Abstract
Influenza virus remains a major health concern worldwide, and there have been continuous efforts to develop effective antivirals despite the use of annual vaccination programs. The purpose of this study was to determine the anti-influenza activity of Bax inhibitor-1 (BI-1). Madin-Darby Canine Kidney (MDCK) cells expressing wild type BI-1 and a non-functional BI-1 mutant, BI-1 ∆C (with the C-terminal 14 amino acids deleted) were prepared and infected with A/PR/8/34 influenza virus. BI-1 overexpression led to the suppression of virus-induced cell death and virus production compared to control Mock or BI-1 ∆C overexpression. In contrast to BI-1 ∆C-overexpressing cells, BI-1-overexpressing cells exhibited markedly reduced virus-induced expression of several viral genes, accompanied by a substantial decrease in ROS production. We found that treatment with a ROS scavenging agent, N-acetyl cysteine (NAC), led to a dramatic decrease in virus production and viral gene expression in control MDCK and BI-1 ∆C-overexpressing cells. In contrast, NAC treatment resulted in the slight additional suppression of virus production and viral gene expression in BI-1-overexpressing cells but was statistically significant. Moreover, the expression of heme oxygenase-1 (HO-1) was also significantly increased following virus infection in BI-1-overexpressing cells compared to control cells. Taken together, our data suggest that BI-1 may act as an anti-influenza protein through the suppression of ROS mediated cell death and upregulation of HO-1 expression in influenza virus infected MDCK cells.
Keywords: Bax inhibitor-1, anti-influenza, reactive oxygen species, heme oxygenase-1
1. Introduction
Influenza viruses, which are enveloped RNA viruses belonging to the family Orthomyxoviridae, are a significant cause of respiratory infections, causing millions of deaths annually and imposing significant economic losses [1]. Influenza virus infection is a serious health problem worldwide, resulting in morbidity and mortality in humans in the form of epidemics or pandemics [2]. Until now, annual immunization and antiviral drugs have represented the main approaches for dealing with influenza. Antiviral drugs have been proven to be effective as a preventive and therapeutic regime over the last several decades [3]. To date, three classes of anti-influenza virus drugs are available for influenza management with different modes of action: the M2 channel blockers such as amantadine and rimantadine, neuraminidase (NA) inhibitors (e.g., oseltamivir) [4], and RNA polymerase inhibitors (e.g., ribavirin) [5]. All of these drugs are effective against virus infection if administered quickly, but concerns have been raised because of their adverse effects and the emergence of drug-resistant influenza A/H1N1 viruses [6,7]. The development of new therapeutic agents is urgently needed to treat influenza infections.
Several novel agents that may be effective against influenza virus are currently under development. Naturally available antiviral nutrients that are usually accessible are now being used as part of the diet to combat diseases, including influenza infection [8,9]. Reactive oxygen species (ROS), which are by-products of natural metabolic pathways, can dramatically increase during periods of cellular stress, inhibiting cellular protein function and promoting cell death. Influenza virus replication in infected cells is influenced by the cellular redox status [10,11]. The inhibition of virus-induced ROS formation using different strategies, including the use of the antioxidant N-acetyl-l-cysteine (NAC), has been shown to inhibit influenza A virus replication [12,13,14]. Identifying cellular molecules that have activities against apoptosis and ROS will be useful to counteract influenza virus infections in cells.
