infection in our minor ethnic groups was 38.1%. The infection rate was lower than previous studies reported in Vietnam (56.1%–78.8%) [9,12,13]. The difference could be due to the difference in the study population (target population; minor ethnic groups, but not the Kinh ethnic group; general population, but not the hospital population), methodology (some studies used only serological test or rapid urine test). However, the result remained in line with the data reported by other countries in the Southeast Asia area, such as Thailand (23.3%–45.9%) [14,15], Laos (36.2%) [16], Myanmar (48%) [17], and Cambodia (approximately 30%, our unpublished data). Thus, it could be hypothesized that the prevalence of H. pylori infection in Vietnam might be not as high as previously reported.
Like many previous observational studies, the prevalence of H. pylori infection in the study differs from country to country, ethnicity to ethnicity, even in the same country [2,6]. Our study showed that the infection rate in Daklak (51%) was significantly higher than that in Lao Cai (29.3%) and also revealed a different risk of H. pylori infection between ethnicities (p < 0.001) (Table 2). Interestingly, the Nung ethnic group resided in both Daklak and Lao Cai; however the prevalence of H. pylori infection of Nung in Daklak was also significantly higher than that of Nung in Lao Cai (66.7% vs. 13.3%, p = 0.02). Indeed, the final model of multivariate analysis revealed that the risk of H. pylori acquisition is related to geographical regions, age groups, and ethnicities. Therefore, further studies based on the general population, and multicenter, multiethnic studies are necessary to generalize the H. pylori prevalence in the entire Vietnamese population, through which am accurate re-evaluation of the burden of H. pylori-caused disease can be made.
Our results indicated that H. pylori increased the risk of PU (OR = 2.7, 95% CI 1.2–5.8). In addition, H. pylori-infected people also increased the risk of precancerous lesions with aging, including intestinal metaplasia (p trend = 0.016) and severe atrophic status (p trend = 0.017). To remain in line with the Kinh ethnicity, as well as the global consensus, the microorganism infection is associated with severe gastroduodenal diseases and H. pylori eradication might contribute to decreasing the burden and risk of H. pylori-caused diseases [1,4].
It was fascinating that, in this study, the histological analysis of the gastric mucosal status exhibited that most histological scores in E De were significantly lower than those in other ethnicities, including the Kinh people (Table 3). According to the cascade pathway of GC proposed by Correa et al., gastritis is a crucial, indispensable step prior to developing GC and the progress is dependent on many factors, including bacterial virulence, and host genetic and environmental factors [4,18]. Many studies showed that a difference in H. pylori virulence factors might explain the variation in H. pylori-caused phenotype traits and we hypothesized that there was not much difference related to host and environmental factors for ethnicities residing in the same territory [2,9,19]. In efforts to clarify the difference, intriguingly only the E De ethnicity harbored Western-type CagA with high prevalence (82%), whereas all remaining studied ethnic groups harbored East Asian-type CagA. Indeed, many epidemiological studies showed that individuals possessing Western-type CagA exhibited lower gastric mucosal inflammation status and were more common in people suffering gastritis, but not in those suffering PU or GC compared to those possessing East Asian-type CagA [19,20,21]. Moreover, in vitro studies also showed that Western-type CagA expressed fewer biological activities, such as hummingbird phenotype, proinflammatory secretion, the disruption of cell junctions, or loss of cell polarity compared to East Asian-type CagA [22]. Thus, it is evident that Western-type CagA is less virulent than East Asian-type CagA. This might be the reason to explain why the gastric mucosal damage was milder in E De mainly harboring Western-type CagA compared to other ethnic groups harboring East Asian-type CagA. The presence of Western-type CagA in E De in Vietnam was similar to that in Okinawa, Japan [23]. To date, H. pylori has been used as a tool for tracing human migration, and further studies speculating where the E De came from is an interesting question.
