Abstract
Non-healing wounds continue to be a clinical challenge for patients and medical staff. These wounds have a heterogeneous etiology, including diabetes and surgical trauma wounds. It is therefore important to decipher molecular signatures that reflect the macroscopic process of wound healing. To this end, we collected wound sponge dressings routinely used in vacuum assisted therapy after surgical trauma to generate wound-derived protein profiles via global mass spectrometry. We confidently identified 311 proteins in exudates. Among them were expected targets belonging to the immunoglobulin superfamily, complement, and skin-derived proteins, such as keratins. Next to several S100 proteins, chaperones, heat shock proteins, and immune modulators, the exudates presented a number of redox proteins as well as a discrete neutrophil proteomic signature, including for example cathepsin G, elastase, myeloperoxidase, CD66c, and lipocalin 2. We mapped over 200 post-translational modifications (PTMs; cysteine/methionine oxidation, tyrosine nitration, cysteine trioxidation) to the proteomic profile, for example, in peroxiredoxin 1. Investigating manually collected exudates, we confirmed presence of neutrophils and their products, such as microparticles and fragments containing myeloperoxidase and DNA. These data confirmed known and identified less known wound proteins and their PTMs, which may serve as resource for future studies on human wound healing.
Keywords: chaperones, damage-associated molecular patterns, heat-shock proteins, mass spectrometry, matrix metalloproteinase, peptidases, post-translational modifications, redox regulation
1. Introduction
Wound healing is the essential process to protect the body from further environmental insults after trauma. It is segmented into discrete phases, namely hemostasis, inflammation, proliferation, and remodeling [1]. The inflammatory phase is central in steering healing into either a physiological or a pathological course. This phase is characterized by swift neutrophil influx, followed by later immigration of macrophage [2]. To oppose any invading pathogen expected during tissue injury, a pro-inflammatory milieu dominates that is controlled via chemokines and cytokines [3]. Once these pro-inflammatory setting is lacking sustained stimuli, e.g., due to clearance of bacteria, the mediator profile switches [4]. This causes macrophage re-programming towards an anti-inflammatory, pro-wound healing phenotype [5]. As consequence, keratinocyte and fibroblast influx is promoted, and the wound is sealed and remodeled [6].
Non-healing wounds and ulcers are a major burden for patients and western health care systems alike [7]. Molecularly, these wounds do not progress from the pro to the anti-inflammatory phase but stay in between them [8]. This is often coined “chronic” inflammation; i.e., an ongoing inflammatory phase that is attenuated but not resolved [9]. Chronic wounds are thought to be a consequences of either endogenous factors, such as impaired angiogenesis as seen in diabetic patients, or exogenous factors, such as excessive presence of pathogens [10]. In both cases, the wounds display distinct molecular signatures that are either a consequence or the driver of impaired healing [11,12,13]. To differentiate between features of pathological healing being hen or egg, studies on physiological healing greatly enhance the understanding of this highly complex, multi-facetted process [14]. Especially wound fluid has added to the understanding of healing in the past as it contains material from wound-resident cell such as neutrophils [15].
Neutrophils are the dominant cell type in inflammation as well as chronic wounds [16]. These cells are armed with an arsenal of molecules toxic to pathogens and host cells [17]. This includes the release of a number of enzymes with proteolytic and antimicrobial activity [18]. Neutrophils are also capable of release extracellular traps; antimicrobial DNA decorated with inflammatory enzymes [19]. Neutrophils have been observed to produce and release microparticles [20]; small double-membraned vesicles encapsulating signaling and enzymatic proteins, small molecules, and messenger RNA [21]. Importantly, the granulocytes are major producers of reactive oxygen and nitrogen species (ROS/RNS) [22]. Two major enzymes are superoxide-producing Flavin reductase (NADPH) oxidases and hypochlorous acid-generating myeloperoxidase [23]. Acute wound healing in rats has been observed to contain peak concentrations of 200 µM hydrogen peroxide [24]. This molecule is a major chemoattractant for neutrophils [25]. Consequently, it has long been proposed that wound healing is subject to redox control [26].
The aim of this study was to investigate wound exudates of acutely healing wounds on a cellular and protein level. Finding and confirming that large parts of cells in exudates are neutrophils as shown by surface marker profiles, we also identified neutrophil-derived small particles in our samples. Adding to and largely confirming a previous study [27], proteomic analysis of protein targets as well as their post-translational modifications (PTMs) revealed a in part unreported signature in the exudates of healing wounds.
2. Results
2.1. Proteomics of Wound Exudates
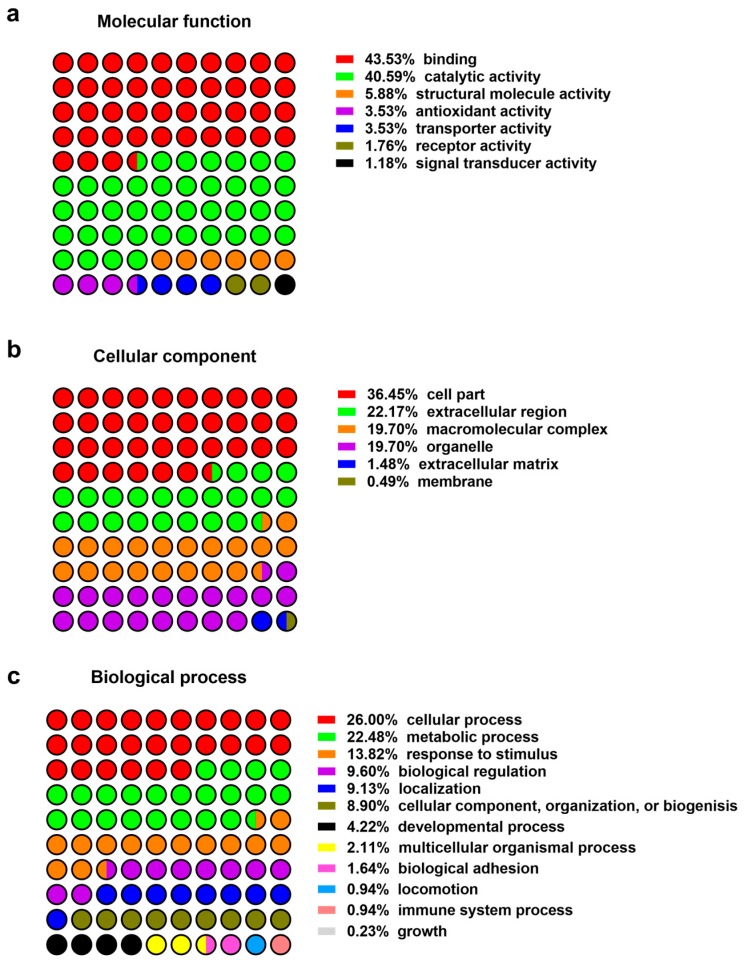
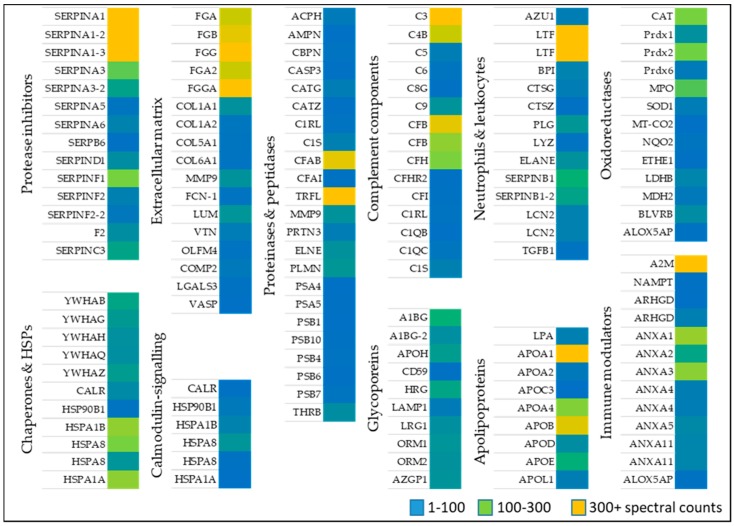
Identified proteins from wounds identified in patients (Table 1) were classified based on their function, compartment, and biological process (Figure 1). Alternatively, proteins were sorted based on their role in wound healing, e.g., activation of anti-oxidant factors such as oxidoreductases (Table 2), immune modulators, chaperone and heat shock proteins (Table 3), neutrophil and leukocytes associated factors (Table 4), extracellular matrix proteins (Table 5) as well as proteinases and peptidases (Table 6). An overview of the relative abundances of these proteins is given in Figure 2.
Table 1.
Patients enrolled in this study.
| Cohort Feature | Value |
|---|---|
| Number of Patients | 11 |
| Median patient age (years ± S.E.) | 54 ± 4 |
| Sex (m/f) | 9/2 |
| Wound type | 11 trauma wounds (1 thoracic, 3 upper extremity, 7 lower extremity) |
| Wound healing response | 11/11 |
| Median time to wound healing (days ± S.E.) | 21 ± 4 |
Figure 1.
Protein classification: (a) Molecular function; (b) cellular compartment; (c) biological process.
Table 2.
Oxidoreductases in wound fluids.
| Protein ID | Acronym | Protein Name | Protein Class |
|---|---|---|---|
| P04040 | CAT | Catalase | Peroxidase |
| Q06830 | Prdx1 | Peroxiredoxin 1 | Peroxidase |
| P32119 | Prdx2 | Peroxiredoxin 2 | Peroxidase |
| P30041 | Prdx6 | Peroxiredoxin 6 | Peroxidase |
| P05164-2 | MPO | Isoform H14 of Myeloperoxidase | Peroxidase |
| P00441 | SOD1 | Superoxide dismutase 1 | Oxidoreductase |
| P00403 | MT-CO2 | Cytochrome C oxidase 2 | Oxidoreductase |
| P16083 | NQO2 | Ribosyldihydronicotinamide dehydrogenase | Oxidoreductase |
| O95571 | ETHE1 | Persulfide dioxygenase | Oxidoreductase |
| P07195 | LDHB | l-lactate dehydrogenase β | Dehydrogenase |
| P40926 | MDH2 | Malate dehydrogenase 2 | Dehydrogenase |
| P30043 | BLVRB | Flavin reductase (NADPH) | Reductase |
| P20292 | ALOX5AP | Arachidonate 5-lipoxygenase activating protein | Transferase |
Table 3.