Characterization of the molecular mechanisms of novel antiviral proteins is also needed to combat influenza infections, as it will aid in developing new therapeutic strategies based on the identified mechanisms. Several identified novel small molecules that have inhibited Bax [15,16] and both Bax and Bak to protect cells from apoptotic cell death [17,18]. Bax inhibitor-1 (BI-1) is a membrane protein containing several transmembrane domains, and it has been reported to be an anti-apoptotic protein that has a protective function against ER stress [14,19,20]. BI-1 is widely conserved in both animal and plant species. BI-1 has been reported to regulate ROS production in the ER by modifying heme oxygenase-1 (HO-1) expression [14,21]. Heme oxygenase-1 is an oxygenase enzyme that blocks ROS activity and is expressed to counteract ROS accumulation, thereby promoting cell survival [22,23,24,25]. HO-1 has also been reported to act as an antiviral factor against porcine reproductive and respiratory syndrome virus infection [26] and to be induced by influenza virus infection [27]. Previous studies reported that a C-terminal mutant of BI-1 (BI-1ΔC) relieved the effect of BI-1 overexpression regarding cell growth in transgenic mice and acted as BI-1 dominant negative mutant [19,28] and enhanced the survival and neural differentiation of embryonic stem cells by differential regulation of ROS production [29]. Thus, in this study, we determined whether BI-1 overexpression would promote cell survival against viral infection by increasing the production of antioxidant enzymes and by destabilizing the complex responsible for ROS production, which will be helpful for the further development of novel antiviral therapeutic strategies.
2. Results and Discussion
2.1. Overexpression of Bax Inhibitor-1 (BI-1) Relieves Virus Induced Cell Death in Madin-Darby Canine Kidney (MDCK) Cells
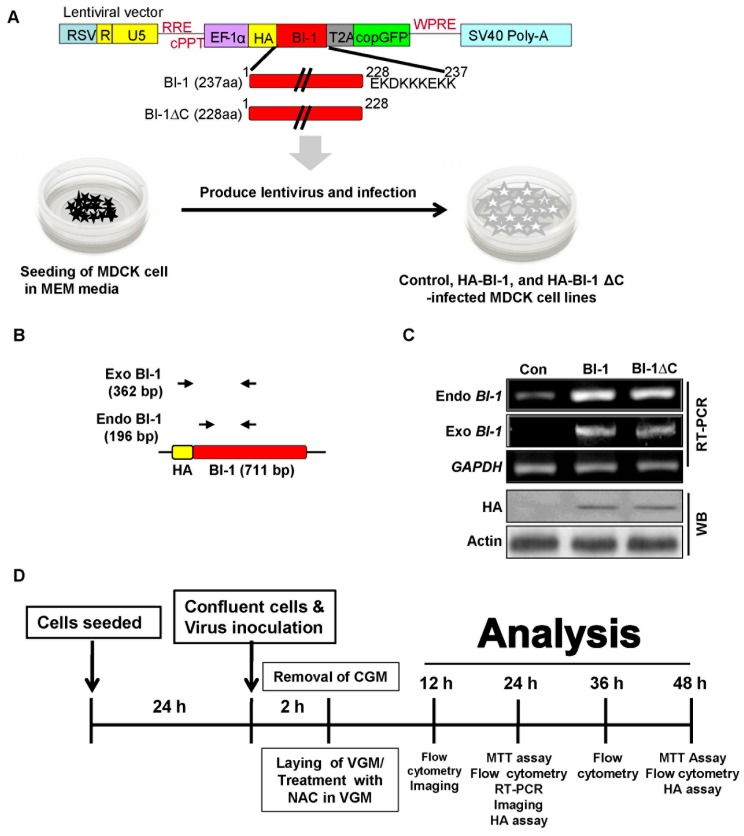
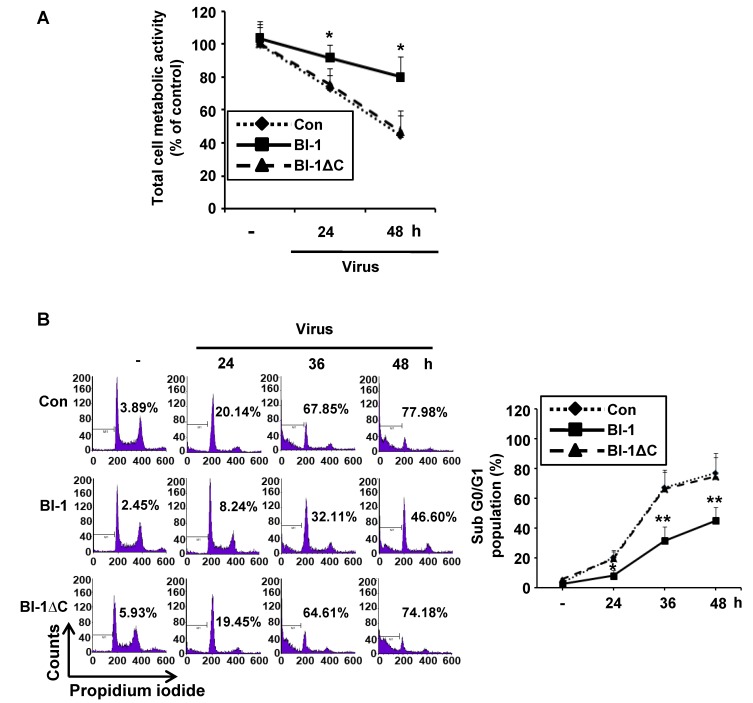
After preparing Madin-Darby Canine Kidney (MDCK) cells expressing the lentiviral construct containing wild type Bax inhibitor-1 (BI-1) or the non-functional deletion mutant form (BI-1 ∆C; deletion of 9 amino acids (EKDKKKEKK) from the C-terminal region of BI-1) (Figure 1A) [19,29], we confirmed the endogenous or exogenous expression of BI-1 from the control, wild type BI-1, and non-functional BI-1 ∆C overexpressing MDCK cells by RT-PCR analysis using endogenous or exogenous specific primer set and overexpression was also confirmed by western blot analysis (Figure 1B,C). Then, the BI-1 or BI-1 ∆C-overexpressing MDCK cells were used in an antiviral experiment against influenza virus A/PR/8/34, as illustrated in Figure 1D. First, the anti-influenza activity of BI-1 overexpressing cells was determined using cell viability assays after influenza virus administration, revealing that the overexpression of BI-1 in MDCK cells led to the significant suppression of virus-induced cell death compared to that in mock or BI-1 ∆C-overexpressing cells (Figure 2A). Flow cytometric analysis of the apoptotic sub G0/G1 populations also confirmed the significant anti-influenza virus activity of BI-1 (Figure 2B), which supported our previous report that C-terminal amino acid deletion of BI-1 acted in a dominant negative fashion [19,29].
Figure 1.
Overexpression and endogenous or exogenous expression of BI-1 or BI-1 ∆C in Madin-Darby Canine Kidney (MDCK) cells. (A) Scheme of the lentiviral constructs for the expression of wild type BI-1 or nonfunctional mutant BI-1 ΔC in MDCK cells. BI-1 or BI-1 ΔC was inserted into the lentiviral pEF vector. The pEF lentivirus containing wild type BI-1 or the non-functional mutant BI-1 ΔC was produced and used to infect MDCK cells. (B) Illustration of specific primer sets which was used to analyze endogenous or exogenous expression of BI-1. (C) The endogenous or exogenous expression of BI-1 was confirmed by RT-PCR analysis in indicated cells; and the expression of HA was confirmed by western blot analysis in indicated cells. The expression of GAPDH and Actin was used as loading control. (D) The illustration of whole experimental protocol used in this study. (Abbreviations: chimeric Rous sarcoma virus (RSV) sequence, Rev response elements (RRE), central polypurine tract (cPPT), elongation factor 1 alpha (EF-1α) promoter region, a copGFP (copepod GFP) tag, a woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), cells growth media (CGM), virus growth media (VGM), and hemagglutinin assay (HA assay)).