Epidemiological and basic studies showed that vacA m1 is more virulent than vacA m2 [9,10,24]. In this study, the frequency of vacA m1 in the E De people was more predominant than other ethnic groups, however, the gastric mucosal damage was milder. This supported the hypothesis that it was better to study the combination or interaction between virulence factors rather than to find which factor is the most virulent [2]. Indeed, when combining cagA and vacA, the more virulent genotype East Asian-type CagA and vacA s1m1 presented a significant association with DU compared to gastritis.
Although East Asian-type CagA is obviously more virulent than Western-type CagA [19,20,21], our results revealed that the histological scores between people infected with Western-type CagA and East Asian-type CagA within E De group were not significantly different (Table 5). Moreover, the histological scores in the E De group possessing East Asian-type CagA were also significantly lower than those in other ethnic groups with East Asian-type CagA. These implicated that although the bacterial factors are more or less virulent, the host susceptibility also plays an important role to modulating the level of gastric mucosal inflammation, irrespective of the virulent level of H. pylori. It suggested that the E De ethnicity has a somewhat specific susceptibility or host factor with a low risk of gastric mucosal inflammation that might be predisposed to a low risk of GC. Our finding was also similar to Thailand [25] and gave evidence to support a novel point of view in the Asian enigma that could be elucidated by specific host susceptibility apart from the presence of less virulent strains. Thus, further studies in the future in Southeast Asia area concentrating on the host factor are essential to verify the enigma.
4. Material and Methods
4.1. Study Design and Study Population
This is a population-based cross-sectional study to characterize the prevalence of H. pylori infection, as well as its virulence factors in a group of minor ethnicities in Vietnam. In the country, minor ethnic groups account for 13% of the general Vietnamese population and mainly reside in the highlands or mountains, especially in the northern mountainous regions and Tay Nguyen Central highlands. The survey was conducted in nine rural areas in Daklak, Tay Nguyen Central highland and Lao Cai Province, northern area, Vietnam from July 2012 to April 2013 (Figure 1). The distribution of minor ethnic groups was Kinh, 67%; E De, 17.2%; Nung, 4.1%; Tay, 3%; and other ethnic groups, less than 1% in Daklak. and Kinh, 35%; H’mong, 24%; Tay, 15.3%; Dao, 14.4%; Nung, 4.2%; Xa Pho 1.5%; and other ethnic groups less than 1% in Lao Cai, respectively (data available at http://www.gso.gov.vn/). Each area was sampled several times and at least two days per time was spent to recruit consecutive participants. The inclusion criteria were as follows: (1) age above 18 years old consenting to participate in the study; (2) no contraindication to upper gastroscopy; (3) no current treatment with proton pump inhibitors, antibiotics, bismuth-containing compounds, aspirin or nonsteroidal anti-inflammatory drugs within four weeks prior to the survey; and (4) no history of gastrectomy. Participants were volunteers in the community called to attend the study and would be given a health check-up, an endoscopy performed, and prescribed and given drugs. All steps were free of charge. All subjects were interviewed by trained medical staff to collect personal information about demographics, lifestyles, medical history, and so on.
Informed consent was obtained from all participants prior to the investigation. Ethical approvals were approved by the Ethics Committee of Daklak and Lao Cai Hospital, Vietnam and Oita University, Faculty of Medicine, Japan.
4.2. Sampling
Four biopsy specimens (three from the antrum and one from the corpus) were taken during endoscopic procedure, of which two antral specimens were used for rapid urease test (RUT) and culture; and one antral and one corporal specimens were adopted for histological evaluation. PU, GC, and gastroesophageal reflux disease (GERD) were diagnosed under endoscopic observation and GC was confirmed by histopathology. Gastritis was defined in the absence of PU or suspicious malignancy in the stomach. After gastroscopy, blood samples were collected and then centrifuged to obtain serum. The obtained sera were stored at –80 °C until processing.
4.3. The Determination of H. pylori Status
To maximize the reliability in the detection ability of H. pylori infection, multiple tests comprising of RUT, culture, serological tests with enzyme-linked immunosorbent assay kit (Eiken Co., Ltd., Tokyo, Japan), and histology confirmed by immunohistochemistry (IHC) were used as previously described [9].
