Abstract
In addition to essential nutrients, human milk contains several classes of bioactive factors such as enzymes, hormones, and growth factors, many of which are implicated in infantile growth and development. Secretory carbonic anhydrase isoenzyme VI (CA VI) has been identified earlier as an essential component of mammalian saliva, and we demonstrate here by using biochemical and immunohistochemical techniques that it is also an elementary component of milk. The 42-kDa glycopolypeptide purified from human milk in CA inhibitor affinity chromatography shared 100% homology with salivary CA VI in the protein sequence analysis (40% coverage), and its digestion with PNGase F resulted in a polypeptide backbone similar in size to salivary CA VI. Quantification of CA VI in milk by using a time-resolved immunofluorometric assay revealed an approximately eight-times-higher concentration in human colostrum than in mature milk, the latter corresponding to the levels previously detected in human saliva. The high concentration in the colostrum, in particular its functional and structural stability in an acidic milieu, and its growth-supporting role in the taste buds suggest that milk CA VI is an essential factor in normal growth and development of the infant alimentary tract.
In addition to essential nutrients, human milk contains several types of bioactive factors, including growth hormones, enzymes, antimicrobial factors, antiinflammatory agents, transporters, and peptide and nonpeptide hormones (1). Many of these factors appear in high concentrations in the colostrum (milk secreted during the first 3–5 days after parturition) and can have multiple functional roles (1–3). The gastrointestinal tract of a newborn infant undergoes pronounced growth, morphological changes, and functional maturation postnatally, and many of these factors have beneficial effects on these changes (2).
The carbonic anhydrases (CAs) are an expanding family of
zinc-containing enzymes, which classically participate in the
maintenance of pH homeostasis in the human body, catalyzing the
reversible reaction: CO2 +
H2O ⇌ HCO +
H+ (4). CA VI is the only known, secreted
isoenzyme of this family (5–7), which has been detected in the saliva
secreted by the serous acinar cells of mammalian parotid and
submandibular glands (8). An intracellular, stress-inducible form of CA
VI recently has been described in mouse fibroblasts (9). However, the
physiological role of this CA VI form has remained undefined. Recent
studies have, in turn, provided new information about the function of
the secretory CA VI. It has been proposed that this isoenzyme may
participate in protecting the teeth from caries (10) and in
neutralizing excess acid in the mucous layer covering the esophageal
and gastric epithelium (11). Another novel role for CA VI became
apparent when Thatcher et al. (12) identified gustin, a
salivary factor implicated in taste bud growth, as CA VI.
+
H+ (4). CA VI is the only known, secreted
isoenzyme of this family (5–7), which has been detected in the saliva
secreted by the serous acinar cells of mammalian parotid and
submandibular glands (8). An intracellular, stress-inducible form of CA
VI recently has been described in mouse fibroblasts (9). However, the
physiological role of this CA VI form has remained undefined. Recent
studies have, in turn, provided new information about the function of
the secretory CA VI. It has been proposed that this isoenzyme may
participate in protecting the teeth from caries (10) and in
neutralizing excess acid in the mucous layer covering the esophageal
and gastric epithelium (11). Another novel role for CA VI became
apparent when Thatcher et al. (12) identified gustin, a
salivary factor implicated in taste bud growth, as CA VI.
Because a high amount of CA VI is supplied to the adult gastrointestinal tract daily in the saliva (13), and the newborn infant's saliva secretion is low because of the immaturity of the salivary glands (14, 15), we set out to ascertain whether there is any other source of CA VI that could ensure a high concentration of the enzyme in the postnatal alimentary canal. Our results show that CA VI is present in milk and that its concentration is approximately eight times higher in the colostrum than in the mature milk, the latter levels corresponding to those reported earlier for adult human saliva. Thus, during the early postnatal period, CA VI is transferred to the infantile alimentary tract in the milk in concentrations that exceed the levels found in adult human saliva several times over. These high concentrations of CA VI found in colostral and mature milk, its functional and structural stability in an acidic milieu (11), and its growth-supporting role in taste buds (12) suggest that milk CA VI not only is linked to the functions of the upper gastrointestinal tract but also may participate in normal growth and development of the whole alimentary tract.
Materials and Methods
Antisera.
The production and characterization of the antisera to human and rat CA VI have been described earlier (8, 16). Both antisera have been characterized by Western blots in which they have shown high isoenzyme specificity.
Collection of Milk, Saliva, and Tissue Samples.