Figure 2.
Overexpression of Bax inhibitor-1 (BI-1) inhibits virus induced cell death and cell cycle arrest in MDCK cells. (A) Control mock-infected MDCK, BI-1-overexpressing MDCK, and BI-1 ΔC-overexpressing MDCK cells were seeded in 96-well cell culture plates and infected with A/PR/8/34 virus at 100 TCID50. The media were changed 2 h after virus infection, and MTT assays were performed 24 h and 48 h after virus infection. Data were shown as the relative expression of cell metabolic activity of BI-1-overexpressing MDCK, and BI-1 ΔC-overexpressing MDCK cells compared to the control infection from three individual experiments (mean ± SEM) (* p < 0.05). (B) Control MDCK, BI-1-overexpressing MDCK, and BI-1 ΔC-overexpressing MDCK cells were seeded in 6-well cell culture plates and infected with A/PR/8/34 virus at 1000 TCID50. Anti-influenza activity was determined three times calculating the apoptotic sub-G0/G1 populations by flow cytometric analysis 24 h, 36 h, and 48 h after virus infection. Data were expressed as relative numbers of apoptotic sub-G0/G1 populations of BI-1-overexpressing MDCK, and BI-1 ΔC-overexpressing MDCK cells compared to the control (mean ± SEM) (* p < 0.05, ** p < 0.01).
2.2. Overexpression of BI-1 Inhibits Viral Replication and Viral Gene Expression in MDCK Cells
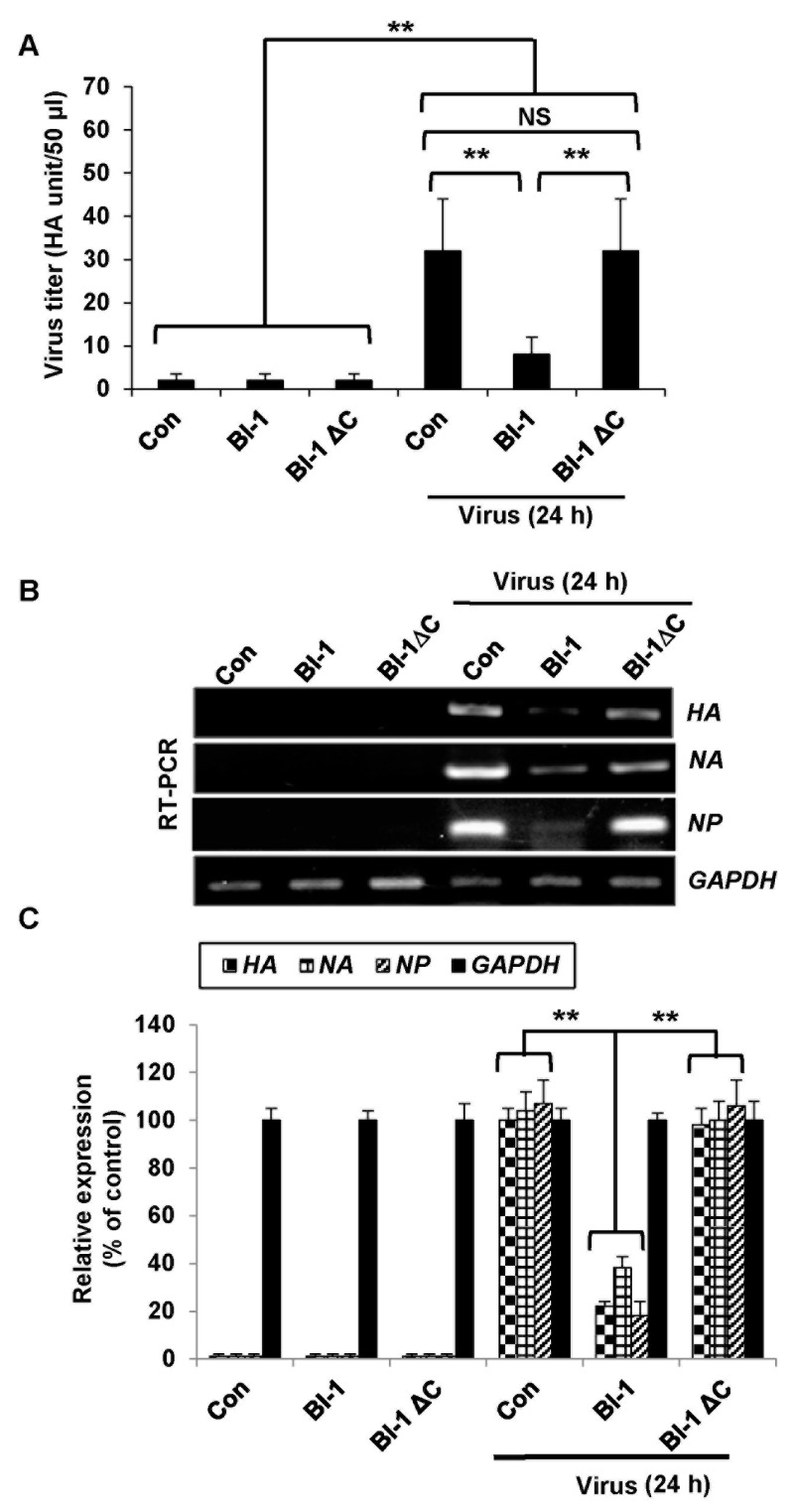
To further analyze the antiviral activity of BI-1 on influenza virus production, we conducted a hemagglutination assay [30] using red blood cells (RBCs). We determined whether overexpression of BI-1 would interfere with viral adsorption to RBCs (Figure 3A). A virus yield reduction HA assay with the media produced in the culture of the virus-infected BI-1- or BI-1 ∆C-overexpressing MDCK cells showed that BI-1 overexpression, but not BI-1 ∆C overexpression, led to a substantial reduction in virus yield compared to the control. More than a four-fold reduction in virus production was observed in BI-1-overexpressing cells compared to that in the mock control or BI-1 ∆C-overexpressing cells. RT-PCR analysis of hemagglutinin (HA), neuraminidase (NA), and nucleoprotein (NP) gene expression revealed that the expression of these viral genes was significantly suppressed with BI-1 overexpression but not mock control and BI-1 ∆C overexpression (Figure 3B,C).
Figure 3.
Overexpression of BI-1 impedes viral replication and viral gene expression in MDCK cells. (A) Control mock MDCK, BI-1-overexpressing MDCK, or BI-1 ΔC-overexpressing MDCK cells were infected with A/PR/8/34 virus at 1000 TCID50, and hemagglutination activity was measured as described in the materials and methods. HA titers were calculated triplicates as hemagglutination units/50 µL (HAU/50 µL) (** p < 0.01). (B) BI-1- or BI-1 ΔC-overexpressing MDCK cells cultured in 6-well cell culture plates were infected with influenza A/PR/8/34 virus at 1000 TCID50, and RT-PCR was performed to determine the expression of the viral HA, NA, and NP genes. (C) Relative expression of the HA, NA, and NP genes was analyzed thrice by real time qRT-PCR and presented as a percentage of the control (** p < 0.01). (Abbreviations: hemagglutinin (HA), neuraminidase (NA), and nucleoprotein (NP)).
2.3. BI-1 Overexpression Suppresses ROS-Mediated Influenza Virus Infection in MDCK Cells
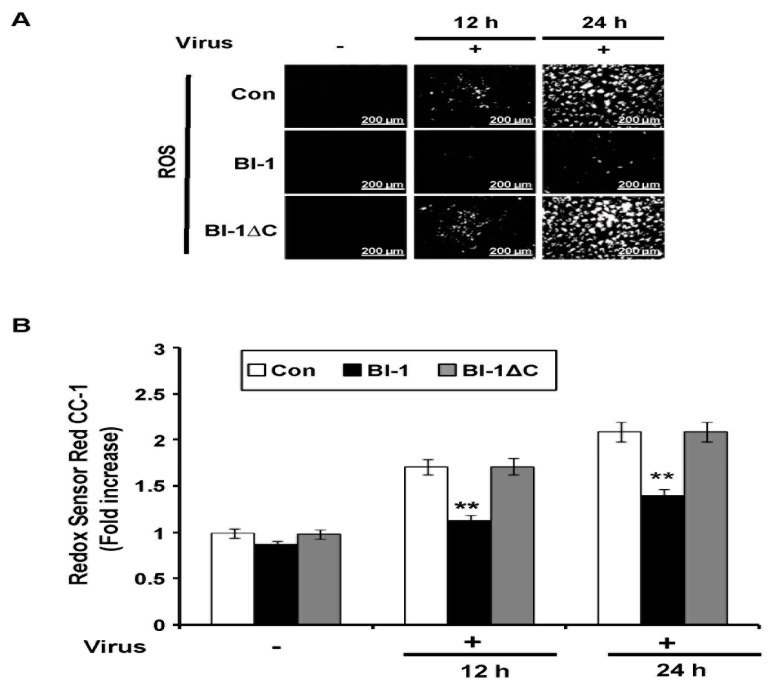
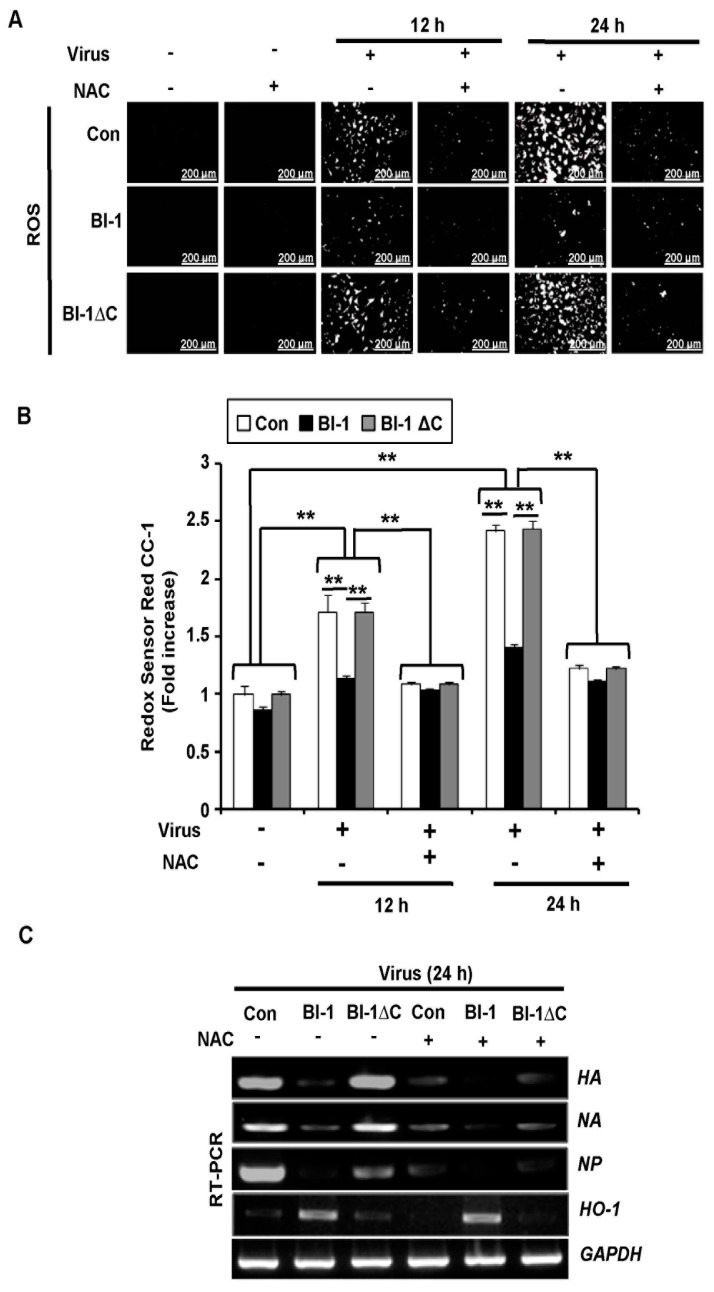
As influenza virus infection modulates ROS production in infected cells [12,13], we evaluated whether BI-1 overexpression provided protection against virus-induced ROS production. Virus-induced ROS production was significantly suppressed by BI-1 overexpression, but not by BI-1 ∆C overexpression (Figure 4A). These observations were confirmed by flow cytometric analysis of the cells stained with Redox Red sensor CC-1 (Figure 4B). In the virus-infected mock control and BI-1 ∆C-overexpressing cells, ROS production obviously increased following virus infection, but the virus-induced ROS production was significantly suppressed in the BI-1-overexpressing MDCK cells. These data indicated that BI-1 would act as anti-influenza virus factor by protecting cells against virus-induced ROS production and apoptosis.