Signaling and immune modulators, chaperones, and heat shock proteins in wound fluids. GDP: Guanosine-5′-triphosphate.
| Protein ID | Acronym | Protein Name |
|---|---|---|
| Calmodulin-signaling molecules | ||
| P26447 | S100A4 | Protein S100 A4 |
| P06703 | S100A6 | Protein S100 A6 |
| P05109 | S100A8 | Protein S100 A8 |
| P06702 | S100A9 | Protein S100 A9 |
| P31949 | S100A11 | Protein S100 A11 |
| P25815 | S100P | Protein S100 P |
| Chaperone and heat shock proteins | ||
| P31946 | YWHAB | 14-3-3 protein β/α |
| P61981 | YWHAG | 14-3-3 protein γ |
| P27348 | YWHAH | 14-3-3 protein τ |
| Q04917 | YWHAQ | 14-3-3 protein ε |
| P63104 | YWHAZ | 14-3-3 protein ζ |
| P27797 | CALR | calreticulin |
| P14625 | HSP90B1 | Endoplasmin |
| P0DMV9 | HSPA1B | Heat shock 70 kDa protein 1B |
| P11142 | HSPA8 | Heat shock cognate 71 kDa protein |
| P11142-2 | HSPA8 | Isoform 2 of Heat shock cognate 71 kDa protein |
| P0DMV8-2 | HSPA1A | Isoform 2 of Heat shock 70 kDa protein 1A |
| Immune modulators | ||
| P01023 | A2M | α2-macroglobulin |
| P43490 | NAMPT | Nicotinamide phosphoribosyltransferase |
| P52565 | ARHGD | Rho GDP-dissociation inhibitor 1 |
| P52566 | ARHGD | Rho GDP-dissociation inhibitor 2 |
| P04083 | ANXA1 | Annexin A1 |
| P07355 | ANXA2 | Annexin A2 |
| P12429 | ANXA3 | Annexin A3 |
| P09525 | ANXA4 | Annexin A4 |
| P09525-2 | ANXA4 | Annexin A4 Isoform 2 |
| P08758 | ANXA5 | Annexin A5 |
| P50995 | ANXA11 | Annexin A11 |
| P50995-2 | ANXA11 | Annexin A11 Isoform 2 |
| P20292 | ALOX5AP | Arachidonate 5-lipoxygenase activating protein |
Table 4.
Neutrophil- and leukocyte-associated factors in wound fluids.
| Protein ID | Acronym | Protein Name |
|---|---|---|
| P20160 | AZU1 | Azurocidin |
| P02788 | LTF | Lactotransferrin |
| P02788-2 | LTF | Isoform δLf of Lactotransferrin |
| P17213 | BPI | Bactericidal permeability-increasing protein |
| P08311 | CTSG | Cathepsin G |
| Q9UBR2 | CTSZ | Cathepsin Z |
| P00747 | PLG | Plasminogen |
| P61626 | LYZ | Lysozyme |
| P08246 | ELANE | Neutrophil elastase |
| P30740 | SERPINB1 | Leukocyte elastase inhibitor |
| P30740-2 | SERPINB1-2 | Leukocyte elastase inhibitor isoform 2 |
| P80188 | LCN2 | Neutrophil gelatinase-associated lipocalin |
| P80188-2 | LCN2 | Isoform 2 of Neutrophil gelatinase-associated lipocalin |
| Q15582 | TGFB1 | Transforming growth factor B1 |
Table 5.
Extracellular matrix proteins in wound fluids.
| Protein ID | Acronym | Protein Name |
|---|---|---|
| P02671 | FGA | Fibrinogen α chain |
| P02675 | FGB | Fibrinogen β chain |
| P02679 | FGG | Fibrinogen γ chain |
| P02671-2 | FGA2 | Isoform 2 of Fibrinogen α chain |
| P02679-2 | FGGA | Isoform γ A of Fibrinogen γ chain |
| P02452 | COL1A1 | Collagen α-1(I) chain |
| P08123 | COL1A2 | Collagen α-2(I) chain |
| P20908 | COL5A1 | Collagen α-1(V) chain |
| P12109 | COL6A1 | Collagen α-1(VI) chain |
| O00602 | FCN-1 | Ficolin 1 |
| P51884 | LUM | Lumican |
| P04004 | VTN | Vitronectin |
| Q6UX06 | OLFM4 | Olfactomedin-4 |
| P49747-2 | COMP2 | Cartilage oligomeric matrix protein 2 |
| P17931 | LGALS3 | Galectin 3 |
| P50552 | VASP | Vasodilator-stimulated phosphoprotein |
Table 6.
Proteinases and peptidases in wound fluids.
| Protein ID | Acronym | Protein Name |
|---|---|---|
| P13798 | ACPH | Acylamino-acid-releasing enzyme |
| P15144 | AMPN | Aminopeptidase N |
| P15169 | CBPN | Carboxypeptidase N catalytic chain |
| P42574 | CASP3 | Caspase-3 |
| P08311 | CATG | Cathepsin G |
| Q9UBR2 | CATZ | Cathepsin Z |
| Q9NZP8 | C1RL | Complement C1r subcomponent-like protein |
| P09871 | C1S | Complement C1s subcomponent |
| P00751 | CFAB | Complement factor B |
| P05156 | CFAI | Complement factor I |
| P02788 | TRFL | Lactotransferrin |
| P14780 | MMP9 | Matrix metalloproteinase-9 |
| P24158 | PRTN3 | Myeloblastin |
| P08246 | ELNE | Neutrophil elastase |
| P00747 | PLMN | Plasminogen |
| P25789 | PSA4 | Proteasome subunit α type-4 |
| P28066 | PSA5 | Proteasome subunit α type-5 |
| P20618 | PSB1 | Proteasome subunit β type-1 |
| P40306 | PSB10 | Proteasome subunit β type-10 |
| P28070 | PSB4 | Proteasome subunit β type-4 |
| P28072 | PSB6 | Proteasome subunit β type-6 |
| Q99436 | PSB7 | Proteasome subunit β type-7 |
| P00734 | THRB | Prothrombin |
Figure 2.
Relative abundance of high confidence proteins as determined in the pooled wound fluid samples by liquid chromatography/mass spectrometry spectral counts.
2.1.1. Oxidoreductases
Anti-oxidative stress factors counteract oxidative stress present during wound healing. A large set of proteins belonging to anti-oxidant down-stream signaling response such as catalase (CAT) several peroxidases (PRDX1, 2, 6, MPO), and superoxide dismutase (SOD1) [28,29,30] was identified in wound fluids. Oxidoreductases such as cytochrome C oxidase (MT-COX2), the terminal enzyme in the mitochondrial respiratory chain, catalyze the reduction of oxygen for energy recovery [31] and its presence in wound fluids prevents an excessive inflammatory response [32]. l-lactate dehydrogenase (LDHB), a marker of tissue destructive microenvironment and hemolysis [33], was also identified. Dehydrogenases represents an additionally protein class of oxidoreductases, of which malate dehydrogenase (MDH2), and flavin reductase (NADPH; BLVRB) was detected (Table 2 and Figure 2). These data indicate the presence of enzymes of the anti-oxidative defense system in wound fluids obtained from the traumatic acute wounds.
2.1.2. Immune Modulators, Chaperones, and Heat Shock Proteins
Several members of the complement factor family (Table A1) as well as numerous members of the S100A protein family (e.g., S100A4, 6, 8, 9, 11, S100P) were found in the exudates of traumatic wounds (Table 3 and Figure 2). Chaperone and heat shock proteins (HSP) are inducible stress proteins promoting wound closure by recruitment of dermal fibroblasts in late stages of wound repair [34]. We identified several HSP family members such as endoplasmin (HSP90B1), HSPA1A, HSPB, and HSP8. Moreover, calreticulin (CALR) and different 14-3-3 protein family members were detected in wound fluids (Table 3, middle part). The detection of α2-macroglobulin (A2M), which is mainly synthesized by macrophages and fibroblasts, suggest a functional inhibition of an enormous variety of proteinases. [35] Additionally, nicotinamide phosphoribosyltransferase (NAMPT) enables NAD+ biosynthesis, and functions as cytokine that promotes B cell maturation as well as inhibition of neutrophil apoptosis [36]. Two members of the Guanosine-5′-triphosphate (GDP)-dissociation inhibitors of Rho proteins (ARHGD1/2) were identified regulating GDP/Guanosine-5′-triphosphate (GTP) exchange and activating oxygen superoxide-generating NADPH oxidase of phagocytes [37]. Based on our proteomic approach, the abundance of several annexin family members (ANX1, 2, 3, 4, 5, 11) was further shown (Table 3, last part) confirming previous results provided by novel proteomic methodology [27].
2.1.3. Neutrophil and Leukocytes Associated Factors
Numerous immune modulators such as lactotransferin (LTF), azurodicin (AZU1), bactericidal permeability-increasing protein (BPI) and lipocalin (LCN2) were identified (Table 4 and Figure 2), which are often associated with host defense against a broad range of microorganisms, immune response, anti-inflammatory activity and regulation of cellular growth as well as differentiation. A balance of the presence of neutrophil elastase (ELANE), inhibitors of leukocyte elastase (SERPINB1) and cathepsin G and Z (CTSG/Z) were found suggesting a strong regulation of a variety of proteolytic events important to tissue repair [27]. Further results also indicate an increased existence of protease inhibitors in the healing wound fluid (Table A2). Additionally, a proteolysis-activated plasminogen (PLG) and an antimicrobial enzyme lysozyme (LYZ) were detected in wound exudates indicating an activated stage of proteases in cellular processes such as wound healing and of innate immune system, respectively. Moreover, the anti-inflammatory transforming growth factor B1 (TGFβ1) activates macrophages and regulates a variety of cellular functions including cell proliferation, differentiation, and apoptosis, which are important during the different stages of wound healing.
2.1.4. Extracellular Matrix Proteins
The extracellular matrix (ECM) is predominantly formed by collagen and is an essential component of the skin among fibroblasts, keratinocytes, endothelial cells, and immune cells [38]. Sixteen ECM molecules were obtained including several collagens (COL1A1, 2, 5, 6), fibrinogens (FGA, FGB, FGG) and oligomeric lectins consisting of both, collagen-like stretches and fibrinogen (e.g., FCN1) [39]. All proteins may function as hallmarks and biomarkers of newly generated granulation tissue and provisional wound matrix (Table 5 and Figure 2). Furthermore, lumican (LUM), vitronectin (VTN), olfactomedin-4 (OLFM4), and cartilage oligomeric matrix protein 2 (COMP2) were identified in wound fluids regulating tissue repair, collagen fibril organization and formation [40]. Galectin 3 (LGALS3) and the vasodilator-stimulated phosphoprotein (VASP), which were also found in wound fluids, are recruit to cell-cell junctions at the wound edge [41].