The gold standard of infection status was a positive culture. In the case of a negative culture, at least two positive tests among the following tests (histology, RUT, and serum-ELISA) were regarded as positive. If only one test was positive apart from culture, it was considered as undetermined H. pylori status. H. pylori was judged as negative when all test gave negative results.
4.4. Histological Status of Chronic Gastritis
All biopsy specimens were fixed in 10% formalin for 24 h and embedded into paraffin. A series of biopsy sections were stained with hematoxylin and eosin, and Giemsa. IHC using anti-H. pylori antibodies was performed as previously described [15]. Histological scores were evaluated based on the updated Sydney system (range 0–3: 0, none; 1, mild; 2, moderate; 3, marked) by an experienced pathologist (T.U) who would not know the sample identities [26]. In addition, the grading of chronic gastritis conveying information about anatomical extent of atrophic-metaplastic changes was also further assessed according to Operative Link for Gastritis Assessment (OLGA) and Operative Link on Gastric Intestinal Metaplasia (OLGIM) [27,28].
4.5. Cytotoxin Associated Gene A (cagA) and Vacuolating Cytoxotin (vacA) Genotype Analysis
An antral tissue specimen was used to isolate H. pylori strains by using standard culture method as previously described [29]. DNA extraction was performed by a commercial kit (QIAGEN DNeasy blood and tissue kit) according to manufacturer’s instruction. Extracted DNA was stored at –20 °C until used as a template for PCR.
cagA and vacA genotyping were performed as previously described [24,30]. Briefly, cagA was amplified using primers cagOMF (5′-AGC AAA AAG CGA CCT TGA AA-3′) and cagOMR (5′-AGT GGC TCA AGC TGC TGA AT-3′) and purified PCR products were sequenced using an AB 3130 genetic analyzer (Applied Biosystems, Foster City, CA, SUA). In silico, the DNA sequence was transformed into amino acids and aligned with MEGA v6 software [31]. Then, CagA types (East-Asian-type CagA and Western-type CagA) were defined according to flanking region of the EPIYA motif. Taken together, the strains possessing EPIYA-A, EPIYA-B, and EPIYA-C segments were considered as Western-type CagA and the strains possessing EPIYA-A, EPIYA-B, and EPIYA-D segments were considered as East Asian-type CagA. In case of untypable cagA, cagA status was confirmed with primers for the cag empty site. As for vacA, vacA s and m genotypes (s1 or s2, m1 or m2) were determined by the size of PCR products as previously described [24].
4.6. Statistical Analysis
Mean ± standard deviation or median was used to present the continuous variables. Frequency and percentage were used to present categorical variables. Data analysis was implemented with χ2 test, Fisher’s exact test, one-way ANOVA test, Mann–Whitney test, and multivariate logistic regression by using the SPSS software package (version 16.0; SPSS Inc., Chicago, USA). Regarding multivariate logistic regression analysis, independent factors with a significance of p < 0.25 were selected in the regression equation. A stepwise backward procedure was used to identify the best fit model. Odds ratio (OR) and 95% confidence interval (CI) were used to estimate the risk. The significance at p < 0.05 was established as statistically significant.
5. Conclusions
The prevalence of H. pylori infection varies based on geographical regions, age groups, and ethnicities, thus, nation-wide studies are necessary to evaluate the H. pylori prevalence in general populations, as well as the burden of H. pylori infection. In addition to H. pylori virulence factors, host factors might also play a crucial role responsible for gastric mucosal damage. This may be a novel insight to clarify the Asian enigma.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (DK62813) and the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan 26640114, 15H02657, and 16H05191) (to Y.Y.). T.T.B. and T.T.H.T. were the PhD students and V.P.T. is the PhD student supported by the Japanese government (Monbukagakusho: MEXT) Scholarship Program for 2010, 2011, and 2015, respectively.