Figure 4.
BI-1 overexpression regulated ROS production in influenza virus-infected MDCK cells. (A) Control mock-MDCK, BI-1-overexpressing MDCK, and BI-1ΔC-overexpressing MDCK cells were seeded in 6-well plates and infected with A/PR/8/34 virus at 100 TCID50. Intracellular ROS levels were measured using Redox Red sensor CC-1. Fluorescence-stained cells were imaged on an inverted fluorescence microscope. (Scale bar- 200 µm). (B) Relative expression of ROS was analyzed thrice by flow cytometer in indicated virus-infected cells (** p < 0.01).
2.4. BI-1 May Independently Act as an Antiviral Protein through the Suppression of ROS Production by Activating the Expression of HO-1 with Additional Effect of the ROS Scavenging Agent NAC
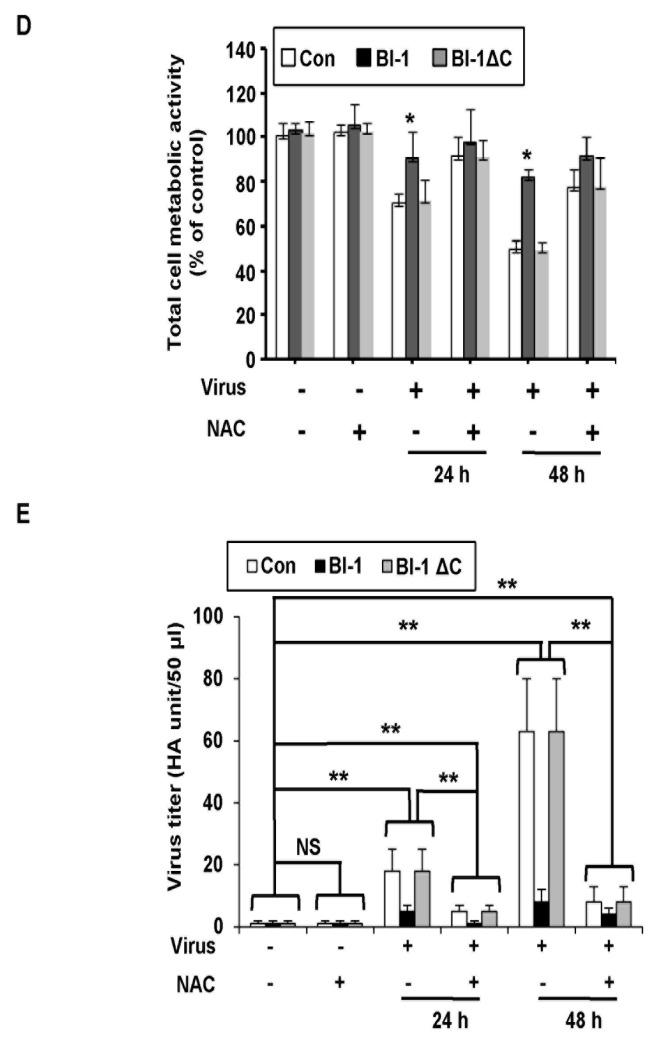
Next, we treated the virus-infected cells with N-acetyl cysteine (NAC) to investigate the effect of ROS on the antiviral function of BI-1. NAC treatment led to the significant suppression of virus-induced ROS production in the mock control and BI-1 ∆C-overexpressing MDCK cells (Figure 5A). The additional suppression of ROS production was also observed by BI-1 overexpressing cells following NAC treatment. These observations were then confirmed by flow cytometric analysis of the intracellular ROS production (Figure 5B). In the virus-infected mock MDCK and BI-1 ∆C-overexpressing cells, ROS production was significantly increased about two-fold compared to the uninfected cells, while BI-1 overexpression led to impede the suppression of ROS production. The treatment of NAC in virus treated cells led to a significant reduction in ROS production in mock control and BI-1 ∆C-overexpressing MDCK cells, while BI-1 overexpressing MDCK cells became like untreated cells after 12 h (Figure 5A,B). After 24 h of virus treatment, ROS level was augmented dramatically in both control and BI-1 ∆C-overexpressing MDCK cells which could be diminished by NAC treatment (Figure 5A,B). In addition, BI-1 overexpressing MDCK cells showed a reduction in virus induced ROS level and the reduction was slightly more but statistically significant in NAC treatment group (Figure 5A,B). The expression of viral genes, such as HA, NA, or NP, was also significantly inhibited by BI-1 overexpression, and the additional reduction of the viral genes expression was also detected by NAC treatment (Figure 5C). Of note, the expression of heme oxygenase-1 (HO-1), which counteracts the accumulation of ROS in stressed cells, was significantly induced following BI-1 overexpression, but the enhanced HO-1 expression in BI-1-overexpressing cells was not suppressed following NAC treatment, suggesting that the BI-1-induced enhancement of HO-1 expression may play an important role in the BI-1 overexpression-induced antiviral activity, probably in a ROS-independent manner which need to be studied in detail. The cell viability and virus titer production results also showed that BI-1 may act as an antiviral protein through the suppression of ROS production and ROS-independent regulation of HO-1 expression in addition to the effect of the ROS scavenging agent NAC (Figure 5D,E).
Figure 5.