2.1.5. Proteinases
Ubiquitous intracellular peptidases belonging to the proteasome (7 proteins: PSMAs/PSMBs; Table 6 and Figure 2) and caspase 3 (CASP3), indicate active inflammatory cellular processes [42] and show that cellular cytoplasmic fractions are present in the collected samples. The serine protease Cathepsin G (CATG) plays an integral part in immune response and inflammatory processes, being associated to NETs [18]. Aminopeptidase N (AMPN) is a peptidase with broad specificity, playing a role in MHCII presented antigen cleavage and in angiogenesis [43]. Apolipoprotein (APOA) inhibiting tissue-type plasminogen activator 1 and matrix metalloprotease 9 (MMP9) indicate a controlled ECM modulation and leukocyte migration [44]. Moreover, presence of prothrombin indicates the presence of blood in the wound bed [45].
2.1.6. Post-Translational Modifications
Besides general annotation of proteins by mass spectrometry (MS), several chemical modifications were searched for in the generated data sets (Table A3). Numerous cysteine oxidations were observed: both cysteine sulfenic acid and, predominantly, cysteine sulfonic acid. Most single oxidations were found at methionines forming methionine sulfoxide, as cysteine sulfenic acid is both partially reduced by Dithiothreitol (DTT) and instable during ionization. In total, oxidative modifications were the most common observed changes compared to nitrosative modifications. Most nitrosative modifications were found to be tyrosine nitration as well as very low amounts of nitrosated cysteine. Additionally, carbamidomethylations were introduced during the reduction/alkylation work step during sample preparation.
2.2. Manually Obtained Wound Material
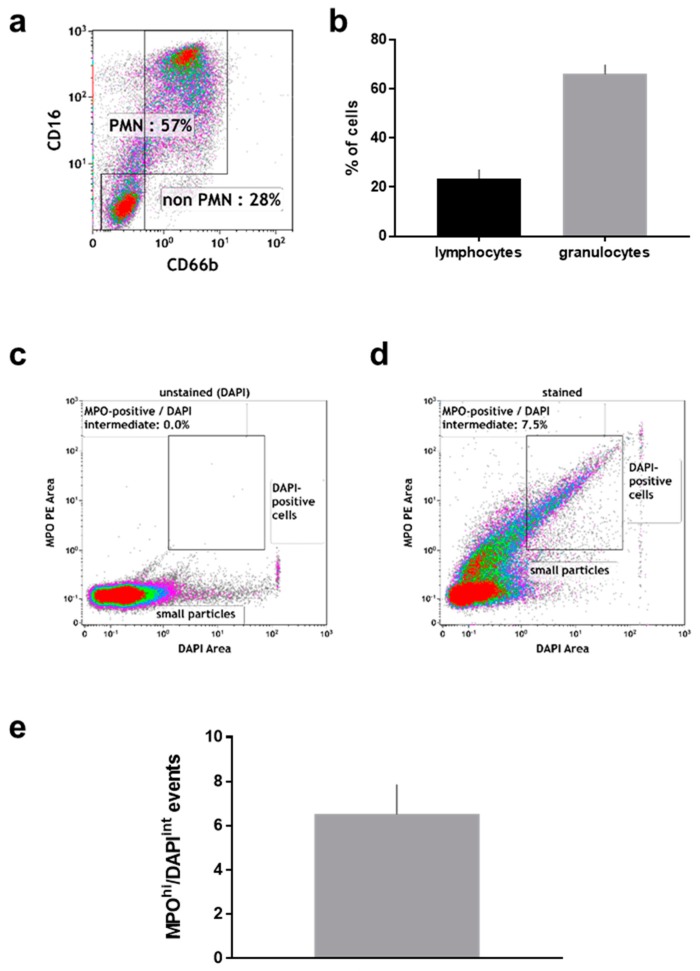
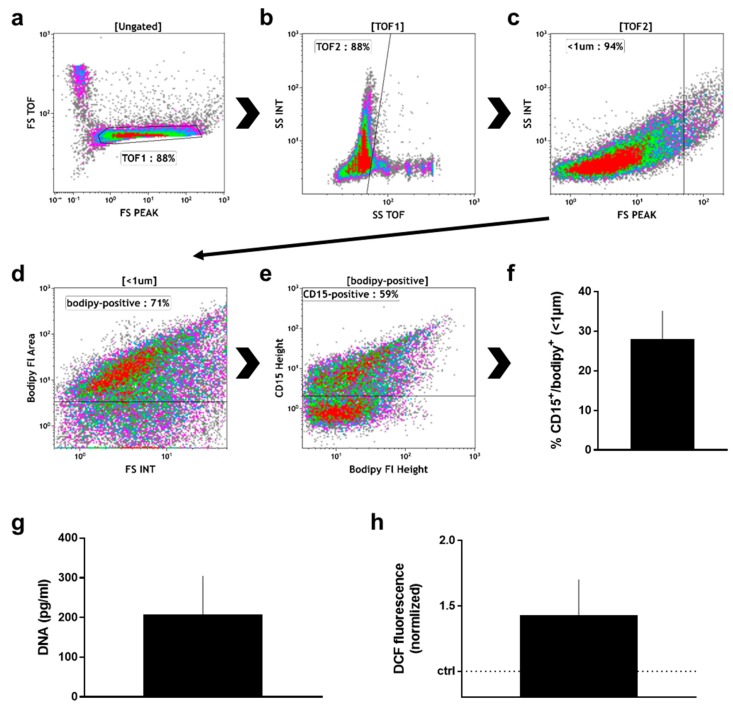
After the wound sponge was removed, from which the material for mass spectrometry analysis was obtained, a swab was used to obtain additional wound material. From this, pelleted material as well as supernatant were analyzed. Within the pelleted material, the percentage (Figure 3a) of neutrophils (CD16+/CD66+) and non-neutrophils (CD16−/CD66−; mostly lymphocytes) among CD45+/DAPI+ cells was calculated (Figure 3b). There was about four times more neutrophils than non-neutrophils in the pelleted fraction. Neutrophils exposed to stimulating agents such as wound-resident bacteria are known to generate sticky DNA decorated with antimicrobial proteins such as myeloperoxidase (MPO), so-called neutrophil extracellular traps (NETs) [19]. To identify such structures, the pelleted material was stained for DNA (DAPI; Figure 3c), and MPO (Figure 3d). A substantial percentage of double-positive events among all acquired events was identified by flow cytometry in the pelleted fraction (Figure 3e). Another feature of eukaryotic cells in general and neutrophils in particular is the release of small vesicles carrying cargo protein and RNA for cell-cell communication and modulation of the tissue microenvironment, so-called microparticles [46]. To identify these small (<1 µm) membraned vesicles in the supernatant of wound material, we applied a stringent flow cytometry gating and staining strategy, tightly discriminating for doublets in the forward scatter (Figure 4a), side scatter (Figure 4b), and size exclusion (<1 µm; Figure 4c) using flow beads. Applying a membrane-stain, we were able to discriminate non-membraned events from microparticles (Figure 4d). Among microparticles, we tested for the percentage of neutrophil-derived CD15+ particles (Figure 4e) that were markedly present in the wound material supernatants (Figure 4f). As DNA+/MPO+ events were identified in the pelleted fraction (Figure 3d), we assayed for DNA (Figure 4g) and oxidants (Figure 4h) in the supernatant as well and found both to be increased.
Figure 3.
Flow cytometric analysis of cell pellets derived from manually removed wound material. (a) representative dot plot and (b) percent of CD16+/CD66b+ (neutrophils) and CD16−/CD66b− cells among CD45+/DAPI+ cells; (c) pelleted material stained with DAPI only; (d) pelleted material stained against DAPI and MPO; (e) quantification of events staining positive for MPO and intermediate for DAPI. Data are from 6–8 patients.
Figure 4.
Supernatant analysis of manually removed wound material. (a) time of flight (TOF) gate in forward scatter (FS) height against FS TOF; (b) TOF gating analogous to (a); (c) size discrimination of small particles as predetermined with beads; (d) gate of membrane-positive particles as predetermined with unstained material; (e) discrimination between CD15+ and CD15− microparticles as predetermined with unstained particles; (f) quantification CD15+ particles among all samples; (g) DNA content in supernatants as determined with sytox green against a known standard; (h) DCF fluorescence of wound material supernatant; data are one representative (a–e) or mean + S.E. of 8 (f), 3 (g), and 2 (h) samples.
3. Discussion
This study was conducted to better understand the proteomic signature paralleling physiological wound healing. While previous studies have identified numerous targets important in this process [47,48,49,50], we also identified so far less reported targets as potential biomarkers that contribute to cell growth, inhibit excessive inflammation, and support matrix as well as granulation tissue formation in normal skin repair. Our findings were complemented by data analysis of protein post-translational modifications and immune-focused analysis of wound exudates by flow cytometry.
Reactive oxygen species (ROS) and wound oxygenation are crucial processes in wound repair, and the oxygen-dependent redox-sensitive signaling represent an integral component of the healing cascade [51]. In wound healing, the initiating and activating of ROS-dependent signaling cascades that promote cellular responses are indispensable. We found indirect evidence of ROS in wound fluids and especially neutrophil-derived oxidants were reported to be crucial in the resolution of inflammation [52]. ROS mainly arise from metabolism via the mitochondrial electron transport chain [53] and a number of oxidases in phagocytes [54]. By proteomics, we identified neutrophil myeloperoxidase (MPO), a producer of potent hypochlorous acid [55]. By flow cytometry, we confirmed the presence of MPO not only in neutrophils from wounds but also on fragments that co-stained for DNA, suggesting the presence of neutrophil extracellular traps (NETs) in wound fluids. These sticky microbicidal DNA-extrusion are only poorly described in wound healing, despite the well-reported abundance of neutrophils in wounds [10]. NET formation is redox-regulated via MPO [56], and NETs form in response to microorganisms [57] and oxidants [58]. NETs were reported to be detrimental in diabetic foot healing [59], can be counteracted in wounds via DNAse treatment [60], and are potent ROS producers themselves [61,62,63]. ROS can be used as a signal for cell proliferation in which oxidoreductases are involved by regulating the redox state of proteins [64]. Among them, we found peroxiredoxins (PRDX1, 2, 6) in wound fluids suggesting a strong control of the wound healing-induced peroxide level [65]. In contrast to our proteomic analysis, PRDX2 was previously detected in exudate obtained from non-healing wounds only [27]. Nevertheless, due to the presence of further oxidoreductases (e.g., SOD1, MT-CO2), acute inflammation is accompanied by metabolic changes and a suppression of mitochondrial respiration. Thus, significant alterations in the anti-oxidant profile accompanied by the presence of an arachidonate 5-lipoxygenase-activating protein (ALOX5AP), a lipid mediator of inflammation [66], and numerous other apolipoproteins (Table A2) may be contributory to healing of traumatic wounds. It was shown in animal studies that re-epithelialization during wound healing was impaired in apolipoprotein (APO) E deficient mice suggesting a beneficial effect of pro-atherogenic lipoproteins on skin fibroblasts and granulation tissue formation, and a direct effect of APOE on wound healing [67]. Next to ALOX5AP as immune-modulator [68], several other factors of inflammation (e.g., ARHGD1/2, NAMPT, etc.) were detected in wound fluids. GDP-dissociation inhibitors of Rho proteins activates NAPDH oxidase (a direct cellular response to redox state) whereas a phosphoribosyltransferase (NAMPT), also known as a pre-B-cell colony enhancing factor 1, enables NAD+ biosynthesis. NAMPT functions as cytokine that promotes B cell maturation as well as inhibition of neutrophil apoptosis [36]. Strong immune modulators and clinical targets in hampered wound healing are chemokines and cytokines [69,70,71]. Despite their undeniable presence in acute wound healing as demonstrated a previous cohort [72], the limit of detection may be too low for detection via mass spectrometry or alternative approach such as targeted proteomics are potentially more suitable for their discovery.