Colostral milk samples were obtained from nine mothers on days 2–4 postpartum, and saliva samples were obtained at the same time from two 3-day-old infants before nursing. Mature milk samples were obtained from four mothers on the 90th day postpartum. The procedures were carried out according to the provisions of the Declaration of Helsinki, and informed consent was obtained from each mother. Rat milk samples were collected from Sprague–Dawley rats anesthetized with fentanyl-fluanisone (3 ml/kg; Janssen) on the 2nd day postpartum and pooled. All of the milk samples were stored frozen before use in the experiments. Mammary gland specimens were taken from a sexually mature, nonpregnant female rat and from another 2 days before and 2 days after parturition. The tissue samples were homogenized in ice-cold 0.1 M Tris⋅SO4 buffer, pH 8.7, containing 1 mM PMSF, 1 mM benzamidine, and 1 mM o-phenanthroline as protease inhibitors, and used for Western blotting or fixed in Carnoy's fluid and embedded in paraffin as described (17).
Purification of CAs.
Human colostrum (15 ml) was centrifuged (35,000 × g) at 4°C for 30 min, and the clear supernatant (10 ml) was collected and mixed with 30 ml of ice-cold 0.1 M Tris⋅SO4 buffer, pH 8.7, containing 1 mM benzamidine as a protease inhibitor, and subjected to affinity purification. The inhibitor affinity chromatography was performed by using CM Bio-Gel A coupled to p-aminomethylbenzenesulfonamide as described (8).
SDS/PAGE and Western Blotting.
The milk samples were centrifuged (15,000 × g) at 4°C for 10 min, and the supernatants were recovered. Samples of CAs purified from human colostrum (0.2 and 0.6 μg), human and rat salivary CA VI (1 μg), human colostrum (10 μl), human saliva (10 μl), rat milk (30 μg), and rat homogenized mammary gland (30 μg) were subjected to SDS/PAGE under reducing conditions according to Laemmli (18). All of the reagents for SDS/PAGE were from Bio-Rad or Sigma. The Western blotting was carried out as described (17).
Protein Sequence Analysis.
The protein sequencing of trypsin-digested polypeptides was carried out by matrix-assisted laser desorption ionization/mass spectrometry followed by analysis with the profound and peptidesearch programs. The sequencing was performed in the Howard Hughes Medical Institute Biopolymer/W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University.
Deglycosylation Studies.
Purified milk and salivary CA VI (1 μg) were digested with PNGase F as described earlier (19), and the deglycosylated and nondeglycosylated proteins were subjected to SDS/PAGE followed by Colloidal Coomassie staining (NOVEX, San Diego).
Fluoroimmunoassay.
The competitive time-resolved fluoroimmunoassay for CA VI was performed on the human milk samples (colostrum, n = 9; mature milk, n = 4) as described in detail previously for saliva samples (13). The mean intraassay coefficient of variation (CV) of the present series was 9.4%, and the interassay CV determined in three assays was 9.7%.
Immunocytochemistry.
Mammary gland samples fixed in Carnoy's fluid were sectioned at 5 μm and placed on gelatin-coated microscope slides. Immunohistochemical staining was performed by using the biotin–streptavidin complex method as described (17). The stained sections were examined and photographed with a Nikon Eclipse E600 microscope.
Results
Documentation of the Presence of CA VI in Human and Rat Milk.