BI-1 may act as an antiviral protein through the suppression of ROS production and ROS-independent regulation of HO-1 expression. (A) Control mock MDCK, BI-1-overexpressing MDCK, or BI-1 ΔC-overexpressing cells were infected with A/PR/8/34 virus at 1000 TCID50 and treated with NAC as an antioxidant agent. Intracellular ROS levels were measured in control mock MDCK, BI-1-overexpressing MDCK, and BI-1 ΔC-overexpressing MDCK cells incubated with Redox Red sensor CC-1. Fluorescence-stained cells were imaged on an inverted fluorescence microscope. (Scale bar- 200 µm). (B) Relative expression of ROS was analyzed thrice by flow cytometer in indicated virus-infected cells with or without NAC treatment (** p < 0.01). (C) RT-PCR analysis of virus-infected cells to determine the expression of viral genes such as HA, NA, and NP and the endogenous HO-1 gene in the host cells. (D) Cells were infected with A/PR/8/34 virus at 100 TCID50 and treated with NAC. MTT assay was performed 24 h and 48 h after virus infection, and the relative expression compared to the control infection results are shown as the mean ± SEM from three individual experiments (* p < 0.05). (E) Hemagglutination assay of the virus-infected cells with and without NAC treatment. HA titers were calculated triplicates as hemagglutination units/50 µL (HAU/50 µL) using 1% chicken RBCs (** p < 0.01). (Abbreviations: hemagglutinin (HA), neuraminidase (NA), nucleoprotein (NP), and heme oxygenase-1 (HO-1)).
The emergence of new strains influenza virus and influenza infections with high morbidity and mortality have been reported in humans, which have a pandemic threat. Although vaccines are used to prevent influenza infections, they take time to impart protection but the viruses may escape immunity induced by vaccination due to antigenic discrepancy between the vaccine strain and the circulating strain or due to antigenic drift [6,7]. Therefore, developing effective antiviral drugs is urgently needed to combat the disease. Influenza virus infection of susceptible host cell causes virus propagation, cell death, and viral gene expression. Notably, following influenza virus infection in the MDCK cell line, the increase in the titer of the influenza virus increases following ROS production [12]. In our previous studies, we found that influenza virus infection, replication, and propagation were prevented by several natural products [31,32]. In this study, we investigated the cell membrane protein BI-1 and found that anti-apoptotic protein BI-1 regulated the cellular responses to influenza virus infection. Importantly, the overexpression of BI-1 in MDCK cells impaired influenza virus production, propagation, and the synthesis of viral genes. BI-1 prevented virus adsorption into the MDCK cells by interfering with viral membrane fusion to the cells. BI-1 overexpression also suppressed NA activity to inhibit virion release, viral RNA synthesis by inhibiting NP, and virus titer production in MDCK cells. Previous studies have reported that BI-1 functions as an anti-apoptotic protein and ROS scavenger [14,20,22], recently in another study we had reported that in human embryonic kidney cells, BI-1 overexpression significantly suppressed ROS production, while BI-1ΔC did not make any significant effect on ROS production [19], indicating that the C-terminal mutant plays in dominant negative fashion. In our studies, we found that the overexpression of BI-1 significantly reduced apoptotic cell death and ROS production in influenza-infected MDCK cells. Thus, BI-1 overexpression in MDCK cells suppressed ROS production and thereby inhibited viral gene and viral titer production, propagation, and cytopathogenicity (Figure 6). Moreover, our study revealed that BI-1-overexpressing MDCK cells were more resistant to influenza virus infection and virus-induced cell death.
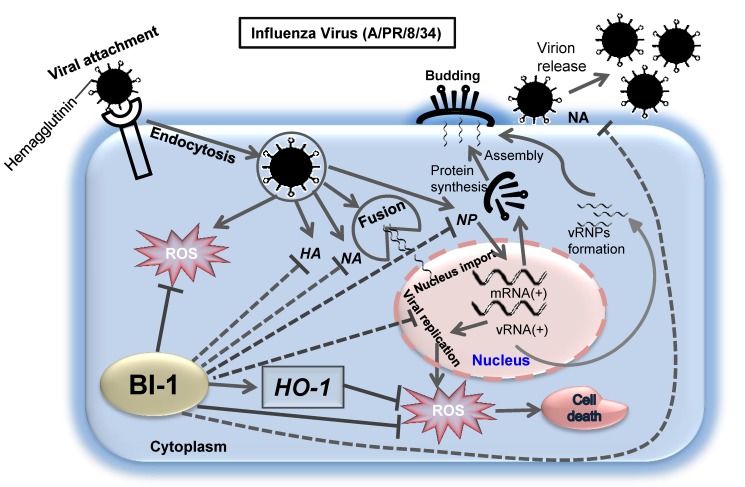
Figure 6.
Mechanism of action of Bax inhibitor-1 to protect MDCK cells from influenza virus infection-induced ROS production and cell death. After attachment to the host cell, the influenza virus induces viral replication and ROS production in the host cells. BI-1 overexpression in influenza virus-infected cells enhances HO-1 expression and suppresses ROS production, accompanied by a reduction in viral replication and propagation. BI-1 may inhibit the new virion assembly and release from the infected cells by inhibiting function of viral genes including HA, NA, and NP. (Abbreviations: hemagglutinin (HA), neuraminidase (NA), nucleoprotein (NP), viral ribonucleoproteins (vRNPs), and heme oxygenase-1 (HO-1)).
The anti-oxidant molecule NAC is known to inhibit ROS production, viral replication, and virus-induced apoptosis in influenza-infected cells [33]. As BI-1 had shown a significant anti-influenza effect against A/PR/8/34 including substantial reduction of ROS production, in our studies we used NAC as ROS scavenger whether it had any notable effect in BI-1 overexpressing cell. In this study, in BI-1-overexpressing MDCK cells, NAC had an additional effect on reducing ROS and viral replication but it was not significant, although, in the mock control and BI-1 ∆C overexpressing cells, it had a significant effect on reducing ROS and inhibiting virus-induced cell death and viral replication and propagation. Moreover, in our studies, we had observed activated expression HO-1 in influenza infected BI-1 overexpressing cells. Although ROS production was significantly reduced but, the BI-1-induced increase in HO-1 expression was not suppressed by NAC treatment, possibly because of the enhanced expression of HO-1 protein in BI-1-overexpressing cells and because HO-1 may play an important role in the suppression of virus-induced ROS production, cell death, virion release from host cells, and the replication and propagation of the viral HA, NA, and NP genes.
BI-1 has been shown to regulate ROS accumulation during cellular stress [22,30,34,35], and here, we present evidence that influenza virus-induced ROS accumulation was suppressed following BI-1 overexpression. HO-1 is known to be activated during cellular stress and influence pro-oxidant signals such as ROS. Interestingly, in our study, we found that HO-1, which is a ROS scavenger, was strongly upregulated in BI-1-overexpressing cells. Thus, highly activated cellular HO-1 in BI-1-overexpressing MDCK cells reduced ROS accumulation following influenza infection. HO-1 may be important for the cytoprotective function of BI-1 as BI-1 overexpressing cells, which had a higher basal level of HO-1, had a survival advantage against influenza virus infection.
Taken together, our findings indicate that the overexpression of BI-1 in the MDCK cell line played a key role in the regulation of host cellular defense mechanisms against influenza virus infection. The overexpression of BI-1 slowed down the ROS-dependent propagation of influenza virus and virus-induced pathogenicity and induced increased expression of HO-1. Our study adds to previous efforts to identify small molecules for use against influenza infection, and our results regarding the anti-apoptotic protein BI-1 as an anti-influenza agent that can suppress ROS production will facilitate the development of novel mechanism-based therapeutics against influenza virus infection, but further studies are needed to elucidate the mechanisms involved.
3. Materials and Methods
3.1. Cells, Virus, Reagents, and Lentiviral Infection
Madin Darby Canine Kidney (MDCK) cells were obtained from the American Type Culture Collection (ATCC CCL-3, Manassas, VA, USA) and maintained in a minimum essential medium (MEM) (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), and 100 U/mL penicillin/streptomycin (Gibco). Influenza virus A Puerto Rico/8/34 was kindly provided by the Immunogenetics Lab., Department of Animal Biotechnology, Konkuk University. MDCK cells were washed with PBS before virus infection and cultured in virus growth medium (MEM without FBS) supplemented with 10% bovine serum albumin, 100 U/mL penicillin/streptomycin, and 2 µg/mL trypsin TPCK (Sigma, St. Louis, MO, USA).