A neutrophil proteomic signature was identified in wound fluids. Neutrophil elastase (ELANE) and cathepsin G (CTSG) are major components of neutrophil granules and participate in digestions of phagocytized microorganisms [73]. ELANE was one of the most abundant neutrophil proteins, and is essential in regulating microbial growth in wounds [74]. The presence of numerous proteins such as lactotransferin (LTF) or azurocidin 1 (AZU1) could be also shown in wound fluids of traumatic wounds in contrast to recently published proteomic studies [27] suggesting an effective impact of antimicrobial acting proteins on physiological wound healing. LTF had the highest abundance among all neutrophil-associated proteins. LTF aids in binding of nucleic acid and iron with a major role in destabilization of microbial membranes [75]. With all wounds ultimately healing properly indicating at least no pathological microbial burden after surgery, we can only speculate that the main role of LTF may not have been antimicrobial one. The second most abundant neutrophil-associated protein was SERPINB1, which acts as ELANE (also among most abundant proteins) inhibitor and is released by neutrophils themselves [76], underlining inflammatory fine-tuning in neutrophil products. Along similar lines, neutrophil gelatinase-associated lipocalin proteins (LCN) are involved in inflammation and detoxification processes caused by immune system activation in humans. Liver-derived PLG is known to be a critical regulator of cutaneous wound healing [77], and neutrophils are major cells targeting PLG [78]. We also identified BPI in highly abundant levels similar to a previous study where it was mildly associated with infection [79]. It is a potent protein antibiotic active against Gram-negative bacteria by binding to the lipopolysaccharide [80].
We identified a broad spectrum of extracellular matrix (ECM) proteins. Modulating the process of wound healing, ECM proteins bind numerous growth factors like TGF and release they after degradation of ECM proteins by proteolytic enzymes [81]. Not only the group members of collagen family is known to be strong associated with physiological wound healing but also the expression of fibrinogens and matrix metalloproteinases. They function as markers of formation of granulation tissue, re-epithelialization and ECM remodeling in dermal repair processes [81]. Proteins such as vitronectin, lumican, olfactomedin-4, a cartilage oligomeric matrix protein 2, and other glycoproteins (Table A2) are basic components of the newly formed provisional matrix at early wound healing stages and reflect—together with the presence of collagens—a physiological healing response. α2-macroglobulin (A2M), an anti-protease inhibiting proteinase, binds to and removes the active forms of gelatinases (e.g., MMP2 or 9) from the circulation via scavenger receptors on the phagocytes. A2M further inhibits plasminogen (PLG) and fibrolysis [82]. The vasodilator-stimulated phosphoprotein (VASP) and galectin 3 (LGALS3) play important roles in cell-cell adhesion and wound closure as well as in cell-matrix interactions, macrophage activation, and angiogenesis [83]. Moreover, calreticulin (CALR) and CD59 were identified, both present on human neutrophils [84]. In addition other 14-3-3 protein family members were detected in wound fluids, which mainly controls renewal of epithelium [85], and stimulation of epithelial cell migration after wounding [86]. Calcium binding proteins such as S100 factors are regulators of calcium homeostasis, inflammation, proliferation, cell cycle progress, and migration. They interact with a variety of target proteins on monocytes/macrophages, neutrophils, and lymphocytes [87]. S100A1 protein has been identified as novel regulator of endothelial angiogenesis suggestive to reflect pro-angiogenetic properties of this protein necessary for wound healing [88]. S100A4 presence is associated with processes of increased cell migration and transcriptional regulation of matrix metalloproteinases, e.g., MMP9, which was also found in our study. S100A6 has a role in cell response to different stressors such as heat shock proteins (e.g., HSP90). Among the S100 proteins, S100A8 and 9 are induced by pro-inflammatory stimuli in macrophages, dendritic cells, epithelial cells, and fibroblasts [89]. Both proteins were also found in wound exudates obtained from normal healing [27] corroborating our results. Additionally, it was recently shown that S100A8/9 overexpression in HaCaT keratinocytes increases NADPH oxidase activity and enhances ROS levels [87] underlining the concept of redox regulation during wound healing [64]. S100P reduces focal cell adhesion [90] emphasizing a support of cell migration. Moreover, the presence of S100A11 indicates a stimulation of keratinocyte cell growth by enhancing the level of several growth factors [91] and we have previously shown a redox regulation of several S100 proteins in human keratinocytes [92]. Based on our proteomic approach, the abundance of several annexins (ANX1, 2, 3, 4, 5, 11) was further demonstrated, which are linked to fibrinolysis, coagulation, inflammation and apoptosis [93], and to trafficking and organization of vesicles, exocytosis, endocytosis, calcium ion channel formation [94]. Trafficking and release of small vesicles, so-called microparticles, is important in neutrophil biology [95]. Neutrophil microparticles stain positive for CD15 [96]. Proteomic analysis of neutrophil-derived microparticles [97] was highly congruent with many targets found in wound fluids, among them annexin A1, A4, A5, and A11, AZU1, ELANE, MPO, BPI, B2M, CALR, CTSG, CTSZ, FCN-1, GRB2, LTF, LYZ, HSP70, HSP71, HSP90, MMP9, PRTN3, PRDX1, S100A9, and others, underlining the strong neutrophil signature in acute wound exudates. On annexins, Eming and colleagues validated its ability remove apoptotic cells to consolidate them as biomarkers to predict healing of traumatic, acute wounds [27].
Regarding chemical modifications, it is well known that oxidative as well as nitrosative modifications have a strong impact on several body functions, e.g., the immune response. Modifications observed by MS are known to be involved in various pathways in inflammation and wound healing. Methionine sulfoxide is the most common modification found by MS besides carbamidomethylation. It is described to attenuate the functions of NFκB and NFTA as well as indirectly influencing the T-cell receptor/CD3 signal transduction pathway [98], indicating its role in immune cell recruitment. In addition, oxidized cysteine residues were observed as the second most occurring modification. Cysteines, and their specific redox state, are key in recruiting leukocytes to freshly wounded tissue, e.g., by the Scr family kinase Lyn [99]. Both modifications can be introduced by hydroxyl radicals as well as hydroxyl radical-forming species, such as hydrogen peroxide, which is also known to be critical affecter of wound healing and immune cell recruitment [25,100,101]. Besides oxidative modification, the formation of nitrotyrosine was observed. Nitrotyrosine is widely regarded as a marker for inflammation [102], and nitrosated peptides can modulate the immune response when presented at MHC complexes [103]. Proteins incorporating nitrotyrosine are discussed to be formed by the impact of the reactive nitrogen species peroxynitrite [104]. Finally, a protein-S-nitrosocysteine was only observed in one instance. This specific PTM is supposed to be physiologically highly active in both innate and adaptive immune system regulation, e.g., by modulating toll-like receptor activity [105]. However, the S-NO moiety is comparably instable, and the occurrence in the wound fluids is likely being underestimated in mass spectrometric approaches. The various oxidative and nitrosative modifications observed are in good agreement with modifications typically associated with the immune response required to trigger wound healing. The redox-based signaling in immune system regulation both by RONS themselves as well as their resulting chemical modifications might be a prime target for clinical application [106]. A controlled regulation of the immune response might allow for faster wound healing with reduced chance of complications, e.g., the formation of chronic wounds. In this regard, first studies using topical applications of S-nitrosocysteine as a donor of the second messenger NO seem to be well-received and have the potential to enhance wound healing [107].
This study had limitations. Regarding the quantification of neutrophils from wound exudates, dead cells were excluded from the analysis only through forward scatter/side scatter profiles but not fluorescence dyes such as propidium iodide because immediate assaying after surgery was not always possible. Moreover, we did not compare surgical wound material with other wound types, such as chronic ulcers or burn wounds, limiting conclusions about potential therapeutic interventions with any of the targets identified. However, there was significant overlap of proteins identified with a previous study that had compared healing and non-healing wounds [27], validating our experimental approach. Nonetheless, this study is rather descriptive, allowing only speculating about the possible mechanistic role of proteins identified in human wound fluids.
In conclusion, we confidently identified hundreds of proteins present during healing of human wounds. Along with data from flow cytometry, this protein signature revealed a major role of neutrophils and their products in wound healing. Moreover, the proteomic study finding oxidoreductases and oxidative post-translational modifications provided evidence of redox control in wound healing. Both corroborates findings and hypothesis of previous studies but in addition resolves the molecular pattern to an extended degree. Together with future studies, this may help to resolve the central processes in human wound healing in physiology and pathology.
4. Materials and Methods
4.1. Wound Material
Wound exudates were collected onsite immediately after sponge removal in the surgical theater as described before [72]. Briefly, two sterile swabs were used to collect material of the wound area. Swabs were rinsed in tubes containing 1 mL of phosphate-buffered saline (PBS) on ice. Tubes were centrifuged, and the supernatant was collected and processed. Cell pellets were fixed with 1% paraformaldehyde until analysis. Wound sponge dressings obtained during surgery were immediately collected into tubes and stored at −80 °C until protein preparation. The sponges are made from polyurethane and are frequently used in vacuum assisted wound therapy. In total, proteins were isolated from 11 patients (Table 1). This study was approved by the local Greifswald ethics committee (BB 113/14, 12 January 2015).
4.2. Protein Preparation from Material in Wound Sponge Dressings
A small cube was cut from each dressing with roughly 5 mm edge length and each cube was weighted on a micro scale as swab loading differed strongly between patients. 50 µL RIPA buffer (1 mM EDTA, 0.5 mM EGTA, 1% (v/v) Triton X-100, 0.1% sodium deoxycholate, 0.1% (w/v) SDS, 140 mM NaCl, 1 mM PMSF, 10 mM Tris/HCl, pH 8) with protease and phosphatase inhibitors (Roche complete mini) was added to each swab per 10 mg wet weight and samples were incubated for 1 h on ice with periodic vortexing. Afterwards, samples were centrifuged at 4 °C for 1 h to remove cell debris and supernatants were decanted into fresh reagent tubes. Proteins were precipitated with acetone overnight and centrifuged for 1 h at 4 °C. To ease re-suspension of proteins, pellets were re-suspended in 500 mM Tris-HCl (pH 7.4), 10% (w/v) SDS, 5% (w/v) β-mercaptoethanol. Afterwards, protein concentrations were measured using a modified Bradford assay (RCDC, Bio-rad, Hercules, CA, USA). As each patient sample was differing strongly, “master” samples were generated by combining 10 µg protein of each sample together. In this way, a more comprehensive overview, which proteins would be present in high abundance in all patients, was possible.