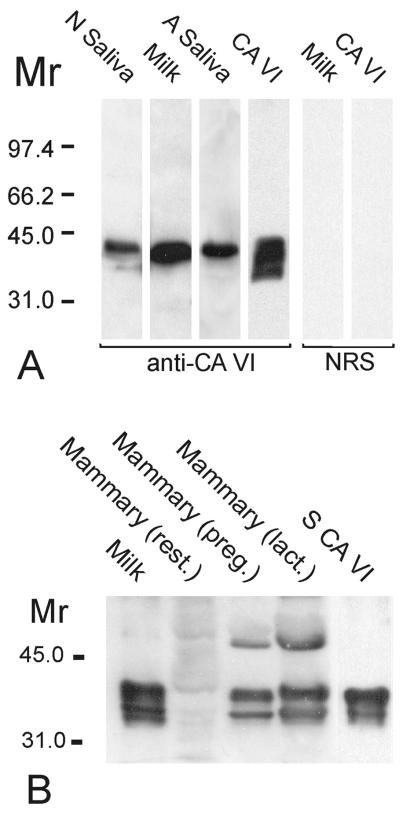
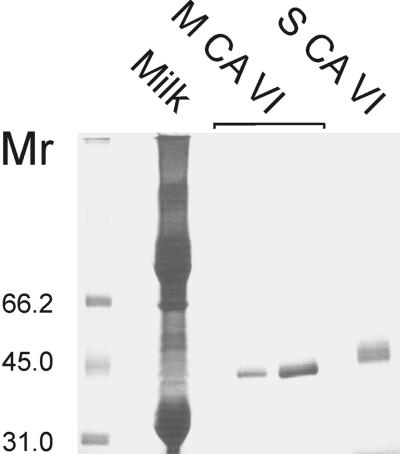
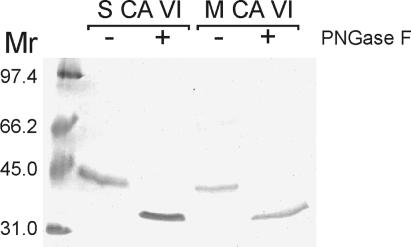
Western blotting of samples of human colostrum, saliva, and purified salivary CA VI with specific antibody to human salivary CA VI revealed a major, 42-kDa polypeptide band in all cases. In addition, a minor, 36-kDa band, representing the deglycosylated form of the enzyme, was detected in the purified salivary CA VI (Fig. 1A). A major, 42-kDa band and minor, 36-kDa polypeptide band also were obtained from rat milk and purified salivary CA VI samples that were subjected to immunoblotting with specific antibody to rat salivary CA VI (Fig. 1B). The 42-kDa polypeptide also was purified effectively from human colostrum in CA inhibitor affinity chromatography (Fig. 2). PNGase F digestion of the purified human milk and salivary CA VI reduced their molecular size from 42 to 36 kDa, indicating that both glycopolypeptides have a similar-sized polypeptide core (Fig. 3). To confirm that the human milk CA is isoenzyme VI, the purified colostral isoenzyme was isolated from a SDS gel followed by trypsin digestion and sequencing with matrix-assisted laser desorption ionization/mass spectrometry. The analysis of the sequence data with the profound and peptidesearch databases revealed a 100% identity with human salivary CA VI. The sequenced polypeptides covered 40% of the full-length CA VI.
Figure 1.
(A) Western blot of the saliva of the human newborn infant (N saliva), human colostral milk (milk), human adult saliva (A saliva), and purified human salivary CA VI (CA VI) by using anti-human CA VI antibody (anti-CA VI) and normal rabbit serum (NRS). The anti-human CA VI antibody recognized the 42-kDa polypeptides of glycosylated CA VI in all of the samples. In addition, the 36-kDa polypeptide of the deglycosylated form of CA VI was visible in the purified human salivary CA VI sample. Control stainings using NRS were negative. (B) Western blot of rat milk (milk), rat mammary glands, and purified rat salivary CA VI (S CA VI) by using antibody raised against rat CA VI. All of the samples showed similar 42- and 36-kDa polypeptide bands. From three different mammary gland specimens, the positive signal was strongest in the lactating gland (lact.), moderate in the gland from pregnant animal (preg.), and faintest in the resting gland (rest.).
Figure 2.
SDS/PAGE and colloidal Coomassie blue staining of total colostral milk (milk), CA purified from colostral milk (M CA VI; 0.2 μg, left lane; 0.6 μg, right lane), and CA purified from saliva (S CA VI). The ≈42-kDa polypeptides are seen in CAs purified from both milk and saliva. A polypeptide of similar size is also visible in the total milk sample.
Figure 3.
PNGase F treatment of human salivary and milk CA VI followed by SDS/PAGE and Colloidal Coomassie blue staining. Without PNGase F treatment (−), the 42-kDa polypeptides for both salivary (S) and milk (M) CA VI are seen, corresponding to the glycosylated form of CA VI, but after digestion (+), the 36-kDa polypeptides for both samples are seen, indicating that the two glycopolypeptides have polypeptide cores of similar sizes.
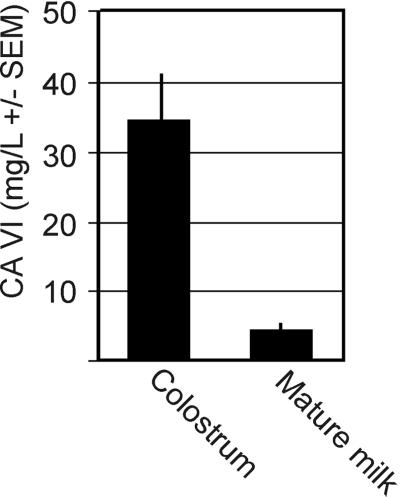
CA VI Concentrations in Human Colostral and Mature Milk.
The mean concentrations of CA VI were 34.7 mg/liter (range, 10.0–78.4 mg/liter, n = 9) in colostrum and 4.5 mg/liter (2.6–6.9 mg/liter, n = 4) in mature milk (Fig. 4). The CA VI levels in the saliva of two infants were 1.9 and 3.6 mg/liter, about half of the concentration in adults (13).
Figure 4.
Mean concentrations of CA VI in human colostrum and mature milk.
Detection of CA VI in the Rat Mammary Gland by Immunohistochemistry.
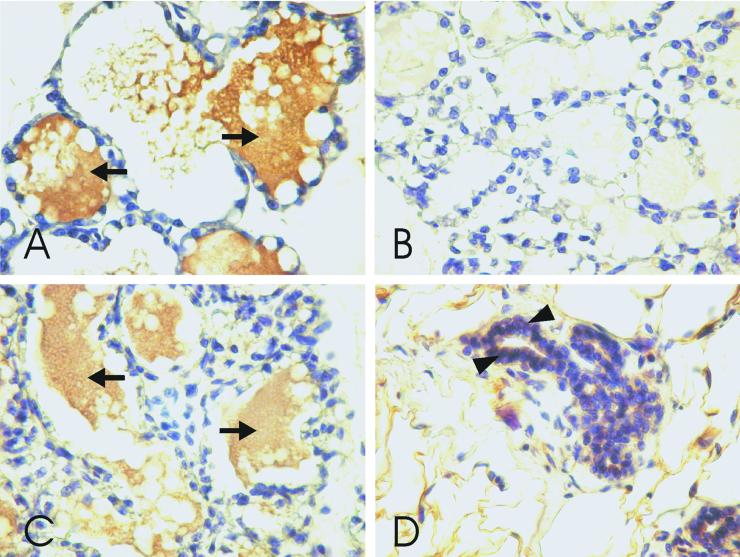
The mammary glands of a sexually mature nonpregnant female rat showed a faint, positive reaction for CA VI in the alveolar epithelia, and the same was observed before and after parturition (Fig. 5 A, C, and D). A strong reaction was seen in the alveolar milk after parturition, with the reaction slightly weaker before parturition (compare Fig. 5 A and C). No reaction was seen in the alveolar lumen of the resting gland (Fig. 5D). Western blotting of mammary gland homogenates showed the most intense band in the lactating gland, a moderate band in glandular tissue before parturition, and a faint band in the resting gland (Fig. 1B).
Figure 5.
Immunohistochemical staining of rat mammary glands by using the anti-rat CA VI antibody (A, C, and D) and normal rabbit serum (NRS) (B). Milk inside the alveoli (arrows) stained strongly for CA VI in the lactating gland (A) and moderately in the gland of the pregnant animal (C). No staining was seen inside the alveoli of the resting gland (D). The cytoplasm of the alveolar epithelium showed a faint staining intensity (D, arrowheads) in all of the glands. Control staining of a lactating mammary gland with NRS was negative (B).
Discussion
CA VI is the only secretory isoform in the CA gene family, identified previously in the saliva of several mammalian species. We demonstrate here that CA VI is also present in milk. There are several lines of evidence to support this conclusion. The specific antibodies to salivary CA VI recognized polypeptides of similar molecular mass (42 kDa) in human and rat saliva and milk, the polypeptide could be purified effectively from human milk on CA inhibitor affinity chromatography, and digestion of the purified human milk 42-kDa polypeptide with PNGase F reduced its molecular mass to 36 kDa, indicating that it is a glycopolypeptide by nature and that its polypeptide backbone probably is identical to that of salivary CA VI. The partial amino acid sequence of the purified 42-kDa polypeptide was identical to salivary CA VI. Although the homologous sequence covered 40% of salivary CA VI sequence, the possibility remains that colostral CA VI represents the product of an additional locus. Immunohistochemical staining of a rat mammary gland showed that the enzyme is present in both the alveolar milk and the glandular epithelia. The fairly faint epithelial staining is likely a result of the rapid secretion rate of the epithelia during late pregnancy and lactation. The immunoblots revealing the major, 42-kDa polypeptide in rat mammary gland samples confirmed that the immunohistochemical results are specific to CA VI.
The mean concentration of CA VI was found to be about eight times higher in the human colostrum than in mature milk, where it corresponded to concentrations reported earlier for adult human saliva (13). This high concentration of CA VI in the colostrum, together with our preliminary observation that the salivary CA VI concentration in newborn babies is low and the fact that their salivary secretion rate also is low (14, 15), suggests that milk CA VI may compensate for this low salivary CA VI as a regulatory factor in the infant alimentary tract, particularly during the early postnatal period.
CA VI initially was discovered as a zinc-binding salivary protein named gustin, which was linked functionally to taste and smell perception (20, 21). It was not until recently that gustin was identified as CA VI by protein sequencing, activity profiles, and other physical data (12). Henkin et al. (21–23) described a clinical disorder in which patients had decreased salivary CA VI concentrations associated with a loss and distortion of taste and smell after an influenza-like illness. The morphological changes in the taste buds presented apoptotic-like features (21). Treatment with zinc normalized the CA VI concentrations and the senses of taste and smell in some cases (24). The taste bud morphology also was normalized in these patients, suggesting that CA VI may function as a trophic factor for the taste bud stem cells (24). Interestingly, CA VI supports in vitro growth in chicken embryo sympathetic ganglia and PC12 cells (24). There is also evidence that CA VI activates calmodulin-dependent bovine brain cAMP phosphodiesterase, which is a factor involved in taste function (25), and that CA inhibitors can cause taste distortions in clinical use (26–28).
Another attractive link between CA VI and growth regulation was provided by observations that CA VI has characteristics similar to nerve growth factor (NGF) (29), which is one of the key growth modulators in the gastrointestinal tract and also is present in milk (30). For example, CA VI displaces 125I-NGF bound to enriched taste bud membrane fractions (24). Although the exact physiological significance of milk CA VI remains undefined here, we suggest that, as an acid-resistant enzyme (11), it may function as a trophic growth factor in the infant alimentary tract, which undergoes pronounced growth and functional maturation during the immediate postnatal period.
Many milk glycoproteins have a multifunctional nature (1, 3), and one also could hypothesize that CA VI as a glycoprotein and an acid–base-modulating enzyme also may possess multifunctional properties such as antimicrobial (31), antiinflammatory, immunomodulating, and mucosa-protecting effects (11). In fact, studies in our laboratory have shown indirectly that CA VI binds to immunoglobulins A and G (8, 32), although its role in immunological responses has not been determined. Future studies will clarify the possible multifunctional nature of CA VI.
Acknowledgments
We thank Ms. Lissu Hukkanen, Ms. Pirkko Peronius, and Mr. Mika Kihlström for their skillful technical assistance. This work was supported by grants to J.L. from the Finnish Cultural Fund and the Finnish Dental Association and by a grant to S.P. from the Sigrid Juselius Foundation.
Abbreviation
- CA
carbonic anhydrase
References
- 1.Kunz C, Rodriguez-Palmero M, Koletzko B, Jensen R. Clin Perinatol. 1999;26:305–333. [PubMed] [Google Scholar]
- 2.Xu R J. Reprod Fertil Dev. 1996;8:35–48. doi: 10.1071/rd9960035. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Palmero M, Koletzko B, Kunz C, Jensen R. Clin Perinatol. 1999;26:335–359. [PubMed] [Google Scholar]
- 4.Sly W S, Hu P Y. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 5.Fernley R T, Wright R D, Coghlan J P. FEBS Lett. 1979;105:299–302. doi: 10.1016/0014-5793(79)80634-1. [DOI] [PubMed] [Google Scholar]
- 6.Feldstein J B, Silverman D N. J Biol Chem. 1984;259:5447–5453. [PubMed] [Google Scholar]
- 7.Murakami H, Sly W S. J Biol Chem. 1987;262:1382–1388. [PubMed] [Google Scholar]
- 8.Parkkila S, Kaunisto K, Rajaniemi L, Kumpulainen T, Jokinen K, Rajaniemi H. J Histochem Cytochem. 1990;38:941–947. doi: 10.1177/38.7.2113069. [DOI] [PubMed] [Google Scholar]
- 9.Sok J, Wang X Z, Batchvarova N, Kuroda M, Harding H, Ron D. Mol Cell Biol. 1999;19:495–504. doi: 10.1128/mcb.19.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kivelä J, Parkkila S, Parkkila A-K, Leinonen J, Rajaniemi H. J Physiol. 1999;520:315–320. doi: 10.1111/j.1469-7793.1999.t01-1-00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkkila S, Parkkila A-K, Lehtola J, Reinilä A, Södervik H-J, Rannisto M, Rajaniemi H. Dig Dis Sci. 1997;42:1013–1019. doi: 10.1023/a:1018889120034. [DOI] [PubMed] [Google Scholar]
- 12.Thatcher B J, Doherty A E, Orvisky E, Martin B M, Henkin R I. Biochem Biophys Res Commun. 1998;250:635–641. doi: 10.1006/bbrc.1998.9356. [DOI] [PubMed] [Google Scholar]
- 13.Parkkila S, Parkkila A-K, Vierjoki T, Ståhlberg T, Rajaniemi H. Clin Chem. 1993;39:2154–2157. [PubMed] [Google Scholar]
- 14.Davidson M. In: Pediatrics. Rudolph A M, Hoffman J I E, Axelrod S, editors. Norwalk, CT: Appleton-Century-Crofts; 1982. pp. 915–919. [Google Scholar]
- 15.Scott J. J Biol Buccale. 1979;7:341–352. [PubMed] [Google Scholar]
- 16.Leinonen J, Parkkila S, Kaunisto K, Koivunen P, Rajaniemi H. J Histochem Cytochem. 2001;49:657–662. doi: 10.1177/002215540104900513. [DOI] [PubMed] [Google Scholar]
- 17.Karhumaa P, Parkkila S, Waheed A, Parkkila A-K, Kaunisto K, Tucker P W, Huang C-J, Sly W S, Rajaniemi H. J Biol Chem. 2000;275:16044–16049. doi: 10.1074/jbc.275.21.16044. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Nature (London) 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Petäjä-Repo U E, Merz W E, Rajaniemi H J. Endocrinology. 1991;128:1209–1217. doi: 10.1210/endo-128-3-1209. [DOI] [PubMed] [Google Scholar]
- 20.Henkin R I, Lippoldt R E, Bilstad J, Edelhoch H. Proc Natl Acad Sci USA. 1975;72:488–492. doi: 10.1073/pnas.72.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henkin R I, Martin B M, Agarwal R P. Am J Med Sci. 1999;318:380–391. doi: 10.1097/00000441-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Henkin R I, Schechter P J, Hoye R C, Mattern C F. J Am Med Assoc. 1971;217:434–440. [PubMed] [Google Scholar]
- 23.Henkin R I, Larson A L, Powell R D. Ann Otol Rhin Laryngol. 1975;84:672–682. doi: 10.1177/000348947508400519. [DOI] [PubMed] [Google Scholar]
- 24.Henkin R I, Martin B M, Agarwal R P. Am J Med Sci. 1999;318:392–405. doi: 10.1097/00000441-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Law J S, Nelson N, Watanabe K, Henkin R I. Proc Natl Acad Sci USA. 1987;84:1674–1678. doi: 10.1073/pnas.84.6.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansson H P J. Nord Med. 1961;65:566–567. [Google Scholar]
- 27.Graber M, Kelleher S. Am J Med. 1988;84:979–980. doi: 10.1016/0002-9343(88)90091-5. [DOI] [PubMed] [Google Scholar]
- 28.Miller L G, Miller S M. J Fam Pract. 1990;31:199–200. [PubMed] [Google Scholar]
- 29.Henkin R I, Law J S, Nelson N R. In: Trace Elements in Man and Animals. Hurley L S, Keen C L, Lönnerdal B, Rucker R B, editors. Vol. 6. New York: Plenum; 1988. pp. 385–387. [Google Scholar]
- 30.Gaull G E, Wright C E, Isaacs C E. Pediatrics. 1985;75:142–145. [PubMed] [Google Scholar]
- 31.Hooper L V, Beranek M C, Manzella S M, Baenziger J U. J Biol Chem. 1995;270:5985–5993. doi: 10.1074/jbc.270.11.5985. [DOI] [PubMed] [Google Scholar]
- 32.Kivelä J, Parkkila S, Waheed A, Parkkila A-K, Sly W S, Rajaniemi H. Clin Chem. 1997;43:2318–2322. [PubMed] [Google Scholar]