It is known that MDCK cells are the best choice to study the influenza virus infection due to rapid virus growth capability and great adaptation in serum free media [36]. BI-1 is naturally present in cells to protect cellular death. According to the result of amino acid sequence alignment, more than 97% of amino acid sequence was same in both human and canine BI-1 protein. In this study, our main purpose is to find the role of BI-1 in influenza virus induced cell death. Thus, we used our available human BI-1 lentivirus expressing construct [19]. Analysis of the amino acid sequences of BI-1 protein was performed using data obtained from Gen Bank (NCBI). The accession number of the protein sequence of BI-1 was NP_003208.1. Scan Prosite (http:www.expasy.org/tools/scanprosite/PROSITE) and NCBI RPS-BLAST (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) were used for conserved domain database screening. The lentiviral construct containing wild type Bax inhibitor-1 (BI-1) or the non-functional deletion mutant form (BI-1 ∆C; deletion of 9 amino acids (EKDKKKEKK) from the C-terminal region of BI-1) was prepared, and the lentiviral vector particles were purified from the supernatant of transiently transfected 293T cells. The cell supernatant was collected 72 h post-transfection, filtered through polyether sulfone membranes (0.45-μm pore size), and concentrated 120-fold by ultracentrifugation (50,000× g for 90 min at 4 °C). The pellet was resuspended in the cell culture medium and subsequently added to the cells. After 1 week of culture, the cells were centrifuged and resuspended in PBS and sorted with a FACSVantage™ flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). The sorted MDCK cells were cultured in virus growth medium (MEM without FBS) supplemented with 10% bovine serum albumin, 100 U/mL penicillin/streptomycin, and 2 µg/mL trypsin TPCK.
For lentiviral transfection, MDCK cells were incubated overnight at a density of 2 × 106 cells per 60-mm culture dish in MEM medium (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS and 100 U/mL penicillin/streptomycin (Gibco) and then infected with the indicated expression vectors using the lentivirus vector. For the selection of MDCK cells that stably expressed wild type BI-1 or the non-functional mutant BI-1 ΔC (with the C-terminal 14 amino acids deleted), cells were transfected with 6 μg pEF HA (control), pEF HA-BI-1, or BI-1ΔC using a central polypurine tract (cPPT), promoter region, and a copGFP (copepod GFP) tag. After selection, surviving MDCK cells were analyzed by RT-PCR using the following primers: BI-1 (F): 5′-GCAGGGGCCTATGTCCAT-3′ BI-1 (R): 5′-AGGATGCTGGGGTTGACAGC-3′, and HA-Exo (F): 5′-TACGATGTTCCAGATTACGCT-3′. A mutant form (BI-1 ΔC) of BI-1 was constructed by deletion of the last nine amino acids at the protein’s C-terminal region [19,29]. The primers for the C-terminal truncation of BI-1 were BI-1 ΔC (F) 5′-GGGAAGAATTCATGAACATATTTGATCGA-3′ and (R) 5′-GGGAACTCGAGTCACTAGGA -CCGGTACTTA-3′.
3.2. Cell Viability and Cell Cycle Analysis
The mock MDCK, BI-1-overexpressing MDCK, and BI-1 ∆C-overexpressing MDCK cells were seeded in 96-well plates for the measurement of cell viability at a concentration of 2 × 104 cells per well. Twenty-four hours after seeding, when the cells were confluent, they were washed twice with PBS and infected with the virus at 100 TCID50 and incubated at 37 °C for 2 h in a 5% CO2 incubator. The virus-containing media were replaced with virus growth media 2 h after infection. MTT assays (Sigma-Aldrich, St. Louis, MI, USA) were performed 24 and 48 h after infection according to the manufacturer’s instructions using a spectrophotometer (Bio-Rad, Hercules, CA, USA) at a wavelength of 490 nm to determine the total cell metabolic activity. For cell cycle analysis, mock, BI-1-overexpressing, and BI-1 ∆C-overexpressing MDCK cells were harvested in 24, 36, and 48 h time frame after virus infection by trypsin-EDTA (Gibco), and stained with propidium iodide (PI) (Sigma-Aldrich) following manufacturer protocol and analyzed by a Beckman Coulter FC-500 flow cytometer (Beckman Coulter, Fullerton, CA, USA) using Cell Quest 3.2 software. The percentage of the apoptotic sub-G0/G1 populations of BI-1-overexpressing MDCK, and BI-1 ΔC-overexpressing MDCK cells were counted and compared to the control.
3.3. Virus Yield Reduction Assay
The mock control cells, overexpressing MDCK-BI-1, and BI-1 ∆C-overexpressing MDCK cells were seeded in 6-well cell culture plates at a concentration of 2–3 × 105 cells per well. When the cells were confluent, the cell growth media were removed, and the cells were washed twice with PBS and infected with the virus at 1000 TCID50 diluted in virus-containing media. The virus-containing media were replaced by virus growth media, and HA assays were performed 48 h after virus infection. Briefly, 25 μL of PBS was added to each well of the 96-well round bottom plates. Then, 25 μL of the cell supernatant containing the virus was diluted by two-fold serial dilution in each well, and 25 μL of PBS was added again to each well. Then, 50 μL of 1% chicken RBCs was added to each well followed by incubation for 1 h at room temperature to observe hemagglutination activity. HA titers were calculated as hemagglutination units/50 µL (HAU/50 µL).
3.4. Intracellular ROS Detection
The mock MDCK, MDCK-BI-1 overexpressing, and MDCK-BI-1 ∆C overexpressing cells were seeded at a concentration of 2–3 × 105 cells per well in 6-well cell culture plates. The media were removed when the cells were confluent, and the cells were washed twice with PBS and infected with the virus at 1000 TCID50 in virus-containing media. Then, 12 h, 24 h and 48 h after virus infection, the cells were exposed to Redox Red sensor CC-1 (Molecular Probes, Inc., Eugene, OR, USA) for 30 min in the dark according to the manufacturer’s instructions to determine ROS levels. The cells were then washed and trypsinized, and the fluorescence intensity was quantified and analyzed with a Beckman Coulter FC-500 flow cytometer (Beckman Coulter, Fullerton, CA, USA) using Cell Quest 3.2 software. At least 10,000 cells were analyzed from each sample. For the measurement of intracellular ROS levels, fluorescence-stained cells were imaged with an inverted fluorescence microscope. To determine ROS scavenging activity, 2 h after virus infection, the cells were treated with 10 mM NAC. Intracellular ROS levels were measured in control MDCK, BI-1-overexpressing MDCK, and BI-1 ΔC-overexpressing MDCK cells using Redox Red sensor CC-1. Fluorescence-stained cells were imaged with an inverted fluorescence microscope to determine the intensity of ROS production, and flow cytometry was performed for ROS quantification.