Two master samples were generated independently and 30 µg protein each was loaded onto a precast Tris-Glycerine gel (Bio-rad) in duplicates. Gels were run at 125 V for about 90 min and stained/destained following standard procedures. Lanes containing samples were cut into 10 slices each. From this point on, all reagents and consumables were of LC/MS grade (e.g., LoBind, Eppendorf, Hamburg, Germany) Gel slices were destained using 400 µL washing solution (20 mM NH4HCO3 in 30% acetonitrile) on a shaker for 15 min. This procedure was repeated with fresh solution until slices were destained. Gel slices were dried in a vacuum centrifuge for about 35 min. Afterwards, proteins in the gel slices were reduced using 50 µL of 10 mM dithiothreitol at 60 °C for 1 h followed by an alkylation step with 50 µL of 50 mM iodoacetamide at 25 °C for 30 min. Afterwards, samples were washed twice with washing solution and supernatant was removed. Gel slices were again dried in a vacuum centrifuge for about 25 min (time adapted independently for each slice) prior to in-gel digestion using trypsin (10 µg/mL, sequencing grade, Promega, Wisconsin-Madison, WI, USA) in a volume adequate to cover the specific gel slices. Digestion was performed for 16 h at 22 °C.
After digestion, peptides were extracted from the gel slices by consecutively using 50 µL of 5% formic acid, 50 µL acetonitrile, and 100 µL acetonitrile. After each solvent addition, samples were toughly vortexted for 15 min, centrifuged for 1 min and supernatants pooled in a fresh reagent tube. Afterwards, the resulting 200 µL were reduced to about 10 µL by vacuum centrifugation and 20 µL of A. dest. added. Samples were stored at −80 °C until measurement.
4.3. Mass Spectrometry
Samples were measured in a randomized order. The LC-MS system consisted of an Ultimate 3000 (Dionex, Sunnyvale, CA, USA) nanoLC using an Acclaim PepMap 100 guard column with an Acclaim PepMap RSLC column (15 cm, 75 µM ID, both heated to 40 °C) and a QExactive (Thermo Scientific, Waltham, MA, USA) MS with a Nanospray Flex (ThermoFischer Scientific, Dreieich, Germany) source using steel emitters. The gradient used for the LC was as followed with the solvents A (A. dest, 0.1% acetic acid) and B (acetonitrile, 0.1% acetic acid): 0–4 min: 2% B, 4–65 min linear ramp to 35% B, 65–75 min linear ramp to 50% B, 75–80 min 50% B, 80–81 min linear ramp to 80% B, 81–85 min 80% B, 85–86 min linear ramp to 2% B, 86–100 min 2% B. Total flow was 300 nL/min. Calibration was performed on a daily basis using Pierce™ LTQ Velos ESI Positive Ion Calibration Solution (Thermo Scientific). QExactive measurements were conducted in positive mode in Full MS → dd-MS2 mode. For survey scans, 70,000 resolution in the range of 300 to 1650 m/z was used with an AGC target of 106 and an ion time of 120 ms. For the Top10 MS/MS scans, a 17,500 resolution with an AGC target of 2 × 105 and a stepped NCE of 27.5. Exclusion time for Top10 picking was 30 s, meaning that the 10 highest signals in a survey scan at any given time were fragmented and MS/MS spectra collected. Afterwards, these exact signals were ignored for determination of the highest signals for the next 30 s.
4.4. Data Analysis
Raw data were analyzed using Proteome Discoverer 2.0 (Thermo Scientific). All 10 gel slices from one lane, which were measured independently, were defined as fractions of a single experiment, resulting in four meta-samples. They were searched against the reviewed human proteome (Uniprot UP000005640) with a FDR of 0.01 (strict) and 0.05 (medium) for both peptides and PSMs. At least 2 unique peptides had to be present for strict annotation. For relative quantification, number of peptide spectral matches normalized by protein length, was used as given by the softwate (#PSM). The following flexible modifications were included: carbamidomethylation at C, single oxidation at C & M, trioxidation at C & W, and nitration at Y. Carbamidomethylation was not set as a fixed modification as wound exposure to ambient conditions might result in oxidative modifications at cysteines. These modifications might not be properly reduced by DTT in the workflow. Results were tested against a mock-up database consisting of randomly generated peptides to determine the false discovery rate (FDR). Annotation procedure was attuned to keep FDR below the 0.05 (medium) and 0.01 (strict) thresholds. Additional information were retrieved using free online PANTHER analysis tools (Figure 1).
4.5. Analysis of Manually Collected Wound Material
To process cell pellets, the fixative was washed off, and cell were stained either with anti-myeloperoxidase (MPO) PE antibodies and 4′,6-diamidin-2-phenylindol (DAPI) to delineate DNA-MPO aggregates, or with antibodies targeted against anti-CD45 PE-Cy7, CD66b PerCP-Cy5.5, and CD16 PE-Dazzle (all BioLegend, San Diego, CA, USA). Cells were washed, and analyzed by multicolor flow cytometry (Beckman-Coulter, Brea, CA, USA). Supernatants obtained after centrifugation of swap material dissolved in PBS were investigated in several ways. To analyze microparticle and their origin, supernatant was centrifuged at 14,000× g, washed, stained with anti-CD15 APC (BioLegend) and bio-maleimide (BODIPY FL N-(2-Aminoethyl; Life Technologies, Carlsbad, CA, USA), washed, and analyzed by flow cytometry (Beckman-Coulter). Beads were utilized to set up the cytometer and to retrieve maximum sensitivity triggering over the side scatter as previously described [97]. Flow analysis was performed with Kaluza software 1.5a (Beckman-Coulter). Graphs were made using prism 7.03 (Graphpad software). Sytox green (Thermo Scientific) was added supernatants, and the DNA concentration was quantified against a DNA standard curve (Thermo Scientific). Fluorescence was read using a microplate reader (Tecan, Männedorf, Switzerland) at λex 485 nm and λem 535 nm. In a similar fashion, fluorescence of H2-DCF (Sigma, Taufkirchen, Germany) added the samples was determined to investigate presence of oxidants and oxidase activity.
Acknowledgments
The authors acknowledge funding by the German Federal Ministry of Education and Research (BMBF, grant numbers 03Z22DN11 and 03Z22DN12).
Appendix A
Table A1.
Apolipoproteins, glycoproteins, and protease inhibitors in wound fluids.
| Acronym | Protein Name | Acronym |
|---|---|---|
| P08519 | LPA | Apolipoprotein(a) |
| P02647 | APOA1 | Apolipoprotein A-I |
| P02652 | APOA2 | Apolipoprotein A-II |
| P02656 | APOC3 | Apolipoprotein C-III |
| P06727 | APOA4 | Apolipoprotein A-IV |
| P04114 | APOB | Apolipoprotein B-100 |
| P05090 | APOD | Apolipoprotein D |
| P02649 | APOE | Apolipoprotein E |
| O14791-3 | APOL1 | Isoform 3 of Apolipoprotein L1 |
| P04217 | A1BG | Alpha-1B-glycoprotein |
| P04217-2 | A1BG-2 | Isoform 2 of Alpha-1B-glycoprotein |
| P02749 | APOH | Beta-2-glycoprotein 1 |
| P13987 | CD59 | CD59 glycoprotein |
| P04196 | HRG | Histidine-rich glycoprotein |
| P11279 | LAMP1 | Lysosome-associated membrane glycoprotein |
| P02750 | LRG1 | Leucine-rich α-2-glycoprotein |
| P02763 | ORM1 | Alpha-1-acid glycoprotein |
| P02765 | ORM2 | Alpha-2-acid glycoprotein |
| P25311 | AZGP1 | Zinc-α-2-glycoprotein |
| P01009 | SERPINA1 | Alpha-1-antitrypsin |
| P01009-2 | SERPINA1-2 | Isoform 2 of α-1-antitrypsin |
| P01009-3 | SERPINA1-3 | Isoform 3 of α-1-antitrypsin |
| P01011 | SERPINA3 | Alpha-1-antichymotrypsin |
| P01011-2 | SERPINA3-2 | Isoform 2 of α-1-antichymotrypsin |
| P05154 | SERPINA5 | Plasma serine protease inhibitor |
| P08185 | SERPINA6 | Corticosteroid-binding globulin |
| P35237 | SERPB6 | Serpin B6 |
| P05546 | SERPIND1 | Heparin cofactor 2 |
| P08670 | SERPINF1 | Pigment epithelium-derived factor |
| P08697 | SERPINF2 | Alpha-2-antiplasmin |
| P08697-2 | SERPINF2-2 | Isoform 2 of α-2-antiplasmin |
| P00734 | F2 | Prothrombin |
| P01008 | SERPINC3 | Antithrombin-III |
Table A2.
Complement components in wound fluids.
| Acronym | Protein Name | Acronym |
|---|---|---|
| P01024 | C3 | Complement C3 |
| P0C0L5 | C4B | Complement C4-B |
| P01031 | C5 | Complement C5 |
| P13671 | C6 | Complement component C6 |
| P07360 | C8G | Complement component C8 γ chain |
| P02748 | C9 | Complement component C9 |
| P00751 | CFB | Complement factor B |
| P00751-2 | CFB | Isoform 2 of Complement factor B |
| P08603 | CFH | Complement factor H |
| P36980 | CFHR2 | Complement factor H-related protein 2 |
| P05156 | CFI | Complement factor I |
| Q9NZP8 | C1RL | Complement C1r subcomponent-like protein |
| P02746 | C1QB | Complement C1q subcomponent subunit B |
| P02747 | C1QC | Complement C1q subcomponent subunit C |
| P09871 | C1S | Complement C1s subcomponent |
Table A3.