Abbreviations
| GC | Gastric cancer |
| Helicobacter pylori | H. Pylori |
| PU | Peptic ulcer |
| ASR | Age-standardized incidence rate |
| DU | Duodenal ulcer |
| GU | Gastric ulcer |
| RUT | Rapid urease test |
| IHC | Immunohistochemistry |
| OLGA | Operative link for gastritis assessment |
| OLGIM | Operative link on gastric intestinal metaplasia |
| cagA | Cytotoxin-associated gene A |
| vacA | Vacuolating cytotoxin |
| OR | Odds ratio |
| CI | Confidence interval |
| IM | Intestinal metaplasia |
Author Contributions
T.T.B., H.D.Q.D., V.V.K., and Y.Y. conceived and designed the study; V.P.T., H.D.Q.D., P.H.T., T.D.T., P.Q.H., V.V.K., L.Q.T., B.C.N., and D.A.G. contributed by collecting samples; T.T.B. and T.T.H.T. performed the experiments; T.T.B., V.P.T., T.T.H.T., S.R., and Y.Y. contributed to the analysis and interpretation; and T.T.B., V.P.T., T.T.H.T., and Y.Y. drafted the manuscript.
Conflicts of Interest
All authors declare that they have no potential conflicts of interest.
References
- 1.Malaty H.M. Epidemiology of Helicobacter pylori infection. Best Pract. Res. Clin. Gastroenterol. 2007;21:205–214. doi: 10.1016/j.bpg.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat. Rev. Gastroenterol. Hepatol. 2010;7:629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall B., Warren J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. The Lancet. 1984;323:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 4.Peek R.M., Jr., Blaser M.J. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 5.Suerbaum S., Michetti P. Helicobacter pylori infection. N. Engl. J. Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 6.Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Graham D.Y., Lu H., Yamaoka Y. African, Asian or Indian enigma, the East Asian Helicobacter pylori: Facts or medical myths. J. Dig. Dis. 2009;10:77–84. doi: 10.1111/j.1751-2980.2009.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uemura N., Okamoto S., Yamamoto S., Matsumura N., Yamaguchi S., Yamakido M., Taniyama K., Sasaki N., Schlemper R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen T.L., Uchida T., Tsukamoto Y., Trinh D.T., Ta L., Mai B.H., Le S.H., Thai K.D., Ho D.D., Hoang H.H., et al. Helicobacter pylori infection and gastroduodenal diseases in Vietnam: A cross-sectional, hospital-based study. BMC Gastroenterol. 2010;10:114. doi: 10.1186/1471-230X-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binh T.T., Tuan V.P., Dung H.D.Q., Tung P.H., Tri T.D., Thuan N.P.M., Khien V.V., Hoan P.Q., Suzuki R., Uchida T., et al. Advanced non-cardia gastric cancer and Helicobacter pylori infection in Vietnam. Gut. Pathogens. 2017;9:46. doi: 10.1186/s13099-017-0195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vietnam Image of The Community of 54 Ethnic Groups. [(accessed on 1 March 2018)]; Available online: http://cema.gov.vn.
- 12.Hoang T.T., Bengtsson C., Phung D.C., Sorberg M., Granstrom M. Seroprevalence of Helicobacter pylori infection in urban and rural Vietnam. Clin. Diagn. Lab. Immunol. 2005;12:81–85. doi: 10.1128/CDLI.12.1.81-85.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen L.T., Uchida T., Tsukamoto Y., Trinh T.D., Ta L., Ho D.Q., Matsuhisa T., Uchida M., Takayama A., Hijiya N., et al. Evaluation of rapid urine test for the detection of Helicobacter pylori infection in the Vietnamese population. Dig. Dis. Sci. 2010;55:89–93. doi: 10.1007/s10620-009-0720-9. [DOI] [PubMed] [Google Scholar]
- 14.Piriyapong K., Tangaroonsanti A., Mahachai V., Vilaichone R.K. Helicobacter pylori infection impacts on functional dyspepsia in Thailand. Asian Pac. J. Cancer Prev. 2014;15:10887–10891. doi: 10.7314/APJCP.2014.15.24.10887. [DOI] [PubMed] [Google Scholar]