3.5. RT-PCR
Confluent mock MDCK, BI-1-overexpressing MDCK, and BI-1∆C-overexpressing MDCK cells grown in 6-well plates were infected with influenza A/PR/8/34 virus at 100 TCID50. The cells were scraped off 24 h after infection, and cell pellets were collected by centrifugation (1500× g for 5 min). Cell pellets were washed two times with PBS, and total cellular and viral RNAs were isolated using Easy Blue total RNA extraction kits (Intron Biotechnology). First-strand cDNA was synthesized from 3 µg of total RNA. PCR reactions were performed using primers for HA, (F): 5’-GAAAGCTCATGGCCCAACCA-3’ and (R): 5’-TCCCAGGGGTGTTTGACACT-3’; NA, (F): 5’-TGCTTGGTCAGCAAGTGCAT-3’ and (R): 5’-GGTTGTCACCGAAAACCCCA-3’; NP, (F): 5’-TGCTTCAAAACAGCCAAGTG-3’ and (R): 5’-GCCCAGTACCTGCTTCTCAG-3’; and HO-1, (F): 5’-ACATCTATGTGGCCCTGGAG-3’ and (R): 5’-CCTGCAACTCCTCAAAGAGC-3’. The amplification conditions were as follows: 95 °C for 10 min, 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, 72 °C, 4 °C (30 cycles). The expression of the cellular housekeeping gene GADPH was used as a control to determine the amount of RNA in the assay.
3.6. Western Blot Analysis
The MDCK, over expressed MDCK-BI-1 and over expressed MDCK-BI-1∆C cells seeded in 60 mm dishes and infected with influenza A/PR/8/34 virus at 1000 TCID50, were washed 3 times in ice-cold PBS, scraped from the dishes, and collected in extraction buffer (1% TritonX-100, 100 mM Tris–HCl, pH 7.5, 10 mM NaCl, 10% glycerol, 50 mM sodium fluoride, and 1 mM phenylmethylsulfonyl fluoride (PMSF)). After the cells had been incubated on ice for 30 min, the lysates were centrifuged and protein containing cleared lysates was quantified using the Bradford Protein Assay Reagent (Bio-Rad). Equal amounts of protein were then separated on 10–12% SDS PAGE gels and electrophoretically transferred on to nitrocellulose membranes (0.2 mm). These membranes were blocked with 3–5% non-fat dry milk and Tris-buffered saline (TBS) and subsequently probed for HA and Actin in TBS containing 3% non-fat dry milk. Antibody–antigen complexes were detected using goat anti-mouse IgG or goat anti-rabbit IgG peroxidase conjugates, followed by use of an enhanced chemiluminescence (ECL) detection kit from Amersham (Buckinghamshire, UK).
3.7. Statistical Analysis
The results were expressed as the mean ± SE. Each value is the mean of at least three independent experiments in each group. The statistical significance of the differences between the two cell populations was determined using the two-tailed Student’s t-test (Origin), and p values equal to or less than 0.05 and 0.01 were considered significant.
Acknowledgments
This paper was supported by Konkuk University in 2016.
Abbreviations
| BI-1 | Bax inhibitor-1 |
| ERK | Extracellular Signal–regulated kinase |
| STAT | Signal transducer and activator of transcription |
| NAC | N-acetyl cysteine |
| ROS | Reactive oxygen species |
| HO-1 | Heme oxygenase-1 |
| HA | Hemagglutinin |
| NA | Neuraminidase |
| M2 | Matrix-2 |
| MDCK | Madin Darby Canine Kidney |
| cPPT | Central polypurine tract |
| TCID50 | Tissue culture infective dose 50 |
| PBS | Phosphate buffered saline |
| IgG | Immunoglobulin G |
| ECL | Enhanced chemiluminescence |
| FBS | Fetal bovine serum |
| GFP | Green fluorescent protein |
| WPRE | Woodchuck hepatitis virus post-transcriptional regulatory element |
Author Contributions
Mohammed Kawser Hossain and Ssang-Goo Cho designed the experiments; Mohammed Kawser Hossain performed most of the cellular and molecular experiments; Mohammed Kawser Hossain and Subbroto Kumar Saha analyzed the results; Subbroto Kumar Saha, Ahmed Abdal Dayem, Jung-Hyun Kim, Kyeongseok Kim, Gwang-Mo Yang, and Hye Yeon Choi partially contributed to the cellular and molecular experiments and analysis of the results; and Mohammed Kawser Hossain, Subbroto Kumar Saha, and Ssang-Goo Cho wrote the manuscript. All authors reviewed and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Palese P. Influenza: Old and new threats. Nat. Med. 2004;10:S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 2.Neumann G., Fujii K., Kino Y., Kawaoka Y. An improved reverse genetics system for influenza A virus generation and its implications for vaccine production. Proc. Natl. Acad. Sci. USA. 2005;102:16825–16829. doi: 10.1073/pnas.0505587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayden F.G. Respiratory viral threats. Curr. Opin. Infect. Dis. 2006;19:169–178. doi: 10.1097/01.qco.0000216628.51563.b1. [DOI] [PubMed] [Google Scholar]
- 4.Meijer A., Rebelo-de-Andrade H., Correia V., Besselaar T., Drager-Dayal R., Fry A., Gregory V., Gubareva L., Kageyama T., Lackenby A., et al. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2012–2013. Antivir. Res. 2014;110:31–41. doi: 10.1016/j.antiviral.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurt A.C., Besselaar T.G., Daniels R.S., Ermetal B., Fry A., Gubareva L., Huang W., Lackenby A., Lee R.T., Lo J., et al. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2014–2015. Antivir. Res. 2016;132:178–185. doi: 10.1016/j.antiviral.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouvier N.M., Lowen A.C., Palese P. Oseltamivir-resistant influenza A viruses are transmitted efficiently among guinea pigs by direct contact but not by aerosol. J. Virol. 2008;82:10052–10058. doi: 10.1128/JVI.01226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Söderholm S., Kainov D.E., Öhman T., Denisova O.V., Schepens B., Kulesskiy E., Imanishi S.Y., Corthals G., Hintsanen P., Aittokallio T. Phosphoproteomics to characterize host response during influenza A virus infection of human macrophages. Mol. Cell. Proteom. 2016;15:3203–3219. doi: 10.1074/mcp.M116.057984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., Jia W., Zhao A. Anti-influenza agents from plants and traditional Chinese medicine. Phytother. Res. 2006;20:335–341. doi: 10.1002/ptr.1892. [DOI] [PubMed] [Google Scholar]
- 9.Choi H.J., Song J.H., Park K.S., Kwon D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur. J. Pharm. Sci. 2009;37:329–333. doi: 10.1016/j.ejps.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Vlahos R., Stambas J., Selemidis S. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharmacol. Sci. 2012;33:3–8. doi: 10.1016/j.tips.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Ye S., Lowther S., Stambas J. Inhibition of reactive oxygen species production ameliorates inflammation induced by influenza A viruses via upregulation of SOCS1 and SOCS3. J. Virol. 2015;89:2672–2683. doi: 10.1128/JVI.03529-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangehei L., Ali M., Zhang W., Chen Z., Wakame K., Haidari M. Oligonol a low molecular weight polyphenol of lychee fruit extract inhibits proliferation of influenza virus by blocking reactive oxygen species-dependent ERK phosphorylation. Phytomedicine. 2010;17:1047–1056. doi: 10.1016/j.phymed.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Uchide N., Ohyama K. Antiviral function of pyrrolidine dithiocarbamate against influenza virus: The inhibition of viral gene replication and transcription. J. Antimicrob. Chemother. 2003;52:8–10. doi: 10.1093/jac/dkg282. [DOI] [PubMed] [Google Scholar]
- 14.Baek D., Nam J., Koo Y.D., Kim D.H., Lee J., Jeong J.C., Kwak S.-S., Chung W.S., Lim C.O., Bahk J.D. Bax-induced cell death of Arabidopsisis meditated through reactive oxygen-dependent and-independent processes. Plant Mol. Biol. 2004;56:15–27. doi: 10.1007/s11103-004-3096-4. [DOI] [PubMed] [Google Scholar]
- 15.Peixoto P.M., Ryu S.-Y., Bombrun A., Antonsson B., Kinnally K.W. MAC inhibitors suppress mitochondrial apoptosis. Biochem. J. 2009;423:381–387. doi: 10.1042/BJ20090664. [DOI] [PubMed] [Google Scholar]
- 16.Bombrun A., Gerber P., Casi G., Terradillos O., Antonsson B., Halazy S. 3,6-dibromocarbazole piperazine derivatives of 2-propanol as first inhibitors of cytochrome c release via Bax channel modulation. J. Med. Chem. 2003;46:4365–4368. doi: 10.1021/jm034107j. [DOI] [PubMed] [Google Scholar]
- 17.Niu X., Brahmbhatt H., Mergenthaler P., Zhang Z., Sang J., Daude M., Ehlert F.G., Diederich W.E., Wong E., Zhu W. A small-molecule inhibitor of Bax and Bak oligomerization prevents genotoxic cell death and promotes neuroprotection. Cell Chem. Biol. 2017;24:493–506. doi: 10.1016/j.chembiol.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peixoto P.M., Teijido O., Mirzalieva O., Dejean L.M., Pavlov E.V., Antonsson B., Kinnally K.W. MAC inhibitors antagonize the pro-apoptotic effects of tBid and disassemble Bax/Bak oligomers. J. Bioenerg. Biomembr. 2017;49:65–74. doi: 10.1007/s10863-015-9635-7. [DOI] [PubMed] [Google Scholar]
- 19.Kim J.H., Lee E.R., Jeon K., Choi H.Y., Lim H., Kim S.J., Chae H.J., Park S.H., Kim S., Seo Y.R., et al. Role of BI-1 (TEGT)-mediated ERK1/2 activation in mitochondria-mediated apoptosis and splenomegaly in BI-1 transgenic mice. Biochim. Biophys. Acta Mol. Cell Res. 1823;1823:876–888. doi: 10.1016/j.bbamcr.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Matsumura H., Nirasawa S., Kiba A., Urasaki N., Saitoh H., Ito M., Kawai-Yamada M., Uchimiya H., Terauchi R. Overexpression of Bax inhibitor suppresses the fungal elicitor-induced cell death in rice (Oryza sativa L.) cells. Plant J. 2003;33:425–434. doi: 10.1046/j.1365-313X.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- 21.Lu B., Li Y., Li H., Zhang Y., Xu J., Ren L., Fu S., Zhou Y. Bax inhibitor-1 is overexpressed in non-small cell lung cancer and promotes its progression and metastasis. Int. J. Clin. Exp. Pathol. 2015;8:1411. [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G.-H., Kim H.-K., Chae S.-W., Kim D.-S., Ha K.-C., Cuddy M., Kress C., Reed J.C., Kim H.-R., Chae H.-J. Bax inhibitor-1 regulates endoplasmic reticulum stress-associated reactive oxygen species and heme oxygenase-1 expression. J. Biol. Chem. 2007;282:21618–21628. doi: 10.1074/jbc.M700053200. [DOI] [PubMed] [Google Scholar]
- 23.Lee M.R., Lee G.H., Lee H.Y., Kim D.S., Chung M.J., Lee Y.C., Kim H.R., Chae H.J. BAX inhibitor-1-associated V-ATPase glycosylation enhances collagen degradation in pulmonary fibrosis. Cell Death Dis. 2014;5:e1113. doi: 10.1038/cddis.2014.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B., Yadav R.K., Jeong G.S., Kim H.R., Chae H.J. The characteristics of Bax inhibitor-1 and its related diseases. Curr. Mol. Med. 2014;14:603–615. doi: 10.2174/1566524014666140603101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson K.S., Clements A., Williams A.C., Berger C.N., Frankel G. Bax inhibitor 1 in apoptosis and disease. Oncogene. 2011;30:2391–2400. doi: 10.1038/onc.2010.636. [DOI] [PubMed] [Google Scholar]
- 26.Xiao S., Zhang A., Zhang C., Ni H., Gao J., Wang C., Zhao Q., Wang X., Wang X., Ma C. Heme oxygenase-1 acts as an antiviral factor for porcine reproductive and respiratory syndrome virus infection and over-expression inhibits virus replication in vitro. Antivir. Res. 2014;110:60–69. doi: 10.1016/j.antiviral.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Espinoza J.A., González P.A., Kalergis A.M. Modulation of antiviral immunity by heme oxygenase-1. Am. J. Pathol. 2017;187:487–493. doi: 10.1016/j.ajpath.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Kawai-Yamada M., Ohori Y., Uchimiya H. Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-, hydrogen peroxide-, and salicylic acid-induced cell death. Plant Cell. 2004;16:21–32. doi: 10.1105/tpc.014613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeon K., Lim H., Kim J.H., Han D., Lee E.R., Yang G.M., Song M.K., Cho S.G. Bax inhibitor-1 enhances survival and neuronal differentiation of embryonic stem cells via differential regulation of mitogen-activated protein kinases activities. Biochim. Biophys. Acta Mol. Cell Res. 2012;1823:2190–2200. doi: 10.1016/j.bbamcr.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Sawai R., Kuroda K., Shibata T., Gomyou R., Osawa K., Shimizu K. Anti-influenza virus activity of Chaenomeles sinensis. J. Ethnopharmacol. 2008;118:108–112. doi: 10.1016/j.jep.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Hossain M.K., Choi H.Y., Hwang J.S., Dayem A.A., Kim J.H., Kim Y.B., Poo H., Cho S.G. Antiviral activity of 3,4′-dihydroxyflavone on influenza a virus. J. Microbiol. 2014;52:521–526. doi: 10.1007/s12275-014-4212-z. [DOI] [PubMed] [Google Scholar]
- 32.Dayem A.A., Choi H.Y., Kim Y.B., Cho S.G. Antiviral Effect of Methylated Flavonol Isorhamnetin against Influenza. PLoS ONE. 2015;10:e0121610. doi: 10.1371/journal.pone.0121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geiler J., Michaelis M., Naczk P., Leutz A., Langer K., Doerr H.-W., Cinatl J. N-acetyl-l-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem. Pharmacol. 2010;79:413–420. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Kim J.-H., Song M., Kang G.-H., Lee E.-R., Choi H.-Y., Lee C., Kim J.-H., Kim Y., Koo B.-N., Cho S.-G. Combined treatment of 3-hydroxyflavone and imatinib mesylate increases apoptotic cell death of imatinib mesylate-resistant leukemia cells. Leuk. Res. 2012;36:1157–1164. doi: 10.1016/j.leukres.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Kim H.-R., Lee G.-H., Cho E.Y., Chae S.-W., Ahn T., Chae H.-J. Bax inhibitor 1 regulates ER-stress-induced ROS accumulation through the regulation of cytochrome P450 2E1. J. Cell Sci. 2009;122:1126–1133. doi: 10.1242/jcs.038430. [DOI] [PubMed] [Google Scholar]
- 36.Lin S.-C., Kappes M.A., Chen M.-C., Lin C.-C., Wang T.T. Distinct susceptibility and applicability of MDCK derivatives for influenza virus research. PLoS ONE. 2017;12:e0172299. doi: 10.1371/journal.pone.0172299. [DOI] [PMC free article] [PubMed] [Google Scholar]