PTMs found in wound fluids (high confidence hits only).
| Protein ID | Acronym | Protein Name | PTMs a |
|---|---|---|---|
| P01834 | IGKC | Immunoglobulin kappa constant | Trioxidation [C87] |
| P15814 | IGLL1 | Immunoglobulin lambda-like polypeptide 1 | Trioxidation [C194]; Oxidation [M197] |
| Q04695 | KRT17 | Keratin, type I cytoskeletal 17 | Oxidation [M88] |
| P30043 | BLVRB | Flavin reductase (NADPH) | Oxidation [M87]; |
| P02549 | SPTA1 | Spectrin α chain, erythrocytic 1 | Oxidation [M647; M881] |
| P02549-2 | SPTA1 | Isoform 2 of Spectrin α chain, erythrocytic | Oxidation [M647; M881] |
| P11142 | HSPA8 | Heat shock cognate 71 kDa protein | Oxidation [M61] |
| P0DMV9 | HSPA1B | Heat shock 70 kDa protein 1B | Oxidation [M549] |
| P0DMV8-2 | HSPA1A | Isoform 2 of Heat shock 70 kDa protein 1A | Oxidation [M494] |
| Q14624 | ITIH4 | Inter-α-trypsin inhibitor heavy chain H4 | Oxidation [M491] |
| Q562R1 | ACTBL2 | Beta-actin-like protein 2 | Oxidation [M45; M48; M191]; |
| P26038 | MSN | Moesin | Oxidation [M433; M451] |
| P12429 | ANXA3 | Annexin A3 | Oxidation [M40] |
| P01871 | IGHM | Immunoglobulin heavy constant mu | Oxidation [M383]; |
| P01871-2 | IGHM | Isoform 2 of Immunoglobulin heavy constant mu | Oxidation [M383]; |
| P04004 | VTN | Vitronectin | Oxidation [M350]; |
| P35542 | SAA4 | Serum amyloid A-4 protein | Oxidation [M35] |
| P30101 | PDIA3 | Protein disulfide-isomerase A3 | Oxidation [M338] |
| P06727 | APOA4 | Apolipoprotein A-IV | Oxidation [M322] |
| P30740 | SERPINB1 | Leukocyte elastase inhibitor | Oxidation [M307] |
| P04114 | APOB | Apolipoprotein B-100 | Oxidation [M306; M3007; M3421] |
| P04264 | KRT1 | Keratin, type II cytoskeletal 1 | Oxidation [M296]; |
| P35237 | SERPINB6 | Serpin B6 | Oxidation [M291] |
| P40121 | CAPG | Macrophage-capping protein | Oxidation [M261] |
| P02671 | FGA | Fibrinogen α chain | Oxidation [M259] |
| P02671-2 | FGA | Isoform 2 of Fibrinogen α chain | Oxidation [M259] |
| P40121-2 | CAPG | Isoform 2 of Macrophage-capping protein | Oxidation [M246] |
| P61981 | YWHAG | 14-3-3 protein γ | Oxidation [M23; M27] |
| P02790 | HPX | Hemopexin | Oxidation [M229; M375; M409]; |
| P47756-2 | CAPZB | Isoform 2 of F-actin-capping protein subunit β | Oxidation [M220] |
| Q06830 | PRDX1 | Peroxiredoxin-1 | Oxidation [M21] |
| P27105 | STOM | Erythrocyte band 7 integral membrane protein | Oxidation [M207; M228] |
| O15145 | ARPC3 | Actin-related protein 2/3 complex subunit 3 | Oxidation [M19] |
| P01860 | IGHG3 | Immunoglobulin heavy constant γ 3 | Oxidation [M182; M288]; Trioxidation [C27; C297]; |
| P09525 | ANXA4 | Annexin A4 | Oxidation [M17] |
| P19823 | ITIH2 | Inter-α-trypsin inhibitor heavy chain H2 | Oxidation [M162] |
| P35527 | KRT9 | Keratin, type I cytoskeletal 9 | Oxidation [M157; M234; M245; M269] |
| P08670 | VIM | Vimentin | Oxidation [M154] |
| P13645 | KRT10 | Keratin, type I cytoskeletal 10 | Oxidation [M150] |
| P00915 | CA1 | Carbonic anhydrase 1 | Oxidation [M149; M242] |
| P01857 | IGHG1 | Immunoglobulin heavy constant γ 1 | Oxidation [M135]; Trioxidation [C27; C250]; Nitro [Y161] |
| P01861 | IGHG4 | Immunoglobulin heavy constant γ 4 | Oxidation [M132]; Trioxidation [C247]; |
| P01859 | IGHG2 | Immunoglobulin heavy constant γ 2 | Oxidation [M131; M237]; Trioxidation [C246]; |
| P02787 | TF | Serotransferrin | Oxidation [M128; M332; C374]; Trioxidation [C374] |
| P08779 | KRT16 | Keratin, type I cytoskeletal 16 | Oxidation [M121]; |
| P02533 | KRT14 | Keratin, type I cytoskeletal 14 | Oxidation [M119]; |
| P02647 | APOA1 | Apolipoprotein A-I | Oxidation [M110; M136; M172] |
| P37837 | TALDO1 | Transaldolase | Oxidation [M11] |
| P02679-2 | FGG | Isoform γ-A of Fibrinogen γ chain | Oxidation [M104; M290]; |
| P02679 | FGG | Fibrinogen γ chain | Oxidation [M104; M290]; |
| P02792 | FTL | Ferritin light chain | Oxidation [M101] |
| A0M8Q6 | IGLC7 | Immunoglobulin lambda constant 7 | Oxidation [C87]; Nitrosyl [C87]; Nitro [Y85] |
| A0A0C4DH72 | IGKV1 | Immunoglobulin kappa variable 1–6 | Oxidation [C22; M26] |
| B9A064 | IGLL5 | Immunoglobulin lambda-like polypeptide 5 | Oxidation [C195]; Trioxidation [C195] |
| P00450 | CP | Ceruloplasmin | Nitro [Y471]; Oxidation [M538] |
| P68871 | HBB | Hemoglobin subunit β | Nitro [Y36]; Oxidation [M56]; |
| P02042 | HBD | Hemoglobin subunit δ | Nitro [Y36] |
| P00738 | HP | Haptoglobin | Nitro [Y280]; Oxidation [M263; M300; M343] |
| P69905 | HBA1 | Hemoglobin subunit α | Nitro [Y25]; Oxidation [M33] |
| P00738-2 | HP | Isoform 2 of Haptoglobin | Nitro [Y221]; Oxidation [M204; M241; M284] |
| P01009 | SERPINA1 | Alpha-1-antitrypsin | Nitro [Y184]; Oxidation [M409] |
| P01009-3 | SERPINA1 | Isoform 3 of Alpha-1-antitrypsin | Nitro [Y184] |
| P01009-2 | SERPINA1 | Isoform 2 of Alpha-1-antitrypsin | Nitro [Y184] |
| P49913 | CAMP | Cathelicidin antimicrobial peptide | Nitro [Y56]; Oxidation [M109] |
| P02768 | ALB | Serum albumin | Oxidation [M111; C125; C289; M353; C500; C501; M572]; Trioxidation [C125; C192; C224; C289; C302; C303; C500; C501; C591]; Nitro [Y164; Y287]; Nitrosyl [C289] |
| P02768-3 | ALB | Isoform 3 of Serum albumin | Oxidation [M111; C125; C287; C288; M359]; Trioxidation [C125; C287; C288; C378] |
| P02768-2 | ALB | Isoform 2 of Serum albumin | Oxidation [C97; M161; C308; C309; M380]; Trioxidation [C97; C110; C111; C308; C309; C399]; Nitro [Y95]; Nitrosyl [C97] |
| P0C0L5 | C4B | Complement C4-B | Oxidation [M393] |
| P01023 | A2M | Alpha-2-macroglobulin | Oxidation [M713; M1378] |
| P01024 | C3 | Complement C3 | Oxidation [M164; M495; M581; M990; M1347; M1384] |
| P08603 | CFH | Complement factor H | Oxidation [M1174] |
| P02788 | LTF | Lactotransferrin | Oxidation [C28; M411]; Trioxidation [C28] |
| P02675 | FGB | Fibrinogen β chain | Oxidation [M254; M272] |
| P02788-2 | LTF | Isoform δLf of Lactotransferrin | Oxidation [M367] |
| P02763 | ORM1 | Alpha-1-acid glycoprotein 1 | Nitro [Y175] |
| P04406 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Oxidation [M328; M331] |
| P07738 | BPGM | Bisphosphoglycerate mutase | Oxidation [M103] |
| P13796 | LCP1 | Plastin-2 | Oxidation [M166] |
| P04406-2 | GAPDH | Isoform 2 of Glyceraldehyde-3-phosphate dehydrogenase | Oxidation [M286; M289] |
| P07738 | BPGM | Bisphosphoglycerate mutase | Oxidation [M103] |
| P13796 | LCP1 | Plastin-2 | Oxidation [M166] |
a Carbamidomethylations were excluded due to their introduction during sample preparation and their ubiquitous presence.
Author Contributions
Sander Bekeschus and Kristian Wende conceived and designed the experiments; Sander Bekeschus and Jan-Wilm Lackmann performed the experiments; Sander Bekeschus and Jan-Wilm Lackmann analyzed the data; Denis Gümbel contributed reagents/materials/analysis tools; Sander Bekeschus, Jan-Wilm Lackmann, Anke Schmidt, and Kristian Wende wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Broughton G., II, Janis J.E., Attinger C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006;117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 2.Hunt T.K., Hopf H., Hussain Z. Physiology of wound healing. Adv. Skin Wound Care. 2000;13:6–11. doi: 10.1016/S0196-0644(88)80351-2. [DOI] [PubMed] [Google Scholar]
- 3.Gillitzer R., Goebeler M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 4.Behm B., Babilas P., Landthaler M., Schreml S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012;26:812–820. doi: 10.1111/j.1468-3083.2011.04415.x. [DOI] [PubMed] [Google Scholar]
- 5.Okizaki S., Ito Y., Hosono K., Oba K., Ohkubo H., Amano H., Shichiri M., Majima M. Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed. Pharmacother. 2015;70:317–325. doi: 10.1016/j.biopha.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Werner S., Krieg T., Smola H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 7.Heyer K., Herberger K., Protz K., Glaeske G., Augustin M. Epidemiology of chronic wounds in Germany: Analysis of statutory health insurance data. Wound Repair Regen. 2016;24:434–442. doi: 10.1111/wrr.12387. [DOI] [PubMed] [Google Scholar]
- 8.Spear M. Acute or chronic? What’s the difference? Plast. Surg. Nurs. 2013;33:98–100. doi: 10.1097/PSN.0b013e3182965e94. [DOI] [PubMed] [Google Scholar]
- 9.Menke N.B., Ward K.R., Witten T.M., Bonchev D.G., Diegelmann R.F. Impaired wound healing. Clin. Dermatol. 2007;25:19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Bowler P.G. Wound pathophysiology, infection and therapeutic options. Ann. Med. 2002;34:419–427. doi: 10.1080/078538902321012360. [DOI] [PubMed] [Google Scholar]
- 11.Eming S.A., Krieg T., Davidson J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 12.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 13.Frank D.N., Wysocki A., Specht-Glick D.D., Rooney A., Feldman R.A., St Amand A.L., Pace N.R., Trent J.D. Microbial diversity in chronic open wounds. Wound Repair Regen. 2009;17:163–172. doi: 10.1111/j.1524-475X.2009.00472.x. [DOI] [PubMed] [Google Scholar]
- 14.Larson B.J., Longaker M.T., Lorenz H.P. Scarless fetal wound healing: A basic science review. Plast. Reconstr. Surg. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yager D.R., Kulina R.A., Gilman L.A. Wound fluids: A window into the wound environment? Int. J. Lower Extrem. Wounds. 2007;6:262–272. doi: 10.1177/1534734607307035. [DOI] [PubMed] [Google Scholar]
- 16.Diegelmann R.F. Excessive neutrophils characterize chronic pressure ulcers. Wound Repair Regen. 2003;11:490–495. doi: 10.1046/j.1524-475X.2003.11617.x. [DOI] [PubMed] [Google Scholar]
- 17.Amulic B., Cazalet C., Hayes G.L., Metzler K.D., Zychlinsky A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 18.Korkmaz B., Moreau T., Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: Physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90:227–242. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 20.Kambas K., Chrysanthopoulou A., Vassilopoulos D., Apostolidou E., Skendros P., Girod A., Arelaki S., Froudarakis M., Nakopoulou L., Giatromanolaki A. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann. Rheum. Dis. 2014;73:1854–1863. doi: 10.1136/annrheumdis-2013-203430. [DOI] [PubMed] [Google Scholar]
- 21.Dalli J., Montero-Melendez T., Norling L.V., Yin X., Hinds C., Haskard D., Mayr M., Perretti M. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol. Cell. Proteom. 2013;12:2205–2219. doi: 10.1074/mcp.M113.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Benna J., Dang P.M., Gougerot-Pocidalo M.A. Priming of the neutrophil NADPH oxidase activation: Role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin. Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 23.Winterbourn C.C., Kettle A.J., Hampton M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016;85:765–792. doi: 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 24.Roy S., Khanna S., Nallu K., Hunt T.K., Sen C.K. Dermal wound healing is subject to redox control. Mol. Ther. 2006;13:211–220. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niethammer P., Grabher C., Look A.T., Mitchison T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sen C.K. The general case for redox control of wound repair. Wound Repair Regen. 2003;11:431–438. doi: 10.1046/j.1524-475X.2003.11607.x. [DOI] [PubMed] [Google Scholar]
- 27.Eming S.A., Koch M., Krieger A., Brachvogel B., Kreft S., Bruckner-Tuderman L., Krieg T., Shannon J.D., Fox J.W. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J. Proteome Res. 2010;9:4758–4766. doi: 10.1021/pr100456d. [DOI] [PubMed] [Google Scholar]
- 28.Ma L., Li P., Shi Z., Hou T., Chen X., Du J. A prospective, randomized, controlled study of hyperbaric oxygen therapy: Effects on healing and oxidative stress of ulcer tissue in patients with a diabetic foot ulcer. Ostomy Wound Manag. 2013;59:18–24. [PubMed] [Google Scholar]
- 29.Kwon J., Wang A., Burke D.J., Boudreau H.E., Lekstrom K.J., Korzeniowska A., Sugamata R., Kim Y.S., Yi L., Ersoy I., et al. Peroxiredoxin 6 (Prdx6) supports NADPH oxidase1 (Nox1)-based superoxide generation and cell migration. Free Radic. Biol. Med. 2016;96:99–115. doi: 10.1016/j.freeradbiomed.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soubhye J., Aldib I., Delporte C., Prévost M., Dufrasne F., Van Antwerpen P. Myeloperoxidase as a target for the treatment of inflammatory syndromes: Mechanisms and structure activity relationships of inhibitors. Curr. Med. Chem. 2016;23:3975–4008. doi: 10.2174/0929867323666160607111806. [DOI] [PubMed] [Google Scholar]
- 31.Tomson F.L., Morgan J.E., Gu G., Barquera B., Vygodina T.V., Gennis R.B. Substitutions for glutamate 101 in subunit II of cytochrome c oxidase from Rhodobacter sphaeroides result in blocking the proton-conducting K-channel. Biochemistry. 2003;42:1711–1717. doi: 10.1021/bi026750y. [DOI] [PubMed] [Google Scholar]
- 32.Wilgus T.A., Bergdall V.K., Tober K.L., Hill K.J., Mitra S., Flavahan N.A., Oberyszyn T.M. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am. J. Pathol. 2004;165:753–761. doi: 10.1016/S0002-9440(10)63338-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato G.J., McGowan V., Machado R.F., Little J.A., Taylor J.T., Morris C.R., Nichols J.S., Wang X., Poljakovic M., Morris S.M., Jr., et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellaye P.S., Burgy O., Causse S., Garrido C., Bonniaud P. Heat shock proteins in fibrosis and wound healing: Good or evil? Pharmacol. Ther. 2014;143:119–132. doi: 10.1016/j.pharmthera.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 36.Revollo J.R., Korner A., Mills K.F., Satoh A., Wang T., Garten A., Dasgupta B., Sasaki Y., Wolberger C., Townsend R.R., et al. Nampt/PBEF/Visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridley A.J. Rho family proteins: Coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/S0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- 38.Watt F.M., Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011;3:a005124. doi: 10.1101/cshperspect.a005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung E.Y., Weijers E.M., Tuk B., Scheffer R., Leebeek F.W., van Neck J.W., Koolwijk P., de Maat M.P. Specific effects of fibrinogen and the γA and γ’-chain fibrinogen variants on angiogenesis and wound healing. Tissue Eng. Part A. 2015;21:106–114. doi: 10.1089/ten.tea.2014.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klaas M., Kangur T., Viil J., Maemets-Allas K., Minajeva A., Vadi K., Antsov M., Lapidus N., Jarvekulg M., Jaks V. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci. Rep. 2016;6:27398. doi: 10.1038/srep27398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pozuelo Rubio M., Geraghty K.M., Wong B.H., Wood N.T., Campbell D.G., Morrice N., Mackintosh C. 14-3-3-affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and trafficking. Biochem. J. 2004;379:395–408. doi: 10.1042/bj20031797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng L., Mohan R., Kwok B.H., Elofsson M., Sin N., Crews C.M. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc. Natl. Acad. Sci. USA. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luan Y., Xu W. The structure and main functions of aminopeptidase N. Curr. Med. Chem. 2007;14:639–647. doi: 10.2174/092986707780059571. [DOI] [PubMed] [Google Scholar]
- 44.Pulakazhi Venu V.K., Uboldi P., Dhyani A., Patrini A., Baetta R., Ferri N., Corsini A., Muro A.F., Catapano A.L., Norata G.D. Fibronectin extra domain A stabilises atherosclerotic plaques in apolipoprotein E and in LDL-receptor-deficient mice. Thromb. Haemost. 2015;114:186–197. doi: 10.1160/TH14-09-0790. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka R., Ichioka S., Sekiya N., Ohura N., Uchino S., Ojima A., Itoh Y., Ishihara O., Nakatsuka T., Ikebuchi K. Elastic plasma protein film blended with platelet releasate accelerates healing of diabetic mouse skin wounds. Vox Sang. 2007;93:49–56. doi: 10.1111/j.1423-0410.2007.00913.x. [DOI] [PubMed] [Google Scholar]
- 46.Herring J.M., McMichael M.A., Smith S.A. Microparticles in health and disease. J. Vet. Intern. Med. 2013;27:1020–1033. doi: 10.1111/jvim.12128. [DOI] [PubMed] [Google Scholar]
- 47.Hsiao C.Y., Tsai T.H., Chak K.F. The molecular basis of wound healing processes induced by lithospermi radix: A proteomics and biochemical analysis. Evid. Based Complement. Alternat. Med. 2012;2012:508972. doi: 10.1155/2012/508972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalkhof S., Forster Y., Schmidt J., Schulz M.C., Baumann S., Weissflog A., Gao W., Hempel U., Eckelt U., Rammelt S., et al. Proteomics and metabolomics for in situ monitoring of wound healing. Biomed. Res. Int. 2014;2014:934848. doi: 10.1155/2014/934848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsiao C.Y., Hung C.Y., Tsai T.H., Chak K.F. A Study of the Wound Healing Mechanism of a Traditional Chinese Medicine, Angelica sinensis, Using a Proteomic Approach. Evid. Based Complement. Alternat. Med. 2012;2012:467531. doi: 10.1155/2012/467531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinstrasser L., Jacobsen F., Hirsch T., Kesting M., Chojnacki C., Krisp C., Wolters D. Immunodepletion of high-abundant proteins from acute and chronic wound fluids to elucidate low-abundant regulators in wound healing. BMC Res. Notes. 2010;3:335. doi: 10.1186/1756-0500-3-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sen C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009;17:1–18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo B., Wang J., Liu Z., Shen Z., Shi R., Liu Y.Q., Liu Y., Jiang M., Wu Y., Zhang Z. Phagocyte respiratory burst activates macrophage erythropoietin signalling to promote acute inflammation resolution. Nat. Commun. 2016;7:12177. doi: 10.1038/ncomms12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hüttemann M., Lee I., Grossman L.I., Doan J.W., Sanderson T.H. Mitochondrial Oxidative Phosphorylation. Springer; Berlin, Germany: 2012. Phosphorylation of mammalian cytochrome c and cytochrome c oxidase in the regulation of cell destiny: Respiration, apoptosis, and human disease; pp. 237–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 55.Winterbourn C.C., Kettle A.J. Reactions of superoxide with myeloperoxidase and its products. Jpn. J. Infect. Dis. 2004;57:S31–S33. [PubMed] [Google Scholar]
- 56.Metzler K.D., Fuchs T.A., Nauseef W.M., Reumaux D., Roesler J., Schulze I., Wahn V., Papayannopoulos V., Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bekeschus S., Winterbourn C.C., Kolata J., Masur K., Hasse S., Broker B.M., Parker H.A. Neutrophil extracellular trap formation is elicited in response to cold physical plasma. J. Leukoc. Biol. 2016;100:791–799. doi: 10.1189/jlb.3A0415-165RR. [DOI] [PubMed] [Google Scholar]
- 59.Wong S.L., Demers M., Martinod K., Gallant M., Wang Y., Goldfine A.B., Kahn C.R., Wagner D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng W., Paunel-Gorgulu A., Flohe S., Witte I., Schadel-Hopfner M., Windolf J., Logters T.T. Deoxyribonuclease is a potential counter regulator of aberrant neutrophil extracellular traps formation after major trauma. Mediat. Inflamm. 2012;2012:149560. doi: 10.1155/2012/149560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akong-Moore K., Chow O.A., von Kockritz-Blickwede M., Nizet V. Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS ONE. 2012;7:e42984. doi: 10.1371/journal.pone.0042984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munafo D.B., Johnson J.L., Brzezinska A.A., Ellis B.A., Wood M.R., Catz S.D. DNase I inhibits a late phase of reactive oxygen species production in neutrophils. J. Innate Immun. 2009;1:527–542. doi: 10.1159/000235860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rada B., Jendrysik M.A., Pang L., Hayes C.P., Yoo D.G., Park J.J., Moskowitz S.M., Malech H.L., Leto T.L. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PLoS ONE. 2013;8:e54205. doi: 10.1371/journal.pone.0054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sen C.K., Roy S. Redox signals in wound healing. Biochim. Biophys. Acta. 2008;1780:1348–1361. doi: 10.1016/j.bbagen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wood Z.A., Schroder E., Robin Harris J., Poole L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28:32–40. doi: 10.1016/S0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 66.Serhan C.N., Chiang N., Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordts S.C., Muthuramu I., Amin R., Jacobs F., de Geest B. The impact of lipoproteins on wound healing: Topical HDL therapy corrects delayed wound healing in apolipoprotein e deficient mice. Pharmaceuticals. 2014;7:419–432. doi: 10.3390/ph7040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu D., Liu L., Song Z., Hu Z.Y., Liu J., Hou D.R. Genetic variations of oxidative stress related genes ALOX5, ALOX5AP and MPO modulate ischemic stroke susceptibility through main effects and epistatic interactions in a Chinese population. Cell. Physiol. Biochem. 2017;43:1588–1602. doi: 10.1159/000482023. [DOI] [PubMed] [Google Scholar]
- 69.Barrientos S., Stojadinovic O., Golinko M.S., Brem H., Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 70.Galkowska H., Wojewodzka U., Olszewski W.L. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006;14:558–565. doi: 10.1111/j.1743-6109.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 71.Barrientos S., Brem H., Stojadinovic O., Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569–578. doi: 10.1111/wrr.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bekeschus S., Schmidt A., Napp M., Kramer A., Kerner W., von Woedtke T., Wende K., Hasse S., Masur K. Distinct cytokine and chemokine patterns in chronic diabetic ulcers and acute wounds. Exp. Dermatol. 2017;26:145–147. doi: 10.1111/exd.13215. [DOI] [PubMed] [Google Scholar]
- 73.Segal A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cole A.M., Shi J., Ceccarelli A., Kim Y.H., Park A., Ganz T. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood. 2001;97:297–304. doi: 10.1182/blood.V97.1.297. [DOI] [PubMed] [Google Scholar]
- 75.Odell E.W., Sarra R., Foxworthy M., Chapple D.S., Evans R.W. Antibacterial activity of peptides homologous to a loop region in human lactoferrin. FEBS Lett. 1996;382:175–178. doi: 10.1016/0014-5793(96)00168-8. [DOI] [PubMed] [Google Scholar]
- 76.Benarafa C., Priebe G.P., Remold-O’Donnell E. The neutrophil serine protease inhibitor serpinb1 preserves lung defense functions in Pseudomonas aeruginosa infection. J. Exp. Med. 2007;204:1901–1909. doi: 10.1084/jem.20070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sulniute R., Shen Y., Guo Y.Z., Fallah M., Ahlskog N., Ny L., Rakhimova O., Broden J., Boija H., Moghaddam A., et al. Plasminogen is a critical regulator of cutaneous wound healing. Thromb. Haemost. 2016;115:1001–1009. doi: 10.1160/TH15-08-0653. [DOI] [PubMed] [Google Scholar]
- 78.Herren T., Burke T.A., Jardi M., Felez J., Plow E.F. Regulation of plasminogen binding to neutrophils. Blood. 2001;97:1070–1078. doi: 10.1182/blood.V97.4.1070. [DOI] [PubMed] [Google Scholar]
- 79.Dentener M.A., Francot G.J.M., Smit F.T., Froon A.H.M., Pennings H.J., Wouters E.F.M., Buurman W.A. Presence of bactericidal/permeability-increasing protein in disease: Detection by elisa. J. Infect. Dis. 1995;171:739–743. doi: 10.1093/infdis/171.3.739. [DOI] [PubMed] [Google Scholar]
- 80.Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J. Biol. Chem. 1978;253:2664–2672. [PubMed] [Google Scholar]
- 81.Xue M., Jackson C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care. 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Boer J., Creasey A., Chang A., Abbink J., Roem D., Eerenberg A., Hack C., Taylor F. Alpha-2-macroglobulin functions as an inhibitor of fibrinolytic, clotting, and neutrophilic proteinases in sepsis: Studies using a baboon model. Infect. Immun. 1993;61:5035–5043. doi: 10.1128/iai.61.12.5035-5043.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguyen T.N., Uemura A., Shih W., Yamada S. Zyxin-mediated actin assembly is required for efficient wound closure. J. Biol. Chem. 2010;285:35439–35445. doi: 10.1074/jbc.M110.119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghiran I., Klickstein L.B., Nicholson-Weller A. Calreticulin is at the surface of circulating neutrophils and uses CD59 as an adaptor molecule. J. Biol. Chem. 2003;278:21024–21031. doi: 10.1074/jbc.M302306200. [DOI] [PubMed] [Google Scholar]
- 85.Lu Q., Xin Y., Ye F., Foulks G., Li Q. 14-3-3sigma controls corneal epithelium homeostasis and wound healing. Investig. Ophthalmol. Vis. Sci. 2011;52:2389–2396. doi: 10.1167/iovs.09-4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hansraj N.Z., Xiao L., Wu J., Chen G., Turner D.J., Wang J.Y., Rao J.N. Posttranscriptional regulation of 14-3-3zeta by RNA-binding protein HuR modulating intestinal epithelial restitution after wounding. Physiol. Rep. 2016;4:e12858. doi: 10.14814/phy2.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donato R., Cannon B.R., Sorci G., Riuzzi F., Hsu K., Weber D.J., Geczy C.L. Functions of S100 proteins. Curr. Mol. Med. 2013;13:24–57. doi: 10.2174/156652413804486214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lerchenmueller C., Rengo G., Katus H.A., Koch W.J., Peppel K., Most P. S100A1 deficiency impairs post-ischemic angiogenesis via compromised proangiogenic endothelial cell function and nitric oxide synthase regulation. Circulation. 2012;126 doi: 10.1161/CIRCRESAHA.112.275156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hsu K., Champaiboon C., Guenther B.D., Sorenson B.S., Khammanivong A., Ross K.F., Geczy C.L., Herzberg M.C. Anti-infective protective properties of S100 calgranulins. Antiinflamm. Antiallergy Agents Med. Chem. 2009;8:290–305. doi: 10.2174/187152309789838975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du M., Wang G., Ismail T.M., Gross S., Fernig D.G., Barraclough R., Rudland P.S. S100P dissociates myosin IIA filaments and focal adhesion sites to reduce cell adhesion and enhance cell migration. J. Biol. Chem. 2012;287:15330–15344. doi: 10.1074/jbc.M112.349787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sakaguchi M., Sonegawa H., Murata H., Kitazoe M., Futami J., Kataoka K., Yamada H., Huh N.H. S100A11, an dual mediator for growth regulation of human keratinocytes. Mol. Biol. Cell. 2008;19:78–85. doi: 10.1091/mbc.E07-07-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt A., von Woedtke T., Bekeschus S. Periodic exposure of keratinocytes to cold physical plasma: an in vitro model for redox-related diseases of the skin. Oxid. Med. Cell. Longev. 2016;2016:9816072. doi: 10.1155/2016/9816072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Genderen H.O., Kenis H., Hofstra L., Narula J., Reutelingsperger C.P. Extracellular annexin A5: Functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim. Biophys. Acta. 2008;1783:953–963. doi: 10.1016/j.bbamcr.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 94.Gerke V., Creutz C.E., Moss S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 95.Gasser O. Ph.D. Thesis. University of Basel; Basel, Switzerland: 2004. Microparticles Released by Ectocytosis from Human Neutrophils: Characterisation, Properties and Functions. [Google Scholar]
- 96.Gasser O., Hess C., Miot S., Deon C., Sanchez J.C., Schifferli J.A. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 2003;285:243–257. doi: 10.1016/S0014-4827(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 97.Bekeschus S., Moritz J., Schmidt A., Wende K. Redox regulation of leukocyte-derived microparticle release and protein content in response to cold physical plasma-derived oxidants. Clin. Plasma Med. 2017;7–8:24–35. doi: 10.1016/j.cpme.2017.07.001. [DOI] [Google Scholar]
- 98.Trushin S.A., Pennington K.N., Algeciras-Schimnich A., Paya C.V. Protein Kinase C and Calcineurin Synergize to Activate IκB Kinase and NF-κB in T Lymphocytes. J. Biol. Chem. 1999;274:22923–22931. doi: 10.1074/jbc.274.33.22923. [DOI] [PubMed] [Google Scholar]
- 99.Yoo S.K., Starnes T.W., Deng Q., Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng Y., Santoriello C., Mione M., Hurlstone A., Martin P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: Parallels between tumor initiation and wound inflammation. PLoS Biol. 2010;8:e1000562. doi: 10.1371/journal.pbio.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moreira S., Stramer B., Evans I., Wood W., Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol. 2010;20:464–470. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 102.Shigenaga M.K., Lee H.H., Blount B.C., Christen S., Shigeno E.T., Yip H., Ames B.N. Inflammation and NOx-induced nitration: Assay for 3-nitrotyrosine by HPLC with electrochemical detection. Proc. Natl. Acad. Sci. USA. 1997;94:3211–3216. doi: 10.1073/pnas.94.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Birnboim H.C., Lemay A.M., Lam D.K.Y., Goldstein R., Webb J.R. Cutting edge: MHC class II-restricted peptides containing the inflammation-associated marker 3-nitrotyrosine evade central tolerance and elicit a robust cell-mediated immune response. J. Immunol. 2014;171:528–532. doi: 10.4049/jimmunol.171.2.528. [DOI] [PubMed] [Google Scholar]
- 104.Nakazawa H., Fukuyama N., Takizawa S., Tsuji C., Yoshitake M., Ishida H. Nitrotyrosine formation and its role in various pathological conditions. Free Radic. Res. 2000;33:771–784. doi: 10.1080/10715760000301291. [DOI] [PubMed] [Google Scholar]
- 105.Hernansanz-Agustin P., Izquierdo-Alvarez A., Garcia-Ortiz A., Ibiza S., Serrador J.M., Martinez-Ruiz A. Nitrosothiols in the immune system: Signaling and protection. Antioxid. Redox Signal. 2013;18:288–308. doi: 10.1089/ars.2012.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gostner J.M., Becker K., Fuchs D., Sucher R. Redox regulation of the immune response. Redox Rep. 2013;18:88–94. doi: 10.1179/1351000213Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krausz A., Friedman A.J. Nitric oxide as a surgical adjuvant. Future Sci. 2015;1 doi: 10.4155/fso.15.56. [DOI] [PMC free article] [PubMed] [Google Scholar]