- 15.Uchida T., Miftahussurur M., Pittayanon R., Vilaichone R.K., Wisedopas N., Ratanachu-Ek T., Kishida T., Moriyama M., Yamaoka Y., Mahachai V. Helicobacter pylori Infection in Thailand: A Nationwide Study of the CagA Phenotype. PLoS ONE. 2015;10:e0136775. doi: 10.1371/journal.pone.0136775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vannarath S., Vilaichone R.K., Rasachak B., Mairiang P., Yamaoka Y., Shiota S., Binh T.T., Mahachai V. Virulence genes of Helicobacter pylori in gastritis, peptic ulcer and gastric cancer in Laos. Asian Pac. J. Cancer Prev. 2014;15:9027–9031. doi: 10.7314/APJCP.2014.15.20.9027. [DOI] [PubMed] [Google Scholar]
- 17.Myint T., Shiota S., Vilaichone R.K., Ni N., Aye T.T., Matsuda M., Tran T.T., Uchida T., Mahachai V., Yamaoka Y. Prevalence of Helicobacter pylori infection and atrophic gastritis in patients with dyspeptic symptoms in Myanmar. World J. Gastroenterol. 2015;21:629–636. doi: 10.3748/wjg.v21.i2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correa P., Haenszel W., Cuello C., Tannenbaum S., Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/S0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 19.Vilaichone R.K., Mahachai V., Tumwasorn S., Wu J.Y., Graham D.Y., Yamaoka Y. Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: a cultural cross roads. Helicobacter. 2004;9:453–459. doi: 10.1111/j.1083-4389.2004.00260.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones K.R., Joo Y.M., Jang S., Yoo Y.J., Lee H.S., Chung I.S., Olsen C.H., Whitmire J.M., Merrell D.S., Cha J.H. Polymorphism in the CagA EPIYA motif impacts development of gastric cancer. J. Clin. Microbiol. 2009;47:959–968. doi: 10.1128/JCM.02330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azuma T., Yamakawa A., Yamazaki S., Ohtani M., Ito Y., Muramatsu A., Suto H., Yamazaki Y., Keida Y., Higashi H., et al. Distinct diversity of the cag pathogenicity island among Helicobacter pylori strains in Japan. J. Clin. Microbiol. 2004;42:2508–2517. doi: 10.1128/JCM.42.6.2508-2517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 23.Matsunari O., Shiota S., Suzuki R., Watada M., Kinjo N., Murakami K., Fujioka T., Kinjo F., Yamaoka Y. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J. Clin. Microbiol. 2012;50:876–883. doi: 10.1128/JCM.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atherton J.C., Cao P., Peek R.M., Tummuru M.K., Blaser M.J., Cover T.L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 25.Subsomwong P., Miftahussurur M., Uchida T., Vilaichone R.K., Ratanachu-Ek T., Mahachai V., Yamaoka Y. Prevalence, risk factors, and virulence genes of Helicobacter pylori among dyspeptic patients in two different gastric cancer risk regions of Thailand. PLoS ONE. 2017;12:e0187113. doi: 10.1371/journal.pone.0187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixon M.F., Genta R.M., Yardley J.H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Rugge M., Correa P., Di Mario F., El-Omar E., Fiocca R., Geboes K., Genta R.M., Graham D.Y., Hattori T., Malfertheiner P., et al. OLGA staging for gastritis: a tutorial. Dig. Liver Dis. 2008;40:650–658. doi: 10.1016/j.dld.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 28.Capelle L.G., de Vries A.C., Haringsma J., Ter Borg F., de Vries R.A., Bruno M.J., van Dekken H., Meijer J., van Grieken N.C., Kuipers E.J. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc. 2010;71:1150–1158. doi: 10.1016/j.gie.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 29.Yamaoka Y., Kodama T., Kita M., Imanishi J., Kashima K., Graham D.Y. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter. 1998;3:241–253. doi: 10.1046/j.1523-5378.1998.08056.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamaoka Y., El–Zimaity H.M.T., Gutierrez O., Figura N., Kim J.K., Kodama T., Kashima K., Graham D.Y. Relationship between the cagA 3′ repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology. 1999;117:342–349. doi: 10.1053/gast.1999.0029900342. [DOI] [PubMed] [Google Scholar]
- 31